Abstract
Aim
Indoleamine 2,3‐dioxygenase 1 (IDO1) metabolizes the essential amino acid tryptophan into kynurenine derivatives, which are involved in neural activity via the kynurenine pathway (KP). IDO1 is an initial rate‐limiting enzyme in the KP and is activated by stress and/or inflammation. The perturbation of IDO1 activity, which causes KP imbalance, is associated with psychiatric and neurological disorders. It has been reported that wild‐type (WT) mice under inflammatory conditions show increased anxiety‐like behavior and decreased novel object recognition, whereas Ido1 knockout (KO) mice do not display these behaviors. However, the behavioral phenotypes of Ido1 KO mice have not yet been fully examined under non‐inflammatory conditions.
Methods
We subjected Ido1 KO mice to a comprehensive behavioral test battery under normal conditions.
Results
Ido1 KO mice and WT mice showed similar locomotor activity, anxiety‐like behavior, social behavior, depression‐like behavior, and fear memory. In the T‐maze test, Ido1 KO mice exhibited weak but nominally significant impairment in the working memory task of the T‐maze, but this result failed to reach study‐wide significance.
Conclusions
Ido1 KO mice did not show any clear behavioral abnormalities under normal conditions. Further studies may be necessary to investigate their behavioral phenotype under inflammatory conditions due to their known roles in inflammation.
Keywords: comprehensive behavioral test battery; indoleamine 2,3‐dioxygenase; kynurenine pathway
It has been reported that wild‐type (WT) mice under inflammatory conditions show increased anxiety‐like behavior and decreased novel object recognition, whereas Ido1 knockout (KO) mice do not display these behaviors. However, the behavioral phenotypes of Ido1 KO mice have not yet been fully examined under non‐inflammatory conditions. Here, we subjected Ido1 KO mice to a comprehensive behavioral test battery under normal conditions.
1. INTRODUCTION
The essential amino acid tryptophan (Trp) is metabolized via the serotonin or kynurenine pathway (KP).1, 2 Along the serotonin pathway, Trp undergoes hydroxylation and decarboxylation to produce the neurotransmitters serotonin and melatonin. These indoleamine derivatives, serotonin and melatonin, are involved in the circadian control of physiological functions, such as sleeping, blood pressure regulation, thermoregulation, and depression. Along the KP, Trp is converted to neuroactive metabolites, such as kynurenine, 3‐hydroxykynurenie,3 kynurenic acid (KYNA),4, 5, 6 and quinolinic acid (QUIN).7, 8 Generally, more than 95% of dietary, Trp is metabolized along the KP to produce neuroactive KYNA and QUIN, and only a small amount of it is converted to serotonin.9 Dysfunction of the KP, which causes an imbalance in the level of kynurenine metabolites in the brain, is associated with neurodegeneration in the brain and psychological illnesses, including schizophrenia and depression.2
Indoleamine 2,3‐dioxygenase 1 (IDO1) and tryptophan 2,3‐dioxygenase 2 (TDO2) are the initial rate‐limiting enzymes in KP.2, 10 These enzymes are activated by stimulation of the immune system. The perturbation of IDO1 or TDO2 activity, which causes KP imbalance, is also reported to be associated with psychiatric and neurological disorders.9 In our previous study, we conducted a comprehensive battery of behavioral tests with Tdo2 KO mice, which showed lower anxiety‐like behavior, higher locomotor activity, and abnormal gait patterns than the controls.11 It has been reported that WT mice under inflammatory conditions show increased anxiety‐like behavior in open field and light/dark transition tests, increased depressive‐like behavior on forced swim and tail suspension tests, and decreased novel object recognition, whereas Ido1 KO mice do not exhibit these behaviors.12, 13 However, the behavioral phenotypes of Ido1 KO mice have not yet been fully examined under non‐inflammatory conditions. Here, we subjected Ido1 KO mice to a battery of comprehensive behavioral tests under normal conditions.
2. MATERIALS AND METHODS
2.1. Animals
Ido1 KO mice were purchased from The Jackson Laboratory (https://www.jax.org/strain/005867) by Asahikawa Medical University and transferred to Kyoto University and Fujita Health University. Ido1 KO mice were derived from 129/SvJ ES cells mated with C57BL/6J (B6) females and backcrossed with more than 10 generations of B6 mice.14 Ido1 KO mice and wild‐type (WT) control littermates were obtained by breeding heterozygotes with a B6 background. Twenty male homozygous mutants, and 19 male WT control mice were used. Mice were group‐housed (2 mutants and 1 or 2 WT mice per cage) in a room with a 12‐hour light/dark cycle (lights on at 7:00 a.m.) with access to food and water ad libitum. The room temperature was kept at 23 ± 2°C.
2.2. Comprehensive behavioral test battery
Most of the behavioral tests were performed as previously described,15, 16, 17, 18 unless otherwise noted. All behavioral tests were carried out with male mice that were at least 13 weeks old at the start of testing. Raw data from the behavioral test and information about each mouse are available at the public database “Mouse Phenotype Database” (http://www.mouse-phenotype.org/). Behavioral testing was performed between 9:00 a.m. and 6:00 p.m. After the tests, the apparatus was cleaned with diluted sodium hypochlorite solution to prevent a bias due to olfactory cues. To minimize the effects of previous tests on subsequent tests, we performed the behavioral test battery in a specific order in which the less stressful tests preceded the more stressful tests. In this study, the tests were performed in the following sequence: neurological screens and neuromuscular strength test, light/dark transition test, open field test, elevated plus maze test, hot plate test, social interaction test, rotarod test, sociability and social novelty preference test, startle response/prepulse inhibition test, Porsolt forced swim test, Barnes maze test, T‐maze test, tail suspension test, and fear conditioning test. Each behavioral test was separated by at least by 1 day. The number and the age of the mice at the time of the experiment are shown in Table S1. All experimental procedures were approved by Institutional Animal Care and Use Committee of Graduate School of Medicine of Kyoto University and Fujita Health University.
2.3. Neurological screen and neuromuscular strength test
Righting, whisker twitch, and ear twitch reflexes were evaluated. Physical features, including the presence of whiskers or bald hair patches, were also recorded. A grip strength meter (O'HARA & Co., Tokyo, Japan) was used to assess forelimb grip strength. Mice were lifted by holding the tail so that their forepaws could grasp a wire grid. The mice were then gently pulled backward by the tail with their posture parallel to the surface of the table until they released the grid. The peak force applied by the forelimbs of the mouse was recorded in Newtons (N). Each mouse was tested 3 times, and the greatest value measured was used for data analysis. In the wire hang test, the mouse was placed on a wire mesh that was then slowly inverted so that the mouse gripped the wire in order to not fall off. Latency to fall (seconds) was recorded with a 60‐seconds cutoff time.
2.4. Light/dark transition test
A light/dark transition test was conducted as previously described.19 The apparatus consisted of a cage (21 × 42 × 25 cm) divided into 2 sections of equal size by a partition with a door (O'HARA & Co.). One chamber was brightly illuminated (390 lux), whereas the other chamber was dark (2 lux). Mice were placed into the dark chamber and allowed to move freely between the 2 chambers with the door open for 10 minutes. The total number of transitions, latency to first enter the lit chamber (seconds), distance traveled (cm), and time spent in each chamber (seconds) were recorded by ImageLD software (see Section, “Data Analysis”).
2.5. Open field test
Each mouse was placed in the corner of the open field apparatus (40 × 40 × 30 cm; Accuscan Instruments, Columbus, OH, USA). The apparatus was illuminated at 100 lux. Total distance traveled (cm), vertical activity (rearing measured by counting the number of photobeam interruptions), time spent (seconds) in the center area (20 × 20 cm), and beam‐break counts for stereotyped behaviors (defined by the number of breaks of the same beam) were recorded. Data were collected for 120 minutes.
2.6. Elevated plus maze test
An elevated plus maze test was conducted as previously described.20 The elevated plus maze consisted of 2 open arms (25 × 5 cm) and 2 enclosed arms of the same size with 15‐cm high transparent walls, and the arms were connected by a central square (5 × 5 cm) (O'HARA & Co.). The open arms were surrounded by a raised ledge (3 mm thick and 3 mm high) to keep mice from falling off the arms. The arms and central square were made of white plastic plates and were elevated 55 cm above the floor. Arms of the same type were located across from each other. Each mouse was placed in the central square of the maze, facing one of the enclosed arms. The number of entries into the open and enclosed arms and the time spent in the open or enclosed arms were recorded during a 10‐min test period. The percentage of entries into open arms (%), time spent in open arms (seconds), number of total arm entries, and total distance traveled (cm) were calculated. Data acquisition and analysis were performed automatically, using ImageEP software (see Section, “Data Analysis”).
2.7. Hot plate test
The hot plate test was used to evaluate mouse sensitivity to a painful stimulus. Mice were placed on a 55.0°C (±0.3°C) hotplate (Columbus Instruments, OH, USA), and latency to the first fore‐ or hind‐paw response (seconds) was recorded. The paw response was defined as either a paw lick or a foot shake.
2.8. Social interaction test
In the social interaction test, 2 mice of identical genotypes that were previously housed in different cages were placed in a box together (40 × 40 × 30 cm) (O'HARA & Co.) and allowed to explore freely for 10 minutes. Behavior was recorded and analyzed automatically using ImageSI program (see Section, “Data Analysis”). The total number of contacts, total duration of active contacts (seconds), total contact duration (seconds), mean duration per contact (seconds), and total distance traveled (cm) were measured. If the 2 mice contacted each other and the distance traveled by either mouse was longer than 10 cm, the behavior was classified as an “active contact.” Images were captured at 3 frames per sec, and distance traveled between 2 successive frames was calculated for each mouse.
2.9. Rotarod test
Motor coordination and balance were tested with the rotarod test. This test, which uses an accelerating rotarod (UGO Basile, Comerio, VA, Italy), was performed by placing mice on rotating drums (3 cm diameter) and measuring how long each animal was able to maintain its balance on the rod. The speed of the rotarod accelerated from 4 to 40 rpm over a 5‐min period.
2.10. Sociability and social novelty preference test
This test is a well‐designed method to investigate the effect of complex genetics on sociability and preference for social novelty.21, 22 The testing apparatus consisted of a rectangular, three‐chambered box and a lid with a video camera (O'HARA & Co.). Each chamber was 20 × 40 × 47 cm, and the dividing walls were made from clear Plexiglas, with small square openings (5 × 3 cm) allowing access into each chamber. We modified the method described by Moy et al22 as follows: a habituation session was performed in the apparatus for 10 minutes on the day before the sociability test, and empty wire cages (11 cm in height, a bottom diameter of 9 cm, and vertical bars 0.5 cm apart) were located in the corner of each side chamber. In the sociability test, an unfamiliar C57BL/6J male mouse that had no prior contact with the test mice was enclosed in the wire cage and placed in one of the side chambers. The wire cage allowed nose contact between the bars, but it prevented the stranger mouse from initiating any social contact and limited the possibility of aggressive behavior. The test mouse was first placed in the middle chamber and allowed to explore the entire box for a 10‐min session to assess sociability. After the first 10‐min session, the test mouse was returned to the holding cage. The wire cage containing the novel unfamiliar mouse was transferred to the chamber that had been empty during the first 10‐min session. In the social novelty preference test, the test mouse was placed in the apparatus for a 10‐min session to quantify social preference for a new stranger. The test mouse thus had a choice between the first, already‐investigated unfamiliar mouse and the novel unfamiliar mouse. The amount of time spent (seconds) in each chamber and time spent around each cage were measured automatically using the ImageCSI program (see Section, “Data Analysis”).
2.11. Startle response/prepulse inhibition test
A startle reflex measurement system (O'HARA & Co.) was used to measure acoustic startle response and prepulse inhibition. A test session began by placing a mouse in a plastic cylinder, where it was left undisturbed for 10 minutes. White noise (40 milliseconds) was used as the startle stimulus for all trial types. The startle response was recorded for 400 milliseconds (measuring the response every 1 milliseconds), starting with the onset of the startle stimulus. The background noise level in each chamber was 70 dB. The peak startle amplitude recorded during the 100‐milliseconds sampling window was used as the dependent variable. A test session consisted of 6 trial types (ie, 2 types for startle stimulus‐only trials, and 4 types for prepulse inhibition trials). The intensity of the startle stimulus was 110 or 120 dB. The prepulse sound was presented 100 milliseconds before the startle stimulus, and its intensity was 74 or 78 dB. Four combinations of prepulse and startle stimuli were used (74‐110, 78‐110, 74‐120, and 78‐120 dB). Six blocks of the 6 trial types were presented in a pseudo‐random order, such that each trial type was presented once within a block. The average inter‐trial interval was 15 seconds (range 10‐20 seconds).
2.12. Porsolt forced swim test
A transparent plastic cylinder (20 cm height × 10 cm diameter) filled with water (21‐23°C) up to a height of 7.5 cm was put in a white plastic chamber (31 × 41 × 41 cm) (O'HARA & Co.). The mouse was placed into the cylinder, and the immobility (%) and distance traveled (cm) were recorded over a 10‐min test period. Images were captured at 2 frames per second. For each pair of successive frames, the area (pixels) within which the mouse moved was measured. When the area was below a certain threshold, the mouse behavior was classified as “immobile.” When the area equaled or exceeded the threshold, the mouse was classified as “moving.” The optimal threshold to judge moving was determined by adjusting it to the immobility measured by human observation. Immobility lasting for less than 2 seconds was not included in the analysis. Data acquisition and analysis were performed automatically using ImagePS software (see Section, “Data Analysis”).
2.13. Barnes maze test
The Barnes maze task was conducted on “dry land,” a white circular surface that was 1.0 m in diameter, with 12 holes equally spaced around the perimeter (O'HARA & Co.). A black Plexiglas escape box (17 × 13 × 7 cm) with paper cage bedding on its bottom was located under one of the holes. The hole above the escape box represented the target, which was analogous to the hidden platform in the Morris water maze task. The location of the escape box (target) was consistent for a given mouse but was randomized across mice. The mice that could not find the box were guided to it and allowed to enter it to remain there for 30 seconds. One or two trials per day were conducted for 19 days. Each trial ended when the mouse entered the escape box or after 5 minutes had elapsed. In each trial, the latency to first reach the target hole (seconds), number of errors to reach the target hole, number of omission errors, and distance travelled to first reach the target hole (cm) were recorded by the ImageBM program. On day 20, a probe test was conducted without the escape box to assess memory based on distal environmental room cues. Another probe trial was conducted 30 days after the last training session to evaluate memory retention. The time spent around the target hole was recorded in these probe tests by the software.
2.14. T‐maze test
The spontaneous alternation task was conducted using an automatic T‐maze apparatus (O'HARA & Co.). It was constructed of white plastic runways with 25‐cm high walls. The maze was partitioned off into 6 areas by sliding doors that can be opened downward. The stem of the T was composed of area S2 (13 × 24 cm), and the arms of T were composed of areas A1 and A2 (11.5 × 20.5 cm). Areas P1 and P2 were the connecting passageways from the respective arm (area A1 or A2) to the start compartment (area S1). Mice were subjected to a spontaneous alternation protocol for 7 days (one session consisting of 10 trials per day; cutoff time, 50 minutes). Each trial had first and second runs. On the sample run, the mouse was forced to choose one of the arms of the T (area A1 or A2). After the mouse stayed more than 10 seconds, the door that separated the arm (area A1 or A2) and the connecting passageway (area P1 or P2) would be opened, and the mouse could return to the starting compartment (area S1) via the connecting passageway. The mouse was then given a 3‐sec delay in area S1, followed by a free choice between both T arms. The percentage of trials in which mice entered the arm opposite to their forced‐choice run during the free choice run was calculated. The location of the sample arm (left or right) varied pseudo‐randomly across trials using the Gellermann schedule so that mice received equal numbers of left and right presentations. A variety of fixed extra‐maze clues surrounded the apparatus. Data acquisition, control of sliding doors, and data analysis were performed by ImageTM software (see Section, “Data Analysis”).
2.15. Tail suspension test
The tail suspension test was performed for a 10‐min test session. Mice were suspended 30 cm above the floor of a white plastic chamber (31 × 41 × 41 cm) (O'HARA & Co.) in a visually isolated area by adhesive tape placed ~1 cm from the tip of the tail, and the behavior was recorded over a 10‐min test period. Images were captured at 2 frames per second. As similar to the Porsolt forced swim test, immobility (%) was judged by the application program according to a certain threshold. Immobility lasting for less than a 2 seconds was not included in the analysis. Data acquisition and analysis were performed automatically using ImageTS software (see Section “Data Analysis”).
2.16. Contextual and cued fear conditioning test
Each mouse was placed in a transparent acrylic chamber (33 × 25 × 28 cm) with a stainless‐steel grid floor (0.2 cm diameter, spaced 0.5 cm apart) (O'HARA & Co.) illuminated at 100 lux and allowed to explore freely for 2 minutes. A 55‐dB white noise, which served as the conditioned stimulus (CS), was presented for 30 seconds, followed by a mild (2 seconds, 0.3 mA) foot shock, which served as the unconditioned stimulus (US). Two more CS‐US pairings were presented with a 2‐minute inter‐stimulus interval. A context test was conducted 24 hours after conditioning in the same chamber. A cued test with altered context was conducted after conditioning in a triangular box (33 × 29 × 32 cm) made of white Plexiglas, which was located in a different room. The chamber of the test was illuminated at 30 lux. Data acquisition, control of stimuli (ie, white noises and shocks), and data analysis were performed automatically using ImageFZ software (see “Data Analysis”). Images were captured at 1 frame per sec. For each pair of successive frames, the area (pixels) in which the mouse moved was measured. When this area was below a certain threshold (ie, 5 pixels), the behavior was judged as “freezing.” When the amount of area equaled or exceeded the threshold, the behavior was considered to be “non‐freezing.” The optimal threshold (amount of pixels) to judge freezing was determined by adjusting it to the amount of freezing measured by human observation. “Freezing” that lasted less than the defined time threshold (ie, 2 seconds) was not included in the analysis.
2.17. Data analysis
Behavioral data were obtained automatically through ImageJ‐based programs, which have been developed and modified by Tsuyoshi Miyakawa (available through O'HARA & Co., and partially available for download at Mouse Phenotype Database). Statistical analysis was conducted using StatView (SAS Institute, Cary, NC, USA). Data were analyzed using either one‐way ANOVA, or two‐way repeated‐measures ANOVA. Values in the graphs are expressed as the mean ± SEM. For the issue of multiple comparisons in the behavioral test battery, we defined “study‐wide significance” as the statistical significance that survived the false discovery rate approach.23, 24 “Nominal significance” was defined as one that achieved a statistical significance in an index (P < .05) but did not survive this approach.
3. RESULTS
3.1. General characterization of Ido1 KO mice
Ido1 KO mice and their WT littermates were subjected to a comprehensive battery of behavioral tests. There were no significant differences between the Ido1 KO and WT mice in body weight (Figure 1A, F 1,37 = 0.267, P = .6088), body temperature (Figure 1B, F 1,37 = 1.076, P = .3062), grip strength score (Figure 1C, F 1,37 = 1.887, P = .1779), or latency to fall off the wire grid (Figure 1D, F 1,37 < 0.001, P = .998). As shown in Figure 1E, no obvious difference between the Ido1 KO and WT mice was found in motor function/learning (F 1,37 = 0.280, P = .6001), as assessed by the rotarod test. There was no significant difference between the Ido1 KO and WT mice in pain sensitivity in the hot plate test (Figure 1F, F 1,36 = 0.89, P = .3517).
Figure 1.
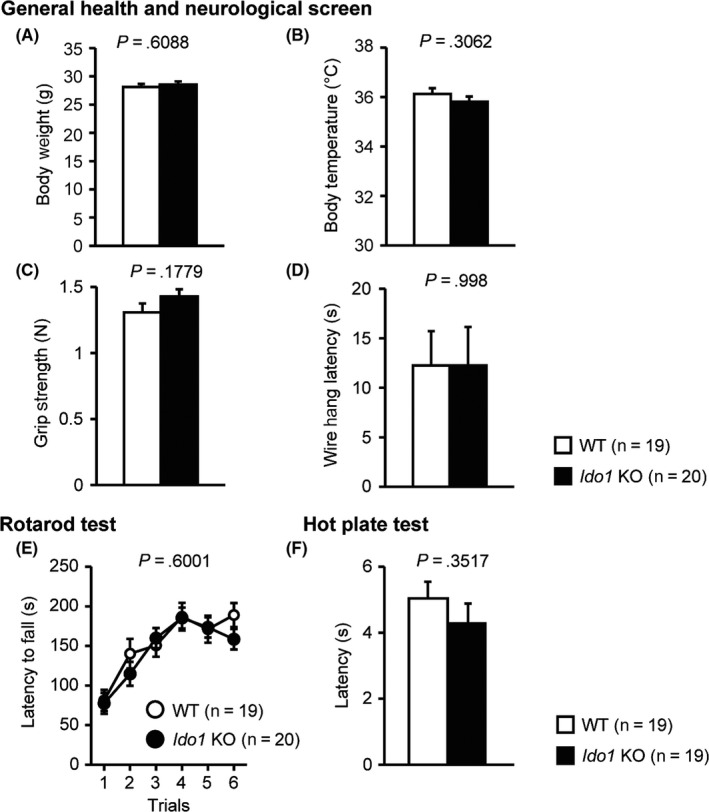
General health and neurological screen, motor function/learning, and pain sensitivity in Ido1 KO mice and WT mice. A, Body weight (g); B, Body temperature (°C); C, Grip strength (N); D, Wire hang latency (s); E, Latency to fall off the rotating rod in the rotarod test (s); F, Latency of the first fore‐ or hind‐paw response in the hot plate test (s). Data represent the mean ± SEM. The P values indicate a genotype effect in one‐way ANOVA (A‐D, F) or two‐way repeated‐measures ANOVA (E)
3.2. Normal locomotor activity and anxiety‐like behavior in Ido1 KO mice
In the light/dark transition test, there were no significant differences between the Ido1 KO and WT mice in distance traveled in the light/dark compartments (Figure 2A, light; F 1,37 = 0.871, P = .3568, dark; F 1,37 = 0.565, P = .4569), time spent in the light compartment (Figure 2B, F 1,37 = 0.489, P = .4885), number of transitions between light/dark compartment (Figure 2C, F 1,37 = 0.789, P = .3801), or latency to enter the light compartment (Figure 2D, F 1,37 = 0.416, P = .523).
Figure 2.
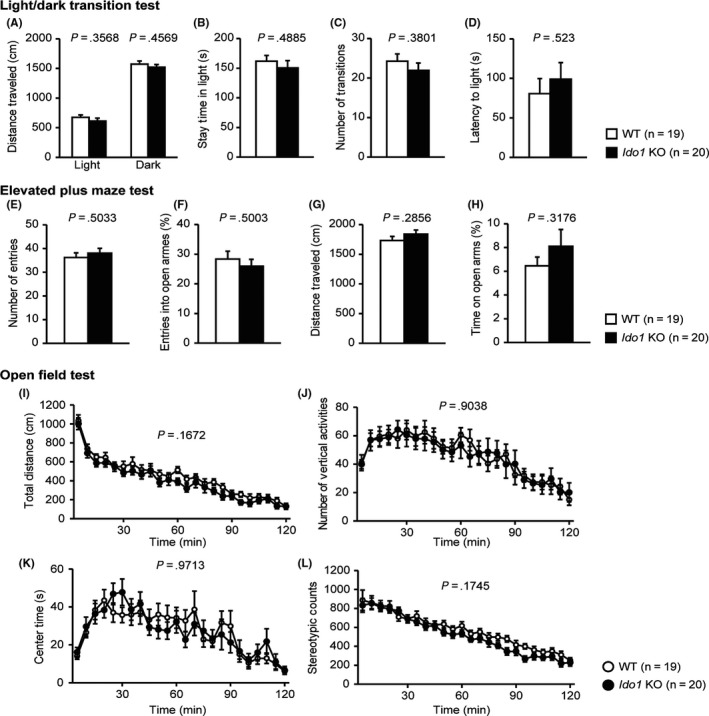
Normal locomotor activity and anxiety‐like behavior in Ido1 KO mice. A‐D, Light/dark transition test: A, Distance traveled in the light/dark compartments (cm); B, Time spent in the light compartment (s); C, Number of light/dark transitions; D, Latency to enter the light compartment (s); E‐H, Elevated plus maze test: E, Number of arm entries; F, Percentage of entries into open arms (%); G, Distance traveled (cm); H, Percentage of time spent in open arms (%); I‐L, Open field test: I, Total distance traveled (cm); J, Vertical activity; K, Time spent in the center area (s); L, Stereotypic behavior counts. Data represent the mean ± SEM. The P values indicate a genotype effect in one‐way ANOVA (A‐H) or two‐way repeated‐measures ANOVA (I‐L)
In the elevated plus maze test, no significant differences between the Ido1 KO and WT mice were found in number of entries (Figure 2E, F 1,37 = 0.457, P = .5033), percentage of entries into open arms (Figure 2F, F 1,37 = 0.463, P = .5003), total distance traveled in arms (Figure 2G, F 1,37 = 1.174, P = .2856), or the percentage of time spent in open arms (Figure 2H, F 1,37 = 1.026, P = .3176).
In the open field test, Ido1 KO and WT mice showed no significant differences in total distance (Figure 2I; F 1,37 = 1.985, P = .1672), number of vertical activities (Figure 2J; F 1,37 = 0.015, P = .9038), center time (Figure 2K; F 1,37 = 0.001, P = .9713), or stereotypic counts (Figure 2L; F 1,37 = 1.917, P = .1745).
3.3. Social behaviors in Ido1 KO mice
In the social interaction test, there were no significant differences between the Ido1 KO and WT mice in total duration of contacts (Figure 3A, F 1,13 = 0.029, P = .8675), number of contacts (Figure 3B, F 1,13 = 0.037, P = .8498), total duration of active contacts (Figure 3C, F 1,13 = 0.058, P = .8133), mean duration per contact (Figure 3D, F 1,13 = 0.255, P = .622), or distance traveled (Figure 3E, F 1,13 = 0.175, P = .6823).
Figure 3.
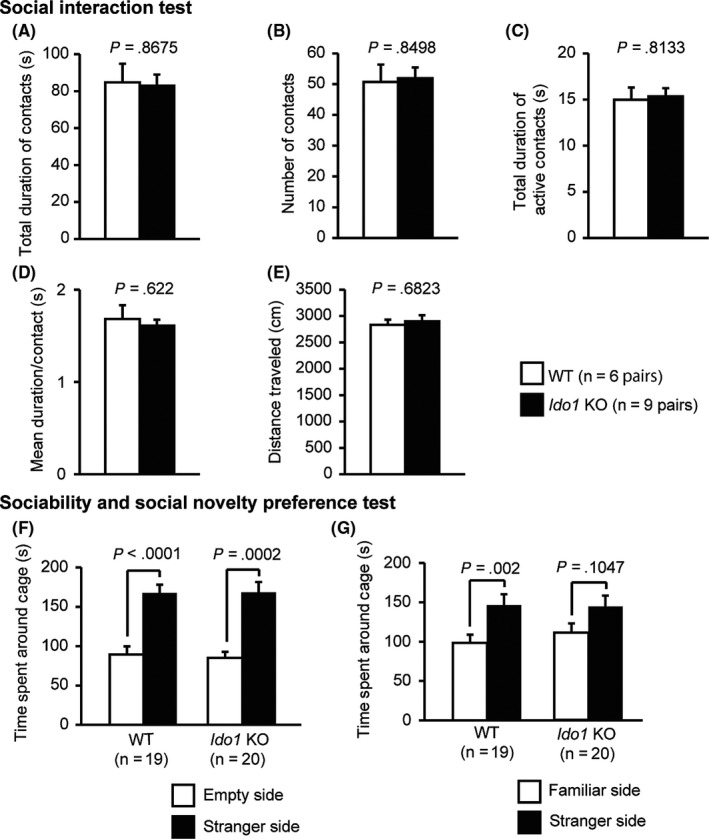
Social behaviors in Ido1 KO mice. A‐E, Social interaction test: A, Total duration of contacts (s); B, Number of contacts; C, Total duration of active contacts (s). (D) Mean duration per contact (s). (E) Total distance traveled (cm); (F, G) Sociability test and social novelty preference test: F, Time spent around the cage in the sociability test (s); G, Time spent around the cage in the social novelty preference test (s). Data represent the mean ± SEM. The P values indicate a genotype effect in one‐way ANOVA
The three‐chamber social approach test is composed of a sociability test and a social novelty preference test. In the sociability test, social behavior can be assessed based on the time spent around a wire cage with an unfamiliar mouse (stranger‐side) vs the time spent around an empty cage (empty side).22 In the sociability test, both the Ido1 KO and WT mice spent more time around the stranger‐side cage than empty side cage (Figure 3F, WT: t 18 = 5.287, P < .0001, Ido1 KO: t 19 = 4.62, P = .0002). In the social novelty preference test, WT mice spent more time around the stranger‐side cage than the familiar‐side cage (Figure 3G, t 18 = 3.62, P = .002), whereas Ido1 KO mice showed no significant differences in the time spent around the familiar‐side cage nor the stranger‐side cage (Figure 3G, t 19 = 1.704, P = .1047). However, the genotype effect failed to reach significance in an index of the ratio of the time spent around the familiar‐side divided by the stranger‐side (F 1,37 = 1.323, P = .2574).
3.4. Normal depression‐like behavior and normal acoustic startle response in Ido1 KO mice
In the Porsolt forced swim test, there were no significant differences between the Ido1 KO and WT mice on the percentage of immobility time on day 1 and day 2 (Figure 4A, F 1,37 = 0.856, P = .3609 and F 1,37 = 2.231, P = .1437, respectively). Likewise, no significant effects due to genotype were found in the distance traveled on day 1 and day 2 (Figure 4B, F 1,37 = 3.425, P = .0722 and F 1,37 = 1.234, P = .2738, respectively).
Figure 4.
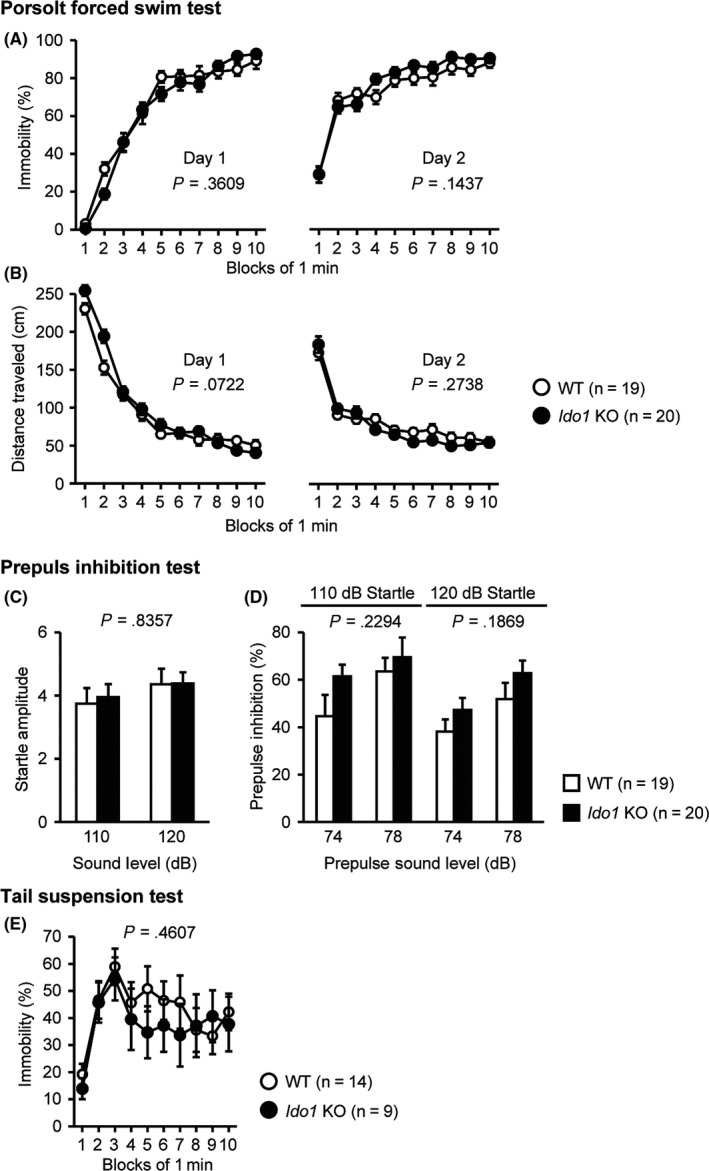
Normal depression‐like behavior and acoustic startle response in Ido1 KO mice. (A, B) Porsolt forced swim test: (A) Percentage of immobility time on day 1 and day 2 (%). (B) Total distance traveled on day 1 and day 2 (cm). (C, D) Prepulse inhibition test: (C) Amplitude of the startle response. (D) Percentage of prepulse inhibition (%). (E) Percentage of immobility time in the tail suspension test (%). Data represent the mean ± SEM. The P values indicate a genotype effect in two‐way repeated‐measures ANOVA
In the prepulse inhibition test, there was no significant difference between Ido1 KO and WT mice on the startle amplitude (F 1,37 = 0.044, P = .8357). No significant effects of genotype were found in the prepulse inhibition of the startle response between Ido1 KO and WT mice (Figure 4D, 110 dB, F 1,37 = 1.494, P = .2294; 120 dB, F 1,37 = 1.809, P = .1869).
In the tail suspension test, the Ido1 KO and WT mice showed no obvious difference in the percentage of immobility time (Figure 4E, F 1,21 = 0.565, P = .4607).
3.5. Normal spatial reference memory in Ido1 KO mice
In the Barnes maze test, there were no significant differences between Ido1 KO and WT mice in latency to reach the target hole (Figure 5A, F 1,37 = 0.705, P = .4064), number of errors to reach the target hole (Figure 5B, F 1,37 = 0.011, P = .9157), distance traveled to reach the target hole (Figure 5C, F 1,37 = 0.18, P = 6737), or number of omission errors (Figure 5D, F 1,37 = 0.074, P = .7877). Probe trials where the escape box was removed were performed 1 day and 1 month after the last day of training. No significant effects due to genotype were found in the time spent around the target during these probe tests (Figure 5E, 1 day, F 1,37 = 1.145, P = .2916; 1 month, F 1,37 = 0.283, P = .5982).
Figure 5.
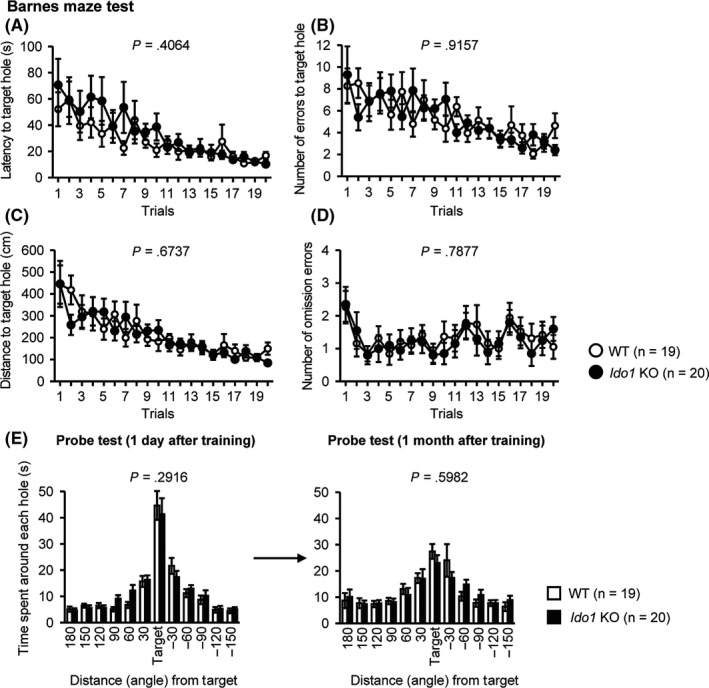
Normal spatial reference memory in Ido1 KO mice. A, Latency to reach the target hole (s); B, Number of errors before reaching the target hole; C, Distance to reach the target hole (cm); D, Number of omission errors before reaching the target hole. Data were analyzed by two‐way repeated‐measures ANOVA. The P values indicate a genotype effect in a two‐way repeated‐measures ANOVA; E, Time spent around each hole in the probe trial conducted 1 day (left) and 1 month (right) after the last training session (s). The P values indicate a genotype effect in one‐way ANOVA
3.6. Normal working and fear memory in Ido1 KO mice
A T‐maze using a spontaneous alternation task is used to evaluate working memory.25 In the T‐maze spontaneous alternation test, the percentages of correct responses of Ido1 KO mice were lower than those of WT mice, but this result failed to reach study‐wide significance after correction for multiple testing (Figure 6A, F 1,21 = 4.954, P = .0371). There was no significant genotype × session interaction (F 6,126 = 0.65, P = .6903). Ido1 KO and WT mice showed no obvious differences in latency to complete a session (Figure 6B, F 1,21 = 1.497, P = .2347) or distance traveled to complete a session (Figure 6C, F 1,21 = 0.007, P = .9333).
Figure 6.
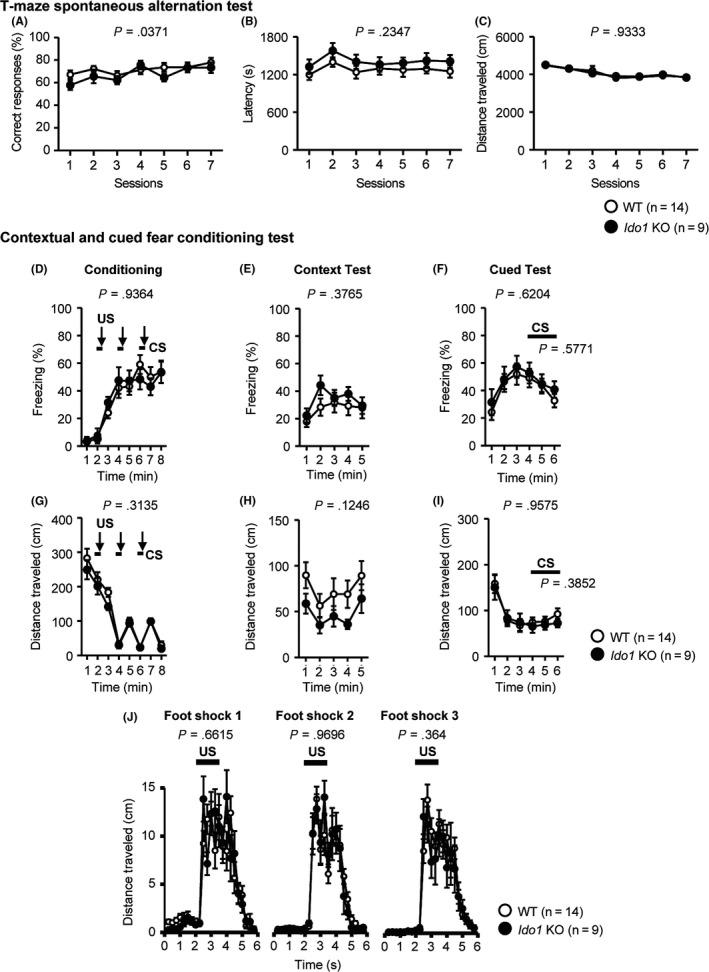
Normal working and fear memory in Ido1 KO mice. A‐C, T‐maze spontaneous alternation test: A, Percentage of correct responses (%); B, Latency to complete a session (s); C, Distance traveled to complete a session (cm); D‐J, Contextual and cued fear conditioning test: D, Percentage of freezing time (%); G, Distance traveled in the conditioning (cm); E, Percentage of freezing time (%); H, Distance traveled in context test (cm); F, Percentage of freezing time (%); I, Distance traveled in cued test (cm); J, Distance traveled during and after shock in the conditioning phase (cm). Data represent the mean ± SEM. The P values indicate a genotype effect in two‐way repeated‐measures ANOVA
In the contextual and cued fear conditioning test, Ido1 KO mice showed no significant differences in the percentages of freezing in the conditioning (Figure 6D, F 1,21 = 0.007, P = .9364), context test (Figure 6E, F 1,21 = 0.817, P = .3765), or cued test (Figure 6F, 1 to 3 min: F 1,21 = 0.253, P = .6204, CS: F 1,21 = 0.321, P = .5771) compared with WT mice. No significant effects due to genotype were found in the distance traveled in the conditioning (Figure 6G, F 1,21 = 1.067, P = .3135), context test (Figure 6H, F 1,21 = 2.559, P = .1246), or cued test (Figure 6I, 1 to 3 min: F 1,21 = 0.003, P = .9575, CS: F 1,21 = 0.786, P = .3852). There were no significant differences between the Ido1 KO mice and WT in the distance traveled during or after each foot shock in the conditioning period (Figure 6J, foot shock 1, F 1,21 = 0.197, P = .6615; foot shock 2, F 1,21 = 0.001, P = .9696; foot shock 3, F 1,21 = 0.861, P = .364).
4. DISCUSSION
In this study, we investigated the behavioral phenotype of Ido1 KO mice by a comprehensive test battery under normal conditions. Ido1 KO mice did not show significant behavioral differences compared to WT control mice in any of the tests, except in the T‐maze spontaneous alternation test. In the T‐maze test, Ido1 KO mice showed a slight but nominally significant deficit in working memory, which failed to reach study‐wide significance after multiple testing correction. In the social novelty preference test, WT mice spent more time around the stranger‐side cage than the familiar‐side cage, whereas Ido1 KO mice showed no significant difference between the spent time around the stranger‐side and familiar‐side cage. However, the genotype effect failed to reach significance.
In a previous study, Too et al26 reported that Ido1 KO mice displayed a decrease in early diurnal exploratory activity. This result is seemingly inconsistent with our results, which did not show hypoactivity in any of the 7 tests that measured distance traveled. The apparent inconsistency between our results and those reported by Too et al might be due to differences in experimental and/or environmental conditions. Too et al analyzed the distance traveled of Ido1 KO using IntelliCage™, which automatically measures distance traveled and frequency of entry into a rewarding water corner in a familiar environment. In contrast, most of our tests were conducted in a novel environment, and this difference might have caused the apparent inconsistency between our results and those reported by Too et al Another possible cause of this inconsistency might be the different genetic background or the number of backcrosses to the B6 background of the mice used in this study.27, 28, 29 Too et al used Ido1 KO mice derived from 129/SvJ ES cells mated with B6 or CBA mice without a sufficient number of backcrossings, whereas we used Ido1 KO mice derived from 129/SvJ ES cells mated with B6 females and backcrossed with more than 10 generations of B6 mice. Genetic background may have a profound influence on behavioral phenotypes, and flanking genes might be responsible for the different phenotypes observed in mutant mice.30, 31
Activated IDO, after immune stimuli, increases KP metabolites,3, 4, 7 and these metabolites cause a behavioral alteration.32, 33 This behavioral alteration is suppressed by treatment with inhibitors of KP metabolism.13, 33 WT mice treated with lipopolysaccharide (LPS) or Bacille Calmette‐Guerin (BCG) showed depression‐like behavior and cognitive impairment, but Ido1‐knockout mice did not show these alterations.12, 32, 33 It is of interest to investigate the behavioral phenotypes of Ido1 KO mice under inflammatory stimuli or treatment with an inhibitor of KP metabolism using our comprehensive behavioral test battery in the future.
The essential amino acid tryptophan is metabolized by TDO2 (expressed in the kidney and liver) and IDO1 (distributed in many tissues, including brain).10 Normally, the oxidizing ability of TDO2, that is its ability to metabolize tryptophan, is approximately threefold greater than that of IDO1.34 Tdo2 deletion disturbs the KP and causes more behavioral alterations, such as lower anxiety‐like behavior and higher locomotor activity under basal conditions, than Ido1 deletion.11, 35 IDO1 activity is quite low in the absence of immune stimuli,36, 37 which may underlie the lack of an obvious behavioral phenotype in Ido1 KO mice.
In conclusion, our comprehensive behavioral analysis showed that no significant differences between Ido1 KO and WT mice in any of the tests, except in the T‐maze spontaneous alternation test, where the KO mice showed nominally significant working memory deficit. These results indicate that Ido1 may not play significant roles in behavior regulation under normal conditions.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
DATA REPOSITORY
Raw data on the behavioral test and the information about each mouse are accessible on the public database “Mouse Phenotype Database” (http://www.mouse-phenotype.org/).
APPROVAL OF THE RESEARCH PROTOCOL BY AN INSTITUTIONAL REVIEWER BOARD
n/a.
INFORMED CONSENT
n/a.
REGISTRY AND THE REGISTRATION NO. OF THE STUDY/TRIAL
n/a.
ANIMAL STUDIES
All behavioral testing procedures were approved by Institutional Animal Care and Use Committee of Graduate School of Medicine of Kyoto University and Fujita Health University.
AUTHOR CONTRIBUTIONS
TM was responsible for the original conception and overall design of the research. HF supplied the mutant mice. SH and HS performed the comprehensive behavioral test battery. NH, SH, HS, and TM analyzed the data. NH and TM wrote the manuscript. All authors read and approved the final manuscript.
Supporting information
ACKNOWLEDGMENT
This work was supported by a Grant‐in‐Aid for Scientific Research (A) (16680015) and Grant‐in‐Aid for Scientific Research on Innovative Areas (Comprehensive Brain Science Network) from the Ministry of Education, Science, Sports and Culture of Japan from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan. This research was also supported by AMED under Grant Number JP17dm0107101, and JSPS KAKENHI Grant Number JP 16H06276. Behavioral analysis was carried out at the Institute for Comprehensive Medical Science, Fujita Health University, by the Joint Usage/Research Center for Genes, Brain and Behavior, which is accredited by MEXT.
Hirata N, Hattori S, Shoji H, Funakoshi H, Miyakawa T. Comprehensive behavioral analysis of indoleamine 2,3‐dioxygenase knockout mice. Neuropsychopharmacol Rep. 2018;38:133–144. 10.1002/npr2.12019
REFERENCES
- 1. Badawy AA‐B. Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects. Int J Tryptophan Res. 2017;10:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schwarcz R, Bruno JP, Muchowski PJ, Wu H‐Q. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13:465–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guidetti P, Hemachandra Reddy P, Tagle DA, Schwarcz R. Early kynurenergic impairment in Huntington's Disease and in a transgenic animal model. Neurosci Lett. 2000;283:233–5. [DOI] [PubMed] [Google Scholar]
- 4. Bagasrawala I, Zecevic N, Radonjić NV. N‐Methyl d‐aspartate receptor antagonist kynurenic acid affects human cortical development. Front Neurosci. 2016;10:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hilmas C, Pereira EFR, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits α7 nicotinic receptor activity and increases non‐α7 nicotinic receptor expression: physiopathological implications. J Neurosci. 2001;21:7463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parsons CG, Danysz W, Quack G, et al. Novel systemically active antagonists of the glycine site of the N‐Methyl‐d‐aspartate receptor: electrophysiological, biochemical and behavioral characterization. J Pharmacol Exp Ther. 1997;283:1264–75. [PubMed] [Google Scholar]
- 7. Guillemin GJ. Quinolinic acid, the inescapable neurotoxin. FEBS J. 2012;279:1356–65. [DOI] [PubMed] [Google Scholar]
- 8. Stone TW, Perkins MN. Quinolinic acid: a potent endogenous excitant at amino acid receptors in CNS. Eur J Pharmacol. 1981;72:411–2. [DOI] [PubMed] [Google Scholar]
- 9. Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dang Y, Dale WE, Brown OR. Comparative effects of oxygen on indoleamine 2,3‐dioxygenase and tryptophan 2,3‐dioxygenase of the kynurenine pathway. Free Radic Biol Med. 2000;28:615–24. [DOI] [PubMed] [Google Scholar]
- 11. Hattori S, Takao K, Funakoshi H, Miyakawa T. Comprehensive behavioral analysis of tryptophan 2,3‐dioxygenase (Tdo2) knockout mice. Neuropsychopharmacol Rep. 2018;38:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O'Connor JC, Lawson MA, André C, et al. Induction of IDO by bacille calmette‐guérin is responsible for development of murine depressive‐like behavior. J Immunol. 2009;182:3202–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O'Connor JC, Lawson MA, André C, et al. Lipopolysaccharide‐induced depressive‐like behavior is mediated by indoleamine 2,3‐dioxygenase activation in mice. Mol Psychiatry. 2009;14:511–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baban B, Chandler P, McCool D, Marshall B, Munn DH, Mellor AL. Indoleamine 2,3‐dioxygenase expression is restricted to fetal trophoblast giant cells during murine gestation and is maternal genome specific. J Reprod Immunol. 2004;61:67–77. [DOI] [PubMed] [Google Scholar]
- 15. Nakao A, Miki T, Shoji H, et al. Comprehensive behavioral analysis of voltage‐gated calcium channel beta‐anchoring and ‐regulatory protein knockout mice. Front Behav Neurosci. 2015;9:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shoji H, Takao K, Hattori S, Tsuyoshi M. Age‐related changes in behavior in C57BL/6J mice from young adulthood to middle age. Mol Brain. 2016;9:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shoji H, Irino Y, Yoshida M, Miyakawa T. Behavioral effects of long‐term oral administration of aluminum ammonium sulfate in male and female C57BL/6J mice. Neuropsychopharmacol Rep. 2018;38:18–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yoshioka N, Miyata S, Tamada A, et al. Abnormalities in perineuronal nets and behavior in mice lacking CSGalNAcT1, a key enzyme in chondroitin sulfate synthesis. Mol Brain. 2017;10:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takao K, Miyakawa T. Light/dark transition test for mice. J Vis Exp. 2006;1:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Komada M, Takao K, Miyakawa T. Elevated plus maze for mice. J Vis Exp. 2008;22:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Crawley JN. Designing mouse behavioral tasks relevant to autistic‐like behaviors. Ment Retard Dev Disabil Res Rev. 2004;10:248–58. [DOI] [PubMed] [Google Scholar]
- 22. Moy SS, Nadler JJ, Perez A, et al. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic‐like behavior in mice. Genes Brain Behav. 2004;3:287–302. [DOI] [PubMed] [Google Scholar]
- 23. Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–84. [DOI] [PubMed] [Google Scholar]
- 24. Kafkafi N, Lipkind D, Benjamini Y, Mayo CL, Elmer GI, Golani I. SEE locomotor behavior test discriminates C57BL/6J and DBA/2J mouse inbred strains across laboratories and protocol conditions. Behav Neurosci. 2003;117:464–77. [DOI] [PubMed] [Google Scholar]
- 25. Shoji H, Hagihara H, Takao K, Hattori S, Miyakawa T. T‐maze forced alternation and left‐right discrimination tasks for assessing working and reference memory in mice. J Vis Exp. 2012;60:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Too LK, Li KM, Suarna C, et al. Deletion of TDO2, IDO‐1 and IDO‐2 differentially affects mouse behavior and cognitive function. Behav Brain Res. 2016;312:102–17. [DOI] [PubMed] [Google Scholar]
- 27. Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–2. [DOI] [PubMed] [Google Scholar]
- 28. Crawley JN, Belknap JK, Collins A, et al. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology. 1997;132:107–24. [DOI] [PubMed] [Google Scholar]
- 29. Matsuo N, Takao K, Nakanishi K, Yamasaki N, Tanda K, Miyakawa T. Behavioral profiles of three C57BL/6 substrains. Front Behav Neurosci. 2010;4:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gerlai R. Gene‐targeting studies of mammalian behavior: is it the mutation or the background genotype? Trends Neurosci. 1996;19:177–81. [DOI] [PubMed] [Google Scholar]
- 31. Gerlai R. Gene targeting using homologous recombination in embryonic stem cells: the future for behavior genetics? Front Genet. 2016;7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heisler JM, O'Connor JC. Indoleamine 2,3‐dioxygenase‐dependent neurotoxic kynurenine metabolism mediates inflammation‐induced deficit in recognition memory. Brain Behav Immun. 2015;50:115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Salazar A, Gonzalez‐Rivera BL, Redus L, Parrott JM, O'Connor JC. Indoleamine 2,3‐dioxygenase mediates anhedonia and anxiety‐like behaviors caused by peripheral lipopolysaccharide immune challenge. Horm Behav. 2012;62:202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maeta A, Sano M, Fukuwatari T, Funakoshi H, Nakamura T, Shibata K. Contributions of tryptophan 2,3‐dioxygenase and indoleamine 2,3‐dioxygenase to the conversion of d‐tryptophan to nicotinamide analyzed by using tryptophan 2,3‐dioxygenase‐knockout mice. Biosci Biotechnol Biochem. 2014;78:878–81. [DOI] [PubMed] [Google Scholar]
- 35. Funakoshi H, Kanai M, Nakamura T. Modulation of tryptophan metabolism, pomotion of neurogenesis and alteration of anxiety‐related behavior in tryptophan 2,3‐dioxygenase‐deficient mice. Int J Tryptophan Res. 2011;4:7–18. [Google Scholar]
- 36. Yoshida R, Hayaishi O. Induction of pulmonary indoleamine 2,3‐dioxygenase by intraperitoneal injection of bacterial lipopolysaccharide. Proc Natl Acad Sci U S A. 1978;75:3998–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yoshida R, Urade Y, Tokuda M, Hayaishi O. Induction of indoleamine 2,3‐dioxygenase in mouse lung during virus infection. Proc Natl Acad Sci U S A. 1979;76:4084–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


