Abstract
Aims
The human NRCAM gene is associated with polysubstance use. Nrcam knockout mice do not acquire a preference for addictive substances. We aimed to elucidate the role of Nrcam in specific neural circuits underlying congenital preference for substances and the acquisition of addiction.
Methods
We analyzed gene expression patterns of neural molecules to find a common addiction pathway dependent on Nrcam function. We examined monoaminergic, glutamatergic, and GABAergic systems in the brains of Nrcam knockout mice following treatment with methamphetamine (METH) or saline (SAL) using micro‐array gene expression analysis, which was replicated using TaqMan gene expression analysis. To find a common addiction pathway, we examined similarities and differences between the expression patterns of molecules in METH‐treated mice and in Nrcam knockout mice treated with cocaine (COC).
Results
Glutaminase expression in brain was reduced in Nrcam heterozygous mice after METH and COC treatment, consistent with our previous study. Metabotropic glutamate receptor 2 expression was reduced in Nrcam heterozygous mice that received either METH or COC treatment. Several other molecules could act in independent addiction pathways involving METH or COC. We also found that GABA receptor subunit g2 expression was reduced in Nrcam heterozygous mice that underwent SAL treatment, and that METH treatment attenuated this reduction.
Conclusion
Nrcam differentially regulates glutamatergic and GABAergic molecules in naive brains and in brains of animals with acquired addiction. Elucidating the complex neural mechanisms underlying polysubstance use will uncover biological features of addiction and may contribute to the development of effective pharmaceutical treatments.
Keywords: alcohol‐ and substance‐related disorders; genetics: animal, psychopharmacology; molecular neurobiology: animal, pharmacogenetics
The human/mice NRCAM is involved in specific neural circuits underlying congenital preference for substances and the acquisition of addiction. Mice Nrcam differentially regulates glutamatergic and GABAergic molecules in naive brains and in brains of animals with acquired addiction. Elucidating the complex neural mechanisms underlying polysubstance use will uncover biological features of addiction and may contribute to the development of effective pharmaceutical treatments.
1. INTRODUCTION
The human neuronal cell adhesion molecule (NrCAM) is a protein encoded by the NRCAM gene. Our previous study revealed that human NRCAM gene polymorphisms and haplotype were associated with reduced gene expression in postmortem human brain tissue from patients with polysubstance use.1 Nrcam knockout mice did not develop conditioned place preference (CPP) to either morphine, amphetamine, or cocaine (COC),1 nor did they develop alcohol preference with free access to a 16%‐32% ethanol solution.2 These findings indicate that NrCAM may be involved in common addiction pathways, although these addictive substances could directly bind to the involved neurons and/or regulate different neural networks underlying the acquisition of addiction. NrCAM appears to work as an anchor of cell connections in neural networks in the adult brain under certain circumstances. These networks are associated with addiction‐related behaviors, including reward and peripheral responses in social/personality behaviors. Indeed, the NRCAM gene is associated with methamphetamine (METH) addiction, as demonstrated by the duration of METH use and number of arrests that predispose individuals to addiction.3 Nrcam knockout mice (both heterozygous and homozygous) showed reduced novelty‐seeking, sociability, anxiety, and repetitive/perseverative responses in a marble burying test, which can be interpreted as obsessive behavior, as marble burying reflects a repetitive and perseverative behavior more than novelty‐induced anxiety does.4 Some molecules regulated by NrCAM in the brain may characterize addiction‐related behaviors, including reward and other social/personality behaviors.2
To elucidate the precise molecular mechanisms that contribute to addiction‐related cognitive and behavioral characteristics, transcriptomic analyses using Nrcam knockout mice could be useful. Using microarray, we evaluated the differences in gene expression in the brain that were related to polysubstance use, either in the naïve brain in which drug preference has not developed, or in the “acquired brain” after the development of drug addiction. Several molecules could act as shared factors in the acquisition of both METH and COC preference; there would also be some nonshared factors. Although many genes have been identified as candidate vulnerability genes by microarray analyses, we focused on monoaminergic, glutamatergic, and GABAergic molecules that have been investigated for associations with the substance use of METH and COC.5, 6, 7, 8, 9, 10, 11
2. MATERIALS AND METHODS
2.1. Subjects
Nrcam knockout mice, developed by Sakurai et al,12 were used in this study. The mice were housed under a 12‐hour light/12‐hour dark cycle (lights off from 19:00 to 07:00) and given access to food and water ad libitum. Heterozygous sibling mating pairs were obtained from the same litter and used as breeding pairs to produce cohorts used in this study. None of the three genotypes was lethal; however, because no human has a complete deficit of the NRCAM gene, Nrcam heterozygous knockout (Het) and wild type (W) mice were analyzed as genetic models. The mice were injected intraperitoneally once a day for 4 days either with saline (SAL), METH (2 mg/kg), or COC (20 mg/kg). This protocol was sufficient to induce the acquisition of addiction to both substances, as shown by the CPP in our previous studies.1, 2 Each experiment was conducted in a separate group of animals. The experiments were performed in the test room from 16:00 to 20:00 under dim lighting conditions.
All animal procedures were performed according to protocols approved by the Institutional Animal Care and Use Committees at University of Yamanashi, in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978).
2.2. Transcriptome analysis in brains from Nrcam knockout mice
Mice were sacrificed 1 hour after the last injection of SAL, METH, or COC. Brains were collected within 30 minutes after the sacrifice to avoid acute effects of drug treatment. The striatum and midbrain were dissected from the brains. Total RNA was extracted from the tissue, and the amount of RNA in each subject was measured. For the first screening, equal amounts (500 ng/μL) of RNA from three mice were pooled for microarray analysis. cDNA for each gene was synthesized from RNA using reverse transcription (ReverTra Ace, TOYOBO Co., Tokyo Japan). Three pooled RNA samples obtained from the tissue of nine mice were analyzed by the Affymetrix Array with GeneChip® Mouse Transcriptome Assay 1.0 to screen for differential gene expression among four groups of mice with different genotypes (W and Het Nrcam knockout) and treatment modalities (METH and SAL treatment). TaqMan gene‐expression analysis was performed to confirm the differences indicated in the first screening with the microarray, using nonpooled cDNA samples, which included some of the samples used for the previous pooled analysis and some samples from additional mice. Replication analysis to confirm the similarities in gene regulation by COC was performed for the candidate genes identified in the METH study (total samples: SAL‐treated mice, 21 W and 21 Het; METH‐treated mice, 19 W and 21 Het; COC‐treated mice, 15 W and 21 Het).
2.3. Statistics
Differentially expressed genes (DEGs) were identified using Student's t test by using Subio Platform software (www.subioplatform.com, Subio Inc., Kagoshima, Japan). Differences in each gene‐expression levels across groups of mice (genotype and treatment) were analyzed by two‐way ANOVA, followed by post hoc analysis using the Tukey HSD test. The difference between COC‐treated mice with different genotypes was analyzed by one‐way ANOVA. ANOVA and Tukey HSD tests were carried out using JMP software version 13 (SAS Institute, Tokyo, Japan). Unless otherwise stated, P < 0.05 was considered statistically significant.
3. RESULTS
Microarray analysis revealed that genes belonging to monoaminergic, glutamatergic, or GABAergic neural circuits showed nominal significant differences between W and Het mice, and between mice undergoing METH and SAL treatments ([Link], [Link], [Link], [Link], [Link], [Link], [Link], [Link], [Link], [Link], [Link], [Link]). Among these candidates, we failed to replicate the differential expression observed in the microarray analysis when we used TaqMan gene‐expression analysis for the following genes: dopamine D5 receptor (Drd5), glutamine receptor and ionotropic kainite 3 (Grik3), and vesicular glutamate transporter (Vglut1, Slc17a7).
Glutaminase (Gls) was expressed at lower levels in brains from Het mice than in those from W naïve mice, as reported in our previous study2 The expression in the striatum of the Het mice was lower than that in the striatum of the W mice after treatment with METH or SAL (F(3,77) = 4.6, P = 0.005; effect of genotype F(1,1) = 12.9, P = 0.0006; effect of treatment F(1,1) = 0.3, P = 0.56; effect of genotype × treatment F(1,1) = 0.17, P = 0.69; post hoc analysis: 20 W vs 21 Het, P = 0.13 in METH‐treated mice, 21 W vs 21 Het, P = 0.026 in SAL‐treated mice, Figure 1A). After treatment with COC, Gls expression in the striatum was lower in Nrcam Het mice than in W mice (14 W vs 20 Het, F(1,32) = 5.4, P = 0.026, Figure 1B).
Figure 1.
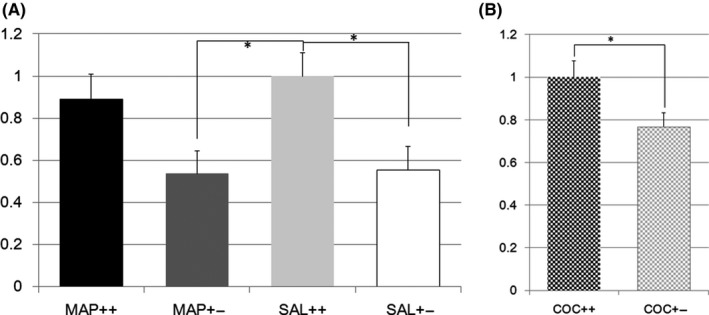
A, Glutaminase gene expression in methamphetamine or saline‐treated Nrcam knockout mice. The vertical axis shows the Gls expression level in the striatum relative to that in Nrcam wild type (++) mice treated with saline (SAL) (light gray bar). The black bar indicates the relative Gls expression in methamphetamine (METH)‐treated wild type (++) mice, the dark gray bar indicates that in METH‐treated Nrcam heterozygous (+−) mice, and the white bar indicates that in SAL‐treated Nrcam heterozygous mice. *A significant difference (P = 0.026) was found between SAL‐treated mice with different Nrcam genotypes, while no difference was found between METH‐treated mice with different Nrcam genotypes. A significant difference (P = 0.018) was also seen between SAL‐treated wild type (++) mice and METH‐treated Nrcam heterozygous (+−) mice; B, Glutaminase gene expression in cocaine‐treated Nrcam knockout mice. The vertical axis shows the Gls expression level in the striatum relative to that in Nrcam wild type (++) mice (checkered black bar). The checkered gray bar indicates the relative Gls expression in heterozygous (+−) mice. *A significant differences (P = 0.026) were found for both comparison
Metabotropic glutamate receptor 2 (Grm2) expression was also differentially regulated (F(3,73) = 1.7, P = 0.16; effect of genotype F(1,1) = 0.7, P = 0.40; effect of treatment F(1,1) = 0.003, P = 0.96; effect of genotype × treatment F(1,1) = 4.7, P = 0.033, Figure 2A). However, post hoc analysis with the Tukey test did not show a significant difference among the groups, as the lowest P value was found for differences between mice with different Nrcam genotypes undergoing METH treatment (P = 0.18). Two‐way ANOVA showed no significant difference in Grm2 expression in the striatum between any of the Nrcam genotype–treatment pairs. One‐way ANOVA indicated a significant reduction in Grm2 expression after METH treatment (16 W vs 20 Het, F(1,35) = 7.5, P = 0.010, Figure 2B) and a trend for reduction after COC treatment (20 W vs 21 Het, F(1,29) = 3.5, P = 0.072 (P = 0.036, single sided), Figure 2B).
Figure 2.
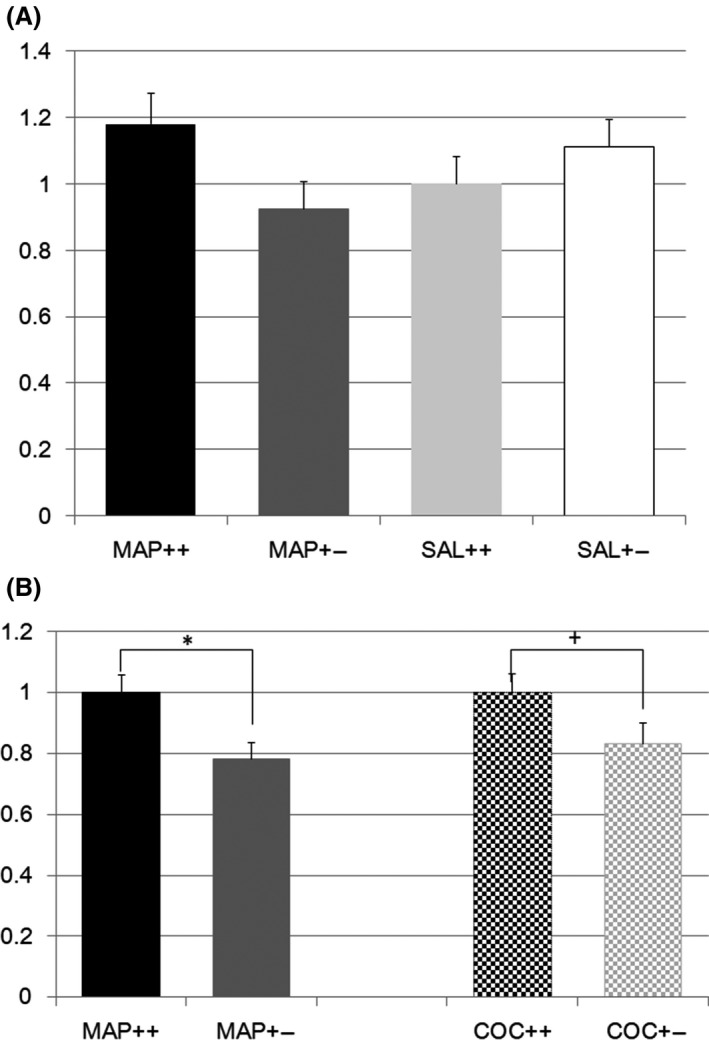
A, Metabotropic glutamate receptor 2 gene expression in methamphetamine or saline‐treated Nrcam knockout mice. The vertical axis shows the Grm2 expression level in the striatum relative to that in Nrcam wild type (++) mice treated with saline (SAL) (light gray bar). The black bar indicates the relative Grm2 expression in methamphetamine (METH)‐treated wild type (++) mice, the dark gray bar indicates that in METH‐treated Nrcam heterozygous (+−) mice, and the white bar indicates that in SAL‐treated Nrcam heterozygous mice; B, Metabotropic glutamate receptor 2 gene expression in methamphetamine and cocaine‐treated Nrcam knockout mice. The vertical axis shows the Grm2 expression level in the striatum relative to that in Nrcam wild type (++) mice treated with methamphetamine (METH) (plain black bar). The plain dark gray bar indicates the relative Grm2 expression in the heterozygous (+−) mice treated with METH. The checkered black bar indicates this expression in Nrcam wild type (++) mice treated with cocaine (COC), and the checkered gray bar indicates the relative Grm2 expression in the heterozygous (+−) mice treated with COC. *A significant difference (P = 0.010) and a +trend of difference (P = 0.072) were found
GABA receptor type g2 (Gabrg2) expression in the striatum seemed to be differentially regulated in Nrcam Het and W mice treated with METH (F(3,77) = 4.0, P = 0.010; effect of genotype F(1,1) = 6.8, P = 0.011; effect of treatment F(1,1) = 4.1, P = 0.047; effect of genotype × treatment F(1,1) = 1.1, P = 0.30, Figure 3A). The Tukey test indicated that Gabrg2 expression was higher in Nrcam Het mice than in W mice when treated with SAL (20 W vs 21 Het, P = 0.052, Figure 3A), but METH treatment attenuated the difference between Nrcam genotypes (19 W vs 21 Het, P = 0.70, Figure 3A). Gabrg2 expression was also significantly higher in Nrcam Het mice than in W mice when treated with SAL (21 Het vs 20 W, P = 0.008, Figure 3A). A differential regulation of Gabrg2 expression after COC vs SAL treatment was observed, with lower expression in the striatum in Nrcam Het mice than in W mice (14 W vs 18 Het, F(1,30) = 5.9, P = 0.022, Figure 3B).
Figure 3.
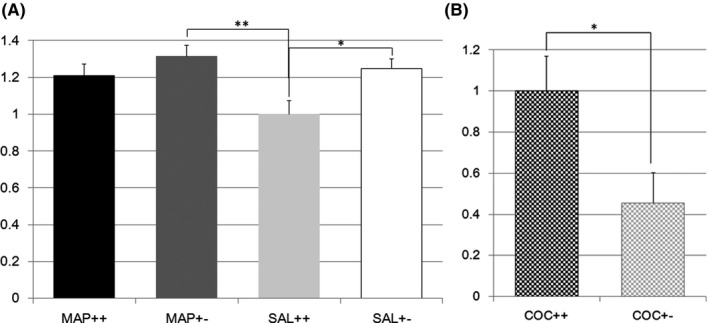
A, GABA receptor type g2 gene expression in methamphetamine or saline‐treated Nrcam knockout mice. The vertical axis shows the Gabrg2 expression level in the striatum relative to that in Nrcam wild type (++) mice treated with saline (SAL) (light gray bar). The black bar indicates the relative Gabrg2 expression in methamphetamine (METH)‐treated wild type (++) mice, the dark gray bar indicates that in METH‐treated Nrcam heterozygous (+−) mice, and the white bar indicates that in SAL‐treated Nrcam heterozygous mice. A non‐significant tendency (P = 0.052) was found between SAL‐treated mice with different Nrcam genotypes. **A significant difference (P = 0.008) was seen between SAL‐treated wild type (++) mice and METH‐treated Nrcam heterozygous (+−) mice; B, GABA receptor type g2 gene expression in cocaine‐treated Nrcam knockout mice. The vertical axis shows the Gabrg2 expression level in the striatum relative to that in Nrcam wild type (++) mice (checkered black bar). The checkered gray bar indicates the relative Gabrg2 expression in heterozygous (+−) mice. *A significant difference (P = 0.022) was found
In the analysis of the midbrain, the first screening using microarrays indicated the upregulation of Slc17a7 gene expression by METH in Nrcam Het mice. However, TaqMan analysis did not reveal any METH‐associated difference in gene expression (Figure 4A). However, TaqMan analysis revealed that COC upregulated Slc17a7 expression in Nrcam Het mice (14 W vs 19 Het, F(1,31) = 8.4, P = 0.0068, Figure 4B).
Figure 4.
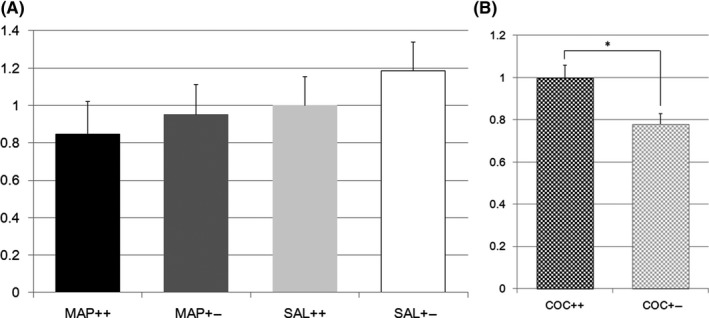
A, Vesicular glutamate transporter gene expression in methamphetamine or saline‐treated Nrcam knockout mice. The vertical axis shows the Slc17a7 expression level in the striatum relative to that in Nrcam wild type (++) mice treated with saline (SAL) (light gray bar). The black bar indicates the relative Slc17a7 expression in methamphetamine (METH)‐treated wild type (++) mice, the dark gray bar indicates that in METH‐treated Nrcam heterozygous (+−) mice, and the white bar indicates that in SAL‐treated Nrcam heterozygous mice; B, Vesicular glutamate transporter gene expression in cocaine‐treated Nrcam knockout mice. The vertical axis shows the Slc17a7 expression level in the striatum relative to that in Nrcam wild type (++) mice (checkered black bar). The checkered gray bar indicates the relative Slc17a7 expression in heterozygous (+−) mice. *A significant difference (P = 0.0068) was found
4. DISCUSSION
NrCAM is a cell adhesion molecule that modulates several neural processes including neurogenesis, neurite outgrowth, and synaptogenesis.13 Although the exact role of NrCAM in cognition and behavior is still unknown, our previous study indicated that NrCAM was involved in addiction to morphine, amphetamine, COC, and alcohol, which could affect different neural systems. These findings imply that NrCAM regulates addiction‐related neural systems, including glutamatergic, GABAergic, and monoaminergic systems, both during development and in the mature brain. Recent studies revealed that glutamate and GABA systems are affected by psychostimulants.11, 14, 15 There may be a common molecular mechanism underlying the addiction to different substances; however, there may also be unique pathways, which may be regulated by NrCAM in the brain. NrCAM has a role in the development of addiction‐related traits in both naive and “acquired brains,” manifesting as behaviors that are associated with responses to novel stimuli, appetitive motivational states, anxiety, and possibly obsessive behavior, as indicated in our previous studies.2
The aversive role of NrCAM dysfunction in addiction may be explained by its regulation of certain neurochemical systems. Repeated exposure to either METH or COC leads to enduring changes in gene expression and subsequent functional CNS plasticity throughout multiple brain regions. The present study aimed to elucidate the neural targets both common and unique to METH and COC use. Changes in the expression of these molecules in the brain may occur following the development of addiction.
Glutamate is the principal excitatory neurotransmitter in the brain and has effects on drug‐seeking behaviors for COC.11, 16 Further, glutamatergic and dopaminergic alterations in the brain are thought to contribute to the relatively long‐lasting and recurrent nature of METH psychosis and depression.17, 18 In previous studies in our laboratory, we found that Gls expression was reduced in cultured cells, mice, and humans that have naturally low Nrcam expression in the brain and that the Gls inhibitor reduced the rewarding effects of substance use. After treatment with either SAL, METH, or COC, Gls expression was differentially regulated. SAL and COC treatment maintained the difference in expression between Het and W mice, similar to what is observed in the naïve condition, while METH treatment seemed to attenuate the difference. A recent study showed that acute COC treatment reduced Gls expression in the striatum but increased GIs expression in the prefrontal cortex,11 while chronic exposure to COC did not change striatal gene expression, as shown in this study. Collectively, as the main glutamate‐producing enzyme, Gls seems to be a common neural molecule involved in the effects of these substances. However, it is still unclear how Gls induces neural network remodeling for drug preference or during the acquisition of addiction.
The antagonism of mGlu2/3 receptors in the NAc reduced METH‐seeking behavior in rats,19 and a recent study indicated that acupuncture reduces METH‐induced locomotion effects related to extracellular DA release in the NAc via the activation of mGluR2/3. Grm2 mutant mice display altered emotionality, impulsivity, and risk‐related behaviors, and show significantly higher levels of alcohol consumption and preference than do wild type control mice.20, 21 The levels of Grm2 expression in both the striatum and hippocampus have been shown to be significantly lower in alcohol preferring (P) rats than in nonpreferring (NP) rats.22 Therefore, we hypothesized that Grm2 expression in the striatum would be lower in Nrcam Het mice than in W mice after METH or COC treatment. The results of the study could support the hypothesis by single‐sided statistical analysis for METH and COC treatment (P < 0.01 and P = 0.03, Figure 2). Thus, the reduction in Grm2 expression appears to be acquired after the development of addiction.
RNA‐Seq analysis revealed that GABRG2 was downregulated in alcoholics and COC addicts,23 while stem cells obtained from alcoholics showed an increase in GABRG2 expression after chronic treatment with alcohol.24 Similar to a previous study indicating that Gabrg2 expression was upregulated in P rats,23 Gabrg2 expression was downregulated by COC in the Nrcam Het mice that showed less preference to addictive substances in this study. When these findings are taken together, Gabrg2 reduction appears to not be a congenital factor, but may instead be an acquired factor during the development of COC and alcoholism. The involvement of Nrcam in METH addiction is still controversial; however, METH treatment eliminates the congenital reduction in Gabrg2 expression in the Nrcam W mice. Because GABRG2 is involved in the suicidal behavior of schizophrenia patients with alcohol use disorder,25 GABRG2 could also have a role in behavioral characteristics related to addiction in addition to its role in reward itself. Future pharmaco‐behavioral experiments may demonstrate the exact role of GABRG2 in the overall biology of addiction.
In addition, the analysis also indicated an involvement of Slc17a7 in COC addiction. A gene‐expression network underlying alcoholism that involves recognized substrates for addiction, including SLC17A7, is known to be present in the hippocampus. However, analyses showed that this network was not conserved in COC users.26 Further studies are needed to identify the exact role of the vesicular glutamate transporter in this type of addiction.
5. CONCLUSION
This study supports the proposed involvement of several glutamatergic and GABAergic molecules, as well as that of NrCAM, in addiction vulnerability. However, this study has several limitations: (a) We used only nine subjects in three pooled samples for the microarray analysis and analyzed only METH and COC addiction models. (b) We focus on monoaminergic, glutamatergic, and GABAergic molecules evaluated from the microarray analysis, although other molecules could have important roles in addiction. (c) While we can observe the development of preference to either METH or COC by series of four daily injections in CPP test, more treatment might be needed to develop addiction‐related behavioral changes including cognitive disfunction. Further studies are necessary to confirm these findings using larger sample sizes and model mice treated longer with METH, COC, and other addictive substances. As demonstrated by Figure 5, some molecules act as congenital factors, whereas others act as factors acquired either during or after the development of addiction. The results of the present study may be consistent with the possible roles of some neural molecules in addiction, which have been indicated by previous studies using different methods such as genetics and transcriptome analyses. Compounds targeting these molecules may be potential therapeutics for polysubstance users. Further analysis may help to develop and identify pharmaceutical molecules targeting these systems for the treatment of addiction.
Figure 5.
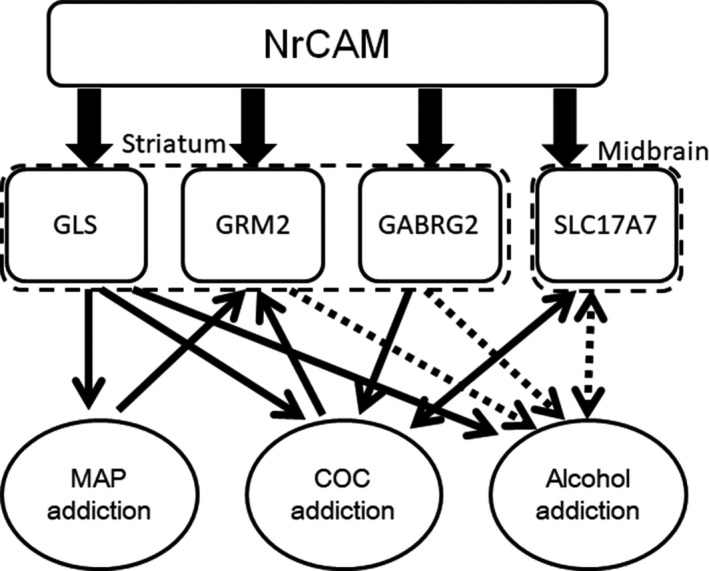
Schema describing the Nrcam‐related molecules and their roles in polysubstance use indicated in this study. Nrcam regulates the gene expression of the glutamatergic and GABAergic molecules Gls, Grm2, and Gabrg2 in the striatum and Slc17a7 in the midbrain. The down arrow indicates an effect for each addiction. The up arrow indicates the regulation of gene expression in the brain of a substance user. Solid lines indicate the findings in the study, while dotted lines show the results from previous studies
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
DATA REPOSITORY
I agree to deposit the data and publish part of it as [Link], [Link], [Link], [Link], [Link], [Link], [Link], [Link], [Link], [Link], [Link], [Link].
APPROVAL OF THE RESEARCH PROTOCOL BY AN INSTITUTIONAL REVIEWER BOARD AND ANIMAL STUDIES
All animal procedures were performed according to protocols approved by the Institutional Animal Care and Use Committees at University of Yamanashi, in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978).
INFORMED CONSENT
N/A.
REGISTRY AND THE REGISTRATION NO. OF THE STUDY/TRIAL
N/A.
Supporting information
Ishiguro H, Miyake K, Tabata K, Mochizuki C, Sakurai T, Onaivi ES. Neuronal cell adhesion molecule regulating neural systems underlying addiction. Neuropsychopharmacol Rep. 2019;39:10–16. 10.1002/npr2.12038
Funding informationThis work was funded by KAKENHI (20375489, 20659177, 23591671, and 26461716), and was supported by William Paterson University. Dr. Hiroki Ishiguro possesses full access to all the data of the present study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1. Ishiguro H, Liu QR, Gong JP, et al. NrCAM in addiction vulnerability: positional cloning, drug‐regulation, haplotype‐specific expression, and altered drug reward in knockout mice. Neuropsychopharmacology. 2006;31(3):572–84. [DOI] [PubMed] [Google Scholar]
- 2. Ishiguro H, Hall FS, Horiuchi Y, et al. NrCAM‐regulating neural systems and addiction‐related behaviors. Addict Biol. 2014;19(3):343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yoo BK, Shim JC, Lee BD, et al. Association of the neuronal cell adhesion molecule (NrCAM) gene variants with personality traits and addictive symptoms in methamphetamine use disorder. Psychiatry Investig. 2013;9(4):400–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thomas A, Burant A, Bui N, Graham D, Yuva‐Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty‐induced anxiety. Psychopharmacology. 2009;204(2):361–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Spencer S, Kalivas PW. Glutamate transport: a new bench to bedside mechanism for treating drug abuse. Int J Neuropsychopharmacol. 2017;20(10):797–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Siniscalchi A, Bonci A, Biagio Mercuri N, et al. The role of topiramate in the management of cocaine addiction: a possible therapeutic option. Curr Neuropharmacol. 2015;13(6):815–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McCreary AC, Muller CP, Filip M. Psychostimulants: basic and clinical pharmacology. Int Rev Neurobiol. 2015;120:41–83. [DOI] [PubMed] [Google Scholar]
- 8. Engin E, Benham RS, Rudolph U. An emerging circuit pharmacology of GABAA receptors. Trends Pharmacol Sci. 2018;39(8):710–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cooper S, Robison AJ, Mazei‐Robison MS. Reward circuitry in addiction. Neurotherapeutics. 2017;14(3):687–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cao DN, Shi JJ, Hao W, Wu N, Li J. Advances and challenges in pharmacotherapeutics for amphetamine‐type stimulants addiction. Eur J Pharmacol. 2016;780:129–35. [DOI] [PubMed] [Google Scholar]
- 11. Marquez J, Campos‐Sandoval JA, Penalver A, et al. Glutamate and brain glutaminases in drug addiction. Neurochem Res. 2017;42(3):846–57. [DOI] [PubMed] [Google Scholar]
- 12. Sakurai T, Lustig M, Babiarz J, et al. Overlapping functions of the cell adhesion molecules Nr‐CAM and L1 in cerebellar granule cell development. J Cell Biol. 2001;154(6):1259–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kayyem JF, Roman JM, de la Rosa EJ, Schwarz U, Dreyer WJ. Bravo/Nr‐CAM is closely related to the cell adhesion molecules L1 and Ng‐CAM and has a similar heterodimer structure. J Cell Biol. 1992;118(5):1259–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu P, Che X, Yu L, et al. Uridine attenuates morphine‐induced conditioned place preference and regulates glutamate/GABA levels in mPFC of mice. Pharmacol Biochem Behav. 2017;163:74–82. [DOI] [PubMed] [Google Scholar]
- 15. Jin W, Kim MS, Jang EY, et al. Acupuncture reduces relapse to cocaine‐seeking behavior via activation of GABA neurons in the ventral tegmental area. Addict Biol. 2017;2017(7):12499. [DOI] [PubMed] [Google Scholar]
- 16. McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine‐induced reinstatement of drug‐seeking behavior. J Neurosci. 2003;23(8):3531–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shin EJ, Dang DK, Tran TV, et al. Current understanding of methamphetamine‐associated dopaminergic neurodegeneration and psychotoxic behaviors. Arch Pharm Res. 2017;40(4):403–28. [DOI] [PubMed] [Google Scholar]
- 18. Tang J, O'Neill J, Alger JR, Shen Z, Johnson MC, London ED. N‐acetyl and glutamatergic neurometabolites in perisylvian brain regions of methamphetamine users. Int J Neuropsychopharmacol. 2018;2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bernheim A, Leong KC, Berini C, Reichel CM. Antagonism of mGlu2/3 receptors in the nucleus accumbens prevents oxytocin from reducing cued methamphetamine seeking in male and female rats. Pharmacol Biochem Behav. 2017;161:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou Z, Karlsson C, Liang T, et al. Loss of metabotropic glutamate receptor 2 escalates alcohol consumption. Proc Natl Acad Sci U S A. 2013;110(42):16963–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wood CM, Nicolas CS, Choi SL, et al. Prevalence and influence of cys407* Grm2 mutation in Hannover‐derived Wistar rats: mGlu2 receptor loss links to alcohol intake, risk taking and emotional behaviour. Neuropharmacology. 2017;115:128–38. [DOI] [PubMed] [Google Scholar]
- 22. Zhou Z, Enoch MA, Goldman D. Gene expression in the addicted brain. Int Rev Neurobiol. 2017;116:251–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Enoch MA, Zhou Z, Kimura M, Mash DC, Yuan Q, Goldman D. GABAergic gene expression in postmortem hippocampus from alcoholics and cocaine addicts; corresponding findings in alcohol‐naive P and NP rats. PLoS ONE. 2012;7(1):e29369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lieberman R, Kranzler HR, Levine ES, Covault J. Examining the effects of alcohol on GABAA receptor mRNA expression and function in neural cultures generated from control and alcohol dependent donor induced pluripotent stem cells. Alcohol. 2018;66:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zai CC, Zai GC, Tiwari AK, et al. Association study of GABRG2 polymorphisms with suicidal behaviour in schizophrenia patients with alcohol use disorder. Neuropsychobiology. 2014;69(3):154–8. [DOI] [PubMed] [Google Scholar]
- 26. Farris SP, Harris RA, Ponomarev I. Epigenetic modulation of brain gene networks for cocaine and alcohol abuse. Front Neurosci. 2015;9(176):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


