Abstract
Aim
The identification of 7,8‐dihydroxyflavone (DHF) as a small molecule agonist for tropomyosin‐related kinase B (TrkB) facilitated understanding of the role of TrkB signaling in regulating higher brain functions. DHF can penetrate the blood‐brain barrier after systemic administration and changes the performance of cognitive and emotional behavioral tasks. However, it is poorly understood how DHF modulates neuronal functions at cellular levels. Aiming to understand the cellular basis underlying DHF‐induced modifications of the brain functions, we examined the effects of DHF on the hippocampal excitatory synaptic transmission.
Methods
Field excitatory postsynaptic potentials were recorded using hippocampal slices prepared from adult male mice. Effects of bath‐applied DHF on the synaptic efficacy were examined.
Results
We found that DHF induced robust synaptic potentiation at the mossy fiber to CA3 synapse. DHF had minimal effects at other hippocampal excitatory synapses or at immature mossy fiber synapse in juvenile mice. The TrkB receptor blockers K252a and ANA‐12 did not affect the DHF‐induced synaptic potentiation. Drug screening revealed that relatively low concentrations of 2‐aminoethoxydiphenylborane blocked the DHF‐induced synaptic potentiation.
Conclusion
Our results demonstrate that DHF selectively potentiates hippocampal mossy fiber synaptic transmission via a TrkB receptor‐independent mechanism. This novel neuromodulatory effect of DHF may influence higher brain functions by itself or together with the activation of the TrkB receptor. The rapid induction of the potentiation implies its potential importance in the acute behavioral effects of DHF.
Keywords: hippocampus, mossy fiber, synaptic modulation, TrkB, 2‐APB
We show that acute 7,8‐dihydroxyflavone (DHF) administration selectively potentiates hippocampal mossy fiber to CA3 synaptic transmission. This DHF‐induced synaptic potentiation was found to be independent of TrkB receptor activation.

1. INTRODUCTION
The brain‐derived neurotrophic factor (BDNF)‐TrkB signaling plays essential roles in the activity‐dependent regulation of developing and mature neural circuits.1 Owing to its neurotrophic and neuromodulatory action, the BDNF‐TrkB signaling pathway has been attracting particular interest as a potential target for therapeutic treatments of neurological and psychiatric disorders.2, 3 However, poor delivery of BDNF into the central nervous system has hindered the development of effective therapeutic treatments targeting this pathway. Jang et al4 have identified 7,8‐dihydroxyflavone (DHF) as a small molecule TrkB agonist that can penetrate the blood‐brain barrier. Systemic administration of DHF can activate TrkB in the brain4 and has been shown to modulate cognitive behavior: DHF can facilitate extinction of fear memory5 and rescue learning and memory impairment in stressed animals,6 aged rats,7 mouse models of Alzheimer's disease,8, 9, 10, 11 a rat model of schizophrenia,12 and a mouse model of fragile X syndrome.13 In addition, DHF has antidepressant‐like effects in mice.14, 15, 16, 17 These behavioral studies using DHF further support the importance of the TrkB signaling in regulating the higher brain functions and suggest a plausible therapeutic potential of DHF in various neuropsychiatric disorders. However, it has not been well characterized how DHF modulates physiological properties of brain neurons at the cellular or synaptic levels. Aiming to understand the cellular mechanism underlying the modification of the brain functions by DHF, in the present study, we examined the effect of DHF on the excitatory synaptic transmission using mouse hippocampal slices. Our results show that DHF has a novel neuromodulatory effect that is hardly explained by activation of the TrkB receptor.
2. METHODS
2.1. Animals
Male C57BL/6J mice were purchased from Japan SLC. Mice were housed in a group of 2‐4 mice per cage in the institutional standard condition (14:10 light/dark cycle; lights on at 6:00 am through 8:00 pm) with ad libitum access to food and water. Animal use and procedures were in accordance with the National Institute of Health guidelines and approved by the Animal Care and Use Committee of Nippon Medical School.
2.2. Electrophysiological analysis
Mice were decapitated under deep halothane anesthesia at the age of 9‐11 weeks, and both hippocampi were isolated. Transverse hippocampal slices (380 μm) were cut using a tissue slicer and maintained in a humidified interface holding chamber at room temperature before use. Electrophysiological recordings were performed as described.18, 19 Recordings were made in a submersion‐type chamber maintained at 27.0‐27.5°C and superfused at 2 mL/min with saline composed of (in mmol/L): NaCl, 125; KCl, 2.5; NaH2PO4, 1.0; NaHCO3, 26.2; glucose, 11; CaCl2, 2.5; MgCl2, 1.3 (equilibrated with 95% O2/5% CO2). In some experiments, the recording chamber was maintained at 37°C and superfused at 2.5 mL/min with the same saline. Excitatory postsynaptic potentials (EPSPs) arising from the mossy fiber (MF) synapses were evoked by stimulating the granule cell layer of the dentate gyrus (DG) and recorded from the stratum lucidum of the CA3 region using a glass pipette filled with 2 mol/L NaCl. The field EPSP amplitude was measured on analysis as described.18 A criterion used to identify the MF input was more than 85% block of EPSP by an agonist of group II metabotropic glutamate receptors, (2S,2′R,3′R)‐2‐(2′,3′‐dicarboxycyclopropyl)glycine (DCG‐IV, 1 μmol/L). Single electrical stimulation was delivered at a frequency of 0.05 Hz unless otherwise specified. For recording field EPSP at CA3 to CA1 synapse, both stimulating and recording electrodes were placed in the stratum radiatum in the CA1 region. For recording field EPSPs at the medial perforant path (MPP) to DG synapse, the stimulating and recording electrodes were placed in the middle third of the dentate molecular layer. The initial slope of field potentials was measured on analysis for CA3 to CA1 and MPP to DG synaptic responses. Triple‐pulse stimulation at an interval of 200 ms was delivered at 0.033 Hz following an experimental protocol described previously.18 All recordings were made using a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA, USA), filtered at 2 kHz, and stored in a personal computer via an interface (digitized at 10 kHz). DHF, K252a, and staurosporine were purchased from Wako Pure Chemical Industries, Ltd (Osaka, Japan). ANA‐12 was from Sigma‐Aldrich. DCG‐IV and 2‐aminoethoxydiphenylborane (2‐APB) were from Tocris Bioscience (Bristol, UK).
2.3. Statistics
All data are presented as means ± SEM. Statistical tests are performed using GraphPad Prism version 7.01. Experiments with two groups were compared with unpaired two‐tailed Student's t test unless otherwise specified in the figure legends, and experiments with more than two groups were subjected to one‐way ANOVA, followed by the Tukey's test or Dunnett's test. Statistical significance was set at P < 0.05. The number of data “n” represents the number of slices.
3. RESULTS
We first examined the effect of DHF at three major excitatory synapses in the hippocampus in adult mice. Bath‐applied DHF (2 μmol/L) induced robust synaptic potentiation at the MF‐CA3 synapse (Figure 1A,B), but had only minor effects at the CA3 to CA1 synapse and MPP to DG synapse (Figure 1C,D). Therefore, in this study, we focused on the investigation of the MF synapse. The potentiating effect of DHF was detectable at a submicromolar concentration, and the magnitude of potentiation increased in a concentration‐dependent manner with an apparent EC50 value of 2 μmol/L (Figure 2A,B). Synaptic facilitation induced by triple‐pulse stimulation was reduced by DHF (Figure 2C), suggesting that the DHF‐induced synaptic potentiation was mediated by presynaptic mechanisms. Since BDNF plays a key role in the development of neuronal circuits,1 we examined the effect of DHF at the immature MF synapse. In 2‐week‐old juvenile mice, DHF had minimal effects on the MF synaptic transmission (Figure 2D). The magnitude of potentiation sharply increased thereafter and reached the mature level at the age of 4 weeks (Figure 2D,E). These results indicate that DHF preferentially potentiates synaptic transmission at the mature MF synapse.
Figure 1.
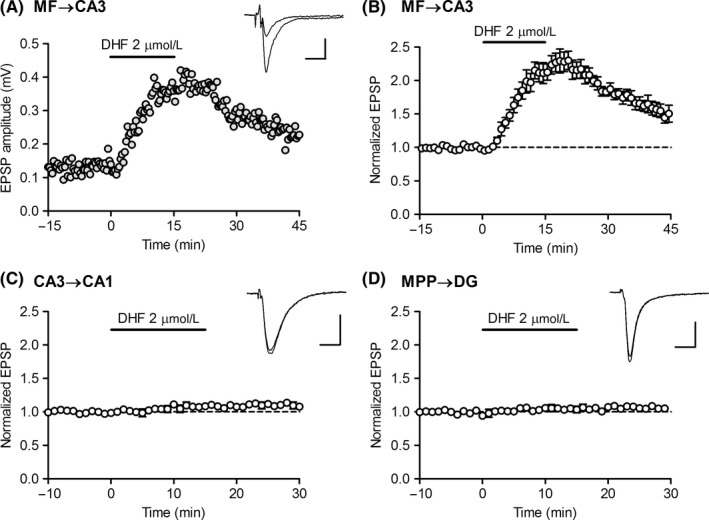
DHF preferentially potentiates mossy fiber‐CA3 synaptic transmission in the hippocampal excitatory circuit. A, DHF‐induced robust synaptic potentiation at the mossy fiber (MF) to CA3 synapse. DHF was applied in bath at the horizontal bar. Sample traces show averages of 15 consecutive EPSPs during baseline and at the peak of potentiation. Scale bar: 10 ms, 0.2 mV. B, Pooled data showing the effect of DHF at the MF‐CA3 synapse (n = 6). C, Small synaptic potentiation induced by DHF at the CA3 to CA1 synapse (n = 4). Scale bar: 10 ms, 0.3 mV. D, Small synaptic potentiation induced by DHF at the medial perforant path (MPP) to dentate gyrus (DG) synapse (n = 4). Scale bar: 10 ms, 0.3 mV
Figure 2.
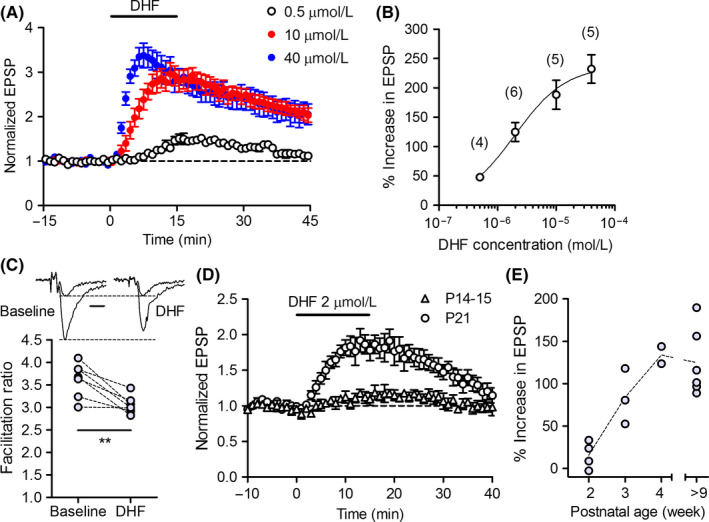
Characterization of DHF‐induced synaptic potentiation at mossy fiber synapse. A, Synaptic potentiation induced by different concentrations of DHF. B, Concentration dependence of DHF‐induced potentiation. The peak magnitude of synaptic potentiation is plotted against DHF concentrations. The number (n) of data is indicated in parenthesis in the graph. C, Reduction in synaptic facilitation by DHF. The magnitude of synaptic facilitation induced by triple‐pulse stimulation before and during DHF (2 μmol/L) application is shown (paired t test, t 6 = 4.187, **P = 0.0058, n = 7). Sample traces show the first and third responses of triple pulse‐evoked EPSPs scaled by the amplitude of the first EPSPs. Scale bar: 5 ms. D, Reduced DHF‐induced synaptic potentiation in juvenile mice. E, Dependence of the magnitude of DHF‐induced potentiation on the postnatal age of mice
Next, we examined the signaling mechanisms involved in the DHF‐induced synaptic potentiation. We first tested the effect of K252a, a commonly used inhibitor of the TrkB receptor tyrosine kinase. Unexpectedly, K252a had no significant effect on the magnitude or time course of the DHF‐induced synaptic potentiation (Figure 3A,B). Another TrkB receptor antagonist, ANA‐12, also did not affect the DHF‐induced potentiation (Figure 3C,D). In the presence of the broad‐spectrum protein kinase inhibitor staurosporine, the DHF‐induced synaptic potentiation initially looked unaffected, but was significantly reduced in magnitude at the peak and during the decay (Figure 3C,D). Although this result suggests that protein kinase activity contributes to the late phase of the DHF‐induced potentiation, the effect of staurosporine was rather small. Hence, drug screening experiments were performed to identify the DHF target essential for the induction of synaptic potentiation. We mainly tested more specific kinase inhibitors and modulators of intracellular Ca2+ signaling and found that a relatively low concentration of 2‐APB entirely suppressed the DHF‐induced synaptic potentiation (see Table S1 for other drugs tested). Bath‐applied 2‐APB at 10 μmol/L had no effect on the basal synaptic transmission (Figure 4A) (102.2 ± 4.7% of baseline, n = 5). Subsequent application of DHF caused slowly developing synaptic potentiation that was reduced in magnitude by about 80% as compared with the control slices (Figure 4A‐C). At 20 μmol/L, 2‐APB tended to increase the basal synaptic transmission (110.7 ± 19.7% of baseline, n = 5) and completely blocked the DHF‐induced potentiation (Figure 4B,C). These results indicate that 2‐APB suppresses the potentiating effect of DHF with an IC50 value less than 10 μmol/L. 2‐APB was originally identified as an antagonist of the inositol 1,4,5‐trisphosphate receptor with the IC50 value of 42 μmol/L.20 Since some of the temperature‐sensitive transient receptor potential (TRP) channels have sensitivity to 2‐APB with the affinity <10 μmol/L,21, 22, 23 we examined the dependence of DHF‐induced potentiation on the bath temperature. We usually perform electrophysiological experiments at 27.0‐27.5°C. Raising the bath temperature to 37.0°C caused a marked increase in the basal transmission efficacy (Figure 4D). At 37.0°C, the DHF‐induced synaptic potentiation was significantly reduced (Figure 4C,E). These results indicate that DHF potentiates MF synaptic transmission via a pathway that is highly sensitive to 2‐APB and temperature.
Figure 3.

Effects of protein kinase inhibitors on DHF‐induced potentiation. A, A lack of effects of K252a on synaptic potentiation induced by DHF (2 μmol/L). Slices were preincubated in the extracellular solution containing K252a (200 nmol/L) for more than 1 h and continuously perfused with the same solution during recordings. Control slices were treated in the same way with the solution containing vehicle (DMSO 0.01%). B, The peak magnitude of DHF‐induced potentiation in control and K252a‐treated slice. C, Effect of staurosporine (1 μmol/L) or ANA‐12 (10 μmol/L) on synaptic potentiation induced by DHF (2 μmol/L). Staurosporine was added in the bath 50‐60 min before DHF application. ANA‐12 was applied 25‐30 min before DHF. D, Reduction in the DHF‐induced potentiation by staurosporine (one‐way ANOVA: F(2,16) = 5.396, P = 0.0162, Dunnett's test **P = 0.0098). The number (n) of data is indicated in parenthesis in the graph
Figure 4.
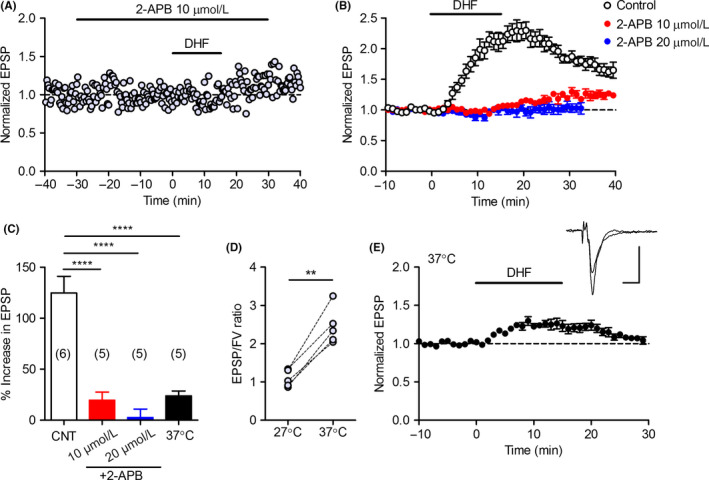
Suppression of DHF‐induced potentiation by 2‐APB and temperature elevation. A, Representative recording showing effects of 2‐APB on the basal transmission and synaptic potentiation induced by DHF (2 μmol/L). B, Block of DHF‐induced synaptic potentiation by 2‐APB. The control data are the same as those shown in Figure 1. C, Summary data showing the reduced effect of DHF (2 μmol/L) in the presence of 2‐APB and at 37°C (one‐way ANOVA: F 3,17 = 27.23, P < 0.0001; Tukey's test: ****P < 0.0001). The number (n) of data is indicated in parenthesis in the graph. D, A temperature‐dependent increase in synaptic efficacy. The ratios of EPSP to fiber volley (FV) amplitude at 27 and 37°C are shown (paired t test, t 4 = 8.388, **P = 0.0011, n = 5). E, Reduced effect of DHF at 37°C. Scale bar: 5 ms, 0.2 mV
4. DISCUSSION
In the present study, we have shown that the TrkB receptor agonist DHF preferentially potentiates hippocampal MF‐CA3 synaptic transmission in a TrkB receptor‐independent manner. In addition to the TrkB agonist action, DHF has been known to have TrkB‐independent antioxidant activity.24, 25, 26 While the robust synaptic potentiation observed here is unlikely to result from the antioxidant effect of DHF, these effects might share signaling pathways. Although the exact mechanism mediating the DHF‐induced synaptic potentiation remains unknown, it was found to be sensitive to 2‐APB below 10 μmol/L. Such a low concentration of 2‐APB can facilitate store‐operated calcium entry,27 activate TRPV3,23 and block TRPM821 and TRPM2.22 We have also found that the DHF‐induced potentiation was suppressed to about 20% in magnitude by raising bath temperature from 27 to 37°C, suggesting that a putative DHF target molecule is thermosensitive. All of the above‐mentioned TRP channels are thermosensitive, and TRPV3 could be a candidate target for DHF, because it can be activated in this temperature range.28, 29 The rise in bath temperature might partially activate TRPV3, thereby augmenting the basal transmission efficacy and occluding the effect of DHF. However, 2‐APB is an activator of TRPV3. Although 2‐APB tended to augment synaptic transmission at 20 μmol/L, it had no effect on the basal transmission at 10 μmol/L. Therefore, the apparent block of the DHF effect by 2‐APB cannot be simply explained by occlusion. Further studies with a more specific methodology would be required to reveal the signaling mechanism involved in the DHF‐induced synaptic potentiation.
The synapse‐selective effect of DHF might be ascribed to synapse‐specific localization of DHF target molecules. However, it is unknown whether the above‐mentioned putative targets are specifically expressed at the MF synapse. While TRPV3 is expressed in the hippocampus,30 its synapse‐specific localization has not been demonstrated. It should be noted that the MF synapse is characterized by prominent presynaptic facilitation and has a low probability of transmitter release.31, 32 In general, at the synapse with the low release probability like the MF synapse, any presynaptic enhancing effect can be more manifest than the synapse with the high release probability. In addition, some neurotransmitter/receptor systems have specific modulatory effects on the MF synaptic transmission, as exemplified by kainate receptor‐dependent synaptic enhancement.33 DHF may indirectly induce synaptic potentiation by activating or augmenting such neurotransmitter/receptor systems.
In many behavioral studies, DHF has been used as a specific TrkB receptor agonist and shown to modulate cognitive and emotional behavior.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 Some of these behavioral changes were correlated with altered synaptic plasticity after chronic DHF treatments.7, 10, 12 The neuromodulatory effect of DHF demonstrated in the present study is supposed to influence such behavior by itself or together with activation of the TrkB receptor. Given the rapid induction of synaptic potentiation by DHF, acute behavioral effects of DHF would be of particular interest. Systemic administration of DHF has been shown to cause acute antidepressant‐like effects.16, 17 The DG‐MF‐CA3 neuronal system has been implicated as a target for antidepressant therapy.34, 35, 36, 37 Both chemical and physical antidepressant treatments can change intrinsic physiological properties of the MF synapse19, 38 and strongly enhance synaptic potentiation caused by monoamines.19, 39, 40 Therefore, the DHF‐induced potentiation at the MF synapse may be involved in acute antidepressant‐like effects of DHF. Synaptic modifications in brain regions other than the hippocampus could also potentially contribute to acute behavioral effects of DHF after systemic administration. Indeed, acute DHF application has been shown to reduce inhibitory synaptic transmission in the visual cortex in a TrkB‐dependent manner41 and potentiate N‐methyl‐d‐aspartate receptor‐mediated excitatory synaptic currents in the infralimbic prefrontal cortex.42 One of the major problems of current pharmacological therapy of depression is the slow onset of action. DHF could be a candidate antidepressant medication with the rapid onset of action supported by TrkB‐dependent and TrkB‐independent dual signaling pathways.
In conclusion, our present study demonstrates that DHF selectively potentiates hippocampal mossy fiber‐CA3 excitatory synaptic transmission. The DHF‐induced potentiation does not require TrkB receptor activation. This novel neuromodulatory effect of DHF should be taken into consideration in interpreting in vivo effects of DHF.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
DATA REPOSITORY
Datasets are available in the Supporting Information.
APPROVAL OF THE RESEARCH PROTOCOL BY AN INSTITUTIONAL REVIEWER BOARD
N/A.
INFORMED CONSENT
N/A.
REGISTRY AND THE REGISTRATION NO. OF THE STUDY/TRIAL
N/A.
ANIMAL STUDIES
Animal use and procedures were approved by the Animal Care and Use Committee of Nippon Medical School.
AUTHOR CONTRIBUTIONS
KK and HS conceived the study. KK performed experiments, analyzed the data, and wrote the manuscript. All authors read and approved the final manuscript.
Supporting information
ACKNOWLEDGMENTS
We thank Kayo Murayama for technical assistance.
Kobayashi K, Suzuki H. Synapse‐selective rapid potentiation of hippocampal synaptic transmission by 7,8‐dihydroxyflavone. Neuropsychopharmacol Rep. 2018;38:197–203. 10.1002/npr2.12033
Funding information
This work was supported by JSPS KAKENHI Grant Number 15H01296 and 17H05964 (to K.K.).
REFERENCES
- 1. Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14:7–23. [DOI] [PubMed] [Google Scholar]
- 2. Liu C, Chan CB, Ye K. 7,8‐dihydroxyflavone, a small molecular TrkB agonist, is useful for treating various BDNF‐implicated human disorders. Transl Neurodegener. 2016;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang JC, Yao W, Hashimoto K. Brain‐derived neurotrophic factor (BDNF)‐TrkB signaling in inflammation‐related depression and potential therapeutic targets. Curr Neuropharmacol. 2016;14:721–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jang SW, Liu X, Yepes M, et al. A selective TrkB agonist with potent neurotrophic activities by 7,8‐dihydroxyflavone. Proc Natl Acad Sci USA. 2010;107:2687–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andero R, Heldt SA, Ye K, Liu X, Armario A, Ressler KJ. Effect of 7,8‐dihydroxyflavone, a small‐molecule TrkB agonist, on emotional learning. Am J Psychiatry. 2011;168:163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andero R, Daviu N, Escorihuela RM, Nadal R, Armario A. 7,8‐dihydroxyflavone, a TrkB receptor agonist, blocks long‐term spatial memory impairment caused by immobilization stress in rats. Hippocampus. 2012;22:399–408. [DOI] [PubMed] [Google Scholar]
- 7. Zeng Y, Lv F, Li L, Yu H, Dong M, Fu Q. 7,8‐dihydroxyflavone rescues spatial memory and synaptic plasticity in cognitively impaired aged rats. J Neurochem. 2012;122:800–11. [DOI] [PubMed] [Google Scholar]
- 8. Devi L, Ohno M. 7,8‐dihydroxyflavone, a small‐molecule TrkB agonist, reverses memory deficits and BACE1 elevation in a mouse model of Alzheimer's disease. Neuropsychopharmacology. 2012;37:434–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Castello NA, Nguyen MH, Tran JD, Cheng D, Green KN, LaFerla FM. 7,8‐Dihydroxyflavone, a small molecule TrkB agonist, improves spatial memory and increases thin spine density in a mouse model of Alzheimer disease‐like neuronal loss. PLoS ONE. 2014;9:e91453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang Z, Liu X, Schroeder JP, et al. 7,8‐dihydroxyflavone prevents synaptic loss and memory deficits in a mouse model of Alzheimer's disease. Neuropsychopharmacology. 2014;39:638–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gao L, Tian M, Zhao HY, et al. TrkB activation by 7, 8‐dihydroxyflavone increases synapse AMPA subunits and ameliorates spatial memory deficits in a mouse model of Alzheimer's disease. J Neurochem. 2016;136:620–36. [DOI] [PubMed] [Google Scholar]
- 12. Yang YJ, Li YK, Wang W, et al. Small‐molecule TrkB agonist 7,8‐dihydroxyflavone reverses cognitive and synaptic plasticity deficits in a rat model of schizophrenia. Pharmacol Biochem Behav. 2014;122:30–6. [DOI] [PubMed] [Google Scholar]
- 13. Tian M, Zeng Y, Hu Y, et al. 7, 8‐Dihydroxyflavone induces synapse expression of AMPA GluA1 and ameliorates cognitive and spine abnormalities in a mouse model of fragile X syndrome. Neuropharmacology. 2015;89:43–53. [DOI] [PubMed] [Google Scholar]
- 14. Liu X, Chan CB, Jang SW, et al. A synthetic 7,8‐dihydroxyflavone derivative promotes neurogenesis and exhibits potent antidepressant effect. J Med Chem. 2010;53:8274–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang MW, Zhang SF, Li ZH, Han F. 7,8‐Dihydroxyflavone reverses the depressive symptoms in mouse chronic mild stress. Neurosci Lett. 2016;635:33–8. [DOI] [PubMed] [Google Scholar]
- 16. Zhang JC, Wu J, Fujita Y, et al. Antidepressant effects of TrkB ligands on depression‐like behavior and dendritic changes in mice after inflammation. Int J Neuropsychopharmacol. 2015;18:pyu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang JC, Yao W, Dong C, et al. Comparison of ketamine, 7,8‐dihydroxyflavone, and ANA‐12 antidepressant effects in the social defeat stress model of depression. Psychopharmacology. 2015;232:4325–35. [DOI] [PubMed] [Google Scholar]
- 18. Kobayashi K, Suzuki H. Dopamine selectively potentiates hippocampal mossy fiber to CA3 synaptic transmission. Neuropharmacology. 2007;52:552–61. [DOI] [PubMed] [Google Scholar]
- 19. Kobayashi K, Ikeda Y, Sakai A, et al. Reversal of hippocampal neuronal maturation by serotonergic antidepressants. Proc Natl Acad Sci USA. 2010;107:8434–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 2APB, 2‐aminoethoxydiphenyl borate, a membrane‐penetrable modulator of Ins(1,4,5)P3‐induced Ca2+ release. J Biochem. 1997;122:498–505. [DOI] [PubMed] [Google Scholar]
- 21. Hu HZ, Gu Q, Wang C, et al. 2‐aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J Biol Chem. 2004;279:35741–8. [DOI] [PubMed] [Google Scholar]
- 22. Togashi K, Inada H, Tominaga M. Inhibition of the transient receptor potential cation channel TRPM2 by 2‐aminoethoxydiphenyl borate (2‐APB). Br J Pharmacol. 2008;153:1324–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hu H, Grandl J, Bandell M, Petrus M, Patapoutian A. Two amino acid residues determine 2‐APB sensitivity of the ion channels TRPV3 and TRPV4. Proc Natl Acad Sci USA. 2009;106:1626–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang R, Kang KA, Piao MJ, et al. Preventive effect of 7,8‐dihydroxyflavone against oxidative stress induced genotoxicity. Biol Pharm Bull. 2009;32:166–71. [DOI] [PubMed] [Google Scholar]
- 25. Chen J, Chua KW, Chua CC, et al. Antioxidant activity of 7,8‐dihydroxyflavone provides neuroprotection against glutamate‐induced toxicity. Neurosci Lett. 2011;499:181–5. [DOI] [PubMed] [Google Scholar]
- 26. Han X, Zhu S, Wang B, et al. Antioxidant action of 7,8‐dihydroxyflavone protects PC12 cells against 6‐hydroxydopamine‐induced cytotoxicity. Neurochem Int. 2014;64:18–23. [DOI] [PubMed] [Google Scholar]
- 27. Prakriya M, Lewis RS. Potentiation and inhibition of Ca2+ release‐activated Ca2+ channels by 2‐aminoethyldiphenyl borate (2‐APB) occurs independently of IP3 receptors. J Physiol. 2001;536:3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu H, Ramsey IS, Kotecha SA, et al. TRPV3 is a calcium‐permeable temperature‐sensitive cation channel. Nature. 2002;418:181–6. [DOI] [PubMed] [Google Scholar]
- 29. Peier AM, Reeve AJ, Andersson DA, et al. A heat‐sensitive TRP channel expressed in keratinocytes. Science. 2002;296:2046–9. [DOI] [PubMed] [Google Scholar]
- 30. Lipski J, Park TI, Li D, et al. Involvement of TRP‐like channels in the acute ischemic response of hippocampal CA1 neurons in brain slices. Brain Res. 2006;1077:187–99. [DOI] [PubMed] [Google Scholar]
- 31. Salin PA, Scanziani M, Malenka RC, Nicoll RA. Distinct short‐term plasticity at two excitatory synapses in the hippocampus. Proc Natl Acad Sci USA. 1996;93:13304–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mori‐Kawakami F, Kobayashi K, Takahashi T. Developmental decrease in synaptic facilitation at the mouse hippocampal mossy fibre synapse. J Physiol. 2003;553:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kobayashi K. Hippocampal mossy fiber synaptic transmission and its modulation. Vitam Horm. 2010;82:65–85. [DOI] [PubMed] [Google Scholar]
- 34. Kobayashi K. Targeting the hippocampal mossy fiber synapse for the treatment of psychiatric disorders. Mol Neurobiol. 2009;39:24–36. [DOI] [PubMed] [Google Scholar]
- 35. Miller BR, Hen R. The current state of the neurogenic theory of depression and anxiety. Curr Opin Neurobiol. 2015;30:51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang X, Zhang D, Lu XY. Dentate gyrus‐CA3 glutamate release/NMDA transmission mediates behavioral despair and antidepressant‐like responses to leptin. Mol Psychiatry. 2015;20:509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yun S, Reynolds RP, Petrof I, et al. Stimulation of entorhinal cortex‐dentate gyrus circuitry is antidepressive. Nat Med. 2018;24:658–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Imoto Y, Segi‐Nishida E, Suzuki H, Kobayashi K. Rapid and stable changes in maturation‐related phenotypes of the adult hippocampal neurons by electroconvulsive treatment. Mol Brain. 2017;10:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kobayashi K, Haneda E, Higuchi M, Suhara T, Suzuki H. Chronic fluoxetine selectively upregulates dopamine D1‐like receptors in the hippocampus. Neuropsychopharmacology. 2012;37:1500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kobayashi K, Imoto Y, Yamamoto F, et al. Rapid and lasting enhancement of dopaminergic modulation at the hippocampal mossy fiber synapse by electroconvulsive treatment. J Neurophysiol. 2017;117:284–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Marongiu D, Imbrosci B, Mittmann T. Modulatory effects of the novel TrkB receptor agonist 7,8‐dihydroxyflavone on synaptic transmission and intrinsic neuronal excitability in mouse visual cortex in vitro. Eur J Pharmacol. 2013;709:64–71. [DOI] [PubMed] [Google Scholar]
- 42. Otis JM, Fitzgerald MK, Mueller D. Infralimbic BDNF/TrkB enhancement of GluN2B currents facilitates extinction of a cocaine‐conditioned place preference. J Neurosci. 2014;34:6057–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


