Abstract
Introduction
Opioid analgesics are widely used as effective analgesics for the treatment of moderate‐to‐severe pain. However, the analgesic efficacy of opioids is well known to vary widely among individuals, and effective pain treatment is hampered by vast individual differences. Although these differences in opioid requirements have been attributed to various factors, genetic factors are becoming increasingly relevant to the development of genome science.
Aim
This review covers the association between opioid analgesic requirements and particularly gene polymorphisms.
Future perspectives
Personalized pain treatment has begun using prediction formulas based on associated gene polymorphisms. Improvements in personalized pain treatment are expected as scientific knowledge further expands in the future.
Keywords: genetic polymorphisms, opioids, pain, personalized medicine, predictive genetic testing
The analgesic efficacy of opioids is well known to vary widely among individuals, and effective pain treatment is hampered by vast individual differences. Although these differences in opioid requirements have been attributed to various factors, genetic factors are becoming increasingly relevant to the development of genome science. This review covers the association between opioid analgesic requirements and particularly gene polymorphisms.

1. INTRODUCTION
Pain is an important physiological mechanism by which humans prevent themselves from developing tissue injury. However, excessive pain can markedly increase psychological health problems and decrease health‐related quality of life.1 Therefore, pain should be managed appropriately by analgesics. Opioid analgesics, such as fentanyl and morphine, are widely used as effective analgesics for the treatment of moderate‐to‐severe pain. In 1986, the World Health Organization proposed a method for the relief of cancer pain.2 The method can be summarized in five phrases: by mouth, by the clock, by the ladder, for the individual, and with attention to detail. Thus, “for the individual” is a critical principle in pain management, demonstrating that there are no standard doses for opioid drugs. Therefore, opioids should be prescribed with proper doses for individual patients. However, the analgesic efficacy of opioids is well known to vary widely among individuals, thus complicating their effective and safe clinical use.3, 4 Therefore, although the same amount of opioid analgesics may be administered to different patients, it may occasionally cause insufficient analgesia or side effects among some individuals, such as nausea, vomiting, constipation, and respiratory depression.5 For example, the minimal effective analgesic concentrations (MEACs) of morphine and fentanyl that are required for adequate analgesia vary from 6.3 to 53.6 ng/mL and 0.2 to 2.0 ng/mL, respectively.6, 7, 8, 9 This suggests that the MEAC is five‐ to tenfold different among individuals. Thus, effective pain treatment is often hampered by significant differences in opioid sensitivity. Empirical methods of administration that are currently performed by trial and error are an imperfect practice that can result in delayed analgesia and possibly overdose (Figure 1).
Figure 1.
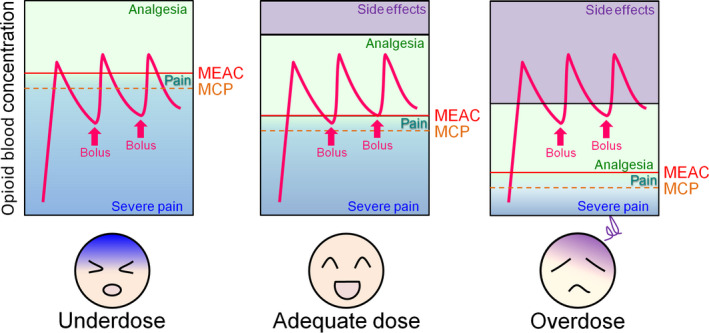
Illustration of individual differences in opioid requirements. Although the blood opioid concentration changes similarly after administration (arrows) and the difference between the minimal effective analgesic concentration (MEAC) (solid line) and maximum concentration with pain (MCP) (dashed line) is low among individuals, the clinical response can differ between patients because of five‐ to tenfold interpatient differences in the MEAC. Therefore, a particular opioid dose that produces satisfactory pain relief in some patients may be either too low (resulting in insufficient analgesia) or too high (resulting in adverse effects) in other patients
2. FACTORS THAT RESULT IN INDIVIDUAL DIFFERENCES
Individual differences have been attributed to environmental, psychological, and genetic factors, including age, gender, patient weight, ethnic origin, hepatic or renal function, type of surgery, surgical methods, duration of surgery, anxiety, and psychological distress.10, 11, 12, 13 In this section, we describe each of these factors.
2.1. Environmental factors
Various environmental factors have been reported. Among these, advancing age has been shown to reduce morphine requirements because age has been suggested to blunt peripheral nociceptive function.11 Additionally, fentanyl use for pain management is more in females than in males.14
2.2. Psychological factors
Many studies have reported associations between depression and pain. The prevalence of pain in depressed cohorts and the prevalence of depression in pain cohorts are higher than the prevalence when these conditions are examined individually.15 Indeed, the prevalence of pain in depressed patients was approximately 60%, and the intensity of depression was generally higher in patients with greater pain.16 Thus, psychological distress, such as depressed mood and negative affect, can increase postoperative analgesic consumption. Moreover, patients who undergo emergency surgery may have less preoperative information and time for psychological preparation, resulting in increased requirements for postoperative analgesia.11
2.3. Genetic factors
Recent studies of individual differences in opioid analgesic requirements have demonstrated the significant involvement of genetic factors. The twin study paradigm is one of the few available designs that provide global estimates of genetic and environmental contributions to complex phenotypes, including interindividual differences in drug responses.17, 18, 19 Genetic effects are thought to be responsible for approximately 12%‐60% of response variability in opioid treatment in twin studies.18, 19, 20 A twin approach generally provides an excellent opportunity to understand the causes and consequences of epigenetic variations. Although the epigenetic modulation of analgesic receptor targets and drug‐metabolizing enzymes may alter the pharmacodynamics and pharmacokinetics of analgesic drugs, respectively,21 to date, no studies have adopted twin approaches to examine the causal effects of epigenetic variations on interindividual differences in opioid responses.
3. PHARMACOGENOMICS
Empirical approaches to the effective treatment of pain are currently limited because the initial drug selection, subsequent dosage titrations, and drug additions are made according to only a few patient‐specific clinical features.22 Therefore, pharmacogenetic studies and in vitro companion diagnostic devices (IVD companion diagnostic devices) are being developed. Pharmacogenetics is defined as the use of pharmacogenomic or pharmacogenetic tests in conjunction with drug therapy. The goal of pharmacogenomic research is to offer personalized medicine to improve the efficacy of medications and patient safety by helping predict the risk of adverse outcomes. Translating pharmacogenetics to clinical practice has been challenging in the context of pain therapies because of the inherent complexity of the study of pain that involves subjective and multifactorial pain perception experiences or responses to pain‐modulating drugs.23, 24 However, the number of United States Food and Drug Administration (FDA)‐approved drug label modifications that contain pharmacogenetic information has significantly increased.25 Additionally, the Clinical Pharmacogenetics Implementation Consortium published guidelines for codeine, clopidogrel, and tacrolimus dosing based on pharmacogenetics testing.26, 27, 28 An IVD companion diagnostic device provides information that is essential for the safe and effective use of a corresponding therapeutic product. The FDA issued draft guidance for IVD companion diagnostic devices in July, 2011.29
4. CANDIDATE GENES THAT PREDICT THE ANALGESIC EFFECT OF OPIOIDS
Opioids exert their effects by binding to opioid peptide receptors (Gi/o protein‐coupled receptors) and triggering signaling transmission to several effector systems, including the inhibition of adenylyl cyclase, activation of G protein‐activated inwardly rectifying potassium (GIRK) channels,30 and inhibition of voltage‐dependent Ca2+ channels. Most opioid drugs are metabolized by cytochrome P450 (CYP) enzymes, including CYP2D6, that are glucuronidated by UDP‐glucuronosyltransferases (UGTs) and transported between the blood and brain by adenosine triphosphate (ATP)‐binding cassette, subfamily B (MDR/TAD), member 1 (ABCB131, 32, 33, 34; Figure 2). Many studies have reported associations between gene polymorphisms in these molecules, some of which are described below, and pain/opioid sensitivity (Table 1).
Figure 2.
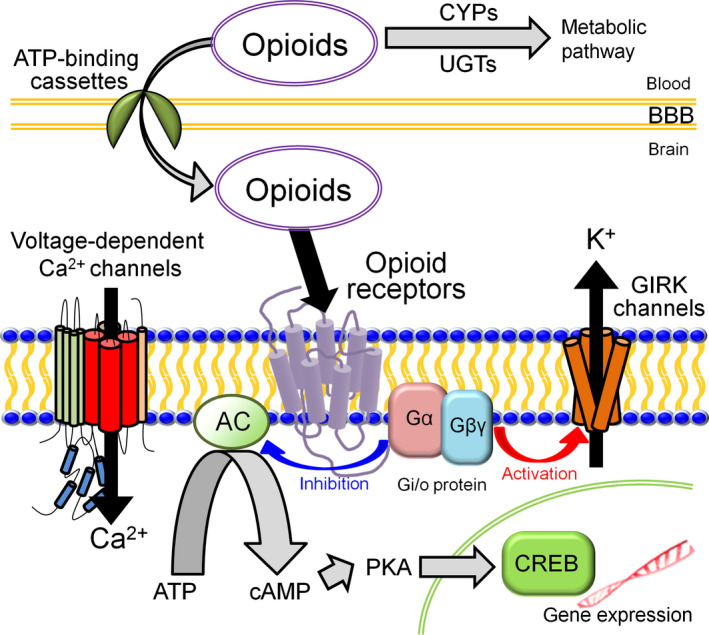
Molecules associated with the action of opioid analgesics. The analgesic effects of opioids depend on such factors as opioid peptide receptors, effector molecules, metabolic enzymes, and transporters. The effector molecules are affected by Gi/o protein include G protein‐activated inwardly rectifying potassium (GIRK) channels, voltage‐dependent Ca2+ channels, and adenylyl cyclase (AC), which influence the activity of cyclic adenosine monophosphate (cAMP)‐responsive element‐binding protein (CREB) through the actions of cAMP and protein kinase A. The metabolic enzymes include cytochrome P450 enzymes (CYPs) and UDP‐glucuronosyltransferases (UGTs). The transporters include adenosine triphosphate (ATP)‐binding cassettes
Table 1.
Summary of candidate genes related to predicting the analgesic effect of opioids
| Gene | Related polymorphism | Subjects | Related phenotype | Reference |
|---|---|---|---|---|
| OPRM1 | rs1799971 (A118G) | Patients undergoing total knee arthroplasty | Analgesic response to morphine for acute postoperative pain relief | Wang et al 37 |
| Female patients undergoing abdominal total hysterectomy | Chou et al38 | |||
| Mice possessing the equivalent substitution | Chou et al39 | |||
| OPRM1 | rs9384179 (IVS3 + A8449G) | Patients undergoing sagittal split ramus osteotomy | Fentanyl requirements for postoperative analgesia | Fukuda et al40 |
| CREB1 | rs2952768 | Patients undergoing sagittal split ramus osteotomy | Fentanyl requirements for postoperative analgesia | Nishizawa et al44 |
| GIRK2 (KCNJ6) | rs2835859 | Patients undergoing sagittal split ramus osteotomy | Fentanyl requirements for postoperative analgesia | Nishizawa et al48 |
| CACNA1E | rs3845446 | Patients undergoing sagittal split ramus osteotomy | Fentanyl requirements for postoperative analgesia | Ide et al14 |
| Patients undergoing laparoscopic colectomy | Fentanyl requirements for postoperative analgesia | Amano et al52 |
4.1. OPRM1
Opioids exert their pharmacological actions through three types of opioid receptors, designated μ, κ, and δ.35 Among these three receptors, morphine and fentanyl particularly interact with μ‐opioid receptors (MOPs). MOPs are essential for the analgesic effects of most clinically effective opioid drugs. Genetic variations in the human MOP gene (OPRM1) that alter the expression of MOPs can affect opioid efficacy. To date, more than 250 single nucleotide polymorphisms (SNPs) have been identified in the human OPRM1 gene.36 The A118G polymorphism (rs1799971) has been the most extensively studied polymorphism because of its clinical associations with opioid responses. This SNP is located in exon 1 and leads to an amino acid substitution that alters the N‐linked glycosylation sites on the receptor. Several clinical studies have shown that subjects with the G118 allele presented a reduction in the analgesic response to morphine for acute postoperative pain relief.37, 38, 39 For example, in Japanese patients who underwent sagittal split ramus osteotomy, the analgesic effects of fentanyl were related to genotypes of the OPRM1 gene. Subjects with the G allele of the OPRM1 A118G SNP were less sensitive to fentanyl. Additionally, subjects with the G allele of the IVS3 + A8449G SNP (rs9384179), representing the complete disequilibrium block, including the 3′ untranslated region (UTR) of the OPRM1 gene, required less fentanyl for postoperative analgesia.40
4.2. CREB1
The inhibition of adenylyl cyclase inhibits the production of cyclic adenosine monophosphate (cAMP), thus decreasing the active form of protein kinase A, phosphorylating cAMP‐responsive element‐binding protein (CREB), decreasing various gene expression in the nucleus, and subsequently affecting analgesia.41 Indeed, the administration of cAMP intracerebrally or intravenously antagonized morphine analgesia, and all of the major behavioral effects of morphine, including analgesia, were attenuated in mice that lacked adenylyl cyclase 5.42, 43 A recent study found that higher CREB1 mRNA expression levels in subjects with the C/C genotype in the rs2952768 SNP may result in elevated CREB function and decreased sensitivity to the rewarding effects of opioids, resulting in greater postoperative opioid analgesic requirements.44
4.3. GIRK2
GIRK channels are expressed in many tissues and activated by several Gi/o protein‐coupled receptors, such as opioid receptors, and thus are known to be involved in the modulation of opioid‐induced analgesia.45 Several studies that used knockout mice showed that opioid‐induced GIRK channel activation, co‐expressed with opioid receptors, inhibited nociceptive transmission and thus opioid‐induced analgesia.46, 47 Recently, an association was found between opioid analgesic sensitivity and the rs2835859 SNP in the GIRK2 (KCNJ6) gene, in which carriers of the C allele of this SNP required less analgesics compared with noncarriers after painful cosmetic surgery.48
4.4. CACNA1E
Voltage‐activated Ca2+ channels (VACCs) mediate Ca2+ entry into cells in response to membrane depolarization and play a crucial role in the nervous system by controlling membrane excitability and calcium signaling.49 Cav2.3 VACCs are reported to be distributed throughout the central and peripheral nervous systems, including pain pathways. Cav2.3 knockout mice have been reported to present functional deficits in pain perception.50, 51 Thus, Cav2.3 (R‐type) VACCs have been especially thought to play critical roles in pain pathways. In Japanese patients who underwent sagittal split ramus osteotomy, the analgesic effects of fentanyl were related to genotype of the CACNA1E gene. Subjects with the minor G allele of the rs3845446 A/G SNP required less fentanyl for adequate postoperative pain control after painful cosmetic surgery,14 although those with the same allele reportedly required greater fentanyl after laparoscopic colectomy.52
5. PERSONALIZED PAIN TREATMENT
Although various factors that are related to individual differences in opioid sensitivity have been identified, a prediction model that calculates appropriate opioid analgesic requirements has not yet been established. Therefore, we sought to construct prediction formulas for individual opioid analgesic requirements based on genetic polymorphisms and clinical data using patients in pain management with opioid drugs.53 Constructing prediction formulas based on data from patients with cancer pain is difficult because the mechanism, severity, and nature of cancer pain can differ substantially between patients.54 Thus, we investigated patients who underwent mandibular sagittal split ramus osteotomy, which is highly standardized at our institute with regard to surgical procedures, the duration of surgery, and the skill of the surgeons. Because these patients are usually young and healthy and expected to experience similar levels of pain after surgery, they may be ideal for evaluating the analgesic effects of opioids.39 Stepwise multiple linear regression analysis was performed to construct prediction formulas and predict postoperative fentanyl requirements based on parameters that may affect the analgesic efficacy of fentanyl (eg, age, gender, height, weight, pain perception latency [PPL], and genotype data for five SNPs that have been reported to be strongly associated with opioid requirements in patients who underwent mandibular sagittal split ramus osteotomy with postoperative pain). These analyses showed that four SNPs (ie, rs2952768, rs2835859, rs9384179, and rs11959113 around the adrenoceptor β2, surface gene [ADRB2]), PPL, and weight were retained as independent predictors of 24‐h postoperative fentanyl use, and two SNPs (ie, rs2952768 and rs3845446) and weight were retained as independent predictors of perioperative fentanyl use. The multiple‐regression equations were the following:
We also validated the utility of the prediction formulas in patients who underwent major open abdominal surgery and found that these prediction formulas may be useful for other types of surgery (Figure 3). Using the prediction formulas and patients' genetic polymorphisms and clinical data, better analgesia could be provided to individual patients.
Figure 3.
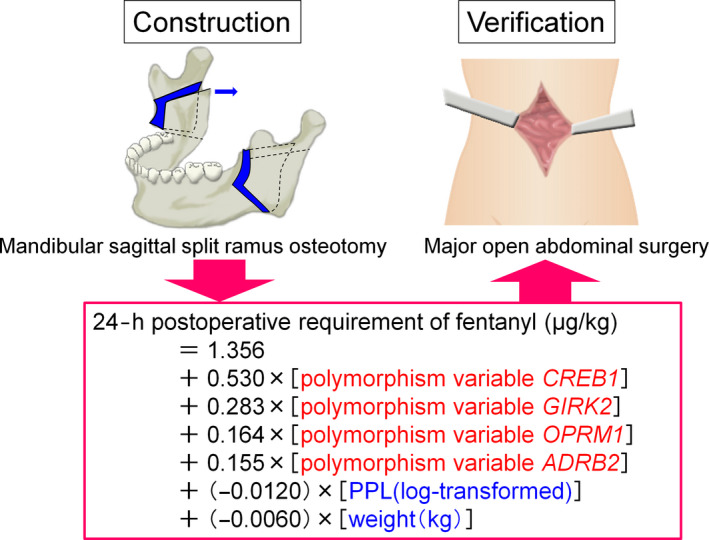
Construction and verification of prediction formula for individual opioid analgesics requirements. We constructed a prediction formula for individual opioid analgesic requirements during the first 24‐h postoperative period using patients who underwent mandibular sagittal split ramus osteotomy and validated the utility of the formula in another type of surgery, major abdominal surgery
6. FUTURE PROSPECTS
Although the precision of our prediction formula for individual opioid analgesic requirements appears to be relatively low, such algorithms are expected to be improved in further studies. For example, genetic factors that are involved in metabolic enzymes and transporters may improve the accuracy of the prediction formulas in the future. Indeed, polymorphisms of the UGT2B7 gene, which encodes one of the UGT subtypes, has recently been reported to be associated with the analgesic effects of fentanyl in the cold pressor‐induced pain test,55 which could also lead to improvement of the accuracy of the prediction formulas. Additionally, the onset of adverse effects (eg, nausea, vomiting, and constipation) may also be a useful and interesting outcome because such effects can cause some patients to stop requesting analgesics despite not actually achieving full analgesia. Gene polymorphisms have also been found to be related to various side effects of other drugs. For example, HLA‐A∗3101 was present in 60.7% (37/61) of patients with carbamazepine‐induced cutaneous adverse drug reactions (cADRs) but in only 12.5% (47/376) of carbamazepine‐tolerant controls (odds ratio = 10.8, 95% confidence interval: 5.9‐19.6, P = 3.64 × 10−15), implying that this allele has 60.7% sensitivity and 87.5% specificity when we apply HLA‐A∗3101 as a risk predictor for carbamazepine‐induced cADRs.56 In the future, we expect that more effective personalized pain treatment may be achieved by taking side effects into account.
7. CONCLUSION
The studies that were presented in this review have shown that individual differences in opioid analgesic requirements are very large, and various factors (eg, genetic factors) are involved in such differences. Although more research is needed, better pain treatment may be provided to patients who suffer from postoperative pain using prediction formulas based on pharmacogenetics research.
CONFLICT OF INTEREST
The authors declare no conflict of interest for this article. [Correction added on 5 March 2018, after first online publication: The word, ‘The’, has been added to the start of the conflict of interest statement.]
AUTHOR CONTRIBUTIONS
KY, DN, SI, and KI conceived and designed the contents. KY, DN, SI, TI, KF, and KI wrote the paper.
ACKNOWLEDGMENTS
We acknowledge Mr. Michael Arends for his assistance with editing the manuscript. Financial support was received from the Japan Society for the Promotion of Science (grant no. 26293347).
Yoshida K, Nishizawa D, Ide S, Ichinohe T, Fukuda K‐I, Ikeda K. A pharmacogenetics approach to pain management. Neuropsychopharmacol Rep. 2018;38:2–8. 10.1002/npr2.12003
Kaori Yoshida and Daisuke Nishizawa contributed equally to this article.
REFERENCES
- 1. Kasai S, Hayashida M, Sora I, et al. Candidate gene polymorphisms predicting individual sensitivity to opioids. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:269–81. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Cancer pain relief. Geneva: World Health Organization; 1986. [Google Scholar]
- 3. Cancer pain relief and palliative care: report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1990;804:1–75. [PubMed] [Google Scholar]
- 4. Ikeda K, Ide S, Han W, et al. How individual sensitivity to opiates can be predicted by gene analyses. Trends Pharmacol Sci. 2005;26:311–7. [DOI] [PubMed] [Google Scholar]
- 5. Goldstein FJ. Adjuncts to opioid therapy. J Am Osteopath Assoc. 2002;102(9 Suppl 3):S15–21. [PubMed] [Google Scholar]
- 6. Pico L, Hernot S, Nègre I, et al. Preoperative titration of morphine improves immediate postoperative analgesia after total hip arthroplasty. Can J Anaesth J Can Anesth. 2000;47:309–14. [DOI] [PubMed] [Google Scholar]
- 7. Takahashi M, Furuya H. Essence of anesthetic control before pain management of PCA. J Jpn Soc Clin Anesth. 2010;30:669–75. [Google Scholar]
- 8. Gourlay GK, Kowalski SR, Plummer JL, et al. Fentanyl blood concentration‐analgesic response relationship in the treatment of postoperative pain. Anesth Analg. 1988;67:329–37. [PubMed] [Google Scholar]
- 9. Iwakiri H, Nagata O, Matsukawa T, et al. Effect‐site concentration of propofol for recovery of consciousness is virtually independent of fentanyl effect‐site concentration. Anesth Analg. 2003;96:1651–5. [DOI] [PubMed] [Google Scholar]
- 10. Coulbault L, Beaussier M, Verstuyft C, et al. Environmental and genetic factors associated with morphine response in the postoperative period. Clin Pharmacol Ther. 2006;79:316–24. [DOI] [PubMed] [Google Scholar]
- 11. Ip HYV, Abrishami A, Peng PWH, et al. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology. 2009;111:657–77. [DOI] [PubMed] [Google Scholar]
- 12. Hajj A, Khabbaz L, Laplanche JL, et al. Pharmacogenetics of opiates in clinical practice: the visible tip of the iceberg. Pharmacogenomics. 2013;14:575–85. [DOI] [PubMed] [Google Scholar]
- 13. Aoki Y, Yoshida K, Nishizawa D, et al. Factors that affect intravenous patient‐controlled analgesia for postoperative pain following orthognathic surgery for mandibular prognathism. PLoS ONE. 2014;9:e98548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ide S, Nishizawa D, Fukuda K, et al. Association between genetic polymorphisms in Cav2.3 (R‐type) Ca2+ channels and fentanyl sensitivity in patients undergoing painful cosmetic surgery. PLoS ONE. 2013;8:e70694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bair MJ, Robinson RL, Katon W, et al. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433–45. [DOI] [PubMed] [Google Scholar]
- 16. Agüera‐Ortiz L, Failde I, Mico JA, et al. Pain as a symptom of depression: prevalence and clinical correlates in patients attending psychiatric clinics. J Affect Disord. 2011;130:106–12. [DOI] [PubMed] [Google Scholar]
- 17. Rahmioğlu N, Ahmadi KR. Classical twin design in modern pharmacogenomics studies. Pharmacogenomics. 2010;11:215–26. [DOI] [PubMed] [Google Scholar]
- 18. Angst MS, Phillips NG, Drover DR, et al. Opioid pharmacogenomics using a twin study paradigm: methods and procedures for determining familial aggregation and heritability. Twin Res Hum Genet. 2010;153:412–25. [DOI] [PubMed] [Google Scholar]
- 19. Angst MS, Phillips NG, Drover DR, et al. Pain sensitivity and opioid analgesia: a pharmacogenomic twin study. Pain. 2012;153:1397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nielsen CS, Stubhaug A, Price DD, et al. Individual differences in pain sensitivity: genetic and environmental contributions. Pain. 2008;136:21–9. [DOI] [PubMed] [Google Scholar]
- 21. Smith MT, Muralidharan A. Pharmacogenetics of pain and analgesia. Clin Genet. 2012;82:321–30. [DOI] [PubMed] [Google Scholar]
- 22. Zineh I, Pacanowski MA. Pharmacogenomics in the assessment of therapeutic risks versus benefits: inside the United States Food and Drug Administration. Pharmacotherapy. 2011;31:729–35. [DOI] [PubMed] [Google Scholar]
- 23. Maliepaard M, Nofziger C, Papaluca M, et al. Pharmacogenetics in the evaluation of new drugs: a multiregional regulatory perspective. Nat Rev Drug Discov. 2013;12:103–15. [DOI] [PubMed] [Google Scholar]
- 24. Vuilleumier PH, Stamer UM, Landau R. Pharmacogenomic considerations in opioid analgesia. Pharmacogenomics Pers Med. 2012;5:73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hong H, Goodsaid F, Shi L, et al. Molecular biomarkers: a US FDA effort. Biomark Med. 2010;4:215–25. [DOI] [PubMed] [Google Scholar]
- 26. Crews KR, Gaedigk A, Dunnenberger HM, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450 2D6 genotype and codeine therapy: 2014 update. Clin Pharmacol Ther. 2014;95:376–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Scott SA, Sangkuhl K, Stein CM, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94:317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Birdwell KA, Decker B, Barbarino JM, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP3A5 genotype and tacrolimus dosing. Clin Pharmacol Ther. 2015;98:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Devices and Radiological Health, Center for Biologics Evaluation and Research, Center for Drug Evaluation and Research . Draft guidance: in vitro companion diagnostic devices [internet]. Rockville: U.S. Food and Drug Administration; 2011. [updated 2014 Aug; cited 2015 Oct 24]. Available from www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM262327.pdf
- 30. Ikeda K, Kobayashi T, Kumanishi T, et al. Molecular mechanisms of analgesia induced by opioids and ethanol: is the GIRK channel one of the keys? Neurosci Res. 2002;44:121–31. [DOI] [PubMed] [Google Scholar]
- 31. Rogers JF, Nafziger AN, Bertino JS. Pharmacogenetics affects dosing, efficacy, and toxicity of cytochrome P450‐metabolized drugs. Am J Med. 2002;113:746–50. [DOI] [PubMed] [Google Scholar]
- 32. King CD, Rios GR, Green MD, et al. UDP‐glucuronosyltransferases. Curr Drug Metab. 2000;1:143–61. [DOI] [PubMed] [Google Scholar]
- 33. D' AS, Grasso KL, editors. Acute pain: causes, effects, and treatment. New York, NY: Nova Science Publishers, 2009. [Google Scholar]
- 34. Dzambazovska‐Trajkovska V, Nojkov J, Kartalov A, et al. Association of single‐nucleotide polymorphism C3435T in the ABCB1 gene with opioid sensitivity in treatment of postoperative pain. Pharmacogenomics. 2016;37:73–80. [DOI] [PubMed] [Google Scholar]
- 35. Inturrisi CE. Clinical pharmacology of opioids for pain. Clin J Pain. 2002;18(4 Suppl):S3–13. [DOI] [PubMed] [Google Scholar]
- 36. Hayashida M, Nagashima M, Satoh Y, et al. Analgesic requirements after major abdominal surgery are associated with OPRM1 gene polymorphism genotype and haplotype. Pharmacogenomics. 2008;9:1605–16. [DOI] [PubMed] [Google Scholar]
- 37. Wang YJ, Huang P, Ung A, et al. Reduced expression of the μ opioid receptor in some, but not all, brain regions in mice with OPRM1 A112G. Neuroscience. 2012;205:178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chou WY, Yang LC, Lu HF, et al. Association of mu‐opioid receptor gene polymorphism (A118G) with variations in morphine consumption for analgesia after total knee arthroplasty. Acta Anaesthesiol Scand. 2006;50:787–92. [DOI] [PubMed] [Google Scholar]
- 39. Chou WY, Wang CH, Liu PH, et al. Human opioid receptor A118G polymorphism affects intravenous patient‐controlled analgesia morphine consumption after total abdominal hysterectomy. Anesthesiology. 2006;105:334–7. [DOI] [PubMed] [Google Scholar]
- 40. Fukuda K, Hayashida M, Ide S, et al. Association between OPRM1 gene polymorphisms and fentanyl sensitivity in patients undergoing painful cosmetic surgery. Pain. 2009;147:194–201. [DOI] [PubMed] [Google Scholar]
- 41. Carlezon WA, Thome J, Olson VG, et al. Regulation of cocaine reward by CREB. Science. 1998;282:2272–5. [DOI] [PubMed] [Google Scholar]
- 42. Ho IK, Loh HH, Way EL. Cyclic adenosine monophosphate antagonism of morphine analgesia. J Pharmacol Exp Ther. 1973;185:336–46. [PubMed] [Google Scholar]
- 43. Kim KS, Lee KW, Lee KW, et al. Adenylyl cyclase type 5 (AC5) is an essential mediator of morphine action. Proc Natl Acad Sci USA. 2006;103:3908–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nishizawa D, Fukuda K, Kasai S, et al. Genome‐wide association study identifies a potent locus associated with human opioid sensitivity. Mol Psychiatry. 2014;19:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ikeda K, Kobayashi T, Kumanishi T, et al. Involvement of G‐protein‐activated inwardly rectifying K+ (GIRK) channels in opioid‐induced analgesia. Neurosci Res. 2000;38:113–6. [DOI] [PubMed] [Google Scholar]
- 46. Marker CL, Cintora SC, Roman MI, et al. Hyperalgesia and blunted morphine analgesia in G protein‐gated potassium channel subunit knockout mice. NeuroReport. 2002;13:2509–13. [DOI] [PubMed] [Google Scholar]
- 47. Mitrovic I, Margeta‐Mitrovic M, Bader S, et al. Contribution of GIRK2‐mediated postsynaptic signaling to opiate and α2‐adrenergic analgesia and analgesic sex differences. Proc Natl Acad Sci USA. 2003;100:271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nishizawa D, Fukuda K, Kasai S, et al. Association between KCNJ6 (GIRK2) gene polymorphism rs2835859 and post‐operative analgesia, pain sensitivity, and nicotine dependence. J Pharmacol Sci. 2014;126:253–63. [DOI] [PubMed] [Google Scholar]
- 49. Catterall WA. Structure and regulation of voltage‐gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–55. [DOI] [PubMed] [Google Scholar]
- 50. Murakami M, Nakagawasai O, Suzuki T, et al. Antinociceptive effect of different types of calcium channel inhibitors and the distribution of various calcium channel α1 subunits in the dorsal horn of spinal cord in mice. Brain Res. 2004;1024:122–9. [DOI] [PubMed] [Google Scholar]
- 51. Saegusa H, Kurihara T, Zong S, et al. Altered pain responses in mice lacking α1E subunit of the voltage‐dependent Ca2+ channel. Proc Natl Acad Sci USA. 2000;97:6132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Amano K, Nishizawa D, Mieda T, et al. Opposite associations between the rs3845446 single‐nucleotide polymorphism of the CACNA1E gene and postoperative pain‐related phenotypes in gastrointestinal surgery versus previously reported orthognathic surgery. J Pain. 2016;17:1126–34. [DOI] [PubMed] [Google Scholar]
- 53. Yoshida K, Nishizawa D, Ichinomiya T, et al. Prediction formulas for individual opioid analgesic requirements based on genetic polymorphism analyses. PLoS ONE. 2015;10:e0116885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Klepstad P, Rakvåg TT, Kaasa S, et al. The 118 A > G polymorphism in the human μ‐opioid receptor gene may increase morphine requirements in patients with pain caused by malignant disease. Acta Anaesthesiol Scand. 2004;48:1232–9. [DOI] [PubMed] [Google Scholar]
- 55. Muraoka W, Nishizawa D, Fukuda K, et al. Association between UGT2B7 gene polymorphisms and fentanyl sensitivity in patients undergoing painful orthognathic surgery. Mol Pain. 2016;12:1744806916683182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ozeki T, Mushiroda T, Yowang A, et al. Genome‐wide association study identifies HLA‐A*3101 allele as a genetic risk factor for carbamazepine‐induced cutaneous adverse drug reactions in Japanese population. Hum Mol Genet. 2011;20:1034–41. [DOI] [PubMed] [Google Scholar]


