Abstract
Aim
Repeated psychostimulant drug treatment, including methamphetamine, in rodents readily produces behavioral sensitization, which reflects altered brain function caused by repeated drug exposure. Dendritic remodeling of medium spiny neurons in the nucleus accumbens is thought to be an essential mechanism underlying behavioral sensitization. We recently showed that chronic methamphetamine treatment did not produce behavioral sensitization in serotonin transporter knockout mice.
Methods
In this study, we report the spine density of medium spiny neurons in the nucleus accumbens after repeated methamphetamine injection to examine morphological alterations in serotonin transporter knockout mice.
Results
Golgi‐COX staining clearly showed that the spine density of medium spiny neurons in the nucleus accumbens increased following repeated methamphetamine treatment in both wild‐type and serotonin transporter knockout mice.
Conclusions
Our results suggested that augmented serotonergic neurotransmission produced by serotonin transporter deletion prevents the development of behavioral sensitization in a manner that is independent of dendritic remodeling in the nucleus accumbens.
1. DESCRIPTION
We recently reported that serotonin transporter (SERT) knockout (KO) mice did not develop behavioral sensitization after repeated injections with methamphetamine (METH).1 Robust sensitization was observed in wild‐type (WT) mice. The repeated administration of psychostimulant drugs, including cocaine and METH, results in many long‐lasting changes in neural mechanisms underlying drug reinforcement and behavioral responses to drugs of abuse. Dendritic remodeling of the nucleus accumbens (NAc) is one of the morphological changes caused by psychostimulant drugs, and these changes have been thought to play key role in psychostimulant‐induced behavioral plasticity.2, 3, 4 Therefore, in this study, we evaluated METH‐induced dendritic changes of medium spiny neurons (MSNs) in the NAc of WT and SERT KO mice. WT and SERT KO mice were injected with saline or METH (1 mg/kg) repeatedly every 1‐2 days (sessions 1‐7). We then measured locomotor activity and analyzed the data as total distance traveled in each session using ANY‐maze software (Stoelting Co., Wood Dale, IL). Analysis of variance (ANOVA) was performed on data from the behavioral sensitization experiment with genotype as a between‐subjects factor and session as a within‐subjects factor. Two‐way repeated ANOVA revealed that there were no significant main effect of genotype (F[1, 4] = 3.261, P = 0.145) and session (F[1, 4] = 5.136, P = 0.086), and no significant genotype × session interaction (F[1, 4] = 1.300, P = 0.318). Because the sample size of our behavioral experiment was very small, we focused on the effect of session in each genotype using paired t test. Similar to our previous report,1 in WT mice, locomotor activity was significantly increased in session 7 compared with session 1 (paired t test, P = 0.045); however, in SERT KO mice, locomotor activity was not altered by repeated treatment with METH (paired t test, P = 0.605; Figure 1A).
Figure 1.
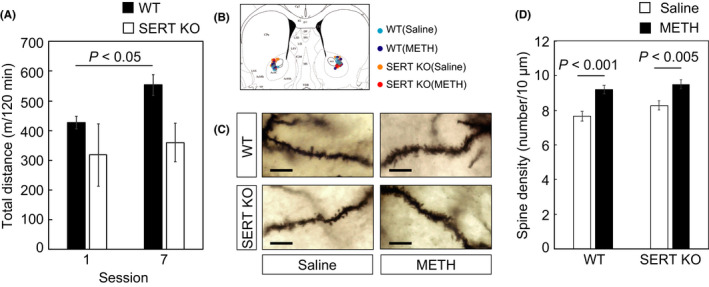
Methamphetamine (METH)‐induced development of behavioral sensitization and increase of spine density in medium spiny neurons (MSNs) in the nucleus accumbens (NAc) of WT and SERT KO mice. METH was repeatedly injected every 1‐2 d, sessions 1‐7. A, Development of behavioral sensitization in WT (n = 3) and SERT KO (n = 3) mice. B, Location of analyzed MSNs in the NAc. Drawing of the coronal sections of the brain are taken from Paxinos and Franklin.10 C, Representative images of Golgi‐COX‐stained dendrites of MSNs in the NAc of WT and SERT KO mice after METH or saline treatments. The scale bar represents 10 μm. D, Spine densities of MSNs in the NAc both in WT and in SERT KO mice significantly increased by repeated treatments of METH compared with those of treatments of saline. Raw data of Figure 1 are shown in Tables S1 and S2 as supporting information
Brains were obtained from WT and SERT KO mice after session 7, and Golgi‐COX staining was conducted to evaluate the changes in spine density of MSNs in the core subregion of the NAc (Figure 1B,C). Detailed methods have been described elsewhere.5 Two‐way ANOVA showed significant main effect of treatment (F[1, 208] = 26.583, P < 0.001), whereas there were no significant effect of genotype (F[1, 208] = 2.954, P = 0.087) and genotype x treatment interaction (F[1, 208] = 0.317, P = 0.574). The Bonferroni post hoc comparison revealed that spine density in the NAc MSNs of WT mice was significantly increased by repeated injections of METH (52 dendrites from 17 neurons, from five animals) compared to values from saline‐injected animals (57 dendrites from 12 neurons, from four animals; P < 0.001). Values of spine densities from the NAc MSNs of SERT KO mice in the METH treatment condition (47 dendrites from 13 neurons, from three animals) were also elevated compared with saline treatment (56 dendrites from 15 neurons, from four animals; P < 0.005; Figure 1D).
These observations clearly indicate that although these mice failed to develop behavioral sensitization, spine density in the NAc MSNs was increased by repeated METH treatment in SERT KO mice to a similar extent to the change observed in WT mice. Overall increases in dendritic spine formation alone thus do not appear sufficient to account for the development of behavioral sensitization. Many studies examining the basis of behavioral sensitization have focused on the mesolimbic dopamine system, which originates from the ventral tegmental area (VTA) projecting mainly to the NAc.4 Our previous report showed that release of extracellular dopamine in the NAc was evoked in both WT and SERT KO mice by acute treatment with METH.1 Thus, repeated dopaminergic stimulation of the NAc in WT and SERT KO mice appears to be sufficient to induce morphological changes in dendritic spine density in the NAc MSNs in both genotypes. It is well known that MSNs play a major role in dopamine signaling through dopamine D1 receptors (D1R) or dopamine D2 receptors (D2R) in the NAc.6 It appears that activity of D1R‐ and D2R‐expressing MSNs may have opposite effects in mediating psychostimulant‐induced locomotion and sensitization. Activity of direct pathway MSNs (D1R‐expressing) stimulates locomotion, whereas activity of indirect pathway MSNs (D2R‐expressing) inhibits locomotion.7, 8 Thus, although we could not distinguish the cell type of MSNs in the NAc, cell‐type‐specific analysis for spine density might be effective to elucidate the function of SERT in MSNs after repeated METH treatment. Although these morphological changes were observed in both genotypes, behavioral sensitization was not in SERT KO mice. The sensitivity of these cells to serotonergic inputs may be critical for the development and/or expression of behavioral sensitization. Excess stimulation of 5‐HT 1B receptor may mitigate the consequences of these changes in SERT KO mice since the development of behavioral sensitization in these animals is restored by treatment with 5‐HT 1B receptor antagonists.1 These observations suggested that augmented serotonergic neurotransmission could prevent the development and/or expression of behavioral sensitization in a manner that is independent of dendritic remodeling in the NAc. Treatments targeting serotonergic neurotransmission might thus have therapeutic potential in psychostimulant addiction.
2. REAGENTS
2.1. Animals
The original line of SERT mutant mice described previously9 was used to produce the congenic mutant line by repeated backcrosses onto a genetic background of C57BL6/N for 20 generations.1 All experiments used male mice. Mice were housed socially (segregated by sex), in a temperature‐ (22‐24°C) and light‐controlled room (light on 08:00‐20:00 hours). Food and water were available ad libitum. All experiments were performed in accordance with the Guidelines for Care of Laboratory Animals of Tohoku University Graduate School of Medicine and conformed to all Japanese federal rules and guidelines.
2.2. Drugs
Methamphetamine hydrochloride (Dainippon‐Sumitomo Pharmaceuticals, Osaka, Japan) was dissolved in 0.9% saline (Otsuka, Tokyo, Japan). Drugs were administered intraperitoneally. All drugs were administered in a 10 mL/kg volume.
CONFLICT OF INTEREST
The authors declare no conflict of interest for this article.
DATA REPOSITORY
Raw data are shown in supporting information.
AUTHOR CONTRIBUTION
YK, YS, and IS involved in the conception and design of the experiments. TH and YS performed the experiments. YK analyzed the data and wrote the manuscript. YS, TH, YM, KPL, FSH, GRU, and IS edited the manuscript. All of the authors read and approved the final manuscript.
APPROVAL OF THE RESEARCH PROTOCOL BY AN INSTITUTIONAL REVIEWER BOARD
n/a.
INFORMED CONSENT
n/a.
REGISTRY AND THE REGISTRATION NO. OF THE STUDY/TRIAL
n/a.
ANIMAL STUDIES
All experiments were performed in accordance with the Guidelines for Care of Laboratory Animals of Tohoku University Graduate School of Medicine and conformed to all Japanese federal rules and guidelines.
Supporting information
ACKNOWLEDGMENT
The authors gratefully thank Dr. Dennis L. Murphy for providing the original SERT KO mice.
Kasahara Y, Sakakibara Y, Hiratsuka T, et al. Repeated methamphetamine treatment increases spine density in the nucleus accumbens of serotonin transporter knockout mice. Neuropsychopharmacol Rep. 2019;39:130–133. 10.1002/npr2.12049
Funding information
This study was supported in part by Research grants from the Ministry of Education, Culture, Sports, Sciences, and Technology (MEXT) of Japan: Grant‐in‐Aid for Health and Labour Science Research (Research on Pharmaceutical and Medical Safety) from MHLW of Japan: Smoking Research Foundation of Japan.
REFERENCES
- 1. Igari M, Shen HW, Hagino Y, et al. Attenuated methamphetamine‐induced locomotor sensitization in serotonin transporter knockout mice is restored by serotonin 1B receptor antagonist treatment. Behav Pharmacol. 2015;26(1‐2):167–79. [DOI] [PubMed] [Google Scholar]
- 2. Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999;11(5):1598–604. [DOI] [PubMed] [Google Scholar]
- 3. Li J, Liu N, Lu K, et al. Cocaine‐induced dendritic remodeling occurs in both D1 and D2 dopamine receptor‐expressing neurons in the nucleus accumbens. Neurosci Lett. 2012;517(2):118–22. [DOI] [PubMed] [Google Scholar]
- 4. Nestler EJ. Molecular basis of long‐term plasticity underlying addiction. Nat Rev Neurosci. 2001;2(2):119–28. [DOI] [PubMed] [Google Scholar]
- 5. Sakakibara Y, Kasahara Y, Hall FS, et al. Developmental alterations in anxiety and cognitive behavior in serotonin transporter mutant mice. Psychopharmacology. 2014;231(21):4119–33. [DOI] [PubMed] [Google Scholar]
- 6. Nagai T, Yoshimoto J, Kannon T, Kuroda K, Kaibuchi K. Phosphorylation signals in striatal medium spiny neurons. Trends Pharmacol Sci. 2016;37(10):858–71. [DOI] [PubMed] [Google Scholar]
- 7. Ferguson SM, Eskenazi D, Ishikawa M, et al. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci. 2011;14(1):22–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beutler LR, Wanat MJ, Quintana A, et al. Balanced NMDA receptor activity in dopamine D1 receptor (D1R)‐ and D2R‐expressing medium spiny neurons is required for amphetamine sensitization. Proc Natl Acad Sci USA. 2011;108(10):4206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bengel D, Murphy DL, Andrews AM, et al. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4‐methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter‐deficient mice. Mol Pharmacol. 1998;53(4):649–55. [DOI] [PubMed] [Google Scholar]
- 10. Paxinos G, Franklin K. The mouse brain in stereotaxic coordinates. 2nd ed San Diego, CA: Academic Press; 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


