Abstract
Aim
We have previously demonstrated that upregulation of CC chemokines through dopamine receptor signaling in the prefrontal cortex (PFC) underlies methamphetamine (Meth)‐induced reward. Given the common pharmacological property of Meth and cocaine (Coca), which are highly addictive psychostimulants, we hypothesized that chemokines may also contribute to Coca‐induced reward. The aim of this study was to identify a key chemokine‐mediating Coca‐induced reward in mice.
Methods
The mRNA expression levels of chemokines were measured by reverse transcription‐quantitative polymerase chain reaction. Coca‐induced reward was evaluated by conditioned place preference test.
Results
We found that mRNA expression levels of CC chemokine ligand 2 (CCL2), CCL7, and CXC chemokine ligand 1 (CXCL1) were upregulated in the PFC after a single administration of Coca (20 mg/kg, s.c.). Upregulation of CXCL1, but not CCL2 and CCL7, mRNA in the PFC was also observed after repeated administration of Coca. A single administration of dopamine D1 receptor agonist SKF 81297 (10 mg/kg, s.c.), but not D2 receptor agonist sumanirole, upregulated CXCL1 mRNA in the PFC. Coca‐induced reward was attenuated by the pretreatment of SB 225002 (5 mg/kg, s.c.), a selective antagonist of CXC chemokine receptor 2 (CXCR2, cognate receptor for CXCL1).
Conclusions
Collectively, we identified CXCL1 as a key regulator in Coca‐induced reward and propose that pharmacological approach targeting CXCL1 could be a novel pharmacotherapy for Coca‐induced reward.
Keywords: addiction, conditioned place preference test, dependence, dopamine receptor, prefrontal cortex
Upregulation of CXCL1 through dopamine D1 receptor signaling in the prefrontal cortex plays an important role in cocaine‐induced reward.
1. INTRODUCTION
We have previously demonstrated that upregulated chemokines (CC chemokine ligand 2 [CCL2] and CCL7) in the prefrontal cortex (PFC) underlie methamphetamine (Meth)‐induced reward estimated by conditioned place preference (CPP) test in mice.1, 2 To date, cocaine (Coca) is widely distributed as a highly addictive psychostimulant as well as Meth, and the mechanisms of Coca‐induced reward have been investigated. Despite there are several lines of evidence, roles of chemokines in Coca‐induced reward are poorly understood. Given the common pharmacological property of Meth and Coca,3 we hypothesized that chemokines may also contribute to Coca‐induced reward.
We performed microarray analysis to identify upregulated chemokines in the PFC after Meth administration. Among all chemokines, CCL2, CCL7, and CXC chemokine ligand 1 (CXCL1) showed significant upregulation by Meth administration in comparison with control saline (data not shown). Because both Meth and Coca administration induce reward via dopaminergic systems,4 we hypothesized that the chemokines may play an important role in Coca‐induced reward as well as Meth‐induced reward. Therefore, we determined whether these chemokines were also upregulated in the PFC after Coca administration.
By reverse transcription‐quantitative polymerase chain reaction (RT‐qPCR), the mRNA expression levels of CCL2 (3.8 ± 0.7), CCL7 (6.7 ± 1.1), and CXCL1 (3.4 ± 0.4) at 60 minutes after a single administration of Coca (20 mg/kg, s.c.) were significantly higher than that after saline (CCL2, 1 ± 0.1; CCL7, 1 ± 0.1; CXCL1; 1 ± 0.1), and the upregulations of these chemokines slightly persisted for at least 120 minutes (CCL2, 2 ± 0.5; CCL7, 2.6 ± 0.6; CXCL1, 4.2 ± 0.5). Interestingly, the upregulation of CXCL1, but not CCL2 and CCL7, mRNA in the PFC was also observed at 120 minutes (CCL2, 1.1 ± 0.2; CCL7, 1.3 ± 0.3; CXCL1, 3.1 ± 0.7) after repeated administration of Coca (once a day for consecutive 3 days), implying the involvement of CXCL1 in Coca‐induced reward (Figure 1).
Figure 1.
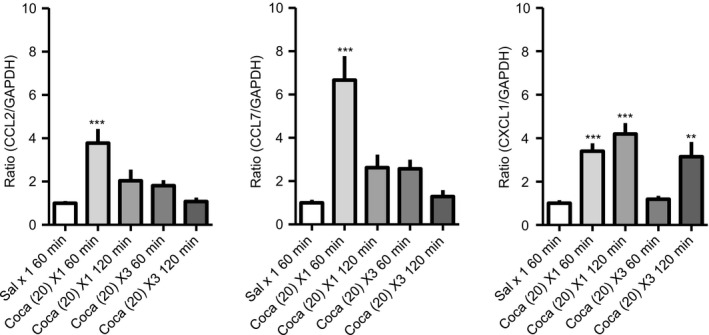
The mRNA expression levels of CC chemokine ligand 2 (CCL2), CCL7, and CXC chemokine ligand 1 (CXCL1) in the prefrontal cortex (PFC) after cocaine (Coca) administration. The PFC samples were collected at indicated time point after a single administration of Coca (20 mg/kg, s.c.) or saline (Sal), and after a repeated administration of Coca (20 mg/kg, s.c., once a day for 3 days). The mRNA expression levels of CCL2, CCL7, and CXCL1 in the PFC were evaluated by RT‐qPCR. Each column shows the mean intensity ratio to GAPDH, and the data are presented as the mean ± SEM of 6‐12 mice. [F(4, 36) = 14.77], **P < .01, ***P < .001 vs Sal
Because dopamine receptor signaling is essential for Coca‐induced reward,5, 6 we next investigated whether dopamine receptor agonists directly upregulate CXCL1 mRNA in the PFC. A single administration of the D1 receptor selective agonist SKF 81297 (10 mg/kg, s.c.) upregulated CXCL1 mRNA in the PFC at 60 minutes (5.8 ± 1.3) in comparison with saline (1 ± 0.2). In contrast, the D2 receptor selective agonist sumanirole (10 and 20 mg/kg, s.c.) had no effect on mRNA expression level of CXCL1 in the PFC (0.97 ± 0.23 and 1.1 ± 0.2, respectively (Figure 2). These results indicate that activation of dopamine D1 receptor, but not D2 receptor, signaling is critical for the CXCL1 expression.
Figure 2.
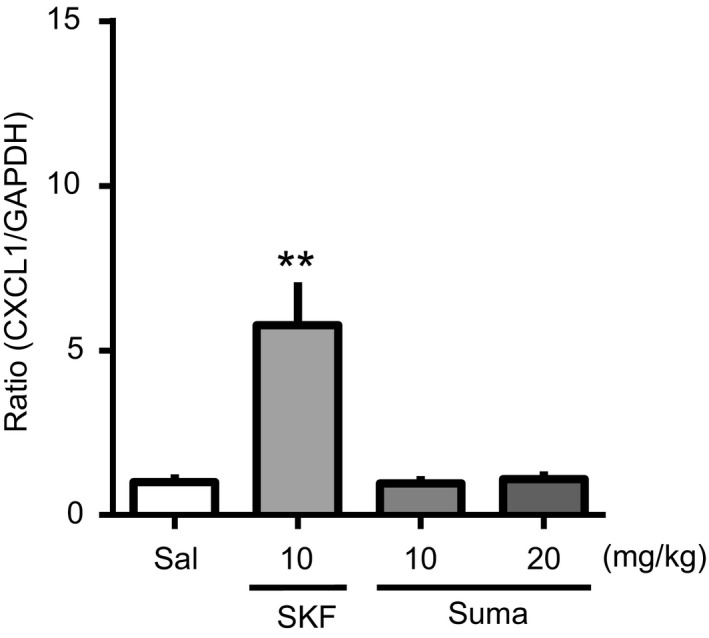
Upregulation of CXCL1 in the PFC induced by dopamine D1 receptor agonist. The PFC samples were collected at 60 min after a single administration of SKF 81297 (SKF; D1 receptor agonist, 10 mg/kg, s.c.), sumanirole (Suma; D2 receptor agonist, 10 and 20 mg/kg, s.c.), or Sal, and the mRNA expression levels of CXCL1 in the PFC were evaluated by RT‐qPCR. Each column shows the mean intensity ratio to GAPDH, and the data are presented as the mean ± SEM of 5‐6 mice. [F(3, 19) = 11.33], **P < .01 vs Sal
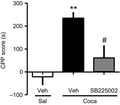
Finally, we determined the pathophysiological roles of upregulated CXCL1 in the Coca‐induced reward. The preventive effect of the selective CXC chemokine receptor 2 (CXCR2) antagonist SB 225002 (5 mg/kg, s.c.) on Coca‐induced reward was assessed by the CPP test. The dose of SB 225002 was determined with reference to the previous reports.7, 8 Pretreatment with SB 225002 15 minutes prior to each Coca conditioning session significantly attenuated Coca‐induced place preference [Veh/Sal (−21.4 ± 36.5), Veh/Coca (233.9 ± 27.4), SB225002/Coca (60 ± 55.6)] (Figure 3). These results support the notion that upregulation of CXCL1 through dopamine D1 receptor signaling in the PFC plays an important role in the pathogenesis of Coca‐induced reward.
Figure 3.
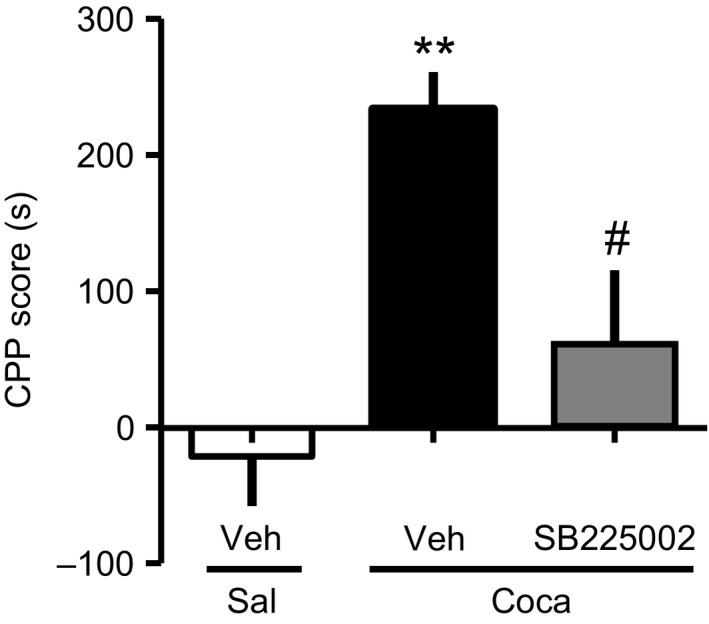
Facilitative role of CXC chemokine receptor 2 (CXCR2) signaling in Coca‐induced conditioned place preference (CPP). Coca (20 mg/kg, s.c.) or Sal was administered to mice, and the Coca‐induced reward was evaluated by CPP test. SB 225002 (a CXCR2 selective antagonist, 5 mg/kg, s.c.) was administered 15 min prior to each Coca conditioning. CXCR2 is a corresponding receptor for CXCL1. Data are presented as the mean ± SEM of 7 mice. [F(2, 18) = 9.867], **P < .01 vs Veh/Sal. # P < .05 vs Veh/Coca
2. MATERIALS AND METHODS
2.1. Animals and drug administration
Male C57BL/6J mice aged 8‐10 weeks (SLC, Hamamatsu, Japan) were used in all experiments approved by the Animal Research Committee of Wakayama Medical University. Mice were housed 5 per plastic cage in a temperature‐controlled room (23°C–24°C, with 60%–70% humidity) with a 12‐hour dark/light cycle (light on 8:00 AM‐8:00 PM) and allowed access to water and food ad libitum. Cocaine (Coca; Takeda Pharma, Osaka, Japan), SB 225002 (Tocris Bioscience, Bristol, UK), SKF 81297 hydrobromide (SKF; Tocris Bioscience), and sumanirole maleate (Suma; Tocris Bioscience) were dissolved in physiological saline, and those were administered to mice by dorsal subcutaneous injection (s.c., 0.1 mL/10 g body weight).
2.2. RT‐qPCR
Drugs were administered to mice without behavioral experiments. Mice were euthanized by decapitation at 60‐120 minutes after administration, and fresh PFC samples collected from 1‐mm‐thick forebrain sections were kept in RNAlater (Ambion, Austin, Texas, USA). Total RNA was isolated using a ReliaPrep RNA tissue miniprep system (Promega, Madison, WI) following the manufacturer's instructions. Total RNA (1 μg) was used for the synthesis of cDNA by reverse transcription (RT) as follows. Total RNA was incubated with Random Primers (Invitrogen, Carlsbad, CA, USA) at 70°C for 5 minutes and then cooled on ice. Samples were converted to cDNA by incubation with M‐MLV Reverse Transcriptase (Promega) and dNTP Mix (Promega) at 37°C for 60 minutes. The synthesized cDNA (10 ng) was used as a template for quantitative polymerase chain reaction (qPCR) with a KAPA SYBR FAST qPCR Kit (KAPA Biosystems, Boston, MA) using an ECO Real‐Time PCR System (AsOne, Osaka, Japan). The primers in this study were designed by Primer 3. The primers for glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH; 5′‐GGG TGT GAA CCA CGA GAA AT‐3′, 5′‐ACT GTG GTC ATG AGC CCT TC‐3′, CCL2; 5′‐AGG TCC CTG TCA TGC TTC TG ‐3′, 5′‐TCA TTG GGA TCA TCT TGC TG‐3′, CCL7; 5′‐ ATC TCT GCC ACG CTT CTG TG ‐3′, 5′‐ CCT CTT GGG GAT CTT TTG TTT C ‐3′ and CXCL1; 5′‐CTG GGA TTC ACC TCA AGA ACA TC‐3′, 5′‐GTG TGG CTA TGA CTT CGG TTT G‐3′) were purchased from Hokkaido System Science (Hokkaido, Japan). Reactions were performed at 95°C for 15 seconds followed by 60°C for 60 seconds. The fluorescence intensity of the intercalated SYBR Green was measured and normalized to GAPDH.
2.3. CPP test
For the assessment of Coca‐induced reward, the CPP test was conducted as previously described1, 2 within a conditioning chamber consisting of 2 equal sized (160 × 160 × 160 mm) compartments made of acrylic resin board. One compartment consisted of white walls and a floor with a rough surface, and the other compartment had black walls with a smooth floor surface. The 2 compartments were separated by a sliding plate door. The experimental schedule was conducted over 10 days and was divided into 3 periods (i.e preconditioning on days 1‐3, Coca conditioning on days 4‐9, and postconditioning on day 10). The dose of Coca was determined in accordance with previous reports.9, 10
2.3.1. Preconditioning
On days 1‐2, mice were placed in the chamber with the door open and allowed to freely move between the 2 compartments for 20 minutes. On day 3, mice were exposed to the same conditions as the previous day, and the time spent in each compartment was measured over 20 minutes (1200 seconds). The compartment that mice spent more time in was designated the preferred compartment.
2.3.2. Conditioning
On day 4, mice were administered Coca (20 mg/kg, s.c.) and were kept in the nonpreferred compartment for 60 minutes. The next day, mice were administered vehicle and were kept in the preferred compartment for 60 minutes. A conditioning treatment was conducted once daily, and these treatments were repeated 3 times over 6 days (day 4‐day 9).
2.3.3. Postconditioning
On day 10, conditioned mice were placed in a chamber with the door open and allowed to freely move between the 2 compartments for 20 minutes, like as in the preconditioning period. The CPP caused by Coca was evaluated by measuring the time spent in each compartment over 20 minutes (1200 seconds). The CPP score reflecting the magnitude of reward was calculated as follows: CPP score (s) = (time spent in the Coca‐paired compartment during the postconditioning test) − (time spent in the same compartment during the preconditioning test).
2.4. Statistical analysis
Data are presented as the mean ± standard error of the mean (SEM). One‐way analysis of variance followed by Tukey's multiple comparisons tests was performed using GraphPad Prism5. Values of P less than .05 were considered statistically significant.
CONFLICT OF INTEREST
The authors have no conflict of interest.
DATA REPOSITORY
Raw data are shown in Table S1.
APPROVAL OF THE RESEARCH PROTOCOL BY AN INSTITUTIONAL REVIEWER BOARD
n/a.
INFORMED CONSENT
n/a.
REGISTRY AND THE REGISTRATION NO. OF THE STUDY/TRIAL
n/a.
ANIMAL STUDIES
All experiments were performed with the approval of the Animal Research Committee of Wakayama Medical University.
AUTHORS CONTRIBUTIONS
FS and NK participated in research design. FS performed experiments. FS, SM, and DK analyzed data. FS, SM, NK, and SK wrote the manuscript.
Supporting information
ACKNOWLEDGMENTS
This work was supported by a Grant‐in‐Aid for Young Scientists (B) from the Japan Society for the Promotion of Science (17K15758).
Saika F, Matsuzaki S, Kobayashi D, Kiguchi N, Kishioka S. Chemokine CXCL1 is responsible for cocaine‐induced reward in mice. Neuropsychopharmacol Rep. 2018;38:145–148. 10.1002/npr2.12018
REFERENCES
- 1. Saika F, Kiguchi N, Wakida N, et al. Upregulation of CCL7 and CCL2 in reward system mediated through dopamine D1 receptor signaling underlies methamphetamine‐induced place preference in mice. Neurosci Lett. 2018;665:33–7. [DOI] [PubMed] [Google Scholar]
- 2. Wakida N, Kiguchi N, Saika F, Nishiue H, Kobayashi Y, Kishioka S. CC‐chemokine ligand 2 facilitates conditioned place preference to methamphetamine through the activation of dopamine systems. J Pharmacol Sci. 2014;125:68–73. [DOI] [PubMed] [Google Scholar]
- 3. Gether U, Andersen PH, Larsson OM, Schousboe A. Neurotransmitter transporters: molecular function of important drug targets. Trends Pharmacol Sci. 2006;27:375–83. [DOI] [PubMed] [Google Scholar]
- 4. Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69:628–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen W, Nong Z, Li Y, Huang J, Chen C, Huang L. Role of dopamine signaling in drug addiction. Curr Top Med Chem. 2017;17:2440–55. [DOI] [PubMed] [Google Scholar]
- 6. Baik JH. Dopamine signaling in reward‐related behaviors. Front Neural Circuits. 2013;7:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Devapatla B, Sharma A, Woo S. CXCR2 inhibition combined with sorafenib improved antitumor and antiangiogenic response in preclinical models of ovarian cancer. PLoS ONE. 2015;10:e0139237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang LY, Tu YF, Lin YC, Huang CC. CXCL5 signaling is a shared pathway of neuroinflammation and blood‐brain barrier injury contributing to white matter injury in the immature brain. J Neuroinflammation. 2016;13:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Itzhak Y, Martin JL. Cocaine‐induced conditioned place preference in mice: induction, extinction and reinstatement by related psychostimulants. Neuropsychopharmacology. 2002;26:130–4. [DOI] [PubMed] [Google Scholar]
- 10. Romieu P, Martin‐Fardon R, Maurice T. Involvement of the sigma1 receptor in the cocaine‐induced conditioned place preference. NeuroReport. 2000;11:2885–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


