Abstract
Aims
Parkinson's disease (PD) is a chronic and progressive neurodegenerative disorder. Although diet may influence the development of PD, the precise mechanisms underlying relationship between diet and PD pathology are unknown. Here, we examined whether dietary intake of glucoraphanin (GF), the precursor of a natural antioxidant sulforaphane in cruciferous vegetables, can affect the reduction of dopamine transporter (DAT) in the mouse striatum after repeated administration of MPTP (1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine).
Methods
Normal food pellet or 0.1% GF food pellet was given into male mice for 28 days from 8‐week‐old. Subsequently, saline (5 mL/kg × 3, 2‐hour interval) or MPTP (10 mg/kg × 3, 2‐hour interval) was injected into mice. Immunohistochemistry of DAT in the striatum was performed 7 days after MPTP injection.
Results
Repeated injections of MPTP significantly decreased the density of DAT‐immunoreactivity in the mouse striatum. In contrast, dietary intake of 0.1% GF food pellet significantly protected against MPTP‐induced reduction of DAT‐immunoreactivity in the striatum.
Conclusion
This study suggests that dietary intake of GF food pellet could prevent MPTP‐induced dopaminergic neurotoxicity in the striatum of adult mice. Therefore, dietary intake of GF‐rich cruciferous vegetables may have beneficial effects on prevention for development of PD.
Keywords: dopamine transporter, glucoraphanin, nutrition, Parkinson disease, sulforaphane
Oxidative stress plays a role in the neurotoxicity of MPTP in the striatum. Dietary intake of glucoraphanin could prevent the MPTP‐induced neurotoxicity in mouse striatum. Dietary intake of glucoraphanin‐rich vegetables may have beneficial effects on prevention of Parkinson's disease.
![]()
1. INTRODUCTION
Parkinson's disease (PD) is a common and progressive neurodegenerative disease that affects predominately dopamine‐producing neurons in the striatum.1, 2 Although the precise mechanisms underlying PD pathology are unknown, environmental factors play a role in the pathology of PD. Importantly, diet is an excellent first step for reducing the risk for PD.3, 4 For example, a high intake of fresh vegetables, fruits, nuts and seeds, fish, olive oil, coconut oil, fresh herbs, and spices is associated with a reduced risk of PD development and slower disease progression.5, 6
Accumulating evidence suggests that the transcription factor Kelch‐like erythroid cell‐derived protein with CNC homology (ECH)‐associated protein 1 (Keap1)‐Nuclear factor (erythroid‐derived 2)‐like 2 (Nrf2) system plays a key role in inflammation which is involved in a number of neurological and psychiatric disorders.7, 8, 9, 10, 11 Multiple lines of evidence suggest a key role of Nrf2 in the pathology of PD.12, 13, 14 Jazwa et al15 reported that sulforaphane (SFN), a potent Nrf2 activator, protects against dopaminergic neurotoxicity in mice after administration of MPTP (1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine). Furthermore, SFN did not protect MPTP‐induced neurotoxicity in Nrf2 knock‐out (KO) mice.15 Furthermore, Zhou et al16 reported that SFN protected against rotenone‐induced neurotoxicity model of PD. Collectively, it is likely that SFN can exert neuroprotective effects in animal models of PD through Nrf2 activation.
Glucoraphanin (GF), which found in cruciferous vegetables, such as broccoli sprout, is a glucosinolate precursor of SFN.17 Previously, we reported that dietary intake of 0.1% GF food pellet could prevent the onset of psychiatric disorders such as depression and psychosis in rodents,18, 19, 20, 21 suggesting that dietary intake of GF has prophylactic effects for development of psychiatric disorders.9
This study was undertaken to investigate whether dietary intake of 0.1% GF food pellet could prevent MPTP‐induced dopaminergic neurotoxicity in the mouse striatum since MPTP‐induced dopaminergic neurotoxicity is widely used as animal model of PD.22
2. METHODS AND MATERIALS
2.1. Animals
Eight weeks old of male adult C57BL/6 mice (body weight 20‐25 g, Japan SLC, Inc) were used in experiments. Animals were housed in 23°C ± 1°C room temperature, 55% ± 5% humidity, and 12‐hour light/dark cycles (lights on between 07:00‐19:00) with libitum food and water. All experiments were done according to the Guide for Animal Experimentation of Chiba University. The experimental protocol was approved by the Chiba University Institutional Animal Care and Use Committee.
2.2. Preparation of 0.1% glucoraphanin (GF) diet
Glucoraphanin food pellets were prepared as previously reported.18, 19, 20, 21 Food pellets (CE‐2; Japan CLEA, Ltd.) containing 0.1% glucoraphanin (GF) were prepared as follows. Broccoli sprout extract powder containing SFN precursor GF was industrially produced by KAGOME CO., LTD. In brief, broccoli sprout was grown from specially selected seeds (Brassica Protection Products LLC.) for 1 day after the germination. The 1‐day broccoli sprout was plunged into boiling water and maintained at 95°C for 30 minutes, and the sprout residues were removed by filtration. The boiling water extract was mixed with a waxy corn starch dextrin and then spray dried to yield the broccoli sprout extract powder containing 135 mg (approx. 0.31 mmol) of GF per gram. For preparing the animal diet containing 0.1% GF (approx. 2.3 mmol GF per 1‐kg diet), the extract powder was mixed with a basal diet CE‐2, and then pelletized at a processing facility (Oriental Yeast Co., Ltd.). The GF content in the diet was determined by high‐performance liquid chromatography as previously described.23, 24
2.3. MPTP‐induced neurotoxicity
The protocol of MPTP‐induced neurotoxicity was used as previously reported.25, 26 Thirty‐nine male mice (8 weeks old) were divided into the following four groups: a normal food + saline (5 mL/kg × 3, 2‐hour interval) group; a normal food pellet + MPTP (10 mg/kg × 3, 2‐hour interval. Tokyo Chemical Industry CO., Ltd.) group; a 0.1% GF food pellet + saline group; a 0.1% GF food pellet + MPTP group. 0.1% GF food was given from day 1 to day 28 (Figure 1A). Normal food was given to all groups from day 29 to day 36. MPTP or saline was injected into mice on day 29. Seven days (day 36) after administration of MPTP, mice were anesthetized with 5% isoflurane and sodium pentobarbital (50 mg/kg), and perfused transcardially with 10 mL of isotonic saline, followed by 30 mL of ice‐cold 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Brains were removed from the skulls and postfixed overnight at 4°C, then brain was used for immunohistochemistry of dopamine transporter (DAT).
Figure 1.
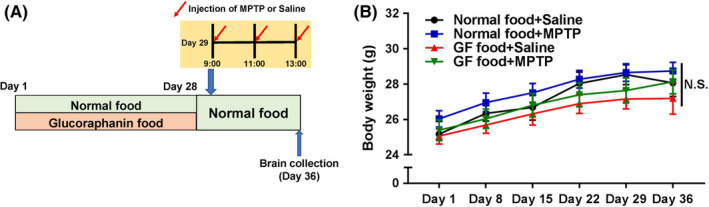
Schedule of dietary intake of 0.1% GF food pellet and MPTP treatment. A, Normal food pellet or 0.1% GF food pellet was given to male mice (8‐week‐old) (day 1‐day 28). Subsequently, normal food pellet was given to all mice (day 29‐day 36). Mice received three injections of MPTP (10 mg/kg at 9:00, 11:00, 13:00, ip) or saline (5 mL/kg at 9:00, 11:00 and 13:00) (day 29). Mice were perfused 7 days after the administration of MPTP (day 36). B, Time course of body weight. There were no statistical changes among the four groups. Each value is the mean ± SEM (n = 9‐11 per group). NS, not significant
2.4. DAT‐immunohistochemistry
Immunohistochemistry of DAT was performed as reported previously.25, 26 The free‐floating mouse brain sections (bregma 0.86–1.54 mm) were put in 0.3% H2O2 in 0.05 M Tris‐HCl saline (TBS) for 30 minutes and blocked in 0.2% Triton X‐100 TBS (TBST) with 1.5% normal serum for 1 hour, at room temperature. Samples were then incubated for 36 hours at 4°C, with rat anti‐DAT antibody (1:10 000, Cat. No: MAB 369, Chemicon International Inc). The sections were then washed three times in TBS and processed according to the avidin‐biotin‐peroxidase method (Vectastain Elite ABC, Vector Laboratories, Inc). Sections were then reacted with 0.15 mg/mL diaminobenzidine, containing 0.06% NiCl and 0.01% H2O2 for 5 minutes. Then, the sections were mounted on gelatinized slides, dehydrated, cleared, and coverslipped under Permount® (Fisher Scientific). Next, sections were imaged, and the staining intensity of DAT‐immunoreactivity in the anterior regions (0.25 mm2) of the striatum was analyzed using a light microscope equipped with a CCD camera (Olympus IX70) and the SCION IMAGE software package. Eight sections from each mouse were used for the quantitative analyses of DAT‐immunoreactivity.
2.5. Statistical analysis
The animal experiment data were expressed as the mean ± standard error of the mean (SEM). The statistical analysis was performed using SPSS Statistics 20 (SPSS). Data of body weight were analyzed using repeated two‐way analyses of variance (ANOVA). Data of DAT were analyzed using two‐way ANOVA, followed post hoc Tukey's multiple comparison test. The P values of less than 0.05 were considered statistically significant.
3. RESULTS
Repeated two‐way ANOVA revealed no statistical difference of body weight among the four groups [MPTP: F (1,35) = 1.122, P = 0.297, 0.1% GF: F (1,35) = 2.203, P = 0.147, interaction (MPTP × 0.1% GF), F (1,35) = 0.021, P = 0.88] (Figure 1B). Repeated dosing with MPTP (10 mg/kg × 3, 2‐hour interval) markedly decreased the density of DAT‐immunoreactivity in the mouse striatum (Figure 2A‐D). Two‐way ANOVA revealed statistical differences among the four groups [MPTP: F (1,35) = 16.73, P < 0.001, 0.1% GF: F (1,35) = 34.34, P < 0.001, interaction (MPTP × 0.1% GF), F (1,35) = 7.234, P = 0.011] (Figure 2E). Post hoc test showed that MPTP significantly (P < 0.001) decreased the density of DAT‐immunoreactivity in the striatum. Furthermore, dietary intake of 0.1% GF significantly (P < 0.001) prevented the MPTP‐induced reduction of DAT‐immunoreactivity in the striatum.
Figure 2.
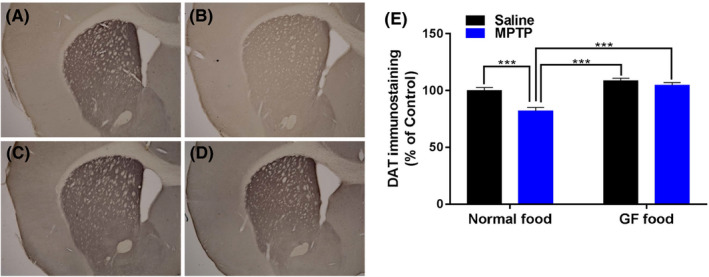
Effects of dietary intake of 0.1% GF on the reduction in the DAT density in the striatum after the repeated administration of MPTP. A, Control food pellet + Saline, B, Control food pellet + MPTP, C, 0.1% GF food pellet + Saline, D, 0.1% GF food pellet + MPTP. Representative photomicrographs showing the DAT‐immunoreactivity in the striatum of mice (A‐D). The mean value for DAT‐immunoreactivity staining was determined for each group and was expressed as a percentage of that of control mice (E). Each value is the mean ± SEM (n = 9‐11 per group). ***P < 0.001 as compared with the normal food + MPTP group
4. DISCUSSION
In this study, we found that dietary intake of 0.1% GF food pellets for 28 days prevented the reduction of DAT‐immunoreactivity in the striatum after repeated administration of MPTP in adult mice, suggesting the potent protective effects of GF for MPTP‐induced neurotoxicity. SFN increased Nrf2 protein levels in the striatum and led to upregulation of phase II antioxidant enzymes heme oxygenase‐1 (HO‐1) and NAD(P)H quinone oxidoreductase (NQO1).15 In addition, SFN protected against MPTP‐induced dopaminergic neurotoxicity in the mouse brain, suggesting that SFN can offer a neuroprotective effect for PD pathology. SFN is a compound derived from a glucosinolate precursor GF found in cruciferous vegetables, such as broccoli sprout.17 Therefore, it is likely that GF‐rich vegetables have prophylactic effects for the development of PD in adulthood.
We found neuroprotective effects of 0.1% GF supplementation for MPTP‐induced neurotoxicity although 0.1% GF food pellet was not given for 7 days after MPTP injection (Figure 1A). Previously, we reported that dietary intake of 0.1% GF food pellet for 28 days could prevent the onset of cognitive deficits and reduction of parvalbumin (PV)‐immunoreactivity in the prefrontal cortex of mouse brain after repeated administration of phencyclidine.18 Furthermore, dietary intake of 0.1% GF food pellet for 28 days prevented the onset of depression‐like phenotypes in mice after chronic social defeat stress19 or inflammation.20 Moreover, dietary intake of 0.1% GF food pellet during juvenile and adolescent stages (P28‐P56) prevented behavioral abnormalities in adult offspring (<P70) after maternal immune activation.21 Collectively, it seems that dietary intake of 0.1% GF food pellet for 28 days has long‐lasting beneficial effects despite 0.1% GF food pellet was not given at the behavioral evaluation. Interestingly, it is known that foods such as fresh vegetables are associated with the reduced rate of PD progression.3, 6 Therefore, it is likely that dietary intake of GF‐rich cruciferous vegetables might play a role in preventing the onset of neuropsychiatric disorders in later life. In addition, the natural antioxidants (ie, the polyphenol‐rich aqueous walnut extract, an extract of Juglandis Semen and pigallocatechin gallate, a major polyphenol in green tea) could protect against MPTP‐induced dopaminergic neurotoxicity in mouse striatum.27, 28 It seems that the natural antioxidants may have beneficial effects in an animal model of PD.
This paper has some limitations. First, we did not measure concentration of SFN in the brain after dietary intake of 0.1% GF food pellet. However, it is reported that dietary intake of 0.1% GF increased levels of SFN in the mouse brain17 and that SFN prepared from 0.1% GF could produce the gene expression of a number of oxidative stress genes through Nrf2 activation.29 It is also reported that SFN could protect against MPTP‐induced neurotoxicity in the mouse brain. Collectively, it is likely that dietary intake of 0.1% GF might have prophylactic effects in MPTP model of PD. Second, we did not confirm specific genes which can contribute to beneficial effects of SFN in this model. It is known that SFN can stimulate the gene expression of a number of ARE (antioxidant response element)‐regulated genes (ie, HO‐1 and NQO1) related with oxidative stress. It is currently unclear whether specific antioxidant genes play a role in the beneficial effects of 0.1% GF food pellet.
In conclusion, the present data suggest that dietary intake of 0.1% GF food pellet for 28 days could prevent the onset of MPTP‐induced reduction of DAT in the striatum of adult mice. Therefore, it is likely that dietary intake of GF‐rich cruciferous vegetables may prevent neurodegenerative disorders such as PD in later life.
CONFLICT OF INTEREST
Dr Ushida and Dr Suganuma are employee of KAGOME which produce SFN supplement. The other authors declare no conflict of interest.
AUTHOR CONTRIBUTION
KH is responsible for the design of the research and experiment and supervised the experimental analyses. YP and KH wrote the paper. YP, YQ, CL, SW, and KZ performed behavioral experiments. YP analyzed the data. YU and HS provided 0.1% GF food pellet. All authors read and approved this paper.
DATA REPOSITORY
All relevant data are included in Supporting Information.
ANIMAL STUDIES
All animal experiments were approved by the Animal Care and Use Committee of Chiba University.
Supporting information
ACKNOWLEDGEMENTS
This study was partly supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (to K.H., 17H04243). Dr. Lijia Chang was supported by The Japan‐China Sasakawa Medical Fellowship (Tokyo, Japan).
Pu Y, Qu Y, Chang L, et al. Dietary intake of glucoraphanin prevents the reduction of dopamine transporter in the mouse striatum after repeated administration of MPTP. Neuropsychopharmacol Rep. 2019;39:247–251. 10.1002/npr2.12060
REFERENCES
- 1. Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896–912. [DOI] [PubMed] [Google Scholar]
- 2. Ascherio PA, Schwarzschild MA. The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol. 2016;15:1257–72. [DOI] [PubMed] [Google Scholar]
- 3. Archer T, Kostrzewa RM. Exercise and nutritional benefits in PD: rodent models and clinical settings. Curr Top Behav Neurosci. 2016;29:333–51. [DOI] [PubMed] [Google Scholar]
- 4. Cassani E, Barichella M, Ferri V, Pinelli G, Iorio L, Bolliri C, et al. Dietary habits in Parkinson's disease: adherence to Mediterranean diet. Parkinsonism Relat Disord. 2017;42:40–6. [DOI] [PubMed] [Google Scholar]
- 5. Gao X, Chen H, Fung TT, Logroscino G, Schwarzschild MA, Hu FB, et al. Prospective study of dietary pattern and risk of Parkinson disease. Am J Clin Nutr. 2007;86:1486–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mischley LK, Lau RC, Bennett RD. Role of diet and nutritional supplements in Parkinson's disease progression. Oxid Med Cell Longev. 2017;2017:6405278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deshmukh P, Unni S, Krishnappa G, Padmanabhan B. The Keap1‐Nrf2 pathway: promising therapeutic target to counteract ROS‐mediated damage in cancers and neurodegenerative diseases. Biophys Rev. 2017;9:41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yamamoto M, Kensler TW, Motohashi H. The KEAP1‐NRF2 system: a thiol‐based sensor‐effector apparatus for maintaining redox homeostasis. Physiol Rev. 2018;98:1169–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hashimoto K. Essential role of Keap1‐Nrf2 signaling in mood disorders: overview and future perspective. Front Pharmacol. 2018;9:1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang JC, Yao W, Dong C, Han M, Shirayama Y, Hashimoto K. Keap1‐Nrf2 signaling pathway confers resilience versus susceptibility to inescapable electric stress. Eur Arch Psychiatry Clin Neurosci. 2018;268:865–70. [DOI] [PubMed] [Google Scholar]
- 11. Vasconcelos AR, Dos Santos NB, Scavone C, Munhoz CD. Nrf2/ARE pathway modulation by dietary energy regulation in neurological disorders. Front Pharmacol. 2019;10:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Innamorato NG, Jazwa A, Rojo AI, García C, Fernández‐Ruiz J, Grochot–Przeczek A, et al. Different susceptibility to the Parkinson's toxin MPTP in mice lacking the redox master regulator Nrf2 or its target gene heme oxygenase‐1. PLoS ONE. 2010;5:e11838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaidery NA, Banerjee R, Yang L, Smirnova NA, Hushpulian DM, Liby KT, et al. Targeting Nrf2‐mediated gene transcription by extremely potent synthetic triterpenoids attenuate dopaminergic neurotoxicity in the MPTP mouse model of Parkinson's disease. Antioxid Redox Signal. 2013;18:139–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Todorovic M, Wood SA, Mellick GD. Nrf2: a modulator of Parkinson's disease? J Neural Transm (Vienna). 2016;123:611–9. [DOI] [PubMed] [Google Scholar]
- 15. Jazwa A, Rojo AI, Innamorato NG, Hesse M, Fernández‐Ruiz J, Cuadrado A. Pharmacological targeting of the transcription factor Nrf2 at the basal ganglia provides disease modifying therapy for experimental parkinsonism. Antioxid Redox Signal. 2011;14:2347–60. [DOI] [PubMed] [Google Scholar]
- 16. Zhou Q, Chen B, Wang X, Wu L, Yang Y, Cheng X, et al. Sulforaphane protects against rotenone‐induced neurotoxicity in vivo: involvement of the mTOR, Nrf2, and autophagy pathways. Sci Rep. 2016;6:32206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fahey JW, Holtzclaw WD, Wehage SL, Wade KL, Stephenson KK, Talalay P. Sulforaphane bioavailability from glucoraphanin‐rich broccoli: control by active endogenous myrosinase. PLoS ONE. 2015;10:e0140963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shirai Y, Fujita Y, Hashimoto R, Ohi K, Yamamori H, Yasuda Y, et al. Dietary intake of sulforaphane‐rich broccoli sprout extracts during juvenile and adolescence can prevent phencyclidine‐induced cognitive deficits at adulthood. PLoS ONE. 2015;10:e0127244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yao W, Zhang J‐C, Ishima T, Dong C, Yang C, Ren Q, et al. Role of Keap1‐Nrf2 signaling in depression and dietary intake of glucoraphanin confers stress resilience in mice. Sci Rep. 2016;6:30659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang J‐C, Yao W, Dong C, Yang C, Ren Q, Ma M, et al. Prophylactic effects of sulforaphane on depression‐like behavior and dendritic changes in mice after inflammation. J Nutr Biochem. 2017;39:134–44. [DOI] [PubMed] [Google Scholar]
- 21. Matsuura A, Ishima T, Fujita Y, Iwayama Y, Hasegawa S, Kawahara‐Miki R, et al. Dietary glucoraphanin prevents the onset of psychosis in the adult offspring after maternal immune activation. Sci Rep. 2018;8:2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jackson‐Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson's disease. Nat Protoc. 2007;2:141–51. [DOI] [PubMed] [Google Scholar]
- 23. Bennett RN, Mellon FA, Rosa EA, Perkins L, Kroon PA. Profiling glucosinolates, flavonoids, alkaloids, and other secondary metabolites in tissues of Azima tetracantha L. (Salvadoraceae) . J Agric Food Chem. 2004;52:5856–62. [DOI] [PubMed] [Google Scholar]
- 24. West LG, Meyer KA, Balch BA, Rossi FJ, Schultz MR, Haas GW. Glucoraphanin and 4‐hydroxyglucobrassicin contents in seeds of 59 cultivars of broccoli, raab, kohlrabi, radish, cauliflower, brussels sprouts, kale, and cabbage. J Agric Food Chem. 2004;52:916–26. [DOI] [PubMed] [Google Scholar]
- 25. Ren Q, Ma M, Yang J, Nonaka R, Yamaguchi A, Ishikawa K‐I, et al. Soluble epoxide hydrolase plays a key role in the pathogenesis of Parkinson's disease. Proc Natl Acad Sci USA. 2018;115:E5815–E5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ren Q, Zhang JC, Ma M, Fujita Y, Wu J, Hashimoto K. 7,8‐Dihydroxyflavone, a TrkB agonist, attenuates behavioral abnormalities and neurotoxicity in mice after administration of methamphetamine. Psychopharmacology. 2014;231:159–66. [DOI] [PubMed] [Google Scholar]
- 27. Choi JG, Park G, Kim HG, Oh DS, Kim H, Oh MS. In vitro and in vivo neuroprotective effects of Walnut (Juglandis Semen) in models of Parkinson's disease. Int J Mol Sci. 2016;17:E108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu Q, Langley M, Kanthasamy AG, Reddy MB. Epigallocatechin gallate has a neurorescue effect in a mouse model of Parkinson disease. J Nutr. 2017;147:1926–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Clarke JD, Hsu A, Williams DE, Dashwood RH, Stevens JF, Yamamoto M, et al. Metabolism and tissue distribution of sulforaphane in Nrf2 knockout and wild‐type mice. Pharm Res. 2011;28:3171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


