Abstract
Aim
This prospective, randomized, controlled, rater‐blinded study investigated the effect of G protein‐activated inwardly rectifying potassium (GIRK) channel inhibitor ifenprodil on alcohol use in patients with alcohol dependence.
Methods
The participants were 68 outpatients with alcohol dependence who were assigned to an ifenprodil group (administered 60 mg ifenprodil per day for 3 months) or control group (administered 600 mg ascorbic acid and calcium pantothenate per day for 3 months). The participants completed a questionnaire that included the frequency of alcohol drinking and presence of heavy drinking before the study period (time 1) and 3 months after the start of the study period (time 2). The alcohol use score was calculated using these two items.
Results
Valid data were obtained from 46 participants (25 in the ifenprodil group and 21 in the control group). The alcohol use score at time 2 in the ifenprodil group was significantly lower than that in the control group after adjusting for the score at time 1 and some covariates. The intention‐to‐treat analysis of multiply imputed datasets indicated similar results. Group differences in the frequency of alcohol drinking were significant in the multiply imputed datasets but not in 46 participants. The ifenprodil group had a significantly lower rate of heavy drinking at time 2 than the control group.
Conclusions
This study found an inhibitory effect of ifenprodil on alcohol use in patients with alcohol dependence. The results support the hypothesis that GIRK channel inhibitors ameliorate alcohol dependence.
Trial registry
This trial was registered in the UMIN clinical trial registry (UMIN000006347).
Keywords: alcohol dependence, alcohol use, G protein‐activated inwardly rectifying potassium channel, ifenprodil, randomized controlled study
The present prospective, randomized, controlled, rater‐blinded study investigated the effect of G protein‐activated inwardly rectifying potassium (GIRK) channel inhibitor ifenprodil on alcohol use in patients with alcohol dependence. This study found an inhibitory effect of ifenprodil on alcohol use in patients with alcohol dependence. The results support the hypothesis that GIRK channel inhibitors ameliorate alcohol dependence.
1. INTRODUCTION
Alcohol dependence is a psychiatric disease, in which the administration of high doses of alcohol or frequent alcohol use causes a clinically significant disorder or distress, together with problems associated with tolerance and withdrawal. The incidence of chronic alcohol abusers is estimated to be 15‐20 million in the United States, and more than 100 000 deaths are ascribed to alcohol dependence annually.1 The United States National Epidemiologic Survey (N = 42 392) showed that the 12‐month prevalence of alcohol dependence was 3.80%, and the lifetime prevalence of alcohol dependence was 12.48%.2 In Japan, approximately 570 000 adults in the general population of 120 million were classified with alcohol dependence in 2012, making this group one of the largest among the various mental disorders.3
The rewarding effects of addictive substances are mediated by various molecules, and much attention has been given to the effects of G protein‐activated inwardly rectifying potassium (GIRK) channels.4 GIRK channels play an important role in signaling that is influenced by addictive substances. Gi/o proteins are activated by neurotransmitters at various Gi/o protein‐coupled receptors, including M2 muscarinic, opioid, α2‐adrenergic, γ‐aminobutyric acid‐B, D2 dopaminergic, and 5‐HT1A serotonergic receptors. G protein βγ subunits dissociate from G protein α subunits to open GIRK channels.5, 6, 7 The opening of GIRK channels hyperpolarizes the cell membrane to modulate neuronal excitability. Ethanol has been found to directly open GIRK channels.8, 9, 10
Nucleotide sequence differences in GIRK channel subunit genes have been reportedly related to alcohol dependence and other substance use disorders. Ethanol‐induced antinociception is reduced in weaver mutant mice that possess an amino acid sequence mutation of the GIRK2 subunit.5, 8 Hill et al11 reported that GIRK2 knockout mice exhibited a reduction in saccharin aversion at a nonhypnotic dose of ethanol in a conditioned taste aversion paradigm and weaker ethanol‐induced conditioned place preference. GIRK3 knockout mice exhibited a reduction in ethanol withdrawal.12 We previously showed that a nucleotide sequence difference in the GIRK2 gene was associated with opioid sensitivity in humans.13
We previously investigated the effects of various drugs that are frequently prescribed by psychiatrists in Xenopus oocytes and mice and found that the selective serotonin reuptake inhibitors (SSRIs) fluoxetine and paroxetine but not another SSRI, fluvoxamine, inhibited GIRK channels and reduced methamphetamine preference.14, 15, 16, 17, 18 Ifenprodil, which is generally prescribed to improve dizziness after brain infarction or hemorrhage in Japan, is a neuroprotectant that reportedly reduces the preference for addictive substances in mice19 and has also been found to inhibit GIRK channels in Xenopus oocytes.20 Ifenprodil has a stronger inhibitory effect on GIRK channels than SSRIs, although it also inhibits GluN2B subunit‐containing N‐methyl‐D‐aspartate (NMDA) receptors, and has fewer side effects (occasionally dry mouth, nausea, headache, or palpitation) than SSRIs.
We previously reported the effect of GIRK channel inhibition on alcohol dependence in humans. GIRK channel inhibition contributed to alcohol abstinence in outpatients with alcohol dependence.21 However, these studies had some limitations. First, the GIRK channel inhibitors (ie, paroxetine, ifenprodil, and haloperidol) that the participants took in our previous studies varied widely. Second, these previous studies were retrospective. Ifenprodil has a stronger effect on GIRK channels compared with SSRIs, and it has fewer side effects, thus justifying investigations of its effect on alcohol dependence. In Japan, ifenprodil has been approved for the treatment of dizziness after brain infarction or hemorrhage but not for the treatment of alcohol dependence. We conducted a prospective randomized controlled study to investigate the effect of the GIRK channel inhibitor ifenprodil on alcohol use in patients with alcohol dependence.
2. METHODS
2.1. Study design
We conducted this randomized, controlled, rater‐blinded study in the Department of Psychiatry at Tokyo Metropolitan Matsuzawa Hospital.
2.2. Ethics statement
The Institutional Review Board of Tokyo Metropolitan Institute of Medical Science and Tokyo Metropolitan Matsuzawa Hospital approved the study, and all of the participants provided written informed consent. This clinical trial was registered in the UMIN clinical trial registry (UMIN000006347).
2.3. Participants
Considering the possibility that some of the participants would be excluded (eg, because of sudden dropout after the research began; Figure 1), we recruited 68 outpatients with alcohol dependence who were treated in the Department of Psychiatry at a hospital in Tokyo, Japan. Alcohol dependence was diagnosed according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, 4th edition.23 The participants in this study were detoxified patients who were able to complete the questionnaire. Importantly, ifenprodil is generally prescribed to improve dizziness (ie, an aftereffect of brain infarction or brain hemorrhage) and is not currently approved by the Japanese national health insurance program for the treatment of alcohol dependence. Many alcohol‐dependent patients have mild cognitive deficiency or brain damage. Thus, patients who were suspected of having mild cognitive deficiency or brain damage (eg, mild brain infarction) were selected as participants in this study to avoid use for a nonapproved indication. Mild cognitive deficiency and brain damage were assessed by a test of cognitive function and the brain imaging data, respectively, evaluated by physicians. We excluded patients who were abstinent for more than 2 years (ie, all of our participants received the treatment as patients with current and lifetime alcohol dependence at the start of the study period), patients who were noncompliant, patients who participated in other clinical trials, patients with comorbid psychiatric disorders (eg, schizophrenia) or serious physical diseases, and patients who exhibited considerably low motivation for treatment at a physician's discretion.
Figure 1.
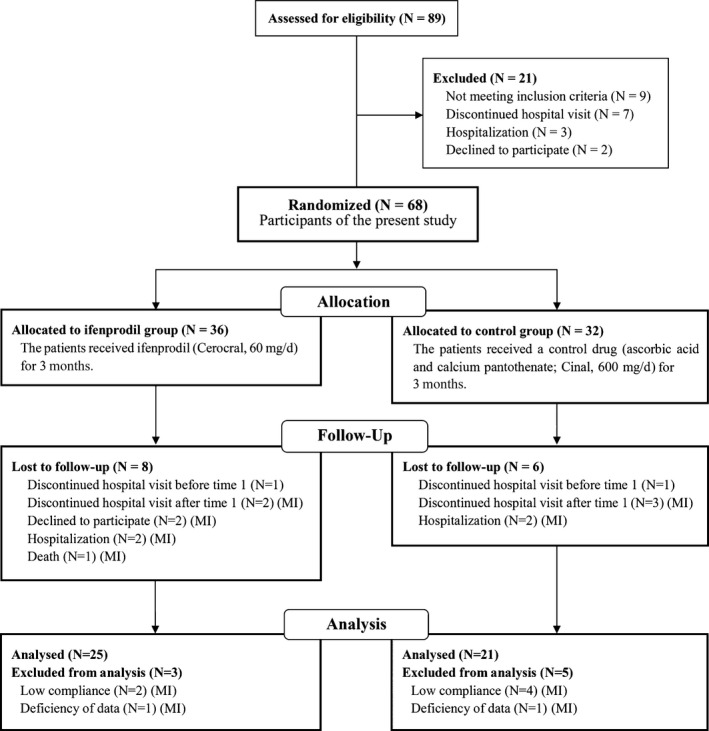
CONSORT 22 diagram for patients with alcohol dependence. (MI): participants applied to multiple imputation analysis
2.4. Randomization and masking
Participants were enrolled by physicians and assigned to two groups, balanced by sex and primary doctor between groups, using stratified permuted block randomization based on computer‐generated random numbers. Person who conducted random assignment informed only physicians to which group participant was assigned. The paper with assigned group and questions regarding other prescribed drugs in sealed envelope was provided to physicians at the beginning of each trial. Raters (clinical psychologists) were unaware of the group assignment.
2.5. Outcomes
2.5.1. Alcohol use disorder identification test
The alcohol use disorder identification test (AUDIT)24, 25 is an instrument that is designed to screen for excessive drinking. The AUDIT consists of three domains and 10 questions. The three domains are “Hazardous alcohol use” (three questions: frequency of drinking, typical quantity, and frequency of heavy drinking), “Dependence symptoms” (three questions: impaired control over drinking, increased salience of drinking, and morning drinking), and “Harmful alcohol use” (four questions: guilt after drinking, blackouts, alcohol‐related injuries, and others concerned about drinking). The internal reliability (α coefficient) was 0.81.
2.5.2. Instrumental activities of daily living
The instrumental activities of daily living (IADL)26 was used to confirm the presence of mild cognitive deficiency by assessing the performance of everyday tasks (eg, using public transportation, managing finances, using the phone, and managing medications). If one of these functions was affected, then it was marked as 1. Disability begins with a score of 2 of 4. The presence of cognitive deficiency was defined by a IADL score of <3 in this study.
2.5.3. Montreal Cognitive Assessment
The Montreal Cognitive Assessment (MoCA)27, 28 is a brief screening tool for mild cognitive impairment. It assesses different cognitive domains: attention and concentration, executive function, memory, language, visuoconstructional skills, conceptual thinking, calculations, and orientation. The administration time for the MoCA is approximately 10 minutes. The total possible score is 30 points. A score ≥ 26 is considered normal. The internal reliability (α coefficient) was 0.74. The presence of cognitive deficiency was defined by a MoCA score of <26 in this study.
2.5.4. Motivation to abstain
The motivation to abstain from alcohol use was assessed as a factor that can possibly influence the effect of the medication on alcohol use. The motivation to abstain during the prior 2 weeks was rated by one statement, “I want to stop drinking” (1 = strongly disagree, 2 = disagree, 3 = neutral, 4 = agree, 5 = strongly agree).
2.5.5. Alcohol use: frequency and amount of alcohol use
The “alcohol use score” was the weighted mean of the frequency of alcohol drinking and presence of heavy drinking, which was the primary endpoint of this study. The frequency of alcohol drinking was rated by one item, “How often did you use alcohol for the past month?” It was answered by selecting one of six choices (0 = none, 1 = once per month, 2 = two to four times per month, 3 = two or three times per week, 4 = four to six times per week, and 5 = every day) for the sake of simplicity. The presence of heavy drinking was rated by one item, “Have you drunk heavily for the past month? (eg, beer, over 1500 mL; Japanese sake, over 540 mL; whiskey, over 180 mL; wine, over 3 glasses per day),” to which the participant answered “Yes” or “No” (1 = Yes, 0 = No). These questions were modified from the AUDIT24, 25. We confirmed the presence of heavy drinking every month, and the participants who answered “Yes” at least once were deemed to have the presence of heavy drinking during the period. Spearman's rank correlation coefficient showed a significant correlation between the frequency of alcohol drinking and presence of heavy drinking (ρ = 0.51), and thus, an integrated index of these two scores was calculated to avoid problems associated with multiple testing. A weighted mean of the frequency of alcohol drinking and presence of heavy drinking, termed the alcohol use score, was calculated using the following formula:
The internal consistency (Cronbach's alpha) of the weighted mean of the frequency of alcohol drinking and presence of heavy drinking was 0.62. This score was the primary endpoint of this study.
2.5.6. Stress experience
Stress experience was assessed as a factor that can possibly cause an individual to drink. Stress experience was rated by one question, “How much did you experience stress during the last 2 weeks? (eg, feeling terrible, sad, down, anxious, or irritable).” It was answered by selecting one of four choices (1 = none, 2 = occasionally, 3 = often, and 4 = almost always).
2.6. Procedure
In the ifenprodil group (N = 36), the patients received ifenprodil (Cerocral, 60 mg/d) for 3 months. In the control group (N = 32), the patients received a control drug (ascorbic acid and calcium pantothenate; Cinal, 600 mg/d) for 3 months. The maximum dosage of prescribed ifenprodil is 60 mg/d in Japan. With regard to the duration of treatment, we decided that a 3‐month duration is suitable for evaluating the effects of the medication on abstinence, based on discussions among the authors and physicians who specialize in alcohol dependence.
The participants in both groups received the same explanation before randomized allocation that they would be administered either Cerocral or Cinal, and the drugs that were administered could potentially improve alcohol dependence symptoms. The participants in this study were administered the AUDIT24, 25, IADL26, and MoCA27, 28 and responded to the item about the motivation to abstain before the study began (time 1). The participants completed questionnaires that included the frequency of alcohol drinking, presence of heavy drinking, and stress experience at time 1 and then 3 months after the start of the study period (time 2). The participants completed the questionnaires in the presence of clinical psychologists who were unaware of the group assignment.
2.7. Sample size
Before participant recruitment, power analysis was performed using G*power 3.0.4 to determine the appropriate sample size for this study.29 The settings for the analysis of covariance (ANCOVA) were the following: effect size (f)30 = 0.4, α = 0.05, 1−β = 0.8, number of groups = 2. This analysis showed that the required number of participants in this study was 52.
2.8. Statistical analysis
Data analysis was performed using SPSS 22.0 software (SPSS, Chicago, IL, USA). The t test was applied to compare ages, AUDIT scores, and MoCA scores before the start of the study period between the ifenprodil group and control group. The Mann‐Whitney U test was applied to compare IADL scores and the motivation to abstain between the ifenprodil group and control group. The χ2 test was used to compare the sex ratio between groups and the presence of heavy drinking at time 2 between groups. Two‐way repeated‐measures analysis of variance (ANOVA) was applied to compare stress experience at times 1 and 2 between groups. Analysis of covariance was used to compare alcohol use scores and the frequency of alcohol drinking at time 2 between groups after adjusting the score at time 1 and other confounding factors, including age, AUDIT score, IADL score, MoCA score, motivation to abstain, and stress experience at time 1. For the ANCOVA of the frequency of alcohol drinking, six choices were converted to the numbers of days per month to adhere to a parametric statistical approach: 0 = 0 d/mo, 1 = 1 d/mo, 2 = 3 d/mo, 3 = 10 d/mo, 4 = 20 d/mo, 5 = 30 d/mo.
Two of the 68 participants dropped out before the start of the study. Twenty of the remaining 66 participants (30.3%) were excluded from the analysis (10 in the ifenprodil group and 10 in the control group; Figure 1). We applied an intention‐to‐treat (ITT) analysis using a multiple imputation technique31 to create and analyze multiply imputed datasets. The incomplete response variables were alcohol use score, frequency of alcohol drinking score, and the presence of heavy drinking at time 2. The observed covariates were these scores at time 1, age, AUDIT score, IADL score, MoCA score, motivation to abstain, and stress experience at time 1. Multiple imputation in R 3.3.0 (R Foundation for Statistical Computing, Vienna, Austria) using the default strings of the mice 2.25 package was estimated using Bayesian linear regression. The results of the analysis of covariance that used a general linear model across 20 imputed datasets were combined by averaging, and standard errors were adjusted to reflect both within‐imputation variability and between‐imputation variability using Rubin's rules. For the presence of heavy drinking, we conducted χ2 tests between groups in multiply imputed datasets. We compared the results of the analysis of multiply imputed cases with the results from complete cases.
3. RESULTS
3.1. Participant attributes
Patient attributes are presented in Table 1. Our investigation was carried out between March 1, 2011, and November 3, 2012, and was ended when the data were collected enough to apply to statistical analysis. Valid data at time 1 and time 2 were obtained for 46 participants (37 males and nine females; 25 participants in the ifenprodil group and 21 participants in the control group) who completed the 3‐month‐long study. No significant difference in the ratio of discontinuation was found between groups (Figure 1). Mild cognitive deficiency in our participants could be identified only by the MoCA or brain imaging data. The IADL did not contribute to the identification of mild cognitive deficiency. The mean age of the participants was 51.7 ± 13.2 years (range = 30‐80 years). The mean scores for the AUDIT, MoCA, 4‐item IADL scale, and motivation to abstain were 21.2 ± 7.9, 22.9 ± 3.2, 3.9 ± 0.3, and 4.5 ± 1.0, respectively. No significant differences in age, sex ratio, AUDIT score, MoCA score, IADL score, or motivation to abstain were found between groups. Two‐way repeated‐measures ANOVA of stress experience did not reveal a significant effect of group or time, with no group × time interaction. Medications that inhibit GIRK channels (ie, sertraline, paroxetine, fluoxetine, maprotiline, and chlorpromazine), with the exception of ifenprodil, were prescribed for six participants in the ifenprodil group and five participants in the control group during the study period, with no significant difference between groups. No participants reported side effects of ifenprodil, including nausea, dry mouth, dizziness, and headache.
Table 1.
Participant attributes
| Ifenprodil group | Control group | Total | |
|---|---|---|---|
| Sex (male/female) | 21/4 | 16/5 | 37/9 |
| Age (years) (mean ± SD) | 52.1 ± 13.8 | 51.2 ± 12.7 | 51.7 ± 13.2 |
| AUDIT score (mean ± SD, N = 45) | 21.8 ± 8.1 | 20.5 ± 7.9 | 21.2 ± 7.9 |
| MoCA score (mean ± SD) | 23.0 ± 3.7 | 22.9 ± 2.6 | 22.9 ± 3.2 |
| IADL score (mean ± SD, N = 43) | 3.9 ± 0.3 | 4.0 ± 0.2 | 3.9 ± 0.3 |
| Motivation to abstain (mean ± SD) | 4.4 ± 1.2 | 4.6 ± 0.7 | 4.5 ± 1.0 |
| Alcohol use score | |||
| Time 1 | 0.2 ± 0.3 | 0.2 ± 0.3 | 0.2 ± 0.3a |
| Time 2 | 0.1 ± 0.0 | 0.2 ± 0.1 | 0.1 ± 0.2 |
| Frequency of alcohol drinking (d/mo) | |||
| Time 1 | 3.9 ± 8.5 | 3.9 ± 7.8 | 3.9 ± 8.1a |
| Time 2 | 2.3 ± 4.9 | 4.8 ± 8.5 | 3.5 ± 6.8 |
| Presence of heavy drinking (%) | |||
| Time 1 | 16.0 | 14.3 | 15.2a |
| Time 2 | 0.0 | 19.0 | 8.7 |
| Stress experience | |||
| Time 1 | 1.0 ± 0.9 | 0.8 ± 0.8 | 0.9 ± 0.9 |
| Time 2 | 1.0 ± 0.9 | 0.9 ± 1.0 | 1.0 ± 1.0 |
| Participants prescribed GIRK channel inhibitors except ifenprodila (%) | 24.0 | 23.8 | 23.9 |
SD, standard deviation; AUDIT, alcohol use disorders identification test; MoCA, Montreal Cognitive Assessment; IADL, instrumental activities of daily living scale; GIRK, G protein‐activated inwardly rectifying potassium.
G protein‐activated inwardly rectifying potassium (GIRK) channel inhibitors except ifenprodil: patients who were prescribed medications that inhibit GIRK channels (ie, sertraline, paroxetine, fluoxetine, maprotiline, and chlorpromazine), with the exception of ifenprodil.
Significant group difference after adjusting for covariates both in 46 participants and in the ITT analysis (N = 66).
Significant group difference after adjusting for covariates in the ITT analysis (N = 66) but not in 46 participants.
Significant group difference both in 46 participants and in the ITT analysis (N = 66).
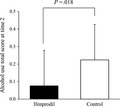
3.2. Comparison of alcohol use score (primary endpoint)
The ANCOVA of alcohol use score was conducted as the primary analysis to investigate the effect of ifenprodil. The alcohol use score at time 2 in the ifenprodil group was significantly lower than in the control group after adjusting for the scores at time 1 (t 43 = 2.5, P = .018, f = 0.4, Cohen's d = 0.33, 95% confidence interval [CI] = 0.03‐0.27; Figure 2 [statistical values of covariate: t 43 = 4.0, P < .001]). After adjusting for alcohol use score, age, AUDIT score, IADL score, MoCA score, motivation to abstain, and stress experience at time 1, the alcohol use score at time 2 was significantly lower in the ifenprodil group than in the control group (t 40 = 2.8, P = .009, f = 0.5, Cohen's d = 0.36, 95% CI = 0.05‐0.33 [statistical values of covariates: t 40 = 2.5, P = .017; t 40 = 0.6, P = .541; t 40 = 0.4, P = .685; t 40 = 1.1, P = .273; t 40 = 1.2, P = .235; t 40 = 0.5, P = .636; t 40 = 0.1, P = .932, respectively]).
Figure 2.
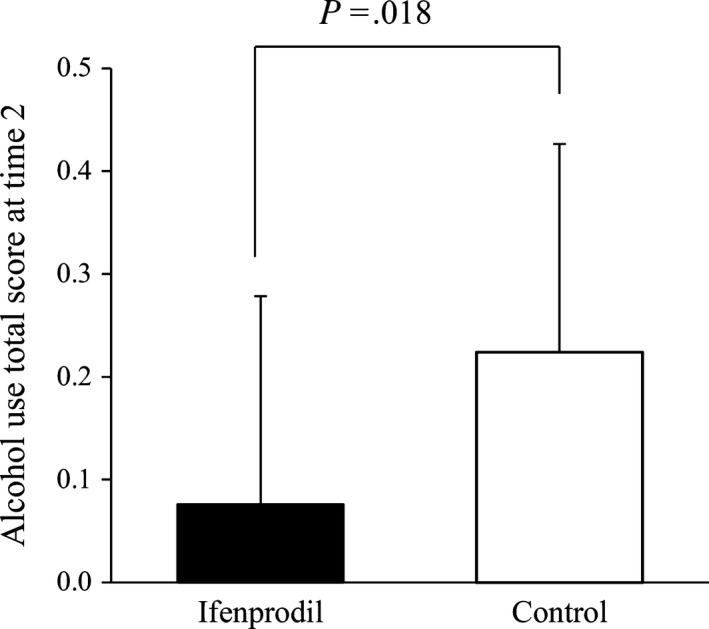
Difference in alcohol use score at time 2 after adjusting the score at baseline (time 1). Ifenprodil group: N = 25. Control group: N = 21. The data were analyzed using analysis of covariance. 95% confidence interval = 0.03‐0.27. Scores are estimated marginal means. Error bars indicate the standard deviations
The ITT analysis of multiply imputed datasets (N = 66) showed that the alcohol use score at time 2 in the ifenprodil group was significantly lower than in the control group (t 60 = 2.5, P = .016, 95% CI = 0.02~0.27) after adjusting alcohol use score (t 60 = 2.8, P = .011), age (t 60 = 0.6, P = .554), AUDIT score (t 60 = 0.03, P = .979), IADL score (t 60 = 1.6, P = .120), MoCA score (t 60 = 0.7, P = .466), motivation to abstain (t 60 = 1.0, P = .319), and stress experience (t 60 = 0.1, P = .889) at time 1.
3.3. Comparison of frequency of alcohol drinking and the presence of heavy drinking
The ANCOVA of frequency of alcohol drinking and χ2 test of the presence of heavy drinking were conducted as the secondary analysis to support the primary analysis of alcohol use score.
After adjusting for alcohol use score, age, AUDIT score, IADL score, MoCA score, motivation to abstain, and stress experience at time 1, no significant group differences in the frequency of alcohol drinking at time 2 were found (P = .088). The ITT analysis of multiply imputed datasets (N = 66) showed that the frequency of alcohol drinking at time 2 in the ifenprodil group was significantly lower than in the control group (t 60 = 2.0, P = .048, 95% CI = 0.03‐7.46) after adjusting the frequency of alcohol drinking (t 60 = 2.2, P = .038), age (t 60 = 0.5, P = .650), AUDIT score (t 60 = 0.3, P = .796), IADL score (t 60 = 2.3, P = .030), MoCA score (t 60 = 0.4, P = .656), motivation to abstain (t 60 = 2.0, P = .050), and stress experience (t 60 = 0.4, P = .671) at time 1.
For the presence of heavy drinking at time 2, the ifenprodil group had a significantly lower rate of heavy drinking than the control group (χ2 1 = 5.5, P = .022). In the ITT analysis of multiply imputed datasets (N = 66), the ifenprodil group had a significantly lower rate of heavy drinking than the control group (χ2 1 = 4.7, P = .031).
4. DISCUSSION
The alcohol use score in the ifenprodil group 3 months after the start of the study period was significantly lower than that in the control group after adjusting for scores before the start of the study period and other confounders, both in 46 participants who completed the study and in multiple imputation with 66 participants. With regard to the frequency of alcohol drinking and presence of heavy drinking, which together comprised the alcohol use score, the frequency of alcohol drinking in the ifenprodil group 3 months after the study began was significantly lower than that in the control group after adjusting for scores before the study began and other confounding factors in multiply imputed datasets but not in 46 participants. The ifenprodil group had a significantly lower rate of the presence of heavy drinking 3 months after the study began compared with the control group. The smaller group difference when only the frequency of alcohol drinking was used as the dependent variable may be attributable to analyzing the data without considering the presence of heavy drinking.
The present study suggests that ifenprodil decreases alcohol use. The present results supported previous results21 that GIRK channel inhibition may decrease alcohol dependence. Most of the GIRK channel inhibitors in the previous study were SSRIs. Ifenprodil has a stronger inhibitory effect on GIRK channels than SSRIs, although it also inhibits GluN2B subunit‐containing NMDA receptors. The number of participants who were prescribed medications that inhibit GIRK channels, with the exception of ifenprodil, during the study period was not significantly different between groups. The present results suggest a possible positive effect of ifenprodil on alcohol use in patients with alcohol dependence. Future investigations should evaluate the long‐term effects of ifenprodil and types of patients who are treated effectively with ifenprodil. Ifenprodil inhibits NMDA receptors and GIRK channels at low micromolar levels and affects brain circulation and metabolism. Tajima et al32 recently reported the mechanism of the inhibitory effect of ifenprodil on NMDA receptors. Acute alcohol exposure inhibits ion flow through NMDA receptor channel complexes,33 whereas chronic alcohol exposure upregulates the number of NMDA receptors and thus increases ion flow.34 Acute withdrawal from alcohol results in hyperexcitability and seizures in the presence of upregulated channels.35 The present results may involve the effect of ifenprodil on NMDA receptors. Future studies should investigate which factors (eg, GIRK channels, NMDA receptors, and brain circulation and metabolism) are associated with the effects of ifenprodil on alcohol use.
Clinical trials and meta‐analyses have demonstrated the efficacy of other medications, including acamprosate,36, 37, 38, 39, 40 naltrexone,41, 42, 43 and disulfiram,44 for alcohol dependence and reported an increase in abstinence rates or duration. However, several other studies failed to show efficacy of these medications for abstinence rates or duration (acamprosate,45, 46, 47 naltrexone,48 and disulfiram49). Thus, the efficacy of these medications for alcohol dependence is still controversial, and investigations of the clinical efficacy of new medications are necessary. In the present study, ifenprodil reduced the severity of alcohol use, suggesting that ifenprodil may be useful for the treatment of alcohol dependence. In the future, the effects of various medications, including ifenprodil, on alcohol use should be compared between studies.
The dropout rates in the present study were 31.6% (11/36) in the ifenprodil group and 34.4% (11/32) in the control group, and the rates of excluded participants from the analysis at time 2 in all participants were 8.3% (3/36, two because of low compliance and one because of deficient data) in the ifenprodil group and 15.6% (5/32, four because of low compliance and one because of deficient data) in the control group (see Figure 1). These results suggest the difficulty that patients with alcohol dependence have in continuing a prospective clinical trial. A previous clinical trial for alcohol dependence50, 51 also reported high rates of participants who did not complete the trials (test drug groups = 39.5‐50.0%, control drug groups = 38.8‐50.0%). Thus, the rates of participant completion in the present study were within the ranges of those of previous clinical trials with alcohol‐dependent patients. The setting of the effect size (f) in the power analysis for the appropriate sample size was 0.4; thus, our results of effect sizes (0.4 and 0.5) fit into the assumption of the power analysis.
The present study has three distinct strengths. First, to our knowledge, this was the first randomized, controlled, rater‐blinded study that investigated the effect of GIRK channel inhibition on alcohol dependence. This randomized controlled study had two groups that were mostly homogeneous, with no significant differences in age, sex ratio, severity of alcohol dependence, or cognitive function at baseline. Second, whereas previous study21 used various anxiolytics, antidepressants, and antipsychotics as GIRK channel inhibitors, the present study investigated the effects of only ifenprodil. Therefore, the present study may provide more reliable evidence than previous studies. Third, ifenprodil is a relatively safe drug that has fewer side effects than SSRIs and has many generic drugs. In the present study, no participants reported side effects of ifenprodil.
The present study also has some limitations. First, the participants knew the name of the medication they took because they were treated according to the health insurance system in Japan. Thus, our participants may have realized whether they were taking the active or control medication. Future double‐blind trials in collaboration with pharmaceutical companies would be ideal. Second, the assessment of heavy drinking in the present study did not consider sex differences because the criteria for heavy drinking, defined by the Ministry of Health, Labour, and Welfare of Japan as the mean volume of pure alcohol consumed that exceeds 60 g/d, are the same for both males and females. Third, we could not control prescribed medications other than Cerocral and Cinal. Fourth, we may not have recruited a sufficient number of subjects. The number of subjects who were recruited in the present study exceeded the suggested number of 52 based on the power analysis, but many participants dropped out of the present clinical trial. We conducted the ITT analysis of multiply imputed datasets in 66 participants to address this limitation. Fifth, we used single, simple questions to assess the frequency of drinking and heavy drinking because answering complicated questions or several questions may be more difficult for patients with alcohol dependence and mild cognitive deficiency. Therefore, the use of these questions may not have sufficient to assess the efficacy of ifenprodil.
5. CONCLUSION
This randomized, rater‐blinded study suggests that ifenprodil has an inhibitory effect on alcohol use in patients with alcohol dependence. The results support the hypothesis that GIRK channel inhibitors ameliorate alcohol dependence. Further clinical studies that utilize a double‐blind, placebo‐controlled, crossover design are worth conducting in the future.
CONFLICT OF INTEREST
The authors declare no conflict of interest for this article. [Correction added on 5 March 2018, after first online publication: The conflict of interest statement has been amended from ‘Nothing declared.’ to the present statement.]
DATA REPOSITORY
We have made our data publicly available in the Data S1 of our article.
APPROVAL OF THE RESEARCH PROTOCOL BY AN INSTITUTIONAL REVIEWER BOARD
The Institutional Review Board of Tokyo Metropolitan Institute OF Medical Science and Tokyo Metropolitan Matsuzawa Hospital approved the study.
INFORMED CONSENT
All of the participants provided written informed consent.
REGISTRY AND THE REGISTRATION NO. OF THE STUDY/TRIAL
This clinical trial was registered in the UMIN clinical trial registry (UMIN000006347).
ANIMAL STUDIES
n/a.
AUTHOR CONTRIBUTIONS
NS collected and entered the data, performed the statistical analysis, and wrote the first draft of the manuscript. YO designed the study, wrote the protocol, collected and entered the data, performed the statistical analysis, and wrote the first draft of the manuscript. YA, YY, MT (Takahama), and MU designed the study, collected the data, and revised the draft of the manuscript. MT (Tanaka) collected, entered, and interpreted the data and revised the draft of the manuscript. AH designed the study, wrote the protocol, collected and entered the data, and revised the draft of the manuscript. KI designed the study, wrote the protocol, and wrote the first draft of the manuscript. All authors approved the final version of the manuscript.
Supporting information
ACKNOWLEDGMENTS
We acknowledge Dr. Jun Horiuchi and Mr. Michael Arends for their assistance with editing the manuscript and Dr. Yasuo Ohashi (Chairman of Board of Directors, Statcom Co, Ltd.) for advice on the statistical analysis. This research was supported by Grants‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan (Tokyo, Japan; no. 22790518, 23390377, 24659549, 24790545, 25116532, and 26293347), Ministry of Health, Labour, and Welfare (MHLW) of Japan (Tokyo, Japan; no. H21‐3jigan‐ippan‐011, H22‐Iyaku‐015, H25‐Iyaku‐020, H26‐Kakushintekigan‐ippan‐060, and 14524680), Smoking Research Foundation (Tokyo, Japan), Japan Agency for Medical Research and Development (AMED) of Japan (Tokyo, Japan; no. JP17dk030707), and Astellas Foundation for Research on Metabolic Disorders (Tokyo, Japan). The funding agencies had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Sugaya N, Ogai Y, Aikawa Y, et al. A randomized controlled study of the effect of ifenprodil on alcohol use in patients with alcohol dependence. Neuropsychopharmacol Rep. 2018;38:9–17. 10.1002/npr2.12001
Nagisa Sugaya and Yasukazu Ogai contributed equally to this study.
REFERENCES
- 1. O'Connor PG, Schottenfeld RS. Patients with alcohol problems. N Engl J Med. 1998;338:592–602. [DOI] [PubMed] [Google Scholar]
- 2. Hasin DS, Grant BF. The co‐occurrence of DSM‐IV alcohol abuse in DSM‐IV alcohol dependence: results of the National Epidemiologic Survey on Alcohol and Related Conditions on heterogeneity that differ by population subgroup. Arch Gen Psychiatry. 2004;61:891–6. [DOI] [PubMed] [Google Scholar]
- 3. Osaki Y, Kinjo A, Higuchi S, et al. Prevalence and trends in alcohol dependence and alcohol use disorders in Japanese adults; results from periodical nationwide surveys. Alcohol Alcohol. 2016;51:465–73. [DOI] [PubMed] [Google Scholar]
- 4. Sugaya N, Kobayashi T, Ikeda K. Role of GIRK channels in addictive substance effects. J Drug Alcohol Res. 2013;2:235823. [Google Scholar]
- 5. Ikeda K, Kobayashi T, Kumanishi T, Yano R, Sora I, Niki H. Molecular mechanisms of analgesia induced by opioids and ethanol: Is the GIRK channel one of the keys? Neurosci Res. 2002;44:121–31. [DOI] [PubMed] [Google Scholar]
- 6. Kobayashi T, Washiyama K, Ikeda K. Inhibition of G protein‐activated inwardly rectifying K+ channels by the antidepressant paroxetine. J Pharmacol Sci. 2006;102:278–87. [DOI] [PubMed] [Google Scholar]
- 7. Nimitvilai S, Lopez MF, Mulholland PJ, Woodward JJ. Ethanol dependence abolishes monoamine and GIRK (Kir3) channel inhibition of orbitofrontal cortex excitability. Neuropsychopharmacology. 2017;42:1800–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kobayashi T, Ikeda K, Kojima H, et al. Ethanol opens G‐protein‐activated inwardly rectifying K+ channels. Nat Neurosci. 1999;2:1091–7. [DOI] [PubMed] [Google Scholar]
- 9. Lewohl JM, Wilson WR, Mayfield RD, Brozowski SJ, Morrisett RA, Harris RA. G‐protein‐coupled inwardly rectifying potassium channels are targets of alcohol action. Nat Neurosci. 1999;2:1084–90. [DOI] [PubMed] [Google Scholar]
- 10. Bodhinathan K, Slesinger PA. Molecular mechanism underlying ethanol activation of G‐protein‐gated inwardly rectifying potassium channels. Proc Natl Acad Sci USA. 2013;110:18309–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hill KG, Alva H, Blednov YA, Cunningham CL. Reduced ethanol‐induced conditioned taste aversion and conditioned place preference in GIRK2 null mutant mice. Psychopharmacology. 2003;169:108–14. [DOI] [PubMed] [Google Scholar]
- 12. Kozell LB, Walter NA, Milner LC, Wickman K, Buck KJ. Mapping a barbiturate withdrawal locus to a 0.44 Mb interval and analysis of a novel null mutant identify a role for Kcnj9 (GIRK3) in withdrawal from pentobarbital, zolpidem, and ethanol. J Neurosci. 2009;29:11662–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nishizawa D, Nagashima M, Katoh R, et al. Association between KCNJ6 (GIRK2) gene polymorphisms and postoperative analgesic requirements after major abdominal surgery. PLoS ONE. 2009;4:e7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kobayashi T, Washiyama K, Ikeda K. Inhibition of G protein‐activated inwardly rectifying K+ channels by fluoxetine (Prozac). Br J Pharmacol. 2003;138:1119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kobayashi T, Washiyama K, Ikeda K. Inhibition of G protein‐activated inwardly rectifying K+ channels by various antidepressant drugs. Neuropsychopharmacology. 2004;29:1841–51. [DOI] [PubMed] [Google Scholar]
- 16. Kobayashi T, Ikeda K. G protein‐activated inwardly rectifying potassium channels as potential therapeutic targets. Curr Pharm Des. 2006;12:4513–23. [DOI] [PubMed] [Google Scholar]
- 17. Takamatsu Y, Yamamoto H, Ogai Y, Hagino Y, Markou A, Ikeda K. Fluoxetine as a potential pharmacotherapy for methamphetamine dependence: studies in mice. Ann N Y Acad Sci. 2006;1074:295–302. [DOI] [PubMed] [Google Scholar]
- 18. Takamatsu Y, Yamamoto H, Hagino Y, Markou A, Ikeda K. The selective serotonin reuptake inhibitor paroxetine, but not fluvoxamine, decreases methamphetamine conditioned place preference in mice. Curr Neuropharmacol. 2011;9:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Suzuki T, Kato H, Tsuda M, Suzuki H, Misawa M. Effects of the non‐competitive NMDA receptor antagonist ifenprodil on the morphine‐induced place preference in mice. Life Sci. 1999;64:PL151–6. [DOI] [PubMed] [Google Scholar]
- 20. Kobayashi T, Washiyama K, Ikeda K. Inhibition of G protein‐activated inwardly rectifying K+ channels by ifenprodil. Neuropsychopharmacology. 2006;31:516–24. [DOI] [PubMed] [Google Scholar]
- 21. Ogai Y, Hori T, Haraguchi A, Asukai N, Senoo E, Ikeda K. [Influence of GIRK channel inhibition on alcohol abstinence and relapse risk in Japanese alcohol‐dependent outpatients]. Nihon Shinkei Seishin Yakurigaku Zasshi. 2011;31:95–6. (In Japanese). [PubMed] [Google Scholar]
- 22. Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. BMC Med. 2010;340:c332. [PMC free article] [PubMed] [Google Scholar]
- 23. American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 24. Hiro H, Shima S. [Availability of the alcohol use disorders identification test (AUDIT) for a complete health examination in Japan]. Nihon Arukoru Yakubutsu Igakkai Zasshi. 1996;31:437–50. (In Japanese). [PubMed] [Google Scholar]
- 25. Babor TF, Higgins‐Biddle JC, Saunders JB, Monteiro MG. AUDIT: the alcohol use disorders identification test: guidelines for use in primary care. 2nd ed Geneva: World Health Organization; 2001. [Google Scholar]
- 26. Lawton MP, Brody EM. Assessment of older people: self‐maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–86. [PubMed] [Google Scholar]
- 27. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–9. [DOI] [PubMed] [Google Scholar]
- 28. Fujiwara Y, Suzuki H, Yasunaga M, et al. Brief screening tool for mild cognitive impairment in older Japanese: validation of the Japanese version of the Montreal Cognitive Assessment. Geriatr Gerontol Int. 2010;10:225–32. [DOI] [PubMed] [Google Scholar]
- 29. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91. [DOI] [PubMed] [Google Scholar]
- 30. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 31. Rubin DB. Multiple imputation for nonresponse in surveys. New York, NY: Wiley; 1987. [Google Scholar]
- 32. Tajima N, Karakas E, Grant T, et al. Activation of NMDA receptors and the mechanism of inhibition by ifenprodil. Nature. 2016;534:63–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA‐activated ion current in hippocampal neurons. Science. 1989;243:1721–4. [DOI] [PubMed] [Google Scholar]
- 34. Trevisan L, Fitzgerald LW, Brose N, et al. Chronic ingestion of ethanol up‐regulates NMDAR1 receptor subunit immunoreactivity in rat hippocampus. J Neurochem. 1994;62:1635–8. [DOI] [PubMed] [Google Scholar]
- 35. Grant KA, Valverius P, Hudspith M, Tabakoff B. Ethanol withdrawal seizures and the NMDA receptor complex. Eur J Pharmacol. 1990;176:289–96. [DOI] [PubMed] [Google Scholar]
- 36. Paille F, Guelfi J, Perkins AC, Royer RJ, Steru L, Parot P. Double‐blind randomized multicentre trial of acamprosate in maintaining abstinence from alcohol. Alcohol Alcohol. 1995;30:239–47. [PubMed] [Google Scholar]
- 37. Sass H, Soyka M, Mann K, Zieglgänsberger W. Relapse prevention by acamprosate: results from a placebo‐controlled study on alcohol dependence. Arch Gen Psychiatry. 1996;53:673–80. [DOI] [PubMed] [Google Scholar]
- 38. Pelc I, Verbanck P, Le Bon O, Gavrilovic M, Lion K, Lehert P. Efficacy and safety of acamprosate in the treatment of detoxified alcohol‐dependent patients: a 90‐day placebo‐controlled dose‐finding study. Br J Psychiatry. 1997;171:73–7. [DOI] [PubMed] [Google Scholar]
- 39. Kranzler HR, Gage A. Acamprosate efficacy in alcohol‐dependent patients: summary of results from three pivotal trials. Am J Addict. 2008;17:70–6. [DOI] [PubMed] [Google Scholar]
- 40. Rösner S, Hackl‐Herrwerth A, Leucht S, Lehert P, Vecchi S, Soyka M. Acamprosate for alcohol dependence. Cochrane Database Syst Rev 2010;9:CD004332. [DOI] [PubMed] [Google Scholar]
- 41. Streeton C, Whelan G. Naltrexone, a relapse prevention maintenance treatment of alcohol dependence: a meta‐analysis of randomized controlled trials. Alcohol Alcohol. 2001;36:544–52. [DOI] [PubMed] [Google Scholar]
- 42. Srisurapanont M, Jarusuraisin N. Naltrexone for the treatment of alcoholism: a meta‐analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2005;8:267–80. [DOI] [PubMed] [Google Scholar]
- 43. Miranda R, Ray L, Blanchard A, et al. Effects of naltrexone on adolescent alcohol cue reactivity and sensitivity: an initial randomized trial. Addict Biol. 2014;19:941–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Niederhofer H, Staffen W. Comparison of disulfiram and placebo in treatment of alcohol dependence of adolescents. Drug Alcohol Rev. 2003;22:295–7. [DOI] [PubMed] [Google Scholar]
- 45. Mason BJ, Goodman AM, Chabac S, Lehert P. Effect of oral acamprosate on abstinence in patients with alcohol dependence in a double‐blind, placebo‐controlled trial: the role of patient motivation. J Psychiatr Res. 2006;40:383–93. [DOI] [PubMed] [Google Scholar]
- 46. Tolliver BK, Desantis SM, Brown DG, Prisciandaro JJ, Brady KT. A randomized, double‐blind, placebo‐controlled clinical trial of acamprosate in alcohol‐dependent individuals with bipolar disorder: a preliminary report. Bipolar Disord. 2012;14:54–63. [DOI] [PubMed] [Google Scholar]
- 47. Berger L, Fisher M, Brondino M, et al. Efficacy of acamprosate for alcohol dependence in a family medicine setting in the United States: a randomized, double‐blind, placebo‐controlled study. Alcohol Clin Exp Res. 2013;37:668–74. [DOI] [PubMed] [Google Scholar]
- 48. Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck R. Naltrexone in the treatment of alcohol dependence. N Engl J Med. 2001;345:1734–9. [DOI] [PubMed] [Google Scholar]
- 49. Jørgensen CH, Pedersen B, Tønnesen H. The efficacy of disulfiram for the treatment of alcohol use disorder. Alcohol Clin Exp Res. 2011;35:1749–58. [DOI] [PubMed] [Google Scholar]
- 50. Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174:70–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wong CJ, Witcher J, Mallinckrodt C, et al. A phase 2, placebo‐controlled study of the opioid receptor antagonist LY2196044 for the treatment of alcohol dependence. Alcohol Clin Exp Res. 2014;38:511–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


