Abstract
Background and Objectives
Altered trafficking of α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid (AMPA) receptors has been reported in postmortem studies and suggested the involvement of AMPA receptors in the pathophysiology underpinning addictive disorders. However, these findings seemed mixed.
Methods
A systematic literature search was conducted, using PubMed and Embase (last search, August 2018), to identify human postmortem studies that examined the expression of proteins and mRNA of AMPA receptor subunits in patients with addictive disorders in comparison with healthy controls.
Results
Twelve (18 studies) out of 954 articles were identified to be relevant. Eight studies included alcohol use disorders, and four studies included heroin/cocaine abusers. The most frequently investigated regions were the hippocampus (three studies), amygdala (three studies), and putamen (three studies). In summary, two out of the three studies showed an increase in the expression of AMPA receptors in the hippocampus, while the other study found no change. Two studies to examine the amygdala demonstrated either a decreased or no change in receptor expression or binding. Concerning putamen, two studies showed no significant change whereas an overexpression of receptors was observed in the other.
Conclusions and Scientific Significance
The hippocampus and amygdala may be pertinent to addictive disorders through their functions on learning and memory, whereas findings in other regions were inconsistent across the studies. Human postmortem studies are prone to degenerative changes after death. Moreover, only qualitative assessment was conducted because of the limited, heterogenous data. These limitations emphasize the need to investigate AMPA receptors in the living human brains.
Keywords: alcohol‐ and substance‐related disorders: basic/clinical
Postmortem studies on AMPA receptors in patients with addiction show that the hippocampus and amygdala may be pertinent to addictive disorders through their functions on learning and memory, whereas findings in other regions were inconsistent across the studies. Human postmortem studies are prone to degenerative changes after death, which emphasizes the need to investigate AMPA receptors in the living human brains.
1. INTRODUCTION
The effectiveness of currently available treatment options for human addictive disorders is far from ideal, in spite of global attention that has been paid to addiction and emerging treatment options. There has been growing interest in glutamatergic abnormalities that have been implicated in various addictions. With regard to alcohol use disorder (AUD) as an example, Tsai et al1 reported an increase in the concentration of glutamate in the cerebrospinal fluid (CSF) and Umhau et al2 also noted a positive association between the severity of illness and glutamate concentration in the CSF. Moreover, lower glutamate and increased glutamine concentration in the bilateral anterior cingulate cortex (ACC) were shown in a small study with the use of magnetic resonance spectroscopy (MRS) in patients with AUD compared with healthy controls.3 Within the glutamatergic system, the N‐methyl‐d‐aspartate (NMDA), a well‐studied ionotropic receptor, is assumed to play a central role in long‐lasting learning and memories via its neurobiological plasticity.4 It is posited that the NMDA receptor may be associated with the development of such disorders5 in light of a possibility that addiction may be a pathological usurpation of mechanisms underlying learning and memory.
On the other hand, evidence has suggested that another glutamatergic receptor, α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid (AMPA) receptor, is clearly associated with the development of addictive disorders.6 Several in vitro studies have shown the alterations in AMPA receptors in the brain of model rodents with alcohol and cocaine dependence: increased expression in the cortical culture7 and nucleus accumbens (NAc)8 and decreased binding in some cortical regions, hippocampus and septohippocampal nucleus9 of alcohol‐dependent models; increased expression10, 11 and potentiated excitatory postsynaptic currents (EPSCs)12 in the NAc and decreased expression in the dorsolateral striatum11 of cocaine‐exposed rodents. Moreover, preclinical studies found, after administration of AMPA receptor antagonists, reductions in substance‐seeking behaviors,13, 14 alcohol withdrawal,15 and alcohol consumption16 in substance‐preferring model of rats. Furthermore, the gene‐based study also noted significant correlation between metabotropic glutamate receptor (mGluR)‐eukaryotic elongation factor 2 (eEF2)‐AMPA receptor pathway (including the glutamate ionotropic receptor AMPA [GRIA] 1 and 4) and addictive behaviors like a frequency of drinking.17 These findings collectively indicate that AMPA receptors are likely involved in the pathophysiology underpinning addiction.
Postmortem studies have provided important insights on the pathophysiology of addictive disorders in human beings. A systematic synthesis of currently available data is helpful to further our understanding on addiction and to underscore what is missing in the field. We therefore systematically reviewed published postmortem studies that investigated AMPA receptor expression in patients with addictive disorders.
2. METHODS
A systematic search of the literature was conducted in order to identify postmortem, case‐control studies that investigated the expression of AMPA receptor subunits or receptor binding in patients with addictive disorders compared with healthy individuals, using PubMed and Embase (last search: August 21, 2018). The search terms were as follows: ((α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid) or AMPA or AMPAR) AND (alcohol* or narco* or addiction). We also performed cross‐reference and manual searches. Original articles in English were included for the purpose of this study. The literature search was performed independently by two of the authors (FU and HU).
3. RESULTS
Figure 1 shows the flow of our literature search, from an initial list of 954 studies; of these, 12 articles were identified to be relevant. All of these studies investigated AMPA receptor binding levels, or AMPA receptor subunit transcripts or protein expression; they compared these indices between patients with substance use disorders and healthy controls. Patients with AUD were targeted in eight studies; five studies used the DSM‐IV for the diagnosis,18, 19, 20, 21, 22 two studies relied on daily consumption of ethanol,23, 24 and one study did not specify any criteria.25 The other four studies included patients with heroin and cocaine addiction, where the bases for the diagnosis were an archived history of abuse in two studies26, 27 and death that resulted from overdose for the other two studies.28, 29 The regions of interest included the hippocampus (three studies in AUD), amygdala (three studies: two in AUD and one in drug abuse), and putamen (three studies: two in drug abuse and one in AUD), followed by the frontal cortex (two studies in AUD), cingulate cortex (two studies in AUD), and NAc (two studies: one in AUD and one in drug abuse).
Figure 1.
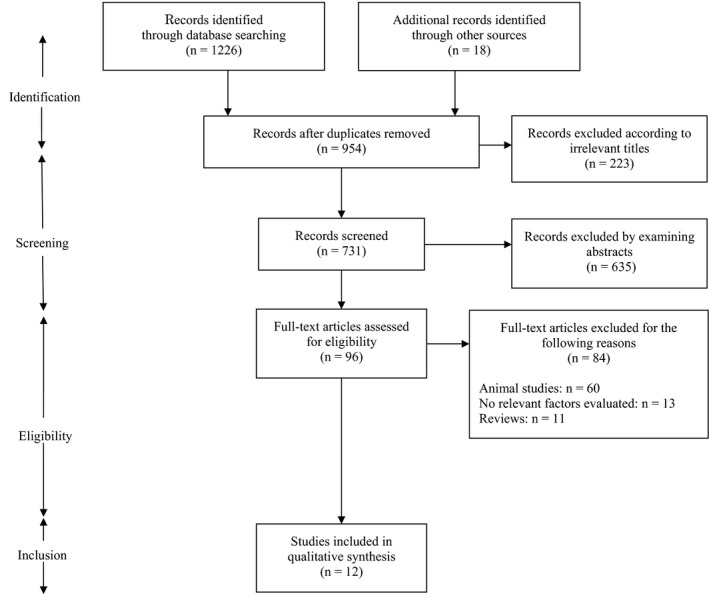
PRISMA flow diagram of the literature search
Table 1 summarizes the methodologies and results of the studies included in our review. Synthesis of the main findings is described in a chronological order below.
Table 1.
AMPA receptor subunit expression or receptor binding in patients with addiction
| 1st author (year) | Subject | N (Male) | Age (±SD) | PMI (h) | Method | Region | Findings | Target molecules |
|---|---|---|---|---|---|---|---|---|
| Cingulate cortex (N = 2) | ||||||||
| Breese (1995) | AUD | 9 (8) | 52.8 (17.3) | 18.8 | Western blot | Cingulate cortex | ns | GRIA2, GRIA3 |
| Kärkkäinen (2013) |
AUD Cloninger type 1 |
9 (7) | 52.7 (12.4) | 11.9 | Autoradiography | ACC | ns | [3H] AMPA binding |
|
AUD Cloninger type 2 |
8 (8) | 34.6 (12.2) | 14.1 | ↑ | ||||
| Frontal Cortex (N = 4) | ||||||||
| Freund (1996) | AUD | 13 (NA) | 64.5 (9.7) | 14.4 | Autoradiography | Frontal cortex | ns |
[3H] AMPA binding Numerically, but not significantly, increased |
| Kärkkäinen (2013) |
AUD Cloninger type 1 |
9 (7) | 52.7 (12.4) | 11.9 | Autoradiography | Frontal cortex | ns | [3H] AMPA binding |
|
AUD Cloninger type 2 |
8 (8) | 34.6 (12.2) | 14.1 | ns | ||||
| Jin (2014a) | AUD | 11 (11) | 58.0 (16.6) | 29 | RT‐qPCR | OFC | ns | GRIA2, GRIA3 |
| 14 (14) | 59.0 (15.0) | 29 | DLPFC | ns | ||||
| Farris (2015) | AUD | 16 (NA) | 53.8 (12.5) | 34.1 | RNA‐Seq | PFC (BA 8) | ↑ |
GRIA1 GM including GRIA1 showed higher gene connectivity in AUD |
| Basal ganglia (N = 5) | ||||||||
| Tang (2003) | Cocaine abuser | 10 (10) | 35.5 (6.5) | 12.3 |
Microarray Western blot |
l‐SN | ↑ | GRIA1‐4 |
| Kärkkäinen (2013) |
AUD Cloninger type 1 |
9 (7) | 52.7 (12.4) | 11.9 | Autoradiography | NAc | ns | [3H] AMPA binding |
|
AUD Cloninger type 2 |
8 (8) | 34.6 (12.2) | 14.1 | ns | ||||
| Hemby (2005) | Cocaine abuser | 8 (8) | 34.4 (7.3) | 12.5 | Western blot | NAc | ↑ |
GRIA2, GRIA3 No statistically significant changes observed for GRIA1 |
| Putamen | ns | GRIA1–3 | ||||||
| Bhandage (2014) | AUD | 29 (29) | 57.5 (9.9) | 35.5 | RT‐qPCR | Caudate nucleus, Putamen | ns | GRIA1–4 |
| Egervari (2017) | Heroin abuser | 48 (40) | 26.2 (4.8) | < 24 | Microarray, Western blot | Putamen | ↑ | GRIA1, GRIA3 |
| Medial temporal lobe (N = 6) | ||||||||
| Breese (1995) | AUD | 9 (8) | 52.8 (17.3) | 18.8 | Western blot | Hippocampus | ↑ | GRIA2, GRIA3 |
| Kärkkäinen (2013) |
AUD Cloninger type 1 |
9 (7) | 52.7 (12.4) | 11.9 | Autoradiography |
Hippocampus Dentate gyrus |
ns | [3H] AMPA binding |
|
AUD Cloninger type 2 |
8 (8) | 34.6 (12.2) | 14.1 | |||||
| Jin (2014a) | AUD | 13 (13) | 56.0 (14.4) | 30 | RT‐qPCR |
Hippocampus Dentate gyrus |
↑ | GRIA2, GRIA3 |
| Kryger (2010) | AUD | 6 (6) | 55.0 (10.7) | 21.7 | Microarray | Basolateral amygdala | ↓ | GRIA2 |
| 10 (10) | 56.4 (14.1) | 22.3 | Western blot | |||||
| Okvist (2011) | Multiple drug abuser (Heroin‐Cocaine) | 7 (5) | 45 (7) | 24 | In situ hybridization, Western blot | Amygdala (lateral, accessory basal, and basal nuclei) | ns | GRIA1 |
| Cocaine abuser | 7 (6) | 45 (9) | ||||||
| Heroin abuser (Study I) | 8 (7) | 41 (10) | ||||||
| Heroin abuser (Study II) | 29 (24) | 27 (5) | <24 | |||||
| Jin (2014b) | AUD | 9 (9) | 55.1 (15.8) | 27 | RT‐qPCR | Central amygdala | ↓ | GRIA1, GRIA4 |
| Brain Stem (N = 1) | ||||||||
| Tang (2003) | Cocaine abuser | 10 (10) | 35.5 (6.5) | 12.3 |
Microarray Western blot |
VTA | ↑ |
GRIA2 No significant alterations observed for GRIA1, 3 or 4 |
[Findings] ↑: increase in patients with addiction compared to healthy individuals, ↓: decrease in patients with addiction compared with healthy individuals, ns: no significant difference between patients with addictive disorders and healthy controls.
Abbreviations: ACC, anterior cingulate cortex; AUD, alcohol use disorder; BA, Brodmann area; DLPFC, dorsolateral prefrontal cortex; GM, gene module; GRIA, glutamate ionotropic receptor AMPA; l‐SN, lateral substantia nigra; N, number of studies; n, number of subjects; NA, not available; NAc, nucleus accumbens; OFC, orbitofrontal cortex; PFC, prefrontal cortex; PMI, postmortem interval; RT‐qPCR, real‐time quantitative polymerase chain reaction; Seq, sequencing; VTA, ventral tegmental area.
4. ALCOHOL USE DISORDER
Protein expressions of AMPA/kainite receptor subtypes were found to be increased in the hippocampus of alcohol users (n = 9) in the study by Breese et al25 Freund and Anderson23 found a numerically, but not significantly, increased [3H]AMPA binding in the frontal cortex of patients with AUD (n = 13). mRNA and protein expression of GRIA 2 were decreased in the basolateral amygdala of patients with AUD (n = 10) in the study by Kryger and Wilce.24 Karkkainen et al18 classified patients with AUD into type 1 (age of onset >25 years, and susceptibility to anxiety, n = 9) and type 2 (age of onset <25, and impulsive and antisocial behaviors, n = 8), in accordance with Cloninger's criteria. The authors found an increased [3H]AMPA binding in the ACC of the type 2 patients compared with healthy controls, whereas no differences were detected in the other brain regions of interest. Jin et al19, 20 demonstrated that mRNA expressions of AMPA receptor subunits were lower in the central amygdala (n = 9) and higher in the hippocampus dentate gyrus (n = 13) in the patient group, while there were no differences in the dorsolateral prefrontal cortex (DLPFC) (n = 14) or the orbitofrontal cortex (OFC) (n = 11). Bhandage et al21 found that mRNAs expression levels were not different among any of the glutamate receptor subunits in the caudate and the putamen (n = 29). Farris et al22 showed higher gene connectivity including GRIA1 in the prefrontal cortex (n = 16).
5. OTHER SUBSTANCE USE DISORDERS
Tang and colleagues28 identified an upregulation of the mRNA and protein level of GRIA2 in the ventral tegmental area (VTA), which was not observed in the lateral substantia nigra (l‐SN), among the victims of cocaine overdose (n = 10). Hemby et al29 showed an increase in the protein expressions of GRIA2/3 in the NAc of cocaine overdose victims, but not in the putamen (n = 8). Okvist et al26 conducted two studies; they compared levels of mRNA expression of AMPA receptor subunits in the amygdala among the four groups (ie, multiple drug abusers [cocaine and heroin, n = 7], cocaine abusers [n = 7], heroin abusers [n = 8], and healthy controls) in one study and those between heroin abusers (n = 29) and healthy controls in the other, only to find no group differences in both studies. An overexpression of GRIA1 and GRIA3 in the putamen was reported among heroin abusers (n = 48) in one study.27
To sum, two out of the three studies showed that the expression of AMPA receptors increased in the hippocampus of the patients with AUD,20, 25 while the other failed to show changes.18 A decreased receptor binding or expression in this region of the patients with AUD was identified in two of three studies that examined the amygdala,19, 24 and no change was found for the cocaine and heroin abusers in the other study.26 With respect to the putamen, two studies to target cocaine abusers and patients with AUD showed no change,21, 29 whereas overexpression of receptors was observed in the study on heroin abusers.27 In the frontal cortex, there was no difference among both two studies that investigated patients with AUD.18, 23 As for the cingulate cortex, binding of AMPA receptor was higher in the Cloninger type 2 AUD patients in one study,18 whereas there was no significant change in the study for the patients with AUD.25 Regarding the NAc, one study found an increment of AMPA receptors expression among cocaine abusers,29 and there was no change in AUD patients compared with healthy controls in the other study.18
6. DISCUSSION
Addictive disorders are prevalent in human being but their pathophysiological underpinnings remain to be elucidated to date. The present systematic review aimed to synthesize the currently available evidence on human postmortem studies that examined AMPA receptor subunit expression and receptor binding in patients with substance use disorders. The results were suggestive of the notion that hippocampus and amygdala may be related to the development of addictive disorders, possibly through their well‐known functions to regulate learning and memory. On the other hand, findings in the other regions were not consistent across the studies, perhaps because of the heterogeneity in the subjects, targeted brain regions, substances of interest, cause of death, and analysis techniques employed. It may further be attributable to variations in degenerative and agonal changes after death to which postmortem studies are almost always susceptible.
Although the currently available data are still very modest, the subunit expression and binding to AMPA receptors are increased in the hippocampus of AUD patients, but decreased in the amygdala in quantity, in comparison with healthy controls. The hippocampus is a vital region for learning and memory; such pivotal functions are postulated to be processed by long‐term potentiation and long‐term depression through glutamate receptors.30 Therefore, a possibility is that the changes in AMPA receptor subunit expression and receptor binding contribute to impaired cognition, including memory function, in association with insobriety of heavy ethanol.31, 32 Interestingly, the finding in the hippocampus of AUD patients is different from the that of the animal study which showed the decreased AMPA receptor binding level in the same region.9 Such a discrepancy may be due to the following limitations of the studies included in the present review; the number of studies identified was small and these postmortem studies were prone to physiological degenerative changes after death. The central amygdala of patients with AUD synthesizes emotional and somatic information gathered from other brain regions and other subregions within the amygdala33 and plays a vital part in regulating alcohol‐drinking behaviors.34 The basolateral amygdala plays an important role in cue‐induced relapse by regulating associative learning,35 which is critical in the regulation of anxiety.36 Thus, AMPA receptor dysfunction in the basolateral amygdala may result in continuous substance‐seeking behavior and withdrawal anxiety.
The NAc8, 10 and VTA37 have been the hot target in previous animal studies. They found an upregulated density of AMPA receptor and revealed a decrease in substance‐seeking behavior by administration of AMPA receptor antagonists, such as 6‐cyano‐7‐nitroquinoxaline‐2,3‐dione (CNQX) and 6,7‐dinitroquinoxaline‐2,3‐dione (NBQX).14, 16, 38 The beneficial results with the use of AMPA receptor antagonists corroborate those of microinjection to basolateral amygdala, to prevent anxiety‐like behavior of withdrawn alcohol‐dependent rats.15, 39 On the other hand, in contrast, human studies reported decreases in AMPA receptors in the amygdala of AUD patients.20, 24 Given the significant impact and burden of substance use disorders around the globe, further investigations are clearly indicated to examine AMPA receptors in the brain regions, hopefully in vivo, in order to devise new therapeutics.
Findings from several studies of antiepileptics collectively suggest that AMPA receptor seems to be a potential target of novel medications against addictive disorders. For example, topiramate is an antiepileptic drug to antagonize glutamate activity at AMPA/kainite receptors.40 This blockade is expected to decrease extracellular dopamine release in the midbrain41 and modulate a nonbenzodiazepine site on the gamma‐amino‐butyric acid‐A (GABA‐A) receptor.42 Previous clinical studies have shown its hope in reducing craving for alcohol, drinking behavior, and withdrawal symptoms.43, 44, 45 Similarly, lamotrigine, which is another antiepileptic medication to inhibit AMPA receptors and decrease glutamate release,46 was found to be promising in reducing cocaine intake and craving in cocaine abusers.47
Several limitations need to be taken into account to interpret the results of this systematic review. First, the number of studies was small. Second, as already mentioned, degrading changes after death render human postmortem studies challenging. Third, subjects included in these studies were not always well‐characterized in terms of the diagnosis and the severity of illness. Forth, not quantitative but just qualitative assessment of the literature was conducted in the present review because of the limited, heterogenous data.
To conclude, while the hippocampus and amygdala may play pivotal roles in the pathophysiology of addiction, the above‐mentioned limitations and the paucity of evidence clearly highlight the necessity for examining AMPA receptors in the living brain of well‐characterized patients by using positron emission tomography (PET). These findings would be expected to inform the development of well‐anticipated novel therapeutics against addictive disorders.
CONFLICT OF INTEREST
Dr Ueno has received a speaker's fee from Meiji‐Seika Pharma in the past three years. Dr Suzuki has received manuscript or speaker's fees from Astellas, Dainippon‐Sumitomo Pharma, Eli Lilly, Elsevier Japan, Janssen Pharmaceuticals, Meiji‐Seika Pharma, Novartis, Otsuka Pharmaceutical, Wiley Japan, and Yoshitomi Yakuhin, and research grants from Eisai, Mochida Pharmaceutical, and Meiji‐Seika Pharma within the past three years. Dr Nakajima has received fellowship grants from Canadian Institute of Health Research, research support from Japan Society for the Promotion of Science, Japan Research Foundation for Clinical Pharmacology, Naito Foundation, Takeda Science Foundation, Daiichi Sankyo, and manuscript fees or speaker's honoraria from Dainippon‐Sumitomo Pharma and Yoshitomi Yakuhin within the past three years. Dr Mimura has received grants and/or speaker's honoraria from Asahi Kasei Pharma, Astellas Pharmaceutical, Daiichi Sankyo, Dainippon‐Sumitomo Pharma, Eisai, Eli Lilly, Fuji Film RI Pharma, Janssen Pharmaceutical, Kracie, Meiji‐Seika Pharma, Mochida Pharmaceutical, MSD, Novartis Pharma, Ono Yakuhin, Otsuka Pharmaceutical, Pfizer, Shionogi, Takeda Yakuhin, Tanabe Mitsubishi Pharma, and Yoshitomi Yakuhin within the past three years. Dr Uchida has received grants from Eisai, Otsuka Pharmaceutical, Dainippon‐Sumitomo Pharma, Mochida Pharmaceutical, Meiji‐Seika Pharmaceutical, and Novartis; speaker's honoraria from Otsuka Pharmaceutical, Eli Lilly, Shionogi, Pfizer, Yoshitomi Yakuhin, Dainippon‐Sumitomo Pharma, Meiji‐Seika Pharma, MSD, and Janssen Pharmaceutical; and advisory panel payments from Dainippon‐Sumitomo Pharma within the past three years.
DATA REPOSITORY
The authors do not deposit any data because this manuscript does not include any original data.
APPROVAL OF THE RESEARCH PROTOCOL BY AN INSTITUTIONAL REVIEWER BOARD
n/a.
INFORMED CONSENT
n/a.
REGISTRY AND THE REGISTRATION NO. OF THE STUDY
n/a.
ANIMAL STUDIES
n/a.
AUTHOR CONTRIBUTION
Dr Ueno and Dr Uchida conducted data acquisition, analysis, and drafted the manuscript. All authors devised the study concept and design, and conducted the critical revisions of the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
This work was supported by Japan Agency for Medical Research and Development, AMED [JP18dm0107125 to MM and HU]. This funding source had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Ueno F, Suzuki T, Nakajima S, et al. Alteration in AMPA receptor subunit expression and receptor binding among patients with addictive disorders: A systematic review of human postmortem studies. Neuropsychopharmacol Rep. 2019;39:148–155. 10.1002/npr2.12058
REFERENCES
- 1. Tsai GE, Ragan P, Chang R, Chen S, Linnoila VM, Coyle JT. Increased glutamatergic neurotransmission and oxidative stress after alcohol withdrawal. Am J Psychiatry. 1998;155:726‐32. [DOI] [PubMed] [Google Scholar]
- 2. Umhau JC, Momenan R, Schwandt ML, Singley E, Lifshitz M, Doty L, et al. Effect of acamprosate on magnetic resonance spectroscopy measures of central glutamate in detoxified alcohol‐dependent individuals: a randomized controlled experimental medicine study. Arch Gen Psychiatry. 2010;67:1069‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thoma R, Mullins P, Ruhl D, Monnig M, Yeo RA, Caprihan A, et al. Perturbation of the glutamate‐glutamine system in alcohol dependence and remission. Neuropsychopharmacology. 2011;36:1359‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morisot N, Ron D. Alcohol‐dependent molecular adaptations of the NMDA receptor system. Genes Brain Behav. 2017;16:139‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162:1414‐22. [DOI] [PubMed] [Google Scholar]
- 6. Holmes A, Spanagel R, Krystal JH. Glutamatergic targets for new alcohol medications. Psychopharmacology. 2013;229:539‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chandler LJ, Norwood D, Sutton G. Chronic ethanol upregulates NMDA and AMPA, but not kainate receptor subunit proteins in rat primary cortical cultures. Alcohol Clin Exp Res. 1999;23:363‐70. [PubMed] [Google Scholar]
- 8. Ary AW, Cozzoli DK, Finn DA, Crabbe JC, Dehoff MH, Worley PF, et al. Ethanol up‐regulates nucleus accumbens neuronal activity dependent pentraxin (Narp): implications for alcohol‐induced behavioral plasticity. Alcohol. 2012;46:377‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen F, Jarrott B, Lawrence AJ. Up‐regulation of cortical AMPA receptor binding in the fawn‐hooded rat following ethanol withdrawal. Eur J Pharmacol. 1999;384:139‐46. [DOI] [PubMed] [Google Scholar]
- 10. Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ferrario CR, Li X, Wang X, Reimers JM, Uejima JL, Wolf ME. The role of glutamate receptor redistribution in locomotor sensitization to cocaine. Neuropsychopharmacology. 2010;35:818‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28:3170‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Czachowski CL, Delory MJ, Pope JD. Behavioral and neurotransmitter specific roles for the ventral tegmental area in reinforcer‐seeking and intake. Alcohol Clin Exp Res. 2012;36:1659‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lack AK, Diaz MR, Chappell A, DuBois DW, McCool BA. Chronic ethanol and withdrawal differentially modulate pre‐ and postsynaptic function at glutamatergic synapses in rat basolateral amygdala. J Neurophysiol. 2007;98:3185‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stephens DN, Brown G. Disruption of operant oral self‐administration of ethanol, sucrose, and saccharin by the AMPA/kainate antagonist, NBQX, but not the AMPA antagonist, GYKI 52466. Alcohol Clin Exp Res. 1999;23:1914‐20. [PubMed] [Google Scholar]
- 17. Meyers JL, Salling MC, Almli LM, Ratanatharathorn A, Uddin M, Galea S, et al. Frequency of alcohol consumption in humans; the role of metabotropic glutamate receptors and downstream signaling pathways. Transl Psychiatry. 2015;5:e586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kärkkäinen O, Kupila J, Häkkinen M, Laukkanen V, Tupala E, Kautiainen H, et al. AMPA receptors in post‐mortem brains of Cloninger type 1 and 2 alcoholics: a whole‐hemisphere autoradiography study. Psychiatry Res. 2013;214:429‐34. [DOI] [PubMed] [Google Scholar]
- 19. Jin Z, Bhandage AK, Bazov I, Kononenko O, Bakalkin G, Korpi ER, et al. Expression of specific ionotropic glutamate and GABA‐A receptor subunits is decreased in central amygdala of alcoholics. Front Cell Neurosci. 2014;8:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jin Z, Bhandage AK, Bazov I, Kononenko O, Bakalkin G, Korpi ER, et al. Selective increases of AMPA, NMDA, and kainate receptor subunit mRNAs in the hippocampus and orbitofrontal cortex but not in prefrontal cortex of human alcoholics. Front Cell Neurosci. 2014;8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bhandage AK, Jin Z, Bazov I, Kononenko O, Bakalkin G, Korpi ER, et al. GABA‐A and NMDA receptor subunit mRNA expression is altered in the caudate but not the putamen of the postmortem brains of alcoholics. Front Cell Neurosci. 2014;8:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Farris SP, Arasappan D, Hunicke‐Smith S, Harris RA, Mayfield RD. Transcriptome organization for chronic alcohol abuse in human brain. Mol Psychiatry. 2015;20:1438‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Freund G, Anderson KJ. Glutamate receptors in the frontal cortex of alcoholics. Alcohol Clin Exp Res. 1996;20:1165‐72. [DOI] [PubMed] [Google Scholar]
- 24. Kryger R, Wilce PA. The effects of alcoholism on the human basolateral amygdala. Neuroscience. 2010;167:361‐71. [DOI] [PubMed] [Google Scholar]
- 25. Breese CR, Freedman R, Leonard SS. Glutamate receptor subtype expression in human postmortem brain tissue from schizophrenics and alcohol abusers. Brain Res. 1995;674:82‐90. [DOI] [PubMed] [Google Scholar]
- 26. Ökvist A, Fagergren P, Whittard J, Garcia‐Osta A, Drakenberg K, Horvath MC, et al. Dysregulated postsynaptic density and endocytic zone in the amygdala of human heroin and cocaine abusers. Biol Psychiatry. 2011;69:245‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Egervari G, Landry J, Callens J, Fullard JF, Roussos P, Keller E, et al. Striatal H3K27 acetylation linked to glutamatergic gene dysregulation in human heroin abusers holds promise as therapeutic target. Biol Psychiatry. 2017;81:585‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tang WX, Fasulo WH, Mash DC, Hemby SE. Molecular profiling of midbrain dopamine regions in cocaine overdose victims. J Neurochem. 2003;85:911‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hemby SE, Tang W, Muly EC, Kuhar MJ, Howell L, Mash DC. Cocaine‐induced alterations in nucleus accumbens ionotropic glutamate receptor subunits in human and non‐human primates. J Neurochem. 2005;95:1785‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nicoll RA, Roche KW. Long‐term potentiation: peeling the onion. Neuropharmacology. 2013;74:18‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heffernan TM. The impact of excessive alcohol use on prospective memory: a brief review. Curr Drug Abuse Rev. 2008;1:36‐41. [DOI] [PubMed] [Google Scholar]
- 32. Loeber S, Duka T, Welzel H, Nakovics H, Heinz A, Flor H, et al. Impairment of cognitive abilities and decision making after chronic use of alcohol: the impact of multiple detoxifications. Alcohol Alcohol. 2009;44:372‐81. [DOI] [PubMed] [Google Scholar]
- 33. Pare D, Duvarci S. Amygdala microcircuits mediating fear expression and extinction. Curr Opin Neurobiol. 2012;22:717‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McBride WJ. Central nucleus of the amygdala and the effects of alcohol and alcohol‐drinking behavior in rodents. Pharmacol Biochem Behav. 2002;71:509‐15. [DOI] [PubMed] [Google Scholar]
- 35. See RE, Fuchs RA, Ledford CC, McLaughlin J. Drug addiction, relapse, and the amygdala. Ann N Y Acad Sci. 2003;985:294‐307. [DOI] [PubMed] [Google Scholar]
- 36. Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A. Corticotrophin releasing factor‐induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci. 2004;24:3471‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Heikkinen AE, Moykkynen TP, Korpi ER. Long‐lasting modulation of glutamatergic transmission in VTA dopamine neurons after a single dose of benzodiazepine agonists. Neuropsychopharmacology. 2009;34:290‐8. [DOI] [PubMed] [Google Scholar]
- 38. Backstrom P, Hyytia P. Ionotropic and metabotropic glutamate receptor antagonism attenuates cue‐induced cocaine seeking. Neuropsychopharmacology. 2006;31:778‐86. [DOI] [PubMed] [Google Scholar]
- 39. Christian DT, Alexander NJ, Diaz MR, Robinson S, McCool BA. Chronic intermittent ethanol and withdrawal differentially modulate basolateral amygdala AMPA‐type glutamate receptor function and trafficking. Neuropharmacology. 2012;62:2430‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Skradski S, White HS. Topiramate blocks kainate‐evoked cobalt influx into cultured neurons. Epilepsia. 2000;41:S45‐47. [DOI] [PubMed] [Google Scholar]
- 41. Moghaddam B, Bolinao ML. Glutamatergic antagonists attenuate ability of dopamine uptake blockers to increase extracellular levels of dopamine: implications for tonic influence of glutamate on dopamine release. Synapse. 1994;18:337‐42. [DOI] [PubMed] [Google Scholar]
- 42. White HS, Brown SD, Woodhead JH, Skeen GA, Wolf HH. Topiramate modulates GABA‐evoked currents in murine cortical neurons by a nonbenzodiazepine mechanism. Epilepsia. 2000;41:S17‐20. [PubMed] [Google Scholar]
- 43. Johnson BA, Ait‐Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361:1677‐85. [DOI] [PubMed] [Google Scholar]
- 44. Krupitsky EM, Rudenko AA, Burakov AM, Slavina TY, Grinenko AA, Pittman B, et al. Antiglutamatergic strategies for ethanol detoxification: comparison with placebo and diazepam. Alcohol Clin Exp Res. 2007;31:604‐11. [DOI] [PubMed] [Google Scholar]
- 45. Miranda Jr R, MacKillop J, Monti PM, Rohsenow DJ, Tidey J, Gwaltney C, et al. Effects of topiramate on urge to drink and the subjective effects of alcohol: a preliminary laboratory study. Alcohol Clin Exp Res. 2008;32:489‐97. [DOI] [PubMed] [Google Scholar]
- 46. Lee CY, Fu WM, Chen CC, Su MJ, Liou HH. Lamotrigine inhibits postsynaptic AMPA receptor and glutamate release in the dentate gyrus. Epilepsia. 2008;49:888‐97. [DOI] [PubMed] [Google Scholar]
- 47. Brown ES, Nejtek VA, Perantie DC, Orsulak PJ, Bobadilla L. Lamotrigine in patients with bipolar disorder and cocaine dependence. J Clin Psychiatry. 2003;64:197‐201. [DOI] [PubMed] [Google Scholar]


