Abstract
Background
Fentanyl, a synthetic opioid categorized as a narcotic analgesic, has a 100‐ to 200‐fold stronger effect than most opioids, such as morphine. Fatal accidents due to chronic use and abuse of fentanyl are a worldwide social problem. One reason for the abuse of fentanyl is its psychostimulant effects that could induce behavioral changes. The effects of chronic fentanyl administration on behavior, however, are unclear.
Methods
Adult male C57BL/6J mice were chronically administered fentanyl (0.03 or 0.3 mg/kg/d i.p.), and various behaviors were assessed using a behavioral test battery.
Results
Mice chronically administered a high dose of fentanyl (0.3 mg/kg/d) exhibited decreased anxiety‐like behavior as assessed by the open field and elevated plus maze tests. On the other hand, interruption of fentanyl administration led to increased anxiety‐like behavior as observed in the light and dark transition test. The hot plate test revealed that chronic administration of fentanyl reduced pain sensitivity. High‐dose chronic fentanyl administration reduced the locomotor stimulatory effects of cocaine. The results, however, failed to reach the threshold for study‐wide statistical significance.
Conclusion
Chronic fentanyl administration induces some behavioral changes in mice. Although further studies are needed to clarify the underlying mechanisms of the behavioral effects of chronic fentanyl administration, our findings suggest that fentanyl is safe under properly controlled conditions.
Keywords: addiction, behavioral test battery, cocaine, fentanyl, opioid
To investigate the effects of long‐term fentanyl use on brain function, adult male C57BL/6J mice were chronically administered fentanyl (0.03 or 0.3 mg/kg/d ip) and analyzed in a behavioral test battery. Chronic fentanyl administration reduced anxiety‐like behavior, pain sensitivity, and the locomotor stimulatory effects of cocaine in mice. The results, however, failed to reach the threshold for study‐wide statistical significance.
![]()
1. INTRODUCTION
Opioids are clinically utilized for the management of severe pain. Synthetic opioids such as fentanyl, methadone, and oxycodone have stronger and shorter‐lasting effects than morphine and are adaptable for intraoperative anesthesia and chronic pain management.1, 2, 3 In addition, because they are easy to synthesize and cheap, synthetic opioids are often used illegally to produce strong feelings of pleasure.4 Fentanyl can also be mixed with other addictive drugs, such as cocaine and heroin, to increase its potency at little cost.5 Chronic use of synthetic opioids, however, carries a high risk of withdrawal symptoms, addiction, and fatal accidents.6 In fact, the number of deaths due to fentanyl in the United States increased 540% from 2013 to 2016, when United States President Donald Trump declared the opioid epidemic a national emergency.7, 8 This situation, called the “Opioid Crisis”, is a critical social problem. The number of inappropriate uses and fatal accidents involving fentanyl has also increased in the United Kingdom and Canada.9, 10 Fentanyl is classified as a narcotic analgesic with strong effects on the central nervous system. Opioids induce psychostimulant effects other than analgesia, such as sedation, delirium, and itching.11, 12 Although fentanyl is a useful analgesic, the use of fentanyl during surgery is associated with a higher incidence of early postoperative negative behavior such as anxiety.13 Chronic fentanyl treatment affects physical performance of rats.14 Such findings indicate that chronic use of fentanyl may also affect behavior, but the specific behavioral effects of chronic synthetic opioid use remain unclear. Therefore, in the present study, C57BL/6J mice chronically treated with fentanyl were assessed using a comprehensive behavioral test battery to investigate how chronic use of the synthetic opioid affects various behaviors.
2. MATERIALS AND METHODS
2.1. Animals and experimental design for comprehensive behavioral analysis
The general procedure of the experiments is illustrated in Figure 1. Thirty‐eight naïve male C57BL/6J mice were transported from Japan SLC, Inc. (Shizuoka, Japan) to the University of Toyama at the age of 6 weeks. After their arrival, the mice were group‐housed (4/cage) in a plastic cage (22.7 × 32.3 × 12.7 cm) in a room maintained at 24 ± 3°C with a 12‐hour light/dark cycle (lights on at 7:00 am) and ad libitum access to food and water. The mice were randomly assigned to either the vehicle‐treated group (n = 12), the low‐dose (0.03 mg/kg) fentanyl‐treated group (n = 13), or the high‐dose (0.3 mg/kg) fentanyl‐treated group (n = 13). Fentanyl administration was started at 7 weeks of age and behaviors were assessed with a battery of behavioral tests starting at 8 weeks of age (Figure 1).
Figure 1.

Schematic diagram of the experimental procedures. Mice were intraperitoneally injected with fentanyl (0.03 or 0.3 mg/kg) or saline (control) once a day. As fentanyl treatment period 1, we continued fentanyl treatment for 28 days. In the first 7 days, no behavioral tests were performed as pretreatment. After the first 7 days, the behavioral test battery was performed. The fentanyl administration was interrupted for 14 days as a withdrawal period, and fentanyl treatment was resumed as treatment period 2
2.2. Fentanyl treatment
Mice were treated with fentanyl (0.03 or 0.3 mg/kg, ip; Janssen Pharmaceutical KK, Tokyo, Japan) in saline or saline only (1% of body weight) once a day. During behavioral testing, fentanyl was administered once a day after completing the behavioral test of the day. Fentanyl treatment was initiated when the mice were 7 weeks of age and continued for 28 days. Fentanyl treatment was then interrupted for 14 days as a withdrawal period. After that, fentanyl treatment was resumed and continued for 75 days. The fentanyl treatment and behavioral test battery schedules are described in Table 1.
Table 1.
Fentanyl treatment schedule and comprehensive test battery of chronical fentanyl‐treated mice
| Order | Test | Age (w) | Fentanyl treatment period | Day of fentanyl treatment during periods (day) | Table/Figure |
|---|---|---|---|---|---|
| 1 | Neurological screens and neuromuscular strength test | 8 | Treatment period 1 (28 days) | 8–9 | Figure 2B‐D |
| 2 | Light/dark transition test | 8–9 | 11 | Table 2 | |
| 3 | Open field test | 9 | 12–13 | Figure 3B‐G | |
| 4 | Elevated plus maze test | 9–10 | 14 | Figure 4C‐E | |
| 5 | Hot plate test | 10 | 15 | Figure 6B | |
| 6 | Social interaction test | 10 | 18 | Table 2 | |
| 7 | Rotarod test | 10–11 | 19–20 | Table 2 | |
| 8 | Three‐chamber social approach test | 10–11 | 21–22 | Table 2 | |
| 9 | Startle response/prepulse inhibition test | 11 | 25 | Table 2 | |
| 10 | Porsolt forced swim test | 11–12 | 26–27 | Figure 4H | |
| 11 | Neurological screens and neuromuscular strength test | 12–13 | Withdrawal period (14 days) | – | Figure 2E‐G |
| 12 | Light/dark transition test | 13 | – | Figure 5B‐F | |
| 13 | Elevated plus maze test | 13 | – | Table 2 | |
| 14 | Hot plate test | 14 | – | Figure 6C | |
| 15 | Open field test (acute fentanyl; 0.03 mg/kg) | 14 | Treatment period 2 (75 days) | 1 | Figure 7B‐E |
| 16 | Y‐maze test | 14–15 | 6 | Table 2 | |
| 17 | Novel Object Location test | 15–16 | 9–13 | Table 2 | |
| 18 | Marble‐burying test | 16–17 | 20 | Table 2 | |
| 19 | Open field test (acute cocaine; 5 mg/kg) | 18 | 30 | Figure 8B‐E | |
| 20 | Open field test (acute cocaine; 15 mg/kg) | 20–21 | 44 | Figure 8F‐I | |
| 21 | Contextual and cued fear conditioning test | 22–23 | 62–68 | Figure 6 D‐I |
2.3. Behavioral tests
The mice were subjected to a battery of behavioral tests in the following sequence: general health and neurologic screening (body weight, body temperature, and grip strength), the light/dark transition, open field, elevated plus maze, hot plate, social interaction, three‐chamber social approach, rotarod, startle response/prepulse inhibition, and Porsolt forced swim tests. During the withdrawal period, the same mice again underwent general health and neurologic screening, and the light/dark transition, elevated plus maze, and hot plate tests. Fentanyl treatment was subsequently resumed and additional behaviors were assessed in the following test sequence: open field test with acute administration of 0.03 mg/kg fentanyl, Y‐maze, object location test, marble‐burying test, open field test with acute administration of cocaine (5 and 15 mg/kg), and contextual/cued fear conditioning. All of the vehicle‐treated and fentanyl‐treated mice went through the same test battery on the same day so that they experienced all of the behavioral tests in the same order. After each test, the floors and walls of the testing apparatuses were cleaned with 70% ethanol solution or super hypochlorous water to prevent a bias caused by olfactory cues. The behavioral tests were performed between 8:30 am and 7:00 pm. Information about each mouse and the behavioral data collected in this study are available in the “Mouse Phenotype Database” (http://www.mouse-phenotype.org/).
2.3.1. Neurological screen and neuromuscular strength test
The righting, whiskers twitch, and ear twitch reflexes were evaluated. A number of physical features, including the presence of whiskers or bald hair patches, were also recorded. Body weight and rectal temperature were measured. Neuromuscular strength was assessed using the grip strength and wire hang tests. A grip strength meter (O'Hara & Co., Tokyo, Japan) was used to assess forelimb grip strength. Mice were lifted and held by their tail so that their forepaws grasp a wire grid. The mice were then gently pulled backward by the tail until they released the grid. The peak force applied by the forelimbs of the mouse was recorded in Newtons (N). Each mouse was tested three times, and the largest value was used for statistical analysis.
2.3.2. Light/dark transition test
The light/dark transition test, developed by Crawley et al,15 was performed as previously described.15 The apparatus comprised a cage (21 × 42 × 25 cm) divided into two sections of equal size divided by a partition with a door (O'Hara & Co.). One chamber was brightly illuminated (390 lux), whereas the other was dark (2 lux). Mice were placed into the dark chamber and were allowed to move freely between the two chambers for 10 minutes with the door open. The distance traveled (cm), the total number of transitions between compartments, latency to first enter the light chamber (seconds), and time spent in the light chamber (seconds) were recorded automatically using the ImageLD program.
2.3.3. Elevated plus maze test
The elevated plus maze test, which is widely used to assess anxiety‐like behavior,17 was performed as previously described.17 The apparatus comprised two arms without walls (open arms, 25 × 5 cm), two arms of the same size with 15‐cm‐high transparent walls (closed arms), and a central square (5 × 5 cm) connecting the arms, which were at 90° to each other (O'Hara & Co.). The arms and central square were made of white plastic plates and were elevated to a height of 55 cm above the floor. The open arms were surrounded by a raised ledge (3 mm thick and 3 mm high) to prevent mice from falling off the open arms. Arms of the same type were located opposite to one another. Each mouse was placed in the central square of the maze facing one of the closed arms. The number of arm entries, distance traveled (cm), percentage of entries into the open arms, and percentage of time spent in the open arms were measured during a 10‐minute test period. Data acquisition and analysis were performed automatically using the ImageEP program.
2.3.4. Social interaction test
The social interaction test was conducted to measure social behavior in a novel environment, as previously described.19 Weight‐matched (within 2 g) mice of the same treatment group that had been housed in different cages were placed together into an acrylic box (40 × 40 × 30 cm) and allowed to explore freely for 10 minutes. The total number of contacts, total duration of contacts (seconds), total duration of active contacts (seconds), mean duration per contact (seconds), and total distance traveled (cm) were recorded and analyzed automatically using the ImageSI program. Active contact was defined as the two mice contacted each other and one or both mice moved with a velocity of at least 10 cm/s.
2.3.5. Three‐chamber social approach test
The three‐chamber social approach test is a well‐designed method to investigate sociability and preference for social novelty in mice.20 The apparatus comprised a rectangular, three‐chambered box and a lid with a video camera (O'Hara & Co.). Each chamber was 20 × 40 × 47 cm, and the dividing walls were made from clear Plexiglas with a small square opening (5 × 3 cm) allowing access into each chamber. The tests were performed as previously described,20 with a slight modification as follows: Subject mice were placed in the three‐chambered box and allowed to explore for 10 minutes before the sociability test was conducted (habituation session), and during the session, empty wire cages (9 cm in diameter, 11 cm in height, with vertical bars 0.5 cm apart) were located in the corner of each outside compartments. During the following session, an unfamiliar C57BL/6J male mouse (stranger 1) that had had no prior contact with the subject mouse was put into a wire cage located in one of the side chambers. The location of the stranger mouse in the left vs right chamber was systematically alternated between trials. The subject mouse was placed in the central compartment and allowed to explore the entire box for 10‐minutes to assess sociability (sociability test). Next, a second stranger male mouse was placed into the wire cage in the other outside compartment that had been empty during the first 10‐minute session to evaluate social preference for a new stranger (social novelty preference test). Thus, the subject mouse had a choice between the first, already‐investigated, now‐familiar mouse (stranger 1) and the novel unfamiliar mouse (stranger 2). The amount of time spent in each chamber and time spent around each cage were automatically calculated from video images using the ImageCSI program.
2.3.6. Rota rod test
Motor coordination and balance were tested with the rotarod test. The rotarod test, using an accelerating rotarod (UGO Basile Accelerating Rotarod, Varese, Italy), was performed by placing the mice on a rotating drum (3 cm diameter), and measuring the time each animal was able to maintain its balance on the drum. The speed of the rotarod accelerated from 4 to 40 rpm over a 5‐minute period.
2.3.7. Hot plate test
The hot plate test was used to evaluate sensitivity to a painful stimulus. The mice were placed on a 55.0 (±0.3) °C hot plate (Columbus Instruments, Columbus, OH, USA), and the latency to the first hind paw response was recorded. The hind paw response was defined as either a foot shake or a paw lick.
2.3.8. Startle response/prepulse inhibition test
The startle response and prepulse inhibition test were performed as previously described.21 A startle reflex measurement system (O'Hara & Co.) was used. The mice were placed in a Plexiglas cylinder and left undisturbed for 10 minutes. The test comprised two test trials with the startle stimulus only and four test trials for prepulse inhibition. White noise (40 ms) was used as the startle stimulus for all trials. The startle response was recorded for 140 ms (measuring the response every 1 ms) starting with the onset of the prepulse stimulus. The background noise level in each chamber was 70 dB. The peak startle amplitude recorded during the 140‐ms sampling window was used as the dependent variable. The intensity of the startle stimulus was 110 or 120 dB. The prepulse sound was presented 100 ms before the startle stimulus, and its intensity was 74 or 78 dB. Four combinations of prepulse and startle stimuli were employed (74‐110, 78‐110, 74‐120, and 78‐120 dB). The mean inter‐trial interval was 15 seconds (range 10‐20 seconds).
2.3.9. Porsolt forced swim test
The Porsolt forced swim test22 was performed to assess depression‐related behavior. Mice were placed into a Plexiglas cylinder (20 cm height × 10 cm diameter, O'Hara & Co.) filled with water (approximately 23°C) up to a height of 7.5 cm for 10 minutes per day for 2 consecutive days. The percentage of time spent immobile was recorded automatically using the ImagePS program. The data of one mouse from the high‐dose group were excluded from statistical analysis because the mouse died by drowning around 7 minutes on the first day of the test.
2.3.10. Y‐maze test
Spatial working memory was evaluated based on spontaneous alternation behavior. The Y‐maze comprised three arms (labeled A, B, and C) diverging at 120° from the central point (O'Hara & Co.). Each mouse was placed at the center of the maze and allowed to move freely for 10 minutes. An alternation was defined as consecutive entries into all three arms. For example, sequential entering into the arms in an ABCBCBCA pattern was counted as two alternations with the first consecutive ABC and the last consecutive BCA of six consecutive arm entries (maximum alternation). The percentage of alternation was calculated as (alternation/maximum alternation) × 100. Data acquisition and analysis were performed automatically using the ImageYM program.23
2.3.11. Contextual and cued fear conditioning test
The fear conditioning test was conducted using an automated video‐analysis system as previously described.24 Mice were placed in a conditioning chamber (26 × 34 × 29 cm) in a sound‐attenuated room and allowed to explore freely for 2 minutes. The animals were presented with an auditory cue (55 dB white noise) that served as a conditioned stimulus (CS) for 30 seconds. During the last 2 seconds of the CS, mice were given a mild footshock (0.3 mA, 2 seconds) as an unconditioned stimulus (US). Two more CS‐US pairings were presented at 120‐seconds intervals. Approximately 24 hours and 7 days after the conditioning session, a context test was performed in the conditioning chamber. A cued test in an altered context was performed after the context test using a triangular box (35 × 35 × 40 cm) made of white opaque plastic, which was located in a different sound‐attenuated room. In the cued test, after the initial 3‐minutes period of no CS presentation, the CS was presented during the last 3‐minutes period of the test. Freezing during each minute of the test was measured automatically using the ImageFZ program in the same manner as previously described.24 Due to technical problems with the video‐analysis system, we failed to obtain cued test data for one mouse in the high‐dose group 7 days after the conditioning session and therefore excluded the data for this mouse from the statistical analysis.
2.3.12. Open field test
Locomotor activity was measured using an open field test. Each mouse was placed in the corner of the open field apparatus (40 × 40 × 30 cm: AccuScan Instruments, Columbus, OH, USA). The center of the floor was illuminated at 100 lux. Total distance traveled (cm), vertical activity (rearing measured by counting the number of photobeam interruptions), time spent in the center area (20 × 20 cm), and beam‐break counts for stereotypic behaviors were recorded. Data were collected for a period of 120 minutes. To investigate the effects of acute administration of fentanyl (0.03 mg/kg) and cocaine (5 and 15 mg/kg), mice in each group were assessed in the open field test for 1 hour of habituation and for 2 hours after acute administration of the drugs dissolved in saline.
2.3.13. Novel object location test
Each mouse was placed in the corner of the open field apparatus (40 × 40 × 30 cm). The center of the apparatus was illuminated at 100 lux. On days 1‐3, the mice were allowed to explore the chamber for 10 minutes as habituation. On day 4, two identical objects were placed centrally 20 cm apart in the chamber and the mice were allowed to explore the chamber for 15 minutes as a training session. On day 5, one of the objects was placed in the same location as on day 4, and the other object was placed in a new location in the open field apparatus. The mice were allowed to explore the chamber for 15 minutes. The time spent exploring the object located in the novel place and the total time spent exploring both objects were measured (O'Hara & Co.).
2.3.14. Marble‐burying test
The marble‐burying test assesses anxiety‐like/compulsive behavior based on spontaneous digging behavior.25 Twenty marbles were distributed equally on top of mouse cage bedding in a 4 × 5 grid pattern (5 cm in depth). After acclimation (30 minutes) in the test room, each mouse was placed in the cage (25.5 × 41 × 18.5 cm) for 30 minutes. The number of buried marbles and the total distance traveled were recorded with a video camera. A buried marble was operationally defined as half or more of the marble covered with bedding.
2.4. Data analysis in behavioral tests
Behavioral data were obtained automatically by applications (ImageLD,16 ImageEP,18 ImageSI,19 ImageCSI,26 ImagePS,19 ImageYM,23 and ImageFZ24) based on the public domain NIH Image program and ImageJ program, and modified for each test.
2.5. Statistical analysis
Statistical analyses were performed using StatView (SAS Institute, Cary, NC, USA). Data were analyzed using one‐way or two‐way ANOVA followed by Fisher's LSD test, two‐way repeated ANOVA, or paired t‐test where appropriate. Values in graphs are presented as mean ± SEM. For multiple comparisons in the behavioral test battery, we defined study‐wide significance as statistical significance after controlling for the false discovery rate (FDR).27, 28 Nominal significance was defined as a statistically significant difference in an index (P < 0.05) that did not survive FDR correction. The results of the statistical analysis are described in Table 2.
Table 2.
Statistical analyses of behavioral data
| Test | Measure | Tables/figures | Means ± SEM | ANOVA (P) | ||||
|---|---|---|---|---|---|---|---|---|
| Control | Low‐dose treatment | High‐dose treatment | Treatment | Treatment × trial/time | ||||
| Neurological screens and neuromuscular strength test | Body weight (g) | Figure 2B | 25.2 ± 0.42 | 25.123 ± 0.289 | 24.915 ± 0.416 | 0.8599 | ||
| Body temperature (˚C) | Figure 2C | 36.392 ± 0.235 | 36.315 ± 0.116 | 36.808 ± 0.223 | 0.1721 | |||
| Grip strength (N)* | Figure 2D | 0.662 ± 0.049 | 0.675 ± 0.039 | 0.513 ± 0.034 | 0.0132 | |||
| Light/dark transition test | Distance traveled (cm) in light chamber | – | 710.625 ± 34.351 | 668.369 ± 41.888 | 689.4 ± 54.113 | 0.8799 | ||
| Distance traveled (cm) in dark chamber | – | 1486.225 ± 45.406 | 1485.523 ± 32.843 | 1469.331 ± 30.305 | ||||
| Transitions | – | 36.917 ± 2.032 | 36.462 ± 1.547 | 34.538 ± 2.186 | 0.6563 | |||
| Latency to light chamber (s) | – | 39.333 ± 5.975 | 38.077 ± 9.576 | 54.308 ± 10.191 | 0.3632 | |||
| Time spent in light chamber (s) | – | 181.625 ± 11.888 | 171.692 ± 9.587 | 179.423 ± 7.307 | 0.7482 | |||
| Open field test | Distance traveled (cm) | Figure 3B | – | – | – | 0.9384 | 0.7918 | |
| Vertical activity | Figure 3C | – | – | – | 0.4245 | 0.2541 | ||
| Center time (s) | Figure 3D | – | – | – | 0.1032 | 0.4306 | ||
| Stereotypic counts | Figure 3E | – | – | – | 0.4591 | 0.1242 | ||
| Elevated plus maze test | Distance traveled (cm) | Figure 4C | 1717.958 ± 87.487 | 1629.746 ± 106.221 | 1623.669 ± 60.703 | 0.701 | ||
| Number of entries | Figure 4B | 40.083 ± 2.491 | 38.538 ± 3.251 | 41.077 ± 1.848 | 0.7818 | |||
| Entries into open arms (%) | Figure 4D | 35.2 ± 2.022 | 32.585 ± 2.019 | 38.938 ± 1.802 | 0.0773 | |||
| Time on open arms (%) | Figure 4E | 10.633 ± 1.549 | 8.346 ± 1.05 | 11.223 ± 1.066 | 0.2224 | |||
| Hot plate test | Hot plate latency (s)* | Figure 6B | 8.138 ± 0.691 | 10.202 ± 0.675 | 10.633 ± 0.632 | 0.0294 | ||
| Social interaction test | Distance traveled (cm) | – | 3164.483 ± 213.194 | 3474.567 ± 219.404 | 2875.65 ± 172.03 | 0.147 | ||
| Number of contacts | – | 52.5 ± 4.71 | 59 ± 7.385 | 52.667 ± 2.186 | 0.6131 | |||
| Total duration of contacts (s) | – | 66.733 ± 4.558 | 76.367 ± 12.26 | 80.717 ± 8.613 | 0.2598 | |||
| Total duration of active contacts (s) | – | 13.333 ± 1.164 | 17.067 ± 2.43 | 13.933 ± 0.951 | 0.5479 | |||
| Mean duration of contact (s) | – | 1.3 ± 0.073 | 1.3 ± 0.144 | 1.55 ± 0.186 | 0.3799 | |||
| Rotarod test | Rotarod latency (s) | – | Trial 1 | 171.75 ± 32.992 | 127.077 ± 24.124 | 164.846 ± 28.257 | 0.4329 | 0.4215 |
| Trial 2 | 222.917 ± 10.618 | 196.615 ± 27.639 | 255.077 ± 16.014 | |||||
| Trial 3 | 223 ± 16.28 | 213 ± 23.731 | 222.692 ± 18.815 | |||||
| Trial 4 | 265.167 ± 14.84 | 246.231 ± 19.917 | 278 ± 11.527 | |||||
| Trial 5 | 264.583 ± 15.935 | 224.385 ± 19.064 | 236.615 ± 17.461 | |||||
| Trial 6 | 254.667 ± 14.744 | 258.308 ± 15.956 | 232.077 ± 22.283 | |||||
| Three‐chamber social approach test (Sociability test) | Distance traveled (cm) | – | 1948.408 ± 69.662 | 1976.569 ± 95.204 | 1931.938 ± 95.096 | 0.93554 | ||
| Time spent around cage (%) | – | 0.706 ± 0.05 | 0.675 ± 0.039 | 0.701 ± 0.043 | 0.7439 | |||
| Three‐chamber social approach test (Social novelty preference test) | Distance traveled (cm) | – | 2044.35 ± 118.128 | 2023.169 ± 135.846 | 2034.2 ± 105.299 | 0.9924 | ||
| Time spent around cage (%) | – | 0.563 ± 0.054 | 0.494 ± 0.051 | 0.594 ± 0.04 | 0.2706 | |||
| Startle response test | Startle response (110 and 120 dB) | – | 110 dB | 2.152 ± 0.174 | 2.078 ± 0.155 | 2.144 ± 0.3 | 0.8828 | |
| 120 dB | 2.687 ± 0.199 | 2.858 ± 0.153 | 3.158 ± 0.406 | |||||
| Prepulse inhibition test | PPI (74‐110, 78‐110 dB) | – | 74‐110 dB | 29.328 ± 4.338 | 29.421 ± 3.003 | 26.205 ± 3.892 | 0.7021 | |
| 78‐110 dB | 30.917 ± 5.158 | 42.383 ± 3.26 | 44.187 ± 3.559 | |||||
| PPI (74‐120, 78‐120 dB) | – | 74‐120 dB | 19.223 ± 3.437 | 18.649 ± 3.783 | 26.041 ± 2.431 | 0.4371 | ||
| 78‐120 dB | 34.389 ± 3.805 | 30.34 ± 2.306 | 36.865 ± 2.094 | |||||
| Porsolt forced swim test | Immobility (%) on Day 1* | Figure 4H | – | – | – | 0.0148 | 0.1137 | |
| Immobility (%) on Day 2 | Figure 4H | – | – | – | 0.2466 | 0.696 | ||
| Neurological screens and neuromuscular strength test | Body weight (g) | Figure 2E | 26.025 ± 0.485 | 26.246 ± 0.424 | 26.233 ± 0.588 | 0.9409 | ||
| Body temperature (˚C) | Figure 2F | 36.642 ± 0.19 | 36.208 ± 0.178 | 36.883 ± 0.243 | 0.0712 | |||
| Grip strength (N)* | Figure 2G | 0.725 ± 0.041 | 0.735 ± 0.058 | 0.537 ± 0.035 | 0.0074 | |||
| Light/dark transition test | Distance traveled (cm) in light chamber | Figure 5B | 643.633 ± 59.141 | 647.085 ± 44.719 | 561.917 ± 52.847 | 0.9478 | ||
| Distance traveled (cm) in dark chamber | Figure 5B | 1285.933 ± 52.53 | 1305.146 ± 79.124 | 1339.308 ± 74.327 | ||||
| Transitions | Figure 5D | 36 ± 4.189 | 38.385 ± 3.339 | 32.417 ± 3.306 | 0.507 | |||
| Latency to light chamber (s) | Figure 5E | 32.417 ± 14.549 | 30.923 ± 16.823 | 52.083 ± 20.796 | 0.6428 | |||
| Time spent in light chamber (s) | Figure 5C | 155.292 ± 13.99 | 159.115 ± 16.684 | 124.25 ± 12.049 | 0.1946 | |||
| Elevated plus maze test | Distance traveled (cm) | – | 1435.35 ± 114.787 | 1399.377 ± 124.055 | 1422.358 ± 109.532 | 0.9756 | ||
| Number of entries | – | 31.333 ± 3.436 | 31.154 ± 2.794 | 32.833 ± 2.855 | 0.9126 | |||
| Entries into open arms (%) | – | 24.467 ± 2.452 | 22.608 ± 2.731 | 23.958 ± 3.595 | 0.898 | |||
| Time in open arms (%) | – | 5.333 ± 1.281 | 4.054 ± 0.745 | 6.592 ± 1.546 | 0.343 | |||
| Hot plate test | Hot plate latency (s) | Figure 6C | 9.776 ± 0.947 | 11.084 ± 1.09 | 12.073 ± 0.879 | 0.2763 | ||
| Open field test (acute fentanyl; 0.03 mg/kg) | Distance traveled (cm) before administration | Figure 7B | – | – | – | 0.758 | 0.9565 | |
| Vertical activity before administration | Figure 7C | – | – | – | 0.5833 | 0.9453 | ||
| Center time (s) before administration | Figure 7D | – | – | – | 0.8979 | 0.8964 | ||
| Stereotypic counts before administration | Figure 7E | – | – | – | 0.1817 | 0.264 | ||
| Distance traveled (cm) post administration | Figure 7B | – | – | – | 0.9823 | 0.9904 | ||
| Vertical activity post administration | Figure 7C | – | – | – | 0.8945 | 0.9967 | ||
| Center time (s) post administration | Figure 7D | – | – | – | 0.7702 | 0.9958 | ||
| Stereotypic counts post administration | Figure 7E | – | – | – | 0.7903 | 0.8516 | ||
| Y‐maze test | Spontaneous alternation (%) | – | 63.425 ± 3.404 | 63.862 ± 2.326 | 69.8 ± 3.311 | 0.2681 | ||
| Distance traveled (cm) | – | 2557.725 ± 112.752 | 2493.885 ± 167.718 | 2133.092 ± 126.396 | 0.0862 | |||
| Novel Object Location test | The time spent exploring the object located in the novel place (%) | – | 0.657 ± 0.022 | 0.719 ± 0.028 | 0.657 ± 0.031 | 0.2432 | ||
| Marble‐burying test | Distance traveled (cm) | – | 4328.1 ± 126.343 | 4656.185 ± 289.225 | 3864.925 ± 218.597 | 0.0574 | ||
| Number of buried marbles | – | 7.583 ± 0.783 | 7.923 ± 1.152 | 8.833 ± 0.842 | 0.6424 | |||
| Open field test (acute cocaine; 5 mg/kg) | Distance traveled (cm) before administration | Figure 8B | – | – | – | 0.7954 | 0.3314 | |
| Vertical activity before administration | Figure 8C | – | – | – | 0.1413 | 0.0599 | ||
| Center time (s) before administration | Figure 8D | – | – | – | 0.1004 | 0.9406 | ||
| Stereotypic counts before administration | Figure 8E | – | – | – | 0.1615 | 0.3779 | ||
| Distance traveled (cm) post administration | Figure 8B | – | – | – | 0.3723 | 0.794 | ||
| Vertical activity post administration | Figure 8C | – | – | – | 0.908 | 0.4391 | ||
| Center time (s) post administration | Figure 8D | – | – | – | 0.7929 | 0.02 | ||
| Stereotypic counts post administration | Figure 8E | – | – | – | 0.7835 | 0.8963 | ||
| Open field test(acute cocaine; 15 mg/kg) | Distance traveled (cm) before administration* | Figure 8F | – | – | – | 0.0088 | 0.0531 | |
| Vertical activity before administration | Figure 8G | – | – | – | 0.4076 | 0.4537 | ||
| Center time (s) before administration | Figure 8H | – | – | – | 0.4827 | 0.0011 | ||
| Stereotypic counts before administration | Figure 8I | – | – | – | 0.1027 | 0.3519 | ||
| Distance traveled (cm) post administration | Figure 8F | – | – | – | 0.0591 | 0.0888 | ||
| Vertical activity post administration | Figure 8G | – | – | – | 0.5466 | 0.1875 | ||
| Center time (s) post administration | Figure 8H | – | – | – | 0.9072 | 0.0078 | ||
| Stereotypic counts post administration | Figure 8I | – | – | – | 0.0641 | 0.1115 | ||
| Fear conditioning test (Conditioning) | Freezing (%) | Figure 6D | – | – | – | 0.7533 | 0.7112 | |
| Fear conditioning test (Context test 1 d after conditioning) | Freezing (%) | Figure 6E | – | – | – | 0.6689 | 0.7352 | |
| Fear conditioning test (Cued test 1 d after conditioning (pre‐CS)) | Freezing (%) | Figure 6F | – | – | – | 0.407 | 0.7152 | |
| Fear conditioning test (Cued test 1 d after conditioning (CS)) | Freezing (%) | Figure 6F | – | – | – | 0.9234 | 0.7842 | |
| Fear conditioning test (Context test 7 d after conditioning) | Freezing (%) | Figure 6G | – | – | – | 0.2246 | 0.5067 | |
| Fear conditioning test (Cued test 7 d after conditioning (pre‐CS)) | Freezing (%) | Figure 6H | – | – | – | 0.922 | 0.8955 | |
| Fear conditioning test (Cued test 7 d after conditioning (CS)) | Freezing (%) | Figure 6H | – | – | – | 0.7056 | 0.5333 | |
| Fear conditioning test (footshock 1) | Distance traveled (cm) | Figure 6I | – | – | – | 0.1092 | 0.0018 | |
| Fear conditioning test (footshock 2) | Distance traveled (cm) | Figure 6I | – | – | – | 0.1333 | 0.4204 | |
| Fear conditioning test (footshock 3) | Distance traveled (cm)* | Figure 6I | – | – | – | 0.0395 | 0.0029 | |
Nominal significance: *P < 0.05.
3. RESULTS
3.1. Effects of chronic fentanyl administration on the general health of the mice
Body weight did not differ significantly among the three groups of mice in either the treatment or the withdrawal periods (Figure 2B,E). In the withdrawal period, the low‐dose group tended to have a lower body temperature (withdrawal period: treatment effect, P = 0.0712; controls vs high‐dose treatments, P = 0.4153, controls vs low‐dose treatments, P = 0.1402, low‐dose treatments vs high‐dose treatments, P = 0.0246; Figure 2F). Neuromuscular strength was measured by grip strength. Grip strength was significantly lower in the high‐dose group than in the other groups in both the treatment and withdrawal periods (Treatment period 1: treatment effect, P = 0.0132; controls vs high‐dose treatments, P = 0.015, low‐dose treatments vs high‐dose treatments, P = 0.0077; withdrawal period: treatment effect, P = 0.0074; controls vs high‐dose treatments, P = 0.0079, low‐dose treatments vs high‐dose treatments, P = 0.0047; Figure 2D,G).
Figure 2.

General health and neurologic screening in chronic fentanyl‐treated mice. We performed neurological screens and neuromuscular strength test twice to examine the effect of fentanyl treatment on the physiologic characteristics of the mice both during fentanyl administration and withdrawal (A). Body weight (B, E), body temperature (C, F), and grip strength (D, G) are shown. In both periods, high‐dose fentanyl treatment led to weaker forelimb muscle function compared with the other treatment groups. Data are presented as means ± SEM for the indicated numbers of animals. The P values indicate treatment effect in one‐way ANOVA. The asterisk indicates a nominally significant difference for comparisons between treatment groups (P < 0.05)
3.2. Mice in the high‐dose fentanyl group exhibited reduced anxiety‐like behavior
In the open field test, we measured the total distance traveled, vertical activity, time spent in the center area, and stereotypic counts for 120 minutes to investigate the locomotor activity and anxiety‐like behavior. Mice in the high‐dose group spent significantly more time in the center of the open field than the other groups during the first and second 30‐minutes periods of the test (0‐30 minutes: controls vs high‐dose group, P = 0.0036, low‐dose group vs high‐dose group, P = 0.0499; 31‐60 minutes: controls vs high‐dose group, P = 0.016, low‐dose group vs high‐dose group, P = 0.0251; Figure 3D). Total distance traveled (Figure 3B), vertical activity (Figure 3C), and stereotypic behaviors (Figure 3E), however, did not differ significantly among groups.
Figure 3.
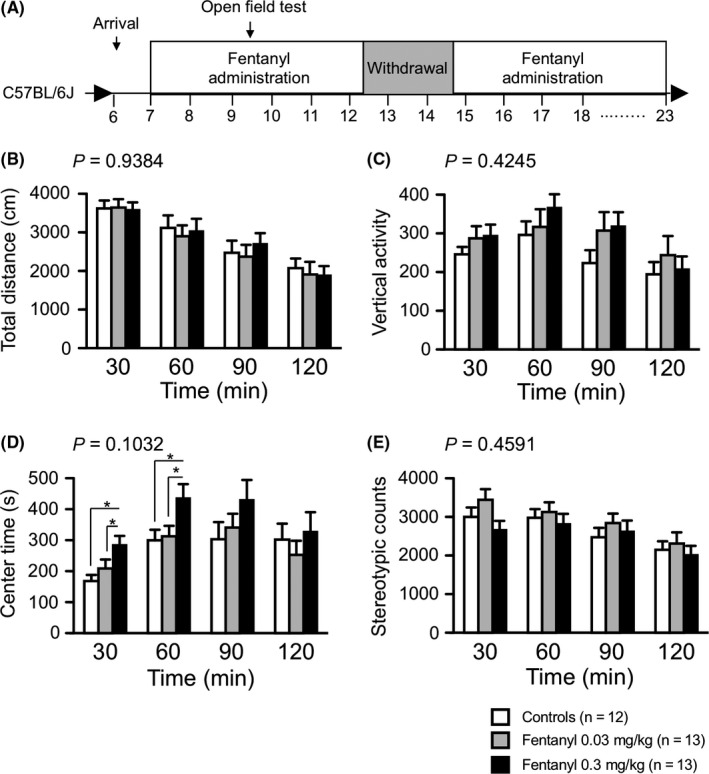
Mice in the high‐dose group exhibited lower anxiety‐like behavior in the open field test. We performed the open field test during the fentanyl treatment period 1 (A). Distance traveled (B), vertical activity (C), time spent in the center of the compartment (D), and stereotypic counts (E) every 30 minutes for 120 minutes are shown. Mice in the high‐dose group spent significantly more time in the center of the open field during the 1st and 2nd 30‐minutes periods than the other groups. Data are presented as means ± SEM for the indicated numbers of animals. The P values indicate treatment effect in two‐way repeated measures ANOVA. The asterisk indicates a nominally significant difference for comparisons between treatment groups (P < 0.05)
In the elevated plus maze test, the high‐dose group tended to have a greater percentage of entries into the open arms compared with the other groups (treatment effect, P = 0.0773; Figure 4D). Additionally, the high‐dose group had significantly more entries into open arms and spent significantly more time on the open arms compared with the controls during minutes 6‐10 of the test (number of entries into open arms during minutes 6‐10: treatment effect, P = 0.033; controls vs high‐dose group, P = 0.0338, low‐dose group vs high‐dose group, P = 0.0172; the spent time in open arms: treatment effect, P = 0.0168; controls vs high‐dose group, P = 0.1351, low‐dose group vs high‐dose group, P = 0.0045; Figure 4F,G). These results suggest that high‐dose fentanyl treatment decreased anxiety‐like behavior in mice. In contrast, during the withdrawal period, there was no significant difference in distance traveled, percentage of entries onto open arms, or percentage of time on open arms among the groups in the elevated plus maze test (Table 2).
Figure 4.
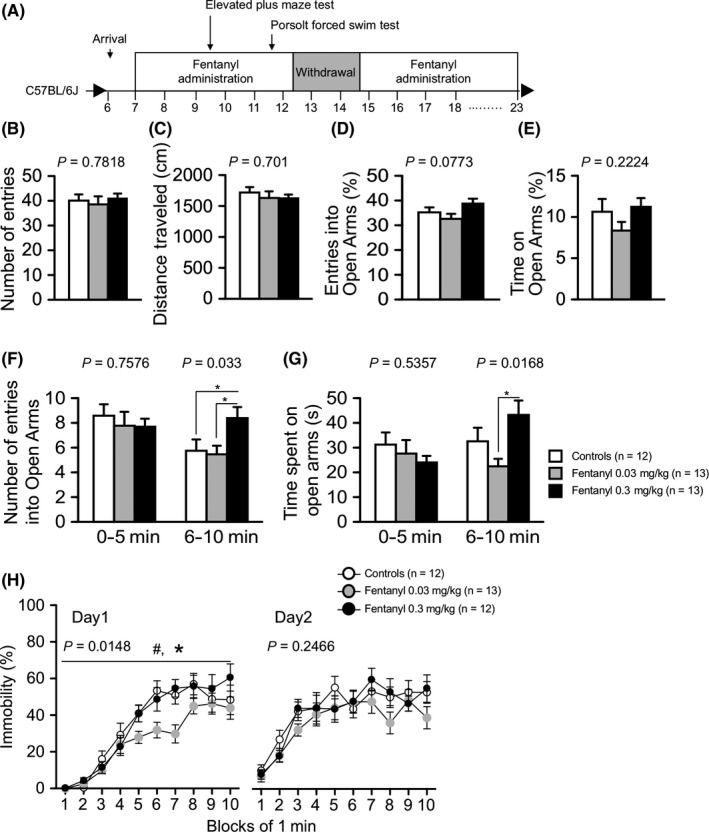
Mice treated with high‐dose fentanyl exhibited reduced anxiety‐like behavior. Mice were tested in the elevated plus maze test and Porsolt forced swim test treatment period 1 (A). The elevated plus maze test: number of arm entries (B), distance traveled (C), percentage of entries into open arms (D), and percentage of time on open arms (E) are shown. In the whole period, there was no significant difference among the three groups (B‐E). In the first and last half of the test, mice treated with high‐dose fentanyl had a significantly greater number of entries into the open arms (F) and spent more time on the open arms (G) than control mice. Porsolt forced swim test: The percentage of time spent immobile on days 1 and 2 was recorded (H). Mice in the low‐dose fentanyl group had a significantly lower immobility ratio than the other groups on day 1. Data are presented as means ± SEM for the indicated numbers of animals. The P values indicate a treatment effect in one‐way ANOVA or two‐way repeated measures ANOVA. In the elevated plus maze test, the asterisk indicates a nominally significant difference for comparisons between treatment groups (P < 0.05). (F‐G). In the Porsolt forced swim test, the asterisk and number sign indicate a nominally significant difference between the high‐dose group and low‐dose group and between the low‐dose group and control group (P < 0.05)
In the Porsolt forced swim test, mice in the low‐dose group exhibited an immobile posture for a significantly shorter time than the other groups (treatment effect, P = 0.0148; controls vs low‐dose group, P = 0.0072, low‐dose group vs high‐dose group, P = 0.0104; Figure 4H). The decreased immobility of the low‐dose group implies that fentanyl has an anti‐depressive effect.
3.3. Mice in the high‐dose fentanyl group exhibited increased anxiety‐like behavior during the withdrawal period
To investigate the effect of withdrawal from chronic fentanyl treatment, we performed a general health and neurologic screening, as well as light/dark transition, elevated plus maze, and hot plate tests during the withdrawal period. In the light/dark transition test, mice in the high‐dose group tended to remain in the light chamber for a shorter period of time than the other groups (Figure 5C). Mice in the high‐dose group spent significantly less time in the light chamber in the first half of the testing period (treatment effect, P = 0.0388; controls vs high‐dose group, P = 0.0472, low‐dose group vs high‐dose group P = 0.0166; Figure 5F). In the elevated plus maze test during the withdrawal period, there were no significant differences between groups (Table 2), although in the fentanyl treatment period, the high‐dose group showed decreased anxiety compared with controls (Figure 4F). Thus, during the withdrawal period, fentanyl interruption may increase anxiety‐like behavior in mice administered high‐dose fentanyl.
Figure 5.
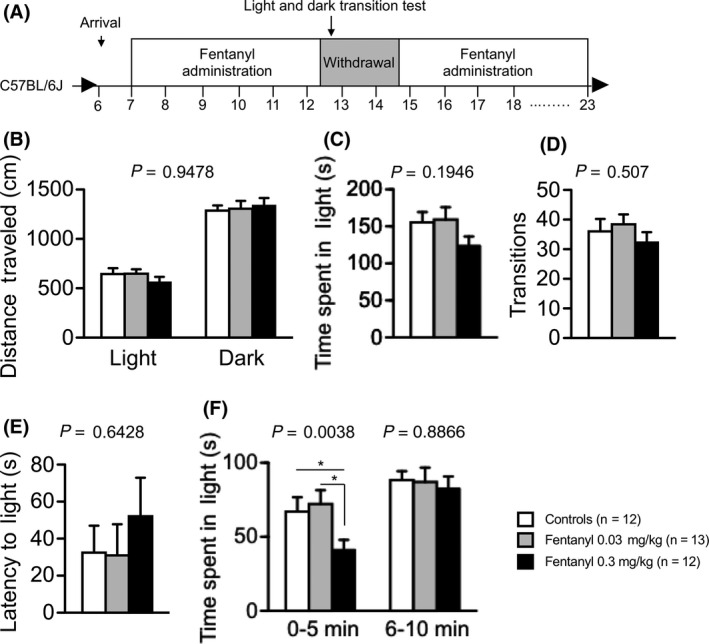
Mice in the high‐dose group exhibited higher anxiety‐like behavior in the withdrawal period. We performed the light and dark transition test during the withdrawal period (A). Distance traveled (B), time spent in the light chamber (C), total number of light/dark transitions (D), and latency to enter the light compartment (E) are shown. There was no significant difference among groups (B‐E). Mice in the high‐dose group remained in the light chamber for a significantly shorter time during the first 0‐5 minutes (F). Data are presented as means ± SEM for the indicated numbers of animals. The P values indicate the treatment effect in one‐way ANOVA or two‐way repeated measures ANOVA. The asterisk indicates a nominally significant difference for comparisons between treatment groups (P < 0.05)
3.4. Mice treated chronically with fentanyl exhibit abnormal pain sensitivity
We performed the hot plate test to assess pain sensitivity in mice with chronic fentanyl treatment. The latency to the first hind paw response on the hot plate (preheated to 55°C) was significantly longer in both the low‐ and high‐dose fentanyl treatment groups than in controls (treatment effect, P = 0.0294; controls vs low‐dose group, P = 0.0364, controls vs high‐dose group, P = 0.0126; Figure 6B), suggesting that fentanyl inhibited sensitivity to painful stimuli. The hot plate test was also performed during the withdrawal period (Figure 6A). The latency to the first hind paw response was not significantly different among the three groups (Figure 6C). After the withdrawal period, we resumed fentanyl treatment and performed the contextual and cued fear conditioning test to assess fear memory (Figure 6A). The freezing ratio during the context and cued tests was not significantly different among the groups (Figure 6D‐H). The distance traveled by the high‐dose group after the third footshock, however, was greater than that of the controls (treatment effect, P = 0.0395; treatment × time, P = 0.0029; controls vs low‐dose group, P = 0.3075, controls vs high‐dose group, P = 0.019; Figure 6I). This finding suggests that high‐dose fentanyl treatment induced higher sensitivity to pain stimuli.
Figure 6.
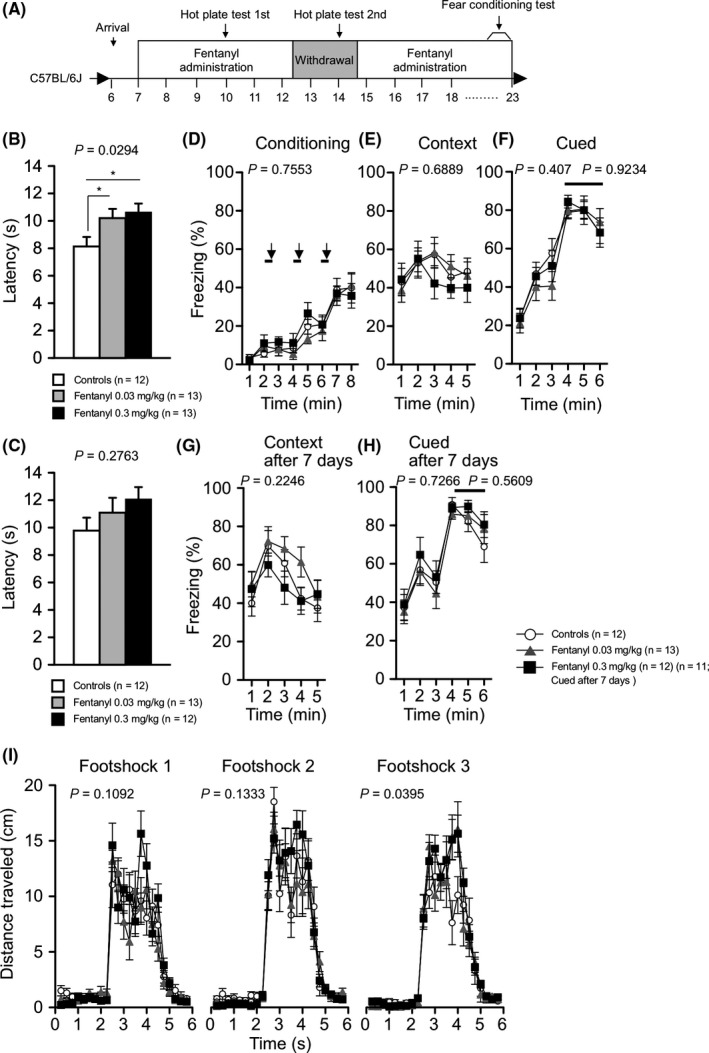
Fentanyl‐treated mice exhibited abnormal pain sensitivity and cognitive function. The hot plate test was performed two times, once during fentanyl treatment period 1 and once during the withdrawal period. To investigate the effect of chronic fentanyl treatment on cognitive function, the fear conditioning test was performed during the fentanyl treatment period 2 (A). The hot plate test: The latency to the first hind paw response on the hot plate at 55°C was recorded (B‐C). During fentanyl treatment, the fentanyl‐treated groups exhibited lower pain sensitivity than the control group. Fear conditioning test: The percentage of time freezing in the conditioning (D), context testing (E, G), and cued testing with altered context (F, H) conditions. Distance traveled during exposure to the three footshocks administered in the conditioning phase was recorded (I). The response to the first and second footshock did not differ significantly among the groups. Mice treated with high‐dose fentanyl traveled a significantly longer distance in response to the third footshock compared with control mice. Data are presented as means ± SEM for the indicated numbers of animals. The P values indicate treatment effect in one‐way ANOVA or two‐way repeated measures ANOVA. The asterisk indicates a significance nominally significant difference for comparisons between treatment groups (P < 0.05)
3.5. Mice treated with high‐dose fentanyl exhibited decreased cocaine‐induced hyperactivity and stereotypic behavior
We performed the open field test with acute administration of low‐dose fentanyl after a 14‐day withdrawal period to evaluate the effect of chronic fentanyl treatment and withdrawal on fentanyl sensitivity (Figure 7A). Locomotor activity, vertical activity, time in the center area, and stereotypic counts were recorded every 5 minutes for 1 hour of habituation and for 2 hours after intraperitoneal administration of fentanyl. No significant differences were detected between groups during the habituation period or after the injection of low‐dose fentanyl (0.03 mg/kg ip) (Figure 7B‐E). Co‐administration of fentanyl with cocaine or heroin enhances the potency,5 suggesting that fentanyl treatment affects the sensitivity of the response to addictive drugs. To examine this point, we performed the open field test with acute administration of low‐ and high‐dose cocaine (5 and 15 mg/kg, respectively; Figure 8A). Mice in all groups showed a progressive increase in locomotor activity and stereotypic counts after the injection of high‐dose cocaine (15 mg/kg), but cocaine‐induced hyperlocomotion and stereotypic behavior were significantly reduced in the high‐dose fentanyl group compared with controls (locomotor activity: treatment effect, P = 0.0591; treatment × time, P = 0.0888; controls vs low‐dose group, P = 0.8839, controls vs high‐dose group P = 0.0222; stereotypic counts: treatment effect, P = 0.0641; treatment × time, P = 0.1115; controls vs low‐dose group, P = 0.9235, controls vs high‐dose group P = 0.0157; Figure 8F,I). These results suggest chronical fentanyl treatment reduces the sensitivity to cocaine.
Figure 7.
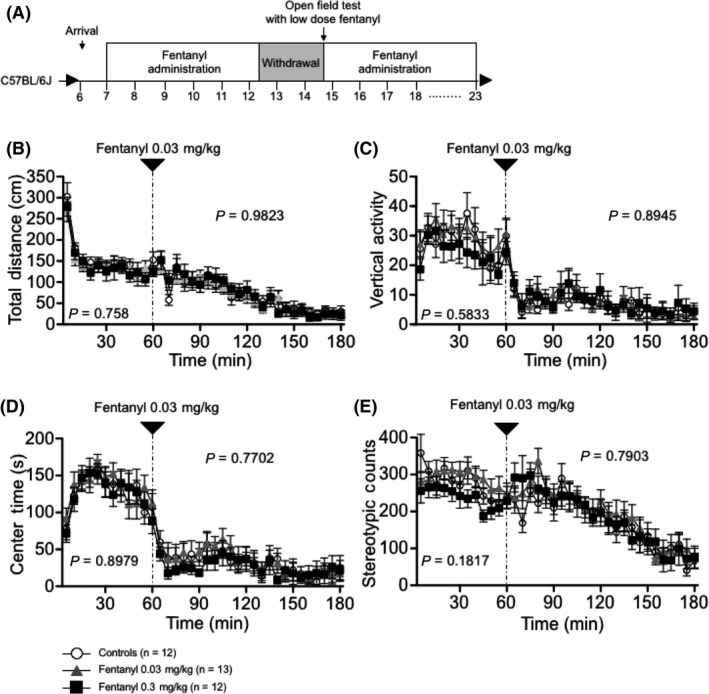
Acute effects of low‐dose fentanyl administration on mouse open field behavior. The open field test was performed on the last day of the withdrawal period following acute administration of low‐dose fentanyl (A). Time‐course of distance traveled (B), vertical activity (C), and time spent in the central area (D) are shown. No significant differences were detected among the three groups. The P values indicate treatment effect in two‐way repeated measures ANOVA
Figure 8.
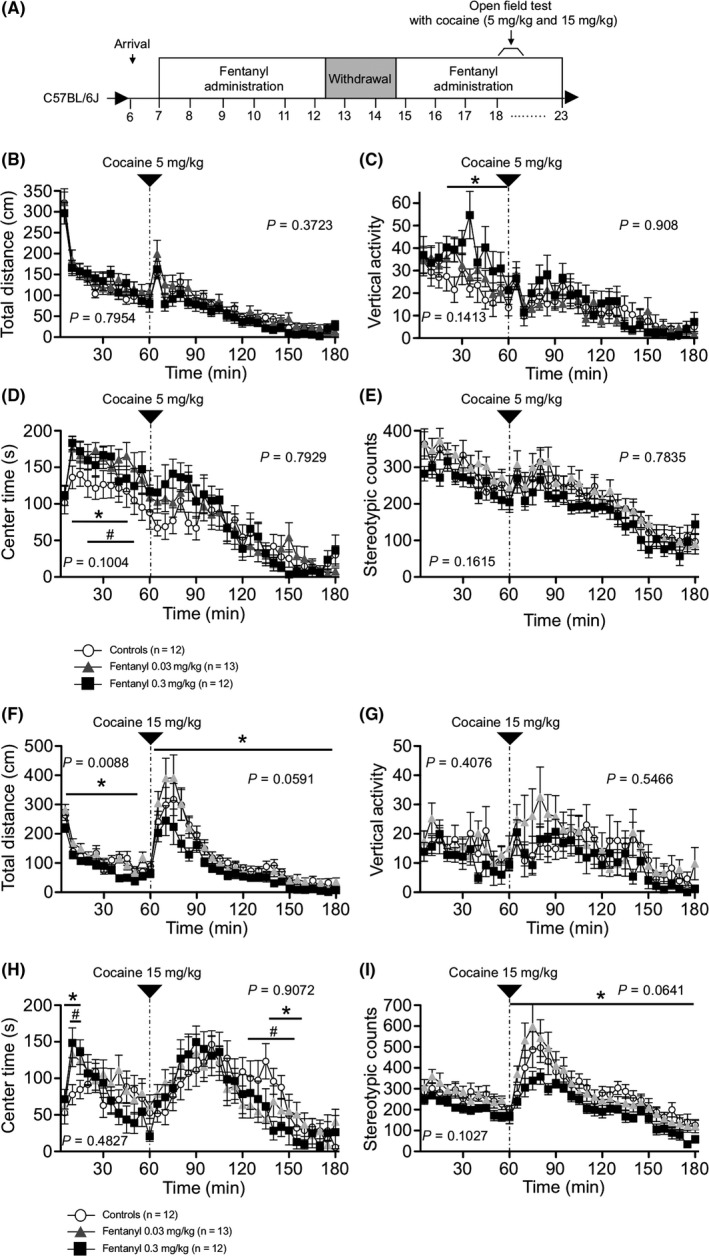
Responses of fentanyl‐treated mice to acute cocaine administration. Open field tests were performed following acute cocaine (5 and 15 mg/kg) administration to investigate the effect of chronic fentanyl treatment on the response to cocaine (A). Time‐course of distance traveled (B, F), vertical activity (C, G), time spent in the central area (D, H), and stereotypic counts (E, I) are shown. Effects of acute administration of low‐dose cocaine (5 mg/kg) did not differ significantly among the three groups (B‐E). In mice with acute administration of high‐dose cocaine (15 mg/kg), cocaine‐induced hyperactivity and stereotypic counts were attenuated compared with control mice (F, I). Data are presented as means ± SEM for the indicated numbers of animals. The P values indicate treatment effect in two‐way repeated measures ANOVA. The asterisk and number sign indicate a nominally significant difference between the high‐dose group and control group or the low‐dose group and control group (P < 0.05)
3.6. Other behaviors
Locomotor activity tended to be lower in the high‐dose fentanyl group compared with the other groups in the Y‐maze test (total distance: controls, 2557.725 ± 112.752 cm; low‐dose treatment, 2493.885 ± 167.718; high‐dose treatment, 2133.092 ± 126.396; treatment effect, P = 0.0911) and the marble‐burying test (total distance: controls, 4328.1 ± 126.343 cm; low‐dose treatment, 4656.185 ± 289.225; high‐dose treatment, 3864.925 ± 218.597; treatment effect, P = 0.0574). Alternations in the Y‐maze and marble‐burying behavior did not differ significantly among the groups. There were also no significant differences among groups in the results of the social interaction, social approach, novel object location, and prepulse inhibition tests (Table 2).
4. DISCUSSION
In the present study, we performed a comprehensive behavioral test battery to assess the effect of chronic fentanyl treatment on mouse behaviors. Our findings revealed lower anxiety‐like behavior in mice treated with high‐dose fentanyl in the open field test and the elevated plus maze test. In addition, grip strength was significantly decreased in the high‐dose fentanyl‐treated mice. There was no significant difference among the three groups in the other behaviors, such as prepulse inhibition or social and cognitive functions.
Chronic treatment with high‐dose fentanyl reduced anxiety‐like behavior in adult C57BL/6J mice. As previously reported, activation of the opioid system has anxiolytic‐like effects.29 Fentanyl treatment positively affects the mental component score of the Short Form 12 Health Survey in cancer patients.30 Our results are consistent with these reports. Conversely, during the withdrawal period, the high‐dose group exhibited more anxiety‐like behavior than controls, observed as a shorter time spent in the light chamber in the light/dark transition test. When fentanyl administration was resumed, the high‐dose group again exhibited lower anxiety‐like behavior than the other groups. These results imply that fentanyl treatment decreases anxiety‐like behavior and maintains the state. In a previous study, 24 hours after a single fentanyl injection, rats exhibited anxiety‐like behavior,31 and therefore abrupt interruption of fentanyl treatment may account for the increased anxiety and the establishment of fentanyl dependence.
Grip strength was lower in the high‐dose group than in the other groups, implying that fentanyl treatment leads to muscle weakness. In an animal model, acute opioid treatment induced limb‐use abnormality.32 Lower grip strength of mice in the high‐dose group was observed during treatment and withdrawal. Our results suggest that chronic use of fentanyl may induce irreversible behavioral effects in mice. In addition, locomotor activity tended to be lower in the high‐dose group compared with the other groups in the Y maze and the marble‐burying tests. Furthermore, the high‐dose group had a lower activity level in the early phase of habituation in the open field test with acute administration of cocaine. Fentanyl induces lethargy in humans.5 The results of the grip strength test imply that mice in the high‐dose group had muscle weakness. The grip strength test is a pure test of strength, but, as for any test, motivational factors could potentially play a role. The reduced grip strength and lower activity in the novel environment suggest that chronic fentanyl treatment may reduce motivation in mice, observed as muscle weakness and decreased activity.
In the hot plate test, mice treated with fentanyl had a longer latency to the first hind paw response. In contrast, during the withdrawal periods, there was no significant difference among the three groups in the hot plate test. These findings suggest that fentanyl attenuated pain sensitivity in mice and are consistent with previous findings in animal pain models.32 In the fear conditioning test, however, the increased locomotor activity after electric shocks in the high‐dose group was greater than that in the control group. This finding implies that fentanyl induces hyperalgesia in mice. Chronic use of opioids sometime induces hyperalgesia.33 Our results suggest that this inverted pain sensitivity of fentanyl treatment may be triggered by long‐term treatment or interruption of fentanyl treatment. Addiction to fentanyl and fatal fentanyl overdoses are emerging social problems in the United States.8 Recent news that a celebrity died of a fentanyl overdose attracted great attention.34 Furthermore, fentanyl co‐administered with other addictive drugs such as cocaine and heroin is intended to enhance the strong feelings of pleasure.35, 36 The resulting mixtures cause high mortality rates.35 In our results, high‐dose fentanyl treatment attenuated cocaine‐induced hyper locomotor activity and stereotypic behavior. Chronic fentanyl treatment has inhibitory effects on cocaine sensitivity. Opioids affect the central nervous system by activating mu, kappa, and delta opioid receptors.37, 38, 39 Natural and synthetic opioids activate these receptors and stimulate analgesia, reward, and/or pleasure.38 A positron emission tomography study revealed that mu opioid receptor binding with [11C] carfentanil is increased in cocaine‐abusing patients.40, 41 To unify these previous reports, we expected that cocaine and fentanyl would have synergistic effects. Contrary to our expectations, mice treated with high‐dose fentanyl had lower sensitivity to cocaine than the control group. Negus's group, however, reported substantial individual differences in the effects of mu agonists in cocaine discrimination tasks in monkeys.42 Cocaine addiction patients are less responsive than healthy controls to mu opioid receptor agonists.43 Characteristic opioid effects are dependent on receptor selectivity and affinity.44, 45 Fentanyl is a strong mu opioid receptor agonist, but it also has the ability to bind two other receptors, kappa and delta receptors.46 Kappa opioid receptor agonists are a candidate treatment for cocaine dependence and inhibit cocaine reward behavior in mice.47, 48 Chronic fentanyl treatment may activate kappa opioid receptors, thereby inhibiting the response to cocaine. Further investigation is needed to reveal the fentanyl‐induced inhibitory effects on the response to cocaine.
Although the mechanisms underlying the behavioral responses induced by chronic fentanyl treatment require further investigation, the distinctive behavioral alterations of mice in this study provide basic data to investigate the side effects of other opioids and will facilitate further in vivo studies of the effects of novel synthetic opioids.
CONFLICT OF INTEREST
The authors declare no conflict of interest for this article.
AUTHORS' CONTRIBUTIONS
Performed experiments—KF, YK, and MA. Experimental design and analysis—KF and KT. Manuscript preparation—KF and KT.
DATA REPOSITORY
Raw data on the behavioral test and information about each mouse are accessible on the public database “Mouse Phenotype Database” (http://www.mouse-phenotype.org/).
ANIMAL STUDIES
All behavioral testing procedures were approved by Institutional Animal Care and Use Committee of University of Toyama.
ACKNOWLEDGMENTS
This work was supported by Ministry of Education, Culture, Sports, Science and Technology, Japan (MEXT)/Japan Society for the Promotion of Science KAKENHI, Grant‐in‐Aid for Scientific Research: 17H06051 and 16H06276. The authors are grateful to the students and teachers from Tonami Senior High School and Uozu High School who participated in “Course D” in the Life Science Toyama Open Lab 2017, for triggering this study. The authors thank Mr. Yu Nakamura, Ms. Hinano Otofuji, Mr. Keita Tsumura, Mr. Daisuke Fukutomi, and Mr. Takahito Maki for technical assistance with the preliminary experiments. We thank the staff at the Division of Animal Resources and Development, Life Science Research Center, University of Toyama for technical support.
Fujii K, Koshidaka Y, Adachi M, Takao K. Effects of chronic fentanyl administration on behavioral characteristics of mice. Neuropsychopharmacol Rep. 2019;39:17–35. 10.1002/npr2.12040
REFERENCE
- 1. Stanley TH. The fentanyl story. J Pain. 2014;15(12):1215–1226. [DOI] [PubMed] [Google Scholar]
- 2. Kamp‐Jensen M, Clausen T, Eriksen J. Methadone as an analgesic. Ugeskr Laeger. 2000;162(2):163–166. [PubMed] [Google Scholar]
- 3. Ordóñez Gallego A, González Barón M, Espinosa Arranz E. Oxycodone: a pharmacological and clinical review. Clin Transl Oncol. 2007;9(5):298–307. [DOI] [PubMed] [Google Scholar]
- 4. O'Donnell JK, Gladden RM, Seth P. Trends in deaths involving heroin and synthetic opioids excluding methadone, and law enforcement drug product reports, by census region – United States, 2006‐2015. MMWR Morb Mortal Wkly Rep. 2017;66(34):897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kuczyńska K, Grzonkowski P, Kacprzak Ł, Zawilska JB. Abuse of fentanyl: an emerging problem to face. Forensic Sci Int. 2018;289:207–214. [DOI] [PubMed] [Google Scholar]
- 6. Brinkley‐Rubinstein L, Macmadu A, Marshall BDL, et al. Risk of fentanyl‐involved overdose among those with past year incarceration: findings from a recent outbreak in 2014 and 2015. Drug Alcohol Depend. 2018;185:189–191. [DOI] [PubMed] [Google Scholar]
- 7. Katz J. Short answers to hard questions about the opioid crisis. The New York Times [Internet]. 2017 Aug 3 [cited 2018 Apr 18]. Available from: https://www.nytimes.com/interactive/2017/08/03/upshot/opioid-drug-overdose-epidemic.html
- 8. Katz J. The first count of fentanyl deaths in 2016: up 540% in three years. The New York Times [Internet]. 2017 Sep 2 [cited 2018 Apr 9]. Available from: https://www.nytimes.com/interactive/2017/09/02/upshot/fentanyl-drug-overdose-deaths.html
- 9. Perks A. Opioid crisis takes personal toll on Washington [Internet]. TheHill. 2018 [update 2018 Jul 2]. Available from: http://thehill.com/policy/healthcare/public-global-health/383075-opioid-crisis-takes-personal-toll-on-washington
- 10. Swanson I. Trump order targets wide swath of public assistance programs [Internet]. TheHill. 2018 [update 2018 Jul 2]. Available from: http://thehill.com/policy/healthcare/383106-trump-order-targets-wide-swathe-of-public-assistance-programs
- 11. McNicol E, Horowicz‐Mehler N, Fisk RA, et al. Management of opioid side effects in cancer‐related and chronic noncancer pain: a systematic review. J Pain. 2003;4(5):231–256. [DOI] [PubMed] [Google Scholar]
- 12. Yamamoto A, Kuyama S, Kamei C, Sugimoto Y. Characterization of scratching behavior induced by intradermal administration of morphine and fentanyl in mice. Eur J Pharmacol. 2010;627(1–3):162–166. [DOI] [PubMed] [Google Scholar]
- 13. Bortone L, Bertolizio G, Engelhardt T, Frawley G, Somaini M, Ingelmo PM. The effect of fentanyl and clonidine on early postoperative negative behavior in children: a double‐blind placebo controlled trial. Paediatr Anaesth. 2014;24(6):614–619. [DOI] [PubMed] [Google Scholar]
- 14. Mitzelfelt JD, Dupree JP, Seo DO, Carter CS, Morgan D. Effects of chronic fentanyl administration on physical performance of aged rats. Exp Gerontol. 2011;46(1):65‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13(2):167–170. [DOI] [PubMed] [Google Scholar]
- 16. Takao K, Miyakawa T. Light/dark transition test for mice. J Vis Exp. 2006;1:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lister RG. The use of a plus‐maze to measure anxiety in the mouse. Psychopharmacology. 1987;92(2):180–185. [DOI] [PubMed] [Google Scholar]
- 18. Komada M, Takao K, Miyakawa T. Elevated plus maze for mice. J Vis Exp. 2008;22:1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shoji H, Takao K, Hattori S, Miyakawa T. Age‐related changes in behavior in C57BL/6J mice from young adulthood to middle age. Mol Brain. 2016;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moy SS, Nadler JJ, Perez A, et al. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic‐like behavior in mice. Genes Brain Behav. 2004;3(5):287–302. [DOI] [PubMed] [Google Scholar]
- 21. Umemura M, Ogura T, Matsuzaki A, et al. Comprehensive behavioral analysis of activating transcription factor 5‐deficient mice. Front Behav Neurosci. 2017;11:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266(5604):730–732. [DOI] [PubMed] [Google Scholar]
- 23. Shoji H, Irino Y, Yoshida M, Miyakawa T. Behavioral effects of long‐term oral administration of aluminum ammonium sulfate in male and female C57BL/6J mice. Neuropsychopharmacol Rep. 2018;38(1):18–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shoji H, Takao K, Hattori S, Miyakawa T. Contextual and cued fear conditioning test using a video analyzing system in mice. J Vis Exp. 2014;(85). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Njung'e K, Handley SL. Evaluation of marble‐burying behavior as a model of anxiety. Pharmacol Biochem Behav. 1991;38(1):63–67. [DOI] [PubMed] [Google Scholar]
- 26. Ohashi R, Takao K, Miyakawa T, Shiina N. Comprehensive behavioral analysis of RNG105 (Caprin1) heterozygous mice: reduced social interaction and attenuated response to novelty. Sci Rep. 2016;6:20775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kafkafi N, Lipkind D, Benjamini Y, Mayo CL, Elmer GI, Golani I. SEE locomotor behavior test discriminates C57BL/6J and DBA/2J mouse inbred strains across laboratories and protocol conditions. Behav Neurosci. 2003;117(3):464–477. [DOI] [PubMed] [Google Scholar]
- 28. Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125(1–2):279–284. [DOI] [PubMed] [Google Scholar]
- 29. Colasanti A, Rabiner EA, Lingford‐Hughes A, Nutt DJ. Opioids and anxiety. J Psychopharmacol. 2011;25(11):1415–1433. [DOI] [PubMed] [Google Scholar]
- 30. Guitart J, Vargas MI, De Sanctis V, et al. Sublingual fentanyl tablets for relief of breakthrough pain in cancer patients and association with quality‐of‐life outcomes. Clin Drug Investig. 2015;35(12):815–822. [DOI] [PubMed] [Google Scholar]
- 31. Bessière B, Laboureyras E, Ben Boujema M, Laulin J‐P, Simonnet G. A high‐dose of fentanyl induced delayed anxiety‐like behavior in rats. Prevention by a NMDA receptor antagonist and nitrous oxide (N(2)O). Pharmacol Biochem Behav. 2012;102(4):562–568. [DOI] [PubMed] [Google Scholar]
- 32. Minami K, Hasegawa M, Ito H, et al. Morphine, oxycodone, and fentanyl exhibit different analgesic profiles in mouse pain models. J Pharmacol Sci. 2009;111(1):60–72. [DOI] [PubMed] [Google Scholar]
- 33. Yi P, Pryzbylkowski P. Opioid induced hyperalgesia. Pain Med. 2015;16(Suppl 1):S32–S36. [DOI] [PubMed] [Google Scholar]
- 34. Press A. Prince had ‘exceedingly high' level of fentanyl in body when he died. The Guardian [Internet]. 2018 Mar 27 [cited 2018 Jul 4]. Available from: http://www.theguardian.com/music/2018/mar/27/prince-had-exceedingly-high-level-of-fentanyl-in-body-when-he-died
- 35. Hull MJ, Juhascik M, Mazur F, Flomenbaum MA, Behonick GS. Fatalities associated with fentanyl and co‐administered cocaine or opiates. J Forensic Sci. 2007;52(6):1383–1388. [DOI] [PubMed] [Google Scholar]
- 36. Marinetti LJ, Ehlers BJ. A series of forensic toxicology and drug seizure cases involving illicit fentanyl alone and in combination with heroin, cocaine or heroin and cocaine. J Anal Toxicol. 2014;38(8):592–598. [DOI] [PubMed] [Google Scholar]
- 37. Chen JC, Smith ER, Cahill M, Cohen R, Fishman JB. The opioid receptor binding of dezocine, morphine, fentanyl, butorphanol and nalbuphine. Life Sci. 1993;52(4):389–396. [DOI] [PubMed] [Google Scholar]
- 38. Klenowski P, Morgan M, Bartlett SE. The role of δ‐opioid receptors in learning and memory underlying the development of addiction. Br J Pharmacol. 2015;172(2):297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Morgan MM, Reid RA, Saville KA. Functionally selective signaling for morphine and fentanyl antinociception and tolerance mediated by the rat periaqueductal gray. PLoS One. 2014;9(12):e114269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zubieta JK, Gorelick DA, Stauffer R, Ravert HT, Dannals RF, Frost JJ. Increased mu opioid receptor binding detected by PET in cocaine‐dependent men is associated with cocaine craving. Nat Med. 1996;2(11):1225–1229. [DOI] [PubMed] [Google Scholar]
- 41. Ghitza UE, Preston KL, Epstein DH, et al. Brain mu‐opioid receptor binding predicts treatment outcome in cocaine‐abusing outpatients. Biol Psychiatry. 2010;68(8):697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Negus SS, Gatch MB, Mello NK. Effects of mu opioid agonists alone and in combination with cocaine and D‐amphetamine in rhesus monkeys trained to discriminate cocaine. Neuropsychopharmacology. 1998;18(5):325–338. [DOI] [PubMed] [Google Scholar]
- 43. Minkowski CP, Epstein D, Frost JJ, Gorelick DA. Differential response to IV carfentanil in chronic cocaine users and healthy controls. Addict Biol. 2012;17(1):149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Delfs JM, Kong H, Mestek A, et al. Expression of mu opioid receptor mRNA in rat brain: an in situ hybridization study at the single cell level. J Comp Neurol. 1994;345(1):46–68. [DOI] [PubMed] [Google Scholar]
- 45. Peng J, Sarkar S, Chang SL. Opioid receptor expression in human brain and peripheral tissues using absolute quantitative real‐time RT‐PCR. Drug Alcohol Depend. 2012;124(3):223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Maguire P, Tsai N, Kamal J, Cometta‐Morini C, Upton C, Loew G. Pharmacological profiles of fentanyl analogs at mu, delta and kappa opiate receptors. Eur J Pharmacol. 1992;213(2):219–225. [DOI] [PubMed] [Google Scholar]
- 47. Bidlack JM. Mixed κ/μ partial opioid agonists as potential treatments for cocaine dependence. Adv Pharmacol. 2014;69:387–418. [DOI] [PubMed] [Google Scholar]
- 48. Váradi A, Marrone GF, Eans SO, et al. Synthesis and characterization of a dual kappa‐delta opioid receptor agonist analgesic blocking cocaine reward behavior. ACS Chem Neurosci. 2015;6(11):1813–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]


