Abstract
Aim
In this review, the author focused on anticraving therapy for alcohol use disorder (AUD) defined by DMS‐5. A comprehensive review was carried out on the available published papers on anticraving drugs for treating AUD patients.
Methods
The author described all drugs with anticraving benefits for treating AUD patients approved by the Food and Drug Administration of the United States (US FDA) and European Medicines Agency of the European Union. Then, the commonly prescribed anticraving drugs and those under development were also described.
Results
The US FDA‐approved anticraving drugs included acamprosate and naltrexone, and those approved by European Medicines Agency were gamma‐hydroxybutyrate and nalmefene. The author also highlighted topiramate, gabapentin, ondansetron, LY196044, ifenprodil, varenicline, ABT‐436, mifepristone, citicoline, and baclofen. The putative mechanisms of action of and the use in clinical practice of those anticraving drugs were also described.
Conclusion
Although slowly developing, the field of anticraving drugs is getting into shape as a promising entity of a pharmaceutical class of drugs. Then, the author addressed on the underused issues of those recommended, and suggested anticraving drugs by the practice guideline of the American Psychiatric Association. The author urges that clinicians should be more “adventurous” in prescribing those promising drugs because benefits of those anticraving drugs are far‐outweighing the possible side effects of anticraving drugs, or the harms of untreated AUD itself.
Keywords: acamprosate, gabapentin, gamma‐hydroxybutyrate, ifenprodil, nalmefene, naltrexone, topiramate
“No, No! The adventures first,” said the Gryphon in an important tone: “Explanations take such a dreadful time” Lewis Carroll, Alice in Wonderland
1. INTRODUCTION
The review on the topic “pharmacotherapy of alcoholism” for my first time was in 1991.1 Upon the invitation of H. Hosaki sensei, then chair professor of Department of Neuropsychiatry at Keio University, the author presented in 1990 a lecture organized by Keio Psychiatrists’ Alumni Organization on this topic. The host's intention was for me to share the state of the arts knowledge of drug treatments for alcoholism of the United States of America to Japanese psychiatric colleagues.
At the 2017 annual meeting of the American College of Neuropsychopharmacology in Palm Springs, California, USA, the author was invited from K. Ikeda sensei, the President of Japanese Society of Neuropsychopharmacology, to write an article for Neuropsychopharmacology Reports. I accepted then the invitation right away. After a 4‐month consideration, I finally decided to choose the topic of anticraving therapy for alcoholism, to give myself a chance to re‐visit the topic that I have been involved at since late 1970s.
Compared to those 27 years ago, all drugs except disulfiram for treating alcohol use disorder (AUD) patients’ “intense drug craving” are new to the author.1, 2 In 1991, disulfiram was the only drug approved by the Food and Drug Administration of the United State of America (US FDA, www.fda.org) specifically for AUD patients.1 But disulfiram is not a drug to give anticraving benefits for AUD patients.
In this review, the author started to describe the diagnosis “AUD” in the 2013 framework of American Psychiatric Association's Diagnostic and Statistical Manual for Mental Disorders, the Fifth Edition (DSM‐5).2 Anticraving drugs include those approved by the US FDA and European Medicines Agency, as well as those commonly prescribed or those in pharmaceutic pipeline for drug development. Finally, the author covered putative mechanism of actions of those drugs, described their prescriptions in clinical practice, and addressed the issue of underuse in prescribing this class of anticraving drugs. This review was intended to promote the awareness of AUD patients, and to urge clinicians to prescribe those underused but readily available anticraving drugs for treating AUD patients.
2. THE DIAGNOSIS OF ALCOHOL USE DISORDER IN DSM‐5
The chapter “substance‐related (and additive) disorders” (pages 481‐589) of APA's DSM‐5,2 describes 11 substance‐related disorders and one nonsubstance related disorder (gambling). On page 482 of DSM‐5,2 a table “diagnoses associated with substance class” lists 12 diagnoses—psychotic, bipolar, depressive, anxiety, obsessive‐compulsive and related, sleep, sexual dysfunctions, delirium, neurocognitive, substance use disorders, as well as substance intoxication, and substance withdrawal. Each diagnosis can be applied to all classes of substances included in the chapter “substance‐related disorders” except caffeine (page 483 of DSM‐5).2 Tea drinking or coffee drinking in DSM‐5, 2 in author's opinion, is recognized as a culturally acceptable human behavior rather than a psychiatric disorder.
Those 12 substance‐related disorders are divided into two groups of diagnoses—substance use disorder (one diagnosis totally) and substance‐induced disorders (11 diagnoses totally). In further description, substance use disorder has “an underlying change in brain circuitry that may persist beyond detoxification, particularly in individuals with severe disorders. The behavioral effects of these brain changes may be exhibited in ‘repeated relapses and intense drug craving’ when the individuals are exposed to drug‐related stimuli” (page 483 of DSM‐5).2
The pharmacologic treatments for substance‐related disorder are intended to manage various symptoms and signs of different diagnoses due to effects or complications from the use of substances. The drug treatments for various “substance use disorder” (listed as a diagnosis number 10 of 12 diagnoses for various substances (page 482 of DSM‐5).2 The diagnosis “AUD” in DSM‐5 is the focus in this review.
3. TWO ANTICRAVING DRUGS APPROVED BY US FDA FOR TREATING PATIENTS WITH AUD
The US FDA (www.fda.org) has so far approved three drugs—disulfiram oral dose in 1951, naltrexone in oral use in 1994 and long‐acting injection use in 2006, and acamprosate in oral use in 2004—for patients with AUD after stopping their use of alcohol.
After ingestion, alcohol is metabolized into acetaldehyde through the oxidation process with the enzyme alcohol dehydrogenase in the presence of zinc ions. This is a rate‐limiting step. Then, the metabolite acetaldehyde is instantly converted into acetic acid through the enzymatic action of acetaldehyde dehydrogenase. But this step can be inhibited by disulfiram. For the patients who are “pre‐medicated” with disulfiram, consumption of alcohol causes rapid accumulation of acetaldehyde in the body, producing disulfiram‐ethanol reaction (DER). The patients with DER experience terribly uncomfortable symptoms and signs, such as facial flush, headache, breathing difficulty, nausea, vomiting, sweating, thirsty feeling, chest pain, dizziness, palpitations of the heart, restlessness, exhaustion, photophobia, confusion in consciousness level, … The severe DER can even cause bradypnea, shock, heart exhaustion, stupor, convulsion, and death.1, 3
The role of disulfiram is to premedicate this compound to create patients’ fear for developing DER to deter them from drinking alcohol. In the sense of behavior science, the treatment mechanism of action is negative reinforcement (i.e., punishment) if the patients decide to drink. Although disulfiram medicated‐patients still have the craving to drink alcohol, they are afraid of drinking due to the threat of developing DER.
The 2018 APA practice guideline suggests (2C) that disulfiram be offered to moderate to severe AUD patients (a) who have a goal of achieving abstinence, (b) who prefer disulfiram or are intolerable to or have not responded to naltrexone and acamprosate, (c) who can understand the risks of alcohol consumption while taking disulfiram, or (d) who have no contraindications to use it.4 To note, the recent APA guideline4 uses the word “suggests,” which is 2C in suggestion level. In my opinion, the APA guideline suggests 2C in suggestion ranking for disulfiram, recognizing that disulfiram is not to be prescribed as the first line drug for AUD patients.
3.1. Acamprosate is intended to be an anticraving drug to stop alcohol drinking by US FDA
Acamprosate, a glutamate agonist, is derived from amino acid, taurine.5 In a double‐blind, placebo‐controlled study in Germany, acamprosate has been found to have anticraving property to prevent recovered alcoholics from drinking alcohol again.5 Under the pressure of families of alcoholic patients, the US FDA‐approved acamprosate for the American market in 2004. At that time of approval, acamprosate had already been on the market of 37 countries in the world.3
The anticraving efficacy of acamprosate has been proven not as good as expected in patients’ tendency for the time to first drink and the time to relapse.6 In the double‐blind, placebo‐controlled comparison study of combining acamprosate and naltrexone for alcoholic patients, the investigators found that anticraving property of acamprosate plus naltrexone is better than acamprosate alone, but not better than naltrexone alone.6 In prospective, 6‐week, randomized double‐blind studies in America7 and in Australia,8 two groups of investigators independently found that naltrexone but not acamprosate has anticraving property against alcohol drinking.
In a recent randomized, double‐blind, placebo‐controlled clinical trial of acamprosate in alcohol‐dependent individuals with bipolar disorder, the investigators found that acamprosate group has not been detected any better treatment difference in alcohol drinking compared to the placebo group, and that no differences exist in depressive symptoms, manic symptoms, or adverse events between acamprosate and control groups.9 In another randomized, double‐blind, placebo‐controlled, parallel group design of acamprosate in two American family medicine settings (North Carolina and Wisconsin), the results of the clinical trial showed that acamprosate has not been found to have anticraving effects in measuring percent days of abstinence and percent heavy drinking days.10
The 2018 APA practice guideline recommends (1B) that acamprosate be offered to patients with moderate to severe AUD, (a) who have a goal of reducing alcohol consumption or achieving abstinence, (b) who prefer pharmacotherapy or have not responded to nonpharmacological treatment alone, or (c) who have no contraindications to use it.4 To note, the use of the word “recommends,” which is 1B in recommendation ranking level.
3.2. Naltrexone as an anticraving agent to stop alcohol drinking by US FDA
Naltrexone, an opioid receptor antagonist, is mainly blocking μ opioid receptors,3 although it also blocks on κ opioid receptors to a lesser extent, but on δ opioid receptors insignificantly. In a 12‐week, randomized, double‐blind, placebo‐controlled study on 72 alcoholic patients,11 patients on 50 mg/d of naltrexone have been found to have less craving, decreased frequency of alcohol drinking time, and decreased frequency of patients’ abnormal hepatic gamma‐glutamyl transpeptidase (gamma GT) enzyme. In another double‐blind, placebo‐controlled study on 97 alcoholic patients,12 patients on 50 mg/d of naltrexone have been found to have less patients’ alcohol drinking days and less problem resulted from their drinking. As stated previously, Anton et al7 in America and Morley et al8 in Australia have independently confirmed the anticraving efficacy of naltrexone against alcohol drinking.
Based on findings of those study results, naltrexone has more reliable anticraving benefit than acamprosate. Similar to acamprosate in recommendation level (1B), naltrexone has also been recommended in the 2018 APA practice guideline for the pharmacological treatment of AUD patients.4 The recent APA practice guideline recommends that naltrexone be offered to patients with moderate to severe AUD, (a) who have a goal of reducing alcohol consumption or achieving abstinence, (b) who prefer pharmacotherapy or have not responded to nonpharmacological treatment alone, or (c) who have no contraindications to use it.4
4. TWO ANTICRAVING DRUGS APPROVED BY EUROPEAN MEDICINES AGENCY FOR TREATING PATIENTS WITH AUD
4.1. Gamma‐hydroxybutyrate
γ‐Hydroxybutyrate (GHB) is thought to be a weak partial agonist at the gamma‐aminobutyric acid B (GABAB) receptors, and is clinically used for treating narcolepsy.13 GHB has been found to be effective for treating AUD in a placebo‐controlled clinical trial conducted in Europe.13 Following a daily oral administration of 50 mg/kg for a 6‐12 month, multicenter, open‐labeled trial, the investigators found that 78% of GHB‐medicated patients have complete abstinence during drug administration and reduced in craving for alcohol.14
γ‐Hydroxybutyrate has been approved for AUD treatment in several European countries.13 Swift and Aston13 in 2015 suggested that GHB acts as an alcohol substitute, reducing craving for alcohol and preventing alcohol withdrawal. But the abuse potential of GHB has been noted in some European studies, and the medication is frequently abused in the United States.13 In prescribing GHB, clinicians need to carefully choose patients who are likely to be adherent to dosing recommendations, and to closely monitor the use of GHB.15, 16
In author's opinion, prescribing GHB for an AUD patient needs to be seen as a kind of replacement therapy, such as methadone for patients with opioid use disorder, or nicotine patch for patients with tobacco use disorder.4 The readers of this review are cautioned in prescribing GHB as an anticraving drug for AUD patients.
4.2. Nalmefene
Nalmefene is a μ‐opioid antagonist and partial κ agonist.17 Nalmefene has been approved by the US FDA only for opioid overdose (www.fda.org). The efficacy of nalmefene for anticraving therapy for AUD patients has not been shown to be any better than placebo in clinical trials in the United States.18 But it did well in three multi‐site trials in Europe, where nalmefene has been approved for AUD patients by the European Medicines Agency.17, 18
In a randomized, double‐blind, placebo‐controlled, efficacy study of nalmefene, as‐needed use, in AUD patients, the efficacy analyses showed a superior effect of nalmefene compared to placebo in having less heavy drinking days from baseline to month 6.19 In a subgroup analysis of patients with high/very high drinking risk levels at screening and at randomization (the target population), nalmefene has been found to have a decreased total alcohol consumption at month 6, and decreased both heavy drinking days and total alcohol consumption at month 13 in nalmefene patients compared to those in placebo group.20
5. PROMISING ANTICRAVING DRUGS NOT APPROVED BY US FDA OR EUROPEAN MEDICINES AGENCY FOR TREATING PATIENTS WITH AUD
5.1. Topiramate
Topiramate, a sulfamate in structure and a fructopyranose derivative,21 was developed serendipitously as an antiepileptic drug (www.fda.org) when it was originally being tested whether it has hypoglycemic properties.3 Besides the antiepileptic property, the US FDA also approved it for the indication of treating migraine headache (www.fda.org),3, 21 Its mechanism of actions is relatively unknown, but has been studied to show that it acts with γ‐aminobutyric acid (GABA). Topiramate also reduces the action of excitatory neurotransmitters through kainate and α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid (AMPA) receptors, and decreasing the action of carbonic anhydrate.3
In a randomized, double‐blind, placebo‐controlled clinical drug trial, topiramate has been found to have anticraving properties against alcohol drinking.22 In a double‐blind, placebo‐controlled drug trial, patients on 300 mg/d of topiramate have been found to have lower drinking quartiles on percentage of heavy drinking days, and improvements in all measures of psychosocial functioning (improved overall well‐being and better overall life satisfaction), compared to those on placebo.23 In another 14‐week, randomized, double‐blind, placebo‐controlled trial, topiramate at the dosage level of 300 mg/d has been found again to have decreased patients’ total drinking days compared to those who were on placebo.24 Furthermore, topiramate in a double‐blind drug trial has shown to have its anticraving effect for patients with comorbid dependence on alcohol and cocaine.25
The recent APA practice guideline suggests (2C) that topiramate be offered to moderate to severe AUD patients (a) who have a goal of reducing alcohol consumption or achieving abstinence, (b) who prefer topiramate or have not responded to naltrexone and acamprosate, or (c) who have no contraindications to use it.4 To note, the APA practice guideline uses the word “suggests” instead of “recommend” with 2C level in suggestion level here. This is probably to reflect topiramate's nonapproval status for AUD from US FDA, although it has been found to have anticraving benefit for AUD patients long time ago.22, 23, 24, 25
5.2. Gabapentin
Gabapentin was originally developed as an analog of GABA, which is the major inhibitory neurotransmitter in the brain.26 Not acting on GABA precursor, agonist or antagonist, gabapentin increases brain and intracellular GABA by an amino acid active transporter at both the blood‐brain barrier and many enzymatic regulation mechanisms. Further glutamate metabolism is also modulated by gabapentin.26 Gabapentin has been approved by the US FDA for the adjunctive treatment of complex partial epilepsy with and without generalized seizures and for treating neuralgia after infection of herpes zoster, diabetic neuropathy, and restless leg syndrome (www.fda.org).
In a 28‐day, placebo‐controlled, randomized, double‐blind clinical trial, investigators found that gabapentin (300 mg, twice daily) can reduce AUD patients’ number of drinks per day, the mean percentage of heavy drinking days, craving for alcohol, and increased percentage of days of abstinence in the gabapentin group than those in the placebo group.27 In a 12‐week, randomized, placebo‐controlled, a dose‐dependent manner of gabapentin (0, 900, 1800 mg/d) and concomitant manual‐guided counseling, gabapentin has been found to improve the rates of abstinence and no heavy drinking, particularly in the 1800 mg/d group.28
According to a medical news report of JAMA,18 Lyon reported that a new 6‐month “pivotal” randomized, double‐blind, placebo‐controlled clinical drug trial of 348 patients at 10 US sites on gabapentin's anticraving efficacy was just completed, and that the full study is to be published in a professional journal. In this trial with two studies, 1200 mg of gabapentin enacarbil in a 4‐week treatment has been reported to have “favorable” no heavy drinking days compared to placebo. In a smaller, 3‐month study of gabapentin was completed with 85 of 150 participants, gabapentin at 1800 mg/d has also been reported to have “favorable” rate of abstinence and no heavy drinking compared to placebo.18
Similar to topiramate in suggestion status (2C), gabapentin has also been suggested in the recent APA practice guideline for the pharmacological treatment of AUD patients.4 The guideline suggests that gabapentin be offered to moderate to severe AUD patients (a) who have a goal of reducing alcohol consumption or achieving abstinence, (b) who prefer gabapentin or have not responded to naltrexone and acamprosate, or (c) who have no contraindications to use it.4 To note, the APA practice guideline uses the word been “suggested” instead of been “recommended” with 2C level in suggestion level here. This is probably to reflect gabapentin's nonapproval status for AUD from US FDA, although it has been reported to have “favorable” anticraving benefits pending for the consideration for an approval by the US FDA.18
5.3. Ondansetron
Ondansetron is a serotonin 5‐HT3 receptor antagonist.3 In a randomized controlled clinical trial, ondansetron has been found to reduce self‐reported drinking.29 Patients who received ondansetron at 4 μg/kg twice per day have been found to have fewer drinks per day, greater percentage of days of abstinence, and total number of days abstinent per study week in AUD patients with early onset of alcohol drinking.29 A meta‐analysis of seven trials showed that selective serotonin reuptake inhibitors do not effectively treat AUD patients who do not have comorbid depression.30
The recent APA practice guideline does not mention any endorsement for the use of any 5‐HT3 antagonist for anticraving use against alcohol drinking.4 At the same time, the guideline also does not recommend for any antidepressant medications for treating AUD patients unless a co‐concurring disorder exists for which an antidepressant is an indicated treatment.4 In the United States, ondansetron is only available in injectable formulation for controlling nausea and vomiting for cancer patients receiving chemotherapy (www.zofran.org).
5.4. LY2196044
LY2196044 is an opioid antagonist.31 In phase 2, 16‐week, multicenter, outpatient, randomized, double‐blind, parallel, and placebo‐controlled trial for patients (N = 375) with alcohol‐dependent, treatment‐seeking adults, the investigators found that LY2196044‐medicated patients have in both decreased mean number of drinks per day and reduced drinks per day than placebo patients.31 But to my best knowledge, I have not seen any further trial pursuing its pharmaceutical development, and I have not found any article on LY2196044 after the publication of the above‐named trial31 in a Pubmed search.
5.5. Ifenprodil
Ifenprodil which is an inhibitor of N‐methy‐d‐aspartate (NMDA) receptor, α‐1 adrenergic receptor, and G protein‐activated inwardly rectifying potassium (GIRK) channel,32, 33 is commonly used as a vasodilator to improve patients with peripheral vascular disease symptoms such as dizziness secondary to brain infarction or brain bleeding in Japan. In 1999, Suzuki et al33 found that pretreatment with ifenprodil (5, 10, 20 mg/kg, intraperitoneally) suppresses the place preference produced by morphine in a dose‐dependent manner in laboratory mice. To paraphrase the study finding, mice have stayed in their own chosen places in the cage under morphine medication, but they do not care much where they stay in the cage after their being premedicated with ifenprodil.
In 2011, a preliminary clinical trial reported that AUD patients treated with GIRK channel inhibitor has been found to improve their alcohol abstinence and negative expectancy for alcohol compared to those treated with non‐GIRK channel inhibitor.34 In a recent study, the investigators found that AUD patients treated with ifenprodil (60 mg/d) for 3 months have lower alcohol use score compared to those treated with Cinal (a control study drug).35 This study was randomized, controlled, rater‐blinded clinical trial. The investigators suggested that double‐blind trials in collaboration with pharmaceutical companies would be important in the future.35
5.6. Additional drugs
The National Institute on Alcohol Abuse and Alcoholism (www.NIAAA.org) of the United States is the lead agency for US research on AUD,18 and other health and developmental effects of alcohol use. The NIAAA also gives grants to support national and international investigators to conduct basic and clinic studies on AUD. In a recent article in the medical news column of JAMA,18 Lyon has highlighted gabapentin and some going or completing clinical trials on anticraving drugs for AUD patients under the support from NIAAA.
5.6.1. Varenicline
Varenicline, a partial agonist for α4β2 nicotinic acetylcholine receptor subtype (nACH),3 has been shown to be the most effective pharmacotherapy for smoking cessation. In a randomized, 12‐week, control trail, varenicline has been found to higher smoking abstinence rate at weeks 1, 12, and 24 in varenicline group compared to placebo group.36 Furthermore, in smokers with AUD, varenicline has also been found to lower mean drinks per drinking day at week 12, suggesting that varenicline can decrease alcohol consumption in this population of smokers.36
5.6.2. ABT‐436
ABT‐436 is an orally active, highly selective vasopressin V1B receptor antagonist which was under development by Abbott Laboratories and AbbVie.37 In phase 2, 4‐site, 12‐week, double‐blind, placebo control clinical trial of ABT‐436, AUD participants (N = 150) received ABT‐436 or placebo, and a computerized behavioral intervention.18, 37 The ABT‐436 (800 mg/d) group has been found to have significantly greater percentage of abstinent days, and to smoke significantly less cigarettes compared to placebo groups.37 But the between‐group differences do not exist in percentage of heavy drinking days, alcohol craving, and alcohol‐related consequences, suggesting there may be value in testing medications targeting the vasopressin receptor in alcohol‐dependent patients with high stress.37
5.6.3. Mifepristone, or RU‐486
Glucocorticoid receptor antagonist better known as mifepristone, or RU‐486, has also been found to reduce alcohol intake in humans. Like ABT‐436, it works on the stress system by regulating the amygdala.18 A clinical trial assessing the efficacy of mifepristone in treating AUD is currently underway at Brown University.18
In a published clinical trial protocol on mifepristone from the United Kingdom,38 the investigators planned to recruit AUD patients after a recent detoxification from alcohol and randomize them to receive 600 mg a day of mifepristone (200 mg morning, afternoon and evening) for 7 days and 400 mg for the subsequent 7 days (200 mg morning and evening) or the equivalent number of placebo tablets for 14 days. Study participants will remain in the trial for 4 weeks (at least 2 weeks as an inpatient) and will be followed up at 3, 6 and 12 months postrandomization. Their plan to measure study patients’ cognitive function at week 3 and 4 after cessation of drinking, symptoms of depression over the 4 weeks after cession of drinking, the acute phase of alcohol withdrawal, alcohol craving, symptoms of protracted withdrawal, maintenance of abstinence, and levels of relapse drinking at follow‐up.38 We are still looking forward to seeing the role of glucocorticoid Type II receptor activation in outcome measures including the anticraving efficacy from mifepristone.
5.6.4. Citicoline
A well‐tolerated, over‐the‐counter supplement, to modulate cholinergic systems. In a previous clinical trial, investigators found that that citicoline is effectively to reduce cocaine use in cocaine use disorder patients with bipolar I disorder.39 Results of a 12‐week, placebo‐controlled trial showed that citicoline reduces heavy drinking, suggesting that it may help not to initiate any substance abuse by enhancing cognitive function and better decision‐making through increased acetylcholine signaling.18
5.6.5. Baclofen
An agonist of metabotropic γ‐aminobutyric acid B (GABAB) receptors that has been approved by the US FDA for muscle spasticity.18 In a recent meta‐analysis of the results of anticraving effects of AUD from 12 randomized clinical trials, baclofen has been found to have good effect on abstinence rates when using intention‐to‐treat analysis, but no difference effects in heavy drinking days and craving score in patients on baclofen compared to those on placebo, suggesting that substantial heterogeneity exists in effect sizes across each analysis.40 The investigators are still doing additional testing and review in the United States and Europe to clarify mixed results in small trials.18
6. PUTATIVE MECHANISMS OF ACTION IMPLICATED IN DRUGS WITH ANTICRAVING BENEFITS FOR TREATING PATIENTS WITH AUD
The mechanisms of action of the above‐listed anticraving drugs in treating AUD patients have captured fascination and imagination of many investigators in the field. The readers are advised to refer to original publications for more detailed explanations. Sampled points of explanations are described briefly.
6.1. Opioid modulation
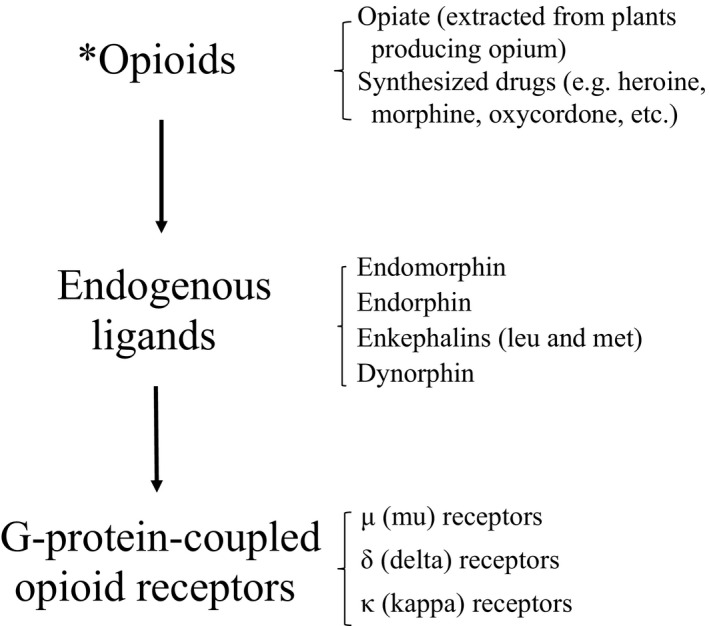
Opioids are referred to products that are extracted from plants or synthesized compounds (e.g., heroin, morphine, and oxycodone), and endogenous ligands include endomorphin, endorphin, enkephalins (Leu and Met), and dynorphin.41, 42 Through three subtypes—μ (mu), δ (delta), and κ (kappa)—of G protein‐coupled opioid receptors, opioids produce inhibitory effects on downstream neurons when activated by endogenous ligands or synthetic receptor agonists.42, 43 The binding affinity of those endogenous ligands depends on different receptor subtypes.42
Many studies showed that opioid antagonists acting either at all opioid receptor subtypes or only at specific subtypes suppress alcohol drinking.44, 45 A complete inactivation (i.e., knockout) of the μ‐opioid receptor blocks alcohol self‐administration in mice.46 Naltrexone, a selective mainly μ opioid antagonist and κ opioid antagonist to a lesser extent, was originally licensed by US FDA for treating patients with opioid overdose (www.fda.org). Later, it was also applied to treat AUD patients,47 because animal studies showed that like opioid, alcohol can activate the endogenous opioid system.48
In translating this study finding,48 an individual who receives naltrexone, cannot experience any extra euphoric effect if he/she takes opioids or drinks alcohol because some important portion of opioid receptors of the brain is blocked by naltrexone. Even though naltrexone blocks away the intense “high” from opioids or alcohol, it does not prevent good feelings that come from other naturally pleasurable activities. The individual still has other natural opioid receptors to bind, for example, endorphins from exercise. Figure 1 is a schematic illustration of how alcohol acts on endogenous opioid system.
Figure 1.
Schematic illustration depicting how alcohol* acts on endogenous opioid system. Originally, the word “opioids” is a term denoting synthetic narcotics resembling opiates but increasingly used to refer to both opiates (produced from opium) and synthetic narcotics
As a peptide, opioid can stimulate inhibitory GABA neurotransmitter of interneuron in ventral tegmental area (VTA or area of Tsai, or A10), resulting in acting on A10 DA neurons. Eventually, DA is transmitted through DA mesolimbic tract to terminate in nucleus accumbens (NAc) as DA projection terminal fibers to have reward expressed.44, 45 Besides the afore‐mentioned DA depending pathway, opioid or alcohol can directly interact on DA projection terminal fibers in NAc to express reward.45 Taken together, naltrexone as an opioid antagonist, can block opioid actions through both DA dependent and DA independent pathways to produce anticraving benefit. More detailed description is as followed in subtitle 6.2 of this article.
6.2. Dopamine neurotransmission
The reward system is transmitted through dopamine mesolimbic neurotransmission, from DA neurons originated from A10 of the midbrain, and terminated in DA projection terminal fibers in NAc.3 The opioid peptide has been thought to be always co‐localized and co‐transmitted with DA neurotransmission in the brain. Opioid modulation action must be through activating DA system.49, 50, 51
DA is released in NAc, after alcohol or drug intake.51 But previous study finding also suggests that DA independent opioid reward is possibly observed in opioid‐naive states, but not in opioid‐dependent states.52 A recent study has further shown that the self‐stimulation response of the brain is increased by stimulants but reduced by morphine,53 suggesting that opioid dependence may change the GABA neurons from inhibitory to excitatory function, resulting in disappearance of DA independent opioid reward pathway.42 These study findings confirm that DA neurotransmission may not always be required for opioid reward responses in AUD patients.54, 55, 56
The mechanisms by which most of the addictive substances, except for alcohol, activated mesolimbic DA systems have been described to involve specific “receptors” for these addictive substances on the DA neurons of the VTA which project to the NAc, or on the projection terminal fibers of the DA neurons in NAc.51 But the current work with alcohol indicates an indirect mechanism which involves the inhibition of GABA function in a particular area of brain.51 This maybe the reason why none of existing anticraving drugs is based on DA hypothesis.47 The clinical pharmaceutic pictures of anticraving drugs are differing from those in antipsychotic and antiparkinsonian drugs that are all based on DA hypothesis57 and DA neurobiology,58 respectively.
Alcohol activates opioid in the dopaminergic circuit of the reward indirectly, and stimulates the release of endogenous opioid peptides possibly at NAc directly.
6.3. GABA neurotransmission
As stated in previous subtitle 6.2, DA transmission per se has not found to have any receptor to be activated by alcohol. The investigators have concentrated on studying two brain major neurotransmission systems—inhibitory GABA and excitatory glutamate neurotransmission systems—which have brought us much closer to identify the main pathways for the addicting characteristics of alcohol.51
After the discovery of benzodiazepines (BZDs), the studies on behavioral similarity of alcohol, BZDs, and GABAA have been increased.51, 59 Alcohol (or meprobamate, or barbiturate) does not compete with the BZDs for activating chloride ion channel, because they do so on different loci although they act on the same receptor (BZD‐GABA‐chloride ionophore receptor complex).3 That is why its effects, in joint administration, are enhanced, as they open up the channel in excess. Therefore, alcohol acts easily through endogenous GABAA, which is regulated by neuroactive steroids. Those neuroactive steroids are potential key modulators in developing alcohol dependence by acting directly at GABAA receptors.51, 60
6.4. Serotonin neurotransmission
Craving is mainly characterized by obsessional thoughts about drugs, causing compulsive drug‐seeking and drug‐taking behavior, therefore, the involvement of brain serotonin in the mechanisms of craving has been proposed.61 Furthermore, the use of a selective serotonin 5‐HT3 antagonist (ondansetron) for patients with early‐onset AUD has been investigated with good results of anticraving benefit.29 The enthusiasm of using serotonergic antidepressants to treat AUD patients came alive62 because the link between serotonin depletion, impulsivity, and alcohol drinking behavior has been observed in rats and humans.63, 64 But the neurobiology of serotonin involvement in anticraving efficacy is not yet clarified.
Antidepressants have been commonly used in all substance abusers due to the potential effect on some underlying mechanisms involved in substance use disorders and to treat comorbid depression. A meta‐analysis was carried out on the studies of the efficacy of antidepressant drugs from randomized, double‐blind, controlled trials in patients with substance use disorders (alcohol, cocaine, nicotine, and opioid), with and without comorbid depression.30 The study result showed that the use of all (including serotonergic) antidepressants do not show any anticraving benefit in AUD patients without depression.30 But a study showed that the G protein‐activated inwardly rectifying potassium (GIRK) channel currents induced by alcohol can be inhibited by sertraline but not by intracellularly applied sertraline, suggesting that GIRK channel inhibition may reveal a novel characteristic of the commonly used antidepressants, particularly sertraline.65 Future attention on serotonin neurotransmission on the role of anticraving benefit should be paid in this aspect.
As stated previously, the recent APA practice guideline does not mention any endorsement for the use of any 5‐HT3 antagonist for anticraving use against alcohol dinking.4 (In fact, ondansetron is only available in intramuscular injection formulary for antiemetic use for cancer patients receiving chemotherapy.) Meanwhile, the APA guideline also does not recommend or suggest for any antidepressant medications for treating AUD patients unless a comorbid disorder exists for which an antidepressant is an indicated treatment.4
6.5. Glutamate neurotransmission
Being an excitatory neurotransmitter, glutamate is an agonist of kainate, NMDA, and AMPA receptors.3 DA transmission system receives excitatory glutamate input at VTA from peduncular pontine tegmentum (PPT) and lateral dorsal tegmentum (LDT) of the amygdala, and at NAc from the cortex of the brain.44, 45
The present data indicate that acamprosate has an extremely weak antagonism of NMDA‐receptors, but its principal anti‐drinking biochemical effect is thought to be from modulating the expression of NMDA‐receptor subunits in specific brain region that is shared with the well‐established NMDA‐antagonists memantine and MK‐801.66 But the investigators suggest the growing importance of NMDA‐receptor plasticity in the AUD research.66
6.5.1. Blockage of GIRK channel
As a postsynaptic transduction system of glutamate receptor, GIRK channel has four subunits.66, 68 Results of cloning study showed that GIRK1, GIRK2, and GIRK3 are distributed in various brain regions (cerebral cortex, amygdala, hippocampus, VTA, locus coeruleus, cerebellum), and GIRK4 is expressed mainly in the heart.66, 68
Investigators found that induced GIRK current is inhibited by ifenprodil using Xenopus oocyte expression assays.66, 69 GIRK2 knockout mice showed reduced alcohol‐induced conditioned taste aversion and conditioned place preference.70 Weaver mutant mice which are missense mutation in channel pore in GIRK2 subunit have been found to have reduced antinociceptive effects of alcohol.71 These studies suggest that ifenprodil is effective for blocking alcohol‐activated GIRK channels.
A recent study showed that combination of pretreated with ifenprodil and cyproheptadine (antagonist of 5‐HT2 receptor) in the mice inhibits alcohol intake and decreases alcohol preference compared to the mice pretreated with saline.72 Furthermore, they also showed the combination of cyproheptadine and prazosin (antagonist of α‐1 adrenergic receptor) also decreases alcohol preference, but separation of administration of these drugs has not found to be effective for alcohol preference. In addition, the antagonist studies show that concurrently activated NMDA and GABA channels each tend to limit the responses of the other.72 These studies suggest that GIRK channel may have a key molecule in alcohol dependence and ifenprodil is one of a candidate drug in medical treatment.
6.6. Summary of mechanisms of action in anticraving drugs
6.6.1. Lists of anticraving drugs
Table 1 lists possible mechanisms implicated for anticraving drugs in treating AUD patients. Expansion of the list is expected if the research in the field is intensified, and the revision is also expected if more new research evidences are accumulated, especially for those anticraving drugs that have been in use to treat nonalcohol other substance use disorders.73, 74
Table 1.
Possible mechanisms implicated for anticraving actions in treating alcohol use disorder patients
| Mechanism of action of anticraving drugs | Applicable drugs |
|---|---|
| Opioid modulation | Naltrexone, Nalmefene |
| Dopamine neurotransmission | Naltrexone, Nalmefene |
| Serotonin neurotransmission | Ondansetron |
| GABA neurotransmission | Gabapentin |
| Glutamate neurotransmission | Acamprosate |
| Blockage of GIRK channel | Ifenprodil |
GABA, γ‐aminobutyric acid; GIRK channel, G protein‐activated inwardly rectifying potassium channel.
6.6.2. Circuitry of brain reward pathway
Nestler44 as well as Gilptin and Koob45 did schematic presentations for brain reward circuitry. Readers should refer to the original graphs for more detailed descriptions. Here, the contents are paraphrased.
DA depending brain reward pathway
From VTA to NAc, DA mesolimbic tract is the main brain reward pathway in carrying alcohol craving/anticraving message. VTA can receive both inhibitory GABAA transmission as interneuron as well as excitatory glutamate transmission from amygdala (PPT and LDT). The entry points for alcohol and opioid to act are at GABAA neurons. Alcohol can possibly activate DA by stimulating the release of opioid peptide at VTA.44, 45
DA independing brain reward
Alcohol and opioid can directly interact on DA projection terminal fibers at NAc to express reward. NAc also receives glutamate input from the brain cortex. Alcohol can also possibly activate DA by stimulating the release of opioid peptide at NAc.44, 45
7. CLINICAL PRACTICE OF PRESCRIBING ANTICRAVING DRUGS FOR TREATING PATIENTS WITH AUD
7.1. Treating AUD patients with anticraving drugs is gratifying
Comparing to only limited armamentarium in treating AUD patients in 1991,1 the author is amazed in writing this current review that several anticraving drugs are available to stop patients’ craving against drinking. My 20‐year clinical experiences in prescribing anticraving drugs have been rewarding.
The author used to prescribe disulfiram (Antabuse®) before the advent of naltrexone in the United States in 1980s and early 1990s in the setting of alcoholism treatment program.75 The disufiram‐medicated patients still have urge to drink alcohol, but they are deterred from drinking because of their fear of developing DER.1, 3 As the stipulation of her employment, a 45‐year‐old female custodian patient at a local elementary school needed to take disulfiram in front of staff in the office of the school principal's office every morning weekdays. She was cut off the chance of alcohol drinking weekdays and the weekend due to her taking disulfiram in the morning weekdays. She did not want to take chance to drink any alcohol because of her fear of having DER. She believed that some of disulfiram was still in her body during the weekend. She was kept to be sober for at least 3 years with disulfiram.
Then, the author stopped prescribing disulfiram in late 1990s when naltrexone (ReVia® or Vivitrol®) became available. I still remember vividly that a naltrexone‐medicated patient of about 50 years of age, a university professor in the United States, could stop all his alcohol drinking completely for the first time in his 6‐month outpatient treatment at my regular psychiatric clinic after the use of naltrexone. He told me and he could forget about alcohol drinking completely, and started to prepare and update his teaching slides of his lecture files for his class at a medical school. In Taiwan, naltrexone had been once unavailable till 2 years ago because only limited prescriptions made the pharmaceutical company to decide not to supply the drug for about 5 years. But naltrexone is currently available (M. C. Huang, personal communication, 2018).
The author has not had the chance to prescribe acamprosate (Campral®). I did prescribe only a couple of patients with gabapentin (Neurontin®) for anticraving use. The patients mentioned sedation and paresthesia as the side effects. They also complained of the cost due to off‐label use.
The author has been offering patients to prescribe topiramate (Topamax®)76 for off‐label use to curb their alcohol drinking if they so desire. The patients have to pay the extra expense of this drug outside of the medication coverage from Taiwan National Insurance Institute due to the lack of official indication in Taiwan. Instead of the dosage used in clinical drug trial of 300 mg/d,22, 23, 24 the dosage of topiramate I have prescribed is 100 mg/d for anticraving therapy.76 But I start with 50 mg for the first night at bedtime. Then, the patient takes 100 mg/d at bedtime from the second day on. I may reduce the dosage to 75 mg nightly if the patient complains of tingling sensation of the arms. I have found that the use of topiramate is more convenient than that of naltrexone because patients’ liver conditions are not a concern. The patient can start to take topiramate on the first day while they are receiving outpatient detoxication with the use of a BZD.3 The patient and his/her family have been warned about the memory problems from using topiramate, BZD, and alcohol.3 All my topiramate medicated‐patients are seen weekly until they are completely sober. Then, they are given monthly prescription for topiramate, which can be combined with any concurrent antidepressants without any concerns of drug‐drug interaction.76, 77, 78 Usually, most of all my patients welcome the use of topiramate because of its superior anticraving benefit to naltrexone.79 What's more, topiramate has good profile in keep the body weight under control.3, 76, 80
7.2. Anticraving drugs can be given at regular outpatient clinics
All the anticraving drugs can be prescribed in regular psychiatric clinic. The prescriptions of anticraving drugs are prescribed in the clinics of family medicine clinics.10, 81 Most of anticraving drugs are available in hospital formulae for indications of other medical conditions. For example, ifenprodil was developed in France and was released from 1979 in Japan as a cerebral circulation/metabolism ameliorator. Original drug is Cerocral that is from Sanofy, France, is sold in Japan. Ifenprodil tartrate is available in France, Korea, Philippines, and Japan.
The urgent and immediate goal is to use an anticraving drug to reduce AUD patients’ craving for alcohol. At this moment, various medications such as naltrexone, topiramate, and gabapentin in the United States, as well as ifenprodil in Japan, are readily available for clinicians to prescribe for those with AUD patients.
Table 2 lists representing anticraving drugs for treating AUD patients. This table is intended to familiarize clinicians with the available drugs for prescription. The more a clinician prescribes for the AUD patients, the more he or she learns about the benefit if the patients are kept sober from drinking.
Table 2.
Representing anticraving drugs for patients with alcohol drinking disorder
| Drug | Drug profile responsible for anticraving properties | Representing human anticraving studies | Other medical use besides anticraving therapy |
|---|---|---|---|
| Naltrexonea | A glutamate agonist, is derived from amino acid, taurine | Weinstein et al (2003)5 | |
| Naltrexonea | A main μ and δ (lesser extent) opioid receptor antagonist |
Volpicelli et al (1992)11 O'Malley et al (1992)12 |
Opioid overdose |
| GHBb | A precursor to GABA, glutamate, and glycine | Addolorato et al (1996)14 | Narcolepsy |
| Nalmefeneb | A μ‐opioid receptor antagonist & κ‐opioid partial agonist |
Gual et al (2013)19 van den Brink et al (2014)20 |
|
| Topiramate | An antagonist for kainate/AMPA, a subtype of the glutamate |
Johnson et al (2003)22 Johnson et al (2004)23 Johnson et al (2007)24 |
Epilepsy Migraine Lennox‐Gastaut syndrome |
| Gabapentin | A drug to facilitate GABA transmission |
Furieri & Nakamura‐Palacio (2007)27 Mason et al (2014)28 |
Epilepsy Nerve pain |
| Ondansetron | A 5‐HT3 reuptake inhibitor | Johnson et al (2000)29 | Antiemetic for cancer patients receiving chemotherapy |
| Ifenprodil | A GIRK channel inhibitor | Sagaya et al (2018)35 | Dizziness in poststroke patients |
The Arab numbers in superscripts denote the reference entries cited in the article.
AMPA receptors, α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid (AMPA) receptors; GABA, gamma‐aminobutyric acid; GHB, gamma‐hydroxybutyate; GIRK channel, G protein‐activated inwardly rectifying potassium channel.
Approved by US Food and Drug Administration for anticraving indication in patients with alcohol use disorder.
Approved by European Medicines Agency for anticraving indication in patients with alcohol use disorder.
8. CONCLUSION
To re‐state, the author is amazed at the availability of various anticraving drugs being described in this review, compared to the drugs to treat AUD patients in 1991,1 Thanks for all diligent researchers to develop all those drugs. But, we need to keep up with the work because all anticraving drugs as “a class of drugs” are still much less than what antipsychotic drugs or antidepressants exist on the market now.
Despite APA practice guideline “recommends” the use of acamprosate and naltrexone, and “suggests” the use of topiramate and gabapentin for AUD patients,4 anticraving drugs are not well‐known and are underused by clinicians. All those drugs mentioned here have ability to curb alcohol drinking to keep patients sober to perform proper societal roles and to be productive citizens. Therefore, we need to write more articles about anticraving drugs,82, 83, 84 to educate clinicians and lay public to promote the awareness of the disease of AUD, treatment and the availability of anticraving drugs. In Japan, the concept of major depressive disorder was popularized as the “cold of the heart” (kokoro no kaza) in 1980s and 1990s, and this promotional language did help the sale of antidepressants.77, 85, 86 Therefore, I think that those articles on anticraving drugs,82, 83, 84 are important to promote the public awareness of AUD and the use of anticraving drugs in Japan.
Let me go to the epigraph, the opening quotation sentence before the introduction section of this article. To agree with Gryphon's sentiments is quite easy, but to understand the “adventure” of the anticraving therapy clinically for AUD patients is essential to explain. The potential benefit of each drug described here in this article is viewed as far‐outweighing the possible side effects of anticraving drugs or the harms of continued use of alcohol. Thus, let's be more adventurous to prescribe an anticraving drug for our next AUD patient!
CONFLICT OF INTEREST
The author declares no potential conflict in writing this review.
DISCLAIMERS
The contents of this review contain information on off‐label use of released licensed medications. The readers need to read package insert on dosage and side effects of each drug mentioned in this review before the prescribing them to patients.
ACKNOWLEDGEMENTS
Chuang C. Chiueh of Taipei Medical University, Taiwan; Francisco López Muñoz of Camilo José Cela University, Spain; Kazutaka Ikeda of Tokyo Metropolitan Institute of Medical Science, Japan; and Ming‐Chyi Huang of Taipei City Psychiatric Center and Taipei Medical University, Taiwan helped in consultative review of an earlier version of the manuscript for the areas of DA, GABAA, GIRK channel, and neurotransmissions in general, respectively. Yukako Nakagami of Department of Psychiatry and Neurology of Kyoto University helped collect related articles in Japanese.
Shen WW. Anticraving therapy for alcohol use disorder: A clinical review. Neuropsychopharmacol Rep. 2018;38:105–116. 10.1002/npr2.12028
REFERENCES
- 1. Shen WW. Pharmacotherapy of alcoholism: the American current status. Keio J Med. 1991;40:9–12. [DOI] [PubMed] [Google Scholar]
- 2. American Psychiatric Association . The diagnostic and statistical manual of mental disorders, fifth edition. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 3. Shen WW. [Clinical psychopharmacology for the 21st century, third edition]. Taipei: Ho‐Chi Publishing Company; 2011. [Google Scholar]
- 4. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175:86–90. [DOI] [PubMed] [Google Scholar]
- 5. Weinstein A, Feldtkeller B, Feeney A, Lingford‐Hughes A, Nutt D. A pilot study on the effects of treatment with acamprosate on craving for alcohol in alcohol‐dependent patients. Addict Biol. 2003;8:229–32. [DOI] [PubMed] [Google Scholar]
- 6. Kiefer F, Jahn H, Tarnaske T, et al. Comparing and combining naltrexone and acamprosate in relapse prevention of alcoholism: a double‐blind, placebo‐controlled study. Arch Gen Psychiatry. 2003;60:92–9. [DOI] [PubMed] [Google Scholar]
- 7. Anton RF, O'Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–17. [DOI] [PubMed] [Google Scholar]
- 8. Morley KC, Teesson M, Reid SC, et al. Naltrexone versus acamprosate in the treatment of alcohol dependence: a multi‐centre, randomized, double‐blind, placebo‐controlled trial. Addiction. 2006;101:1451–62. [DOI] [PubMed] [Google Scholar]
- 9. Tolliver BK, DeSantis SM, Brown DG, Prisciandaro JJ, Brady KT. A randomized, double‐ blind, placebo‐controlled clinical trial of acamprosate in alcohol‐dependent individuals with bipolar disorder: a preliminary report. Bipolar Disord. 2012;14:54–63. [DOI] [PubMed] [Google Scholar]
- 10. Berger L, Fisher M, Brondino M, et al. Efficacy of acamprosate for alcohol dependence in a family medicine setting in the United States: a randomized, double‐blind, placebo‐controlled study. Alcohol Clin Exp Res. 2013;37:668–74. [DOI] [PubMed] [Google Scholar]
- 11. Volpicelli JR, Alterman AI, Hayashida M, O'Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876–80. [DOI] [PubMed] [Google Scholar]
- 12. O'Malley SS, Jaffe AJ, Chang G, et al. Six‐month follow‐up of naltrexone and psychotherapy for alcohol dependence. Arch Gen Psychiatry. 1996;53:217–24. [DOI] [PubMed] [Google Scholar]
- 13. Swift RM, Aston ER. Pharmacotherapy for alcohol use disorder: current and emerging therapies. Harv Rev Psychiatry. 2015;23:122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Addolorato G, Castelli E, Stefanini GF, et al. An open multicentric study evaluating 4‐hydroxybutyric acid sodium salt in the medium‐term treatment of 179 alcohol dependent subjects. Alcohol Alcohol. 1996;31:341–5. [DOI] [PubMed] [Google Scholar]
- 15. Caputo F. Gamma‐hydroxybutyrate (GHB) for the treatment of alcohol dependence: a call for further understanding. Alcohol Alcohol. 2011;46:3. [DOI] [PubMed] [Google Scholar]
- 16. Addolorato G, Caputo F, Capristo E, Stefanini GF, Gasbarrini G. Gamma‐hydroxybutyric acid efficacy, potential abuse, and dependence in the treatment of alcohol addiction. Alcohol. 2000;20:217–22. [DOI] [PubMed] [Google Scholar]
- 17. Soyka M, Lieb M. Recent developments in pharmacotherapy of alcoholism. Pharmacopsychiatry. 2015;48:123–35. [DOI] [PubMed] [Google Scholar]
- 18. Lyon J. More treatments on deck for alcohol use disorder. JAMA. 2017;317:2267–9. [DOI] [PubMed] [Google Scholar]
- 19. Gual A, He Y, Torup L, van den Brink W, Mann K; ESENSE 2 Study Group . A randomised, double‐blind, placebo‐controlled, efficacy study of nalmefene, as‐needed use, in patients with alcohol dependence. Eur Neuropsychopharmacol. 2013;23:1432–42. [DOI] [PubMed] [Google Scholar]
- 20. van den Brink W, Sørensen P, Torup L, Mann K, Gual A; SENSE Study Group . Long‐term efficacy, tolerability and safety of nalmefene as‐needed in patients with alcohol dependence: a 1‐year, randomised controlled study. J Psychopharmacol. 2014;28:733–44. [DOI] [PubMed] [Google Scholar]
- 21. Glauser TA. Topiramate. Epilepsia. 1999;40(Suppl 5):S71–80. [DOI] [PubMed] [Google Scholar]
- 22. Johnson BA, Ait‐Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361:1677–85. [DOI] [PubMed] [Google Scholar]
- 23. Johnson BA, Ait‐Daoud N, Akhtar FZ, Ma JZ. Oral topiramate reduces the consequences of drinking and improves the quality of life of alcohol‐dependent individuals: a randomized controlled trial. Arch Gen Psychiatry. 2004;61:905–12. [DOI] [PubMed] [Google Scholar]
- 24. Johnson BA, Rosenthal N, Capece JA, et al. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298:1641–51. [DOI] [PubMed] [Google Scholar]
- 25. Kampman KM, Pettinati HM, Lynch KG, Spratt K, Wierzbicki MR, O'Brien CP. A double‐blind, placebo‐controlled trial of topiramate for the treatment of comorbid cocaine and alcohol dependence. Drug Alcohol Depend. 2013;133:94–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taylor CP, Gee NS, Su TZ, et al. A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Res. 1998;29:233–49. [DOI] [PubMed] [Google Scholar]
- 27. Furieri FA, Nakamura‐Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double‐blind, placebo‐controlled trial. J Clin Psychiatry. 2007;68:1691–700. [DOI] [PubMed] [Google Scholar]
- 28. Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized controlled trial. JAMA Intern Med. 2014;174:70–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johnson BA, Roache JD, Javors MA, et al. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: a randomized controlled trial. JAMA. 2000;284:963–71. [DOI] [PubMed] [Google Scholar]
- 30. Torrens M, Fonseca F, Mateu G, Farré M. Efficacy of antidepressants in substance use disorders with and without comorbid depression: a systematic review and meta‐analysis. Drug Alcohol Depend. 2005;78:1–22. [DOI] [PubMed] [Google Scholar]
- 31. Wong CJ, Witcher J, Mallinckrodt C, et al. A phase 2, placebo‐controlled study of the opioid receptor antagonist LY2196044 for the treatment of alcohol dependence. Alcohol Clin Exp Res. 2014;38:511–20. [DOI] [PubMed] [Google Scholar]
- 32. Tajima N, Karakas E, Grant T, et al. Activation of NMDA receptors and the mechanism of inhibition by ifenprodil. Nature. 2016;534:63–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Suzuki T, Kato H, Tsuda M, Suzuki H, Misawa M. Effects of the non‐competitive NMDA receptor antagonist ifenprodil on the morphine‐induced place preference in mice. Life Sci. 1999;64:PL151‐6.2. [DOI] [PubMed] [Google Scholar]
- 34. Ogai Y, Hori T, Haraguchi A, Asukai N, Senoo E, Ikeda K. Influence of GIRK channel inhibition on alcohol abstinence and relapse risk in Japanese alcohol‐dependence outpatients. Jpn J Neuropsychopharmcol. 2011;31:95–6. [PubMed] [Google Scholar]
- 35. Sugaya N, Ogai Y, Aikawa Y, et al. A randomized controlled study of the effect of ifenprodil on alcohol use in patients with alcohol dependence. Neuropsychopharmacol Rep. 2018;38:e12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hurt RT, Ebbert JO, Croghan IT, Schroeder DR, Hurt RD, Hays JT. Varenicline for tobacco‐dependence treatment in alcohol‐dependent smokers: a randomized controlled trial. Drug Alcohol Depend. 2018;184:12–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ryan ML, Falk DE, Fertig JB, et al. (Litten RZ.) A phase 2, double‐blind, placebo‐controlled randomized trial assessing the efficacy of ABT‐436, a novel V1b receptor antagonist, for alcohol dependence. Neuropsychopharmacology. 2017;42:1012–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Donoghue K, Rose A, Coulton S, et al. Double‐blind, 12 month follow‐up, placebo‐controlled trial of mifepristone on cognition in alcoholics: the MIFCOG trial protocol. BMC Psychiatry. 2016;16:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brown ES, Todd JP, Hu LT, et al. A Randomized, double‐blind, placebo‐controlled trial of citicoline for cocaine dependence in bipolar I disorder. Am J Psychiatry. 2015;172:1014–21. [DOI] [PubMed] [Google Scholar]
- 40. Rose AK, Jones A. Baclofen: its effectiveness in reducing harmful drinking, craving, and negative mood: a meta‐analysis. Addiction. 2018;113:1396–406. [DOI] [PubMed] [Google Scholar]
- 41. Trigo JM, Martin‐Garcia E, Berrendero F, et al. The endogenous opioid system: a common substrate in drug addiction. Drug Alcohol Depend. 2010;108:183–94. [DOI] [PubMed] [Google Scholar]
- 42. Fujita M, Ide S, Ikeda K. Opioid and nondopamine reward circuitry and state‐dependent mechanisms. Ann N Y Acad Sci. 2018; 10.1111/nyas.13605 (forthcoming). [DOI] [PubMed] [Google Scholar]
- 43. Kieffer BL. C.J. Evans CJ. Opioid receptors: from binding sites to visible molecules in vivo. Neuropharmacology. 2009;56(Suppl 1):205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–9. [DOI] [PubMed] [Google Scholar]
- 45. Gilpin NW, Koob GE. Neurobiology of alcohol dependence. Alcohol Res Health. 2008;31:185–95. [PMC free article] [PubMed] [Google Scholar]
- 46. Roberts AJ, McDonald JS, Heyser CJ, et al. μ‐Opioid receptor knockout mice do not self‐administer alcohol. J Pharmacol Exp Ther. 2000;293:1002–8. [PubMed] [Google Scholar]
- 47. O'Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry. 2005;162:1423–31. [DOI] [PubMed] [Google Scholar]
- 48. Altshuler HL, Phillips PE, Feinhandler DA. Alteration of ethanol self‐administration by naltrexone. Life Sci. 1980;26:679–88. [DOI] [PubMed] [Google Scholar]
- 49. Volkow ND, Wang GJ, Fowler JS, et al. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res. 1996;20:1594–8. [DOI] [PubMed] [Google Scholar]
- 50. Nutt DJ, Lingford‐Hughes A, Erritzoe D, Stokes PR. The dopamine theory of addiction: 40 years of highs and lows. Nat Rev Neurosci. 2015;16:305–12. [DOI] [PubMed] [Google Scholar]
- 51. Tabakoff B, Hoffman PL. The neurobiology of alcohol consumption and alcoholism: an integrative history. Pharmacol Biochem Behav. 2013;113:1–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–8. [DOI] [PubMed] [Google Scholar]
- 53. Ide S, Takahashi T, Takamatsu Y, et al. Distinctroles of opioid and dopamine systems in lateral hypothalamic intracranialself‐stimulation. Int J Neuropsychopharmacol. 2017;20:403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. [DOI] [PubMed] [Google Scholar]
- 56. Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res. 2008;14:169–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr Bull. 2009;35:549–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Burns RS, Chiueh CC, Markey SP, Ebert MH, Jacobowitz DM, Kopin IJ. A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine. PNAS. 1983;80:4546–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lopez‐Munoz F, Alamo C, Garcia‐Garcia P. The discovery of chlordiazepoxide and the clinical introduction of benzodiazepines: half a century of anxiolytic drugs. J Anxiety Disord. 2011;25:554–62. [DOI] [PubMed] [Google Scholar]
- 60. Sanna E, Talani G, Busonero F, et al. Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. J Neurosci. 2004;24:6521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ciccocioppo R. The role of serotonin in craving: from basic research to human studies. Alcohol Alcohol. 1999;34:244–53. [DOI] [PubMed] [Google Scholar]
- 62. Johnson BA. Serotonergic agents and alcoholism treatment: rebirth of serotonin subtype concept — a hypothesis. Alcohol Clin Exp Res. 2000;24:1597–601. [PubMed] [Google Scholar]
- 63. Myers RD, Veale WL. Alcohol preference in the rat: reduction following depletion of brain serotonin. Science. 1968;160:1469–71. [DOI] [PubMed] [Google Scholar]
- 64. Virkkunen M, Linnoila M. Serotonin in early onset, male alcoholics with violent behaviour. Ann Med. 1990;22:327–31. [DOI] [PubMed] [Google Scholar]
- 65. Kobayashi T, Washiyama K, Ikeda K. Inhibition of G protein‐activated inwardly rectifying K+ channels by different classes of antidepressants. PLoS ONE. 2011;6:e28208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rammes G, Mahal B, Putzke J, et al. The anti‐craving compound acamprosate acts as a weak NMDA‐receptor antagonist, but modulates NMDA‐receptor subunit expression similar to memantine and MK‐801. Neuropharmacology. 2001;40:749–60. [DOI] [PubMed] [Google Scholar]
- 67. Kobayashi T, Ikeda K, Ichikawa T, Abe S, Togashi S, Kumanishi T. Molecular cloning of a mouse G‐protein‐activated K+ channel (mGIRK1) and distinct distributions of three GIRK (GIRK1, 2 and 3) mRNAs in mouse brain. Biochem Biophys Res Commun. 1995;208:1166–73. [DOI] [PubMed] [Google Scholar]
- 68. Krapivinsky G, Gordon EA, Wickman K, Velimirović B, Krapivinsky L, Clapham DE. The G‐protein‐gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K+‐ channel proteins. Nature. 1995;374:135–41. [DOI] [PubMed] [Google Scholar]
- 69. Kobayashi T. Ikeda K.G protein‐activated inwardly rectifying potassium channels as potential therapeutic targets. Curr Pharm Des. 2006;12:4513–23. [DOI] [PubMed] [Google Scholar]
- 70. Hill RH, Svensson E, Dewael Y, Grillner S. 5‐HT inhibits N‐type but not L‐type Ca (2+) channels via 5‐HT1A receptors in lamprey spinal neurons. Eur J Neurosci. 2003;18:2919–24. [DOI] [PubMed] [Google Scholar]
- 71. Kobayashi T, Ikeda K, Kojima H, et al. Ethanol opens G‐protein‐activated inwardly rectifying K+ channels. Nat Neurosci. 1999;2:1091–7. [DOI] [PubMed] [Google Scholar]
- 72. Trovero F, David S, Bernard P, Puech A, Bizot JC, Tassin JP. The combination of marketed antagonists of α1b‐adrenergic and 5‐HT2A receptors inhibits behavioral sensitization and preference to alcohol in mice: a promising approach for the treatment of alcohol dependence. PLoS ONE. 2016;11:e0151242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Marszalec W, Aistrup GL, Narahashi T. Ethanol modulation of excitatory and inhibitory synaptic interactions in cultured cortical neurons. Alcohol Clin Exp Res. 1998;22:1516–24. [PubMed] [Google Scholar]
- 74. Lin SH. Pharmacological means of reducing human drug dependence: a selective and narrative review of the clinical literature. Br J Clin Pharmacol. 2014;77:242–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Shen WW, Chavez CE, Huang TD. Which detoxified alcoholic keeps the first clinic appointment? Drug Alcohol Depend. 1983;11:353–8. [DOI] [PubMed] [Google Scholar]
- 76. Chiu YH, Lee TH, Shen WW. Use of low‐dose topiramate in substance use disorder and body weight control. Psychiatry Clin Neurosci. 2007;61:630–3. [DOI] [PubMed] [Google Scholar]
- 77. Shen WW. Antidepressant therapy. Aino J (Osaka). 2016;15:1–13. [Google Scholar]
- 78. Wu‐Chou AI, Liu YL, Shen WW. Genetic polymorphisms of cytochrome P 450 and antidepressants In: Srinivasan V, López‐Muñoz F, De Berardis D, Alamo C, Takahiro A, Kato TA, editors. Melatonin, neuroprotective agents and antidepressant therapy. New Delhi, India: Springer India, 2016; p. 533–43. [Google Scholar]
- 79. Flórez G, Saiz PA, García‐Portilla P, Alvarez S, Nogueiras L, Bobes J. Topiramate for the treatment of alcohol dependence: comparison with naltrexone. Eur Addict Res. 2011;17:29–36. [DOI] [PubMed] [Google Scholar]
- 80. Goh KK, Chen CH, Lu ML. Topiramate mitigates weight gain in antipsychotic‐treated patients with schizophrenia: meta‐analysis of randomised controlled trials. Int J Psychiatry Clin Pract. 2018; 10.1080/13651501.2018.1449864 (forthcoming). [DOI] [PubMed] [Google Scholar]
- 81. Williams SH. Medications for treating alcohol dependence. Am Fam Physician. 2005;72:1776–80. [PubMed] [Google Scholar]
- 82. Sugaya N, Ikeda K. [Roles of GIRK channels in the reward system]. Jpn J Biol Psychiatry. 2011;22:263–7. [Google Scholar]
- 83. Hironaka N. How does alcohol molecule act on the brain. Med Ayumi. 2015;254:944–8. [Google Scholar]
- 84. Takamatsu Y, Ikeda K. Role of GIRK channel in drug dependence. Brain. 2016;21:13–7. [Google Scholar]
- 85. Schulz K. Did antidepressants depress Japan? New York Time Magazine, August 22, 2004.
- 86. Ingrams E. Long‐taboo ‘mood disorder’ is now being seen as the common and crippling disease it is. The Japan Times. July 10, 2005.


