Abstract
Background
Early detection and treatment of cardiometabolic diseases (CMD) in high-risk patients is a promising preventive strategy to anticipate the increasing burden of CMD. The Dutch guideline ‘the prevention consultation’ provides a framework for stepwise CMD risk assessment and detection in primary care. The aim of this study was to assess the outcome of this program in terms of newly diagnosed CMD.
Methods
A cohort study among 30 934 patients, aged 45–70 years without known CMD or CMD risk factors, who were invited for the CMD detection program within 37 general practices. Patients filled out a CMD risk score (step 1), were referred for additional risk profiling in case of high risk (step 2) and received lifestyle advice and (pharmacological) treatment if indicated (step 3). During 1-year follow-up newly diagnosed CMD, prescriptions and abnormal diagnostic tests were assessed.
Results
Twelve thousand seven hundred and thirty-eight patients filled out the risk score of which 865, 6665 and 5208 had a low, intermediate and high CMD risk, respectively. One thousand seven hundred and fifty-five high-risk patients consulted the general practitioner, in 346 of whom a new CMD was diagnosed. In an additional 422 patients a new prescription and/or abnormal diagnostic test were found.
Conclusions
Implementation of the CMD detection program resulted in a new CMD diagnosis in one-fifth of high-risk patients who attended the practice for completion of their risk profile. However, the potential yield of the program could be higher given the considerable number of additional risk factors—such as elevated glucose, blood pressure and cholesterol levels—found, requiring active follow-up and presumably treatment in the future.
Introduction
Cardiometabolic diseases (CMD) defined as cardiovascular disease (CVD), diabetes type 2 (DM2) and chronic kidney disease are the leading cause of death and of a reduced quality of life worldwide.1,2 CMD are causally related to modifiable lifestyle factors such as smoking, physical inactivity, unhealthy diet and overweight3,4 which can be reduced through a healthy lifestyle. About a quarter of the Dutch population smokes and almost half of the people are overweight or obese.5 Due to an increasing prevalence of obesity,5 the related risk factors such as hypertension, dyslipidaemia and an impaired fasting glucose will rise; indivertibly leading to increasing rates of CMD.
Early detection and treatment of CMD risk factors could diminish overall CMD risk and a combined approach targeted at case finding of high-risk individuals with subsequent CMD screening might be an efficient preventive strategy.6 This is supported by the European Society of Cardiology considering targeted systematic risk assessment for men ≥40 and women ≥50 without known CMD risk factors.4
Although programs for systematic CMD risk assessment7–9 have been implemented in several countries, early detection of CMD in Dutch primary care is still non-programmatic and mainly directed at individual case finding.7,10
In 2011, the Dutch College of General Practitioners (DCGPs) developed a clinical practice guideline to provide a framework for structured stepwise CMD risk assessment and detection in primary care (‘the prevention consultation’).11 It focuses on all individuals aged 45–70 without known CMD or CMD risk factors. This stepwise program entails the self-assessment of CMD risk through a risk score (first step) and—in case of high risk—a referral to the practice for further risk profiling (second step) and individualized treatment if indicated (third step). Pilot studies evaluating precursors of this program showed participation rates between 33% and 75% and found a new CMD in about one-fifth of high-risk patients who attended the practice.12–14 As the CMD detection program is not yet widespread implemented, its overall impact is unknown. Therefore, the aim of the present cohort study was to assess the yield of implementing this stepwise CMD detection program in terms of uptake and detection rate of newly diagnosed CMD in 37 general practices across the Netherlands.
Methods
Design
We performed a cohort study within the framework of the INTEGRATE study among 12 738 patients in the Dutch CMD detection program. The INTEGRATE study is a stepped-wedge randomized controlled trial that was conducted in 37 general practices in the Netherlands. The design of the study has been described previously.15 The study was considered by the UMC Utrecht Institutional Review Board and exempted from full ethical assessment.
Participants
Patients, aged 45–70 years without recorded CMD, CMD risk factors or treatment with antihypertensive, lipid-lowering or antidiabetic drugs were invited through a personal letter by their GP in a time frame of 2 years.
The Dutch CMD detection program
The Dutch CMD detection program has a stepwise approach.11 The first step is an online risk score (paper version available), consisting of questions regarding sex, age, smoking status, body mass index (BMI) (increased if ≥25 kg/m2), waist circumference (increased if ≥80 cm for women and ≥94 cm for men) and a family history of premature CVD (age <65 years) and DM2. The risk score incorporates components of the widely accepted FINDRISK score and the SCORE Risk Charts, and is externally validated.6,16–18 On the basis of the risk score, patients are categorized as having low, intermediate or high risk. A high risk is defined as a chance to develop CMD in the next 7 years of ≥23% for men and ≥19% for women.6 Patients with a score below threshold are categorized as having a low risk (no risk factors present) or an intermediate risk (one or several risk factors present). These patients receive tailored lifestyle advice online. In case of high risk, patients are referred to their GP for additional risk profiling (step 2)—including blood pressure measurement and laboratory tests on fasting glucose, total cholesterol, high-density lipoprotein (HDL) and low-density lipoprotein (LDL) levels—and appropriate follow-up treatment (step 3).
Outcome variables
The primary outcome was newly diagnosed International Classification of Primary Care (ICPC)-coded CMD recorded in the electronic health record (EHR) (see table 1) in high-risk patients who completed the two-step risk assessment.
Table 1.
CMD, prescriptions, abnormal diagnostic test results
| ICPC-codes of CMD: |
| K74: angina pectoris |
| K75: acute myocardial infarction |
| K76: other chronic ischemic heart disease |
| K77: heart failure |
| K86: uncomplicated hypertension |
| K87: hypertension with secondary organ damage |
| K89: transient cerebral ischemia |
| K90: stroke/cerebrovascular accident |
| K91: atherosclerosis |
| K92: peripheral vascular diseases |
| T90: diabetes mellitus |
| T93: lipid metabolism disorder |
| ATC clusters of prescriptions: |
| A10: antidiabetic drugs |
| C02–03, C07–C09: antihypertensive drugs |
| C10: lipid-lowering drugs |
| Abnormal diagnostic test results: |
| Blood pressure ≥140/90 mmHg |
| Total cholesterol/HDL ratio ≥5–8 |
| Total cholesterol ≥8 mmol/l or total cholesterol/HDL ratio ≥8 |
| Fasting glucose ≥6–7 mmol/l (prediabetes) |
| Fasting glucose ≥7 mmol/l |
CMD, cardiometabolic disease; ICPC, International Classification of Primary Care; ATC, Anatomical Therapeutic Chemical Classification System.
Secondary outcomes were (i) new prescriptions of antihypertensive, lipid-lowering or antidiabetic drugs without a CMD diagnosis during 1 year follow-up; (ii) abnormal diagnostic test results reported during the first GP visit [blood pressure ≥140/90 mmHg, total cholesterol/HDL ratio ≥5–8, total cholesterol level ≥8 mmol/l and/or total cholesterol/HDL ratio ≥8, fasting glucose ≥6-7 mmol/l (prediabetes) or fasting glucose levels ≥7 mmol/l] without a CMD diagnosis or prescription and (iii) newly diagnosed ICPC-coded CMD and new prescriptions in patients with a risk score below threshold.
Measurements
All patients completed the risk score and filled out additional online questionnaires at baseline and 1-year follow-up including topics on demographic characteristics and CMD risk factors. Measurements have been described in detail elsewhere.15
Data collection
Baseline data on CMD risk factors (sex, age, smoking status, BMI, waist circumference and a family history of premature CVD and DM2) were derived from the CMD risk score.
For high-risk patients who attended the practice—as confirmed in the EHR, case report forms or self-report—we collected data on newly diagnosed ICPC-coded CMD and prescriptions of antihypertensive, lipid-lowering and antidiabetic drugs during 1-year follow-up (see table 1). In addition, we collected data on abnormal diagnostic test results during the first GP visit (see table 1). Abnormal diagnostic test results were defined according to thresholds for hypertension and impaired fasting glucose levels and treatment thresholds for hypercholesterolemia in Dutch and/or European guidelines.4,19,20 For low- and intermediate-risk patients, we collected data on newly diagnosed ICPC-coded CMD and new prescriptions from the EHR during 1-year follow-up.
Analysis
Demographic characteristics and CMD risk factors were tabulated for all patients.
The yield of the program was based on the number of high-risk patients (i) who attended general practice and (ii) were identified with a new ICPC-coded CMD diagnosis during 1-year follow-up.
We calculated the number needed to screen (NNS) as the inverse of the proportion of high-risk patients with a new CMD diagnosis to all invitees.
In order to estimate the potential additional yield of the program, we examined the number of new prescriptions without a CMD diagnosis during 1-year follow-up and abnormal diagnostic test results reported during the first GP visit without a CMD diagnosis or prescription recorded in the EHR.
For the low- and intermediate-risk groups, we tabulated newly diagnosed ICPC-coded CMD and new prescriptions recorded during 1-year follow-up. Analyses were performed using STATA version 15.
Results
Participants
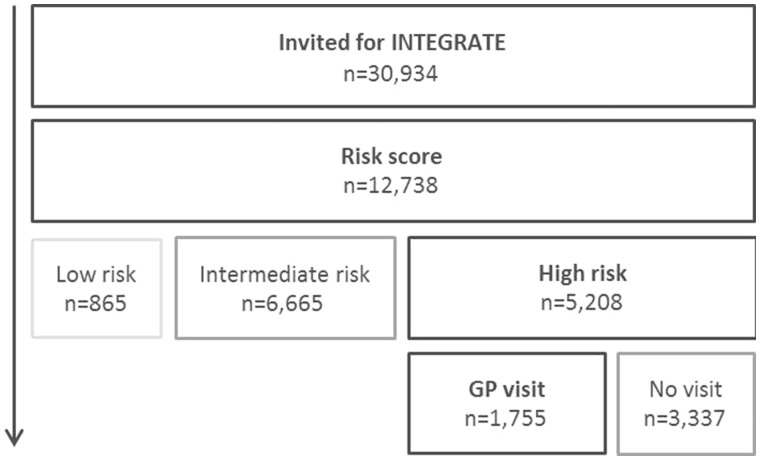
In total 30 934 eligible patients were approached, of whom 12 738 (41%) consented to participate and completed the risk score as first step of the program. Of those 67% was below the age of 60 years, and 54% were female (5-year age categories displayed in table 2). Of those who completed the risk score 7% (n = 865) was categorized as having a low CMD risk, 52% (n = 6665) as having an intermediate risk and 41% (n = 5208) as having a high risk. Detailed description of CMD risk factors per risk category is summarized in table 2.
Table 2.
Baseline characteristics
| Risk category | |||||
|---|---|---|---|---|---|
| Low | Intermediate | High | Total group | ||
| N = 865 | N = 6665 | N = 5208 | N = 12 738 | ||
| Demographics | |||||
| Sex (%) | |||||
| Female | 39.4 | 58.7 | 49.4 | 53.6 | |
| Male | 60.6 | 41.3 | 50.6 | 46.4 | |
| Age (5-year categories) (%) | |||||
| 45–49 years | 35.8 | 36.3 | 1.6 | 22.1 | |
| 50–54 years | 36.8 | 37.2 | 6.5 | 24.6 | |
| 55–59 years | 26.2 | 23.0 | 17.1 | 20.8 | |
| 60–64 years | 1.2 | 3.5 | 36.1 | 16.7 | |
| 65+ years | – | – | 38.8 | 15.9 | |
| CMD risk factors | |||||
| Positive CVD family history (%) | 0 | 29.0 | 36.0 | 29.9 | |
| Positive DM2 family history (%) | 0 | 17.9 | 21.5 | 18.1 | |
| Current smoker (%) | 0 | 9.3 | 21.6 | 13.7 | |
| BMI (categories) (%) | |||||
| <25 kg/m2 | 100 | 57.7 | 45.4 | 55.5 | |
| 25–30 kg/m2 | – | 37.2 | 41.5 | 36.5 | |
| >30 kg/m2 | – | 5.1 | 13.1 | 8.0 | |
| Waist circumference (categories) (%) | |||||
| Women | <80 cm | 98.8 | 9.5 | 5.7 | 12.6 |
| 80–88 cm | 0.3 | 32.6 | 15.4 | 24.5 | |
| >88 cm | 0.9 | 57.9 | 79.0 | 63.0 | |
| Men | <94 cm | 100 | 20.3 | 20.2 | 27.3 |
| >94 cm | – | 79.8 | 79.8 | 72.7 | |
| Additional CMD risk factors of high-risk participants who consulted their GP [mean (SD)] | |||||
| N = 1755 | |||||
| Systolic blood pressure in mmHg (n = 1477) | 134.4 (17.6) | ||||
| Diastolic blood pressure in mmHg (n = 1461) | 79.9 (9.8) | ||||
| Total cholesterol in mmol/l (n = 1411) | 5.8 (1.0) | ||||
| Total cholesterol/HDL ratio (n = 1407) | 3.9 (1.1) | ||||
| LDL in mmol/l (n = 1334) | 3.7 (0.9) | ||||
| Fasting glucose in mmol/l (n = 1283) | 5.4 (0.9) | ||||
| SCORE Risk Chartsa (%) (n = 1285) | 3.1 (2.6) | ||||
Note: Total of percentages may not equal 100% due to rounding.
a10 years CVD mortality risk, The Netherlands is considered as a ‘low-risk’ country.17
CMD, cardiometabolic disease; CVD, cardiovascular disease; DM2, diabetes mellitus type 2; BMI, body mass index; GP, general practitioner; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Of the 5208 high-risk patients, 1755 (34%) consulted their GP (see figure 1). These patients had a mean systolic blood pressure of 134.4 (SD 17.6) mmHg, a total cholesterol/HDL ratio of 3.9 (SD 1.1), LDL of 3.7 (SD 0.9) mmol/l and a fasting glucose of 5.4 (SD 0.9) mmol/l. Their mean 10 years CVD mortality risk (SCORE Risk Charts) was 3.1% (SD 2.6) (table 2).
Figure 1.
Flowchart of participants
Detection rate of the program
EHR data were available for 12 393 (97%) patients. Table 3 shows that in about one in five at least one CMD (19.7%) was newly diagnosed. In total, 9.2% was diagnosed with hypertension, 9.6% with hypercholesterolemia and 1.6% with diabetes. In addition, we found new prescriptions for antihypertensive and lipid-lowering drugs in absence of an EHR recorded CMD diagnosis in 1.3% and 1.4% of the patients, respectively. No antidiabetic prescriptions were found without a DM2 diagnosis. In an additional 21.9% of patients in whom no CMD diagnosis or prescription was recorded, we found abnormal diagnostic test results for CMD; elevated blood pressure (≥140/90 mmHg) in 18.1%, abnormal cholesterol levels (total cholesterol/HDL ratio ≥5 or total cholesterol ≥8 mmol/l) in 8.4% and an increased fasting glucose level (≥6 mmol/l) in 22.2%. In 43.8.% of patients, either a new CMD diagnosis, a new prescription or an abnormal diagnostic test result was found.
Table 3.
Detection rate and potential yield of stepwise CMD risk assessment
| High-risk category | NNS | |
|---|---|---|
| GP visit | ||
| N = 1755 | N = 30 934 | |
| Newly diagnosed: % (n) | ||
| Hypertensiona | 9.2 (n = 161) | |
| Hypercholesterolemiab | 9.6 (n = 169) | |
| Diabetes mellitusc | 1.6 (n = 28) | |
| Newly prescribed without recorded diagnosis: % (n) | ||
| Antihypertensivesd | 1.3 (n = 23) | |
| Lipid-lowering drugse | 1.4 (n = 25) | |
| Antidiabeticsf | 0 (n = 0) | |
| Abnormal diagnostic test without recorded diagnosis or prescription: % (n) | ||
| Blood pressure ≥140/90 mmHg | 18.1 (n = 318) | |
| Total cholesterol/HDL ratio ≥5–8 | 8.0 (n = 140) | |
| Total cholesterol ≥8 mmol/l or total cholesterol/HDL ratio ≥8 | 0.4 (n = 7) | |
| Fasting glucose ≥6–7 mmol/l (prediabetes) | 21.9 (n = 385) | |
| Fasting glucose ≥7 mmol/l | 0.3 (n = 5) | |
| Newly diagnosed CMD, newly prescribed or abnormal diagnostic test result % (n) | ||
| No. of participants with newly diagnosed CMDg | 19.7 (n = 346) | 89 |
| No. of participants with newly diagnosed CMD or prescriptionh | 21.9 (n = 385) | 80 |
| No. of participants with new CMD, prescription or abnormal diagnostic test | 43.8 (n = 768) | 40 |
aICPC codes: K86/K87.
bICPC code: T93.
cICPC code: T90.
dATC cluster: C02–03, C07–C09.
eATC cluster: C10.
fATC cluster: A10.
gICPC codes: K74, K75, K76, K77, K86, K87, K89, K90, K91, K92, T90 and T93.
hICPC codes + ATC cluster: A10 and C02–03, C07–C10.
CMD, cardiometabolic disease; GP, general practitioner; NNS, number needed to screen; ICPC, International Classification of Primary Care; ATC, Anatomical Therapeutic Chemical Classification System.
Number needed to screen
The calculated NNS among all invitees (n = 30 934) to find a newly confirmed CMD diagnosis was 89 (table 3). Although a detailed and thorough cost-effectiveness analysis is required, a first estimation demonstrates that costs per newly diagnosed individual with CMD would be €489. For this estimation, direct medical costs were taken into account: €2 per patient for invitation, €40 per high-risk patient who attended the general practice (two standard consultations and laboratory costs) and an estimated €1000 per practice for implementation (15–20 h of time investment at €50/h). Taking a broader definition of new CMD (confirmed diagnosis, prescription or an abnormal diagnostic test result), the NNS would decrease to 40.
Newly diagnosed CMD in low- and intermediate-risk categories
A new ICPC-coded CMD diagnosis was found in 1.6% of patients with a low risk and in 4.3% of patients with an intermediate risk (Supplementary table S3).
Discussion
Summary of results
Implementation of a structured stepwise CMD detection program in general practice results in a participation rate of 41%, and new diagnosis of CMD in 20% of the high-risk-patients (NNS 89). Over 40% of patients required active follow-up, receiving either a new diagnosis, a new prescription or had an abnormal diagnostic test result during their GP visit. In low- and intermediate-risk categories, small numbers of new CMD diagnoses were found (2% and 4%, respectively).
Strengths and limitations
This is the first large study evaluating the uptake and detection rate of the Dutch CMD detection program in a real-life clinical setting. The roll-out of the ‘prevention consultation’ was coordinated and implemented by the local staff of each practice. This resulted in a pragmatic and feasible implementation in each practice. With this approach, we have tackled some earlier identified challenges such as good preparation of involved staff and the integration of the program within everyday practice.21 Another strength was that we were able to collect the EHR data of 97% of the patients, instead of the anticipated 90%.15 The small number of missing data (3%) was equally distributed among patients of different risk categories and therefore we assume these data were missing at random and did not influence our results.
The risk score we used was recently externally validated among 3544 patients of the Australian Diabetes, Obesity and Lifestyle Study, showing robust discriminative performance across populations, though recalibration was recommended to account for disease incidence per region.6,18
However, some limitations should be considered.
Due to the stepwise nature of the program, we anticipated non-response.15 This was 59% on the initial invitation and 66% on the second step of the risk assessment. In case of non-response, we did sent reminders after 2 weeks as recommended in the guideline. The response and accompanying detection rate of the program may have been larger if we had incorporated more labour-intensive strategies for enhancing the response (e.g. telephone reminders or reminders by email)14,22
Another limitation was that our primary outcome was based on ICPC-coded diagnoses in the EHR. Under-registration may have differed between professionals and practices. However, even if under-registration did play a role, this would have resulted in an underestimation of the total estimated yield.
Interpretation of results and comparison with existing literature
We found a new CMD diagnosis in 20% of high-risk patients attending general practice. This is comparable with the results of previous Dutch pilot studies.12,13 A population-based cohort study estimating the yield of the UK NHS health check identified 18.4% active smokers, 22.7% obese patients (BMI ≥30 kg/m2), 30.1% patients with blood pressure levels ≥140/90 mmHg and 66.1% with total cholesterol levels ≥5 mmol/l.23 However, it is hard to compare our results with those from international equivalents, since variable selection criteria for participation in structured CMD risk assessment are used in different countries.7,9,24 For example, the NHS health check targets all patients 40–75 without known CMD or CMD risk factors for complete screening and does not use a stepwise approach.25
A remarkable result is that we found abnormal diagnostic test results recorded in an additional 22% of the high-risk patients who attended general practice, without a CMD diagnosis or prescription recorded in the EHR. In some patients (e.g. with a total cholesterol ≥8 mmol/l, a total cholesterol/HDL ratio ≥8 or fasting glucose levels ≥7 mmol/l), these abnormal diagnostic test results may reflect under-registration of a diagnosis. However single abnormal test results do not always implicate the presence of CMD. For example, in case of high blood pressure, they may reflect a ‘white coat’ effect or a transient deviation of the norm due to stress or temporary illnesses. In addition, single abnormal test results do not always require treatment, because treatment indications are frequently based on the overall CMD risk instead of single risk factors.4,19 Nevertheless, abnormal diagnostic test results often require active follow-up and one could argue that at least a part of these individuals will develop CMD in the (near) future. For example, it is estimated that one- to two-third of those with prediabetes (fasting glucose between 6 and 7 mmol/l) will develop diabetes within 6 years.26 Moreover, impaired fasting glucose levels are associated with an increased risk for CMD.20 Taking this into account, the program has the potential to identify additional patients who are likely to develop CMD in the future.
Implications for research and practice
Stepwise screening methods—such as in the Dutch CMD detection program—are preferred, selecting people at high risk—who are likely to benefit most from interventions—reducing the number of people that needs to be screened.27 In addition, previous studies have shown that this stepwise program is positively evaluated by GPs and patients.28,29 To further optimize acceptance, compliance and participation rates of the program, additional analyses of non-response and response-enhancing strategies are warranted.
The cost-effectiveness of CMD detection programs has not yet been established;24,30 however, prevention of CMD either by lifestyle changes or medication is considered cost-effective in many scenario’s.4 Future economic evaluation of this program will add to the evidence on this topic.15 It is important to establish the cost-effectiveness in order to justify and create wider acceptance for large-scale implementation of stepwise CMD detection programs in primary care.
Conclusion
The Dutch CMD detection program proved adequate in identifying high-risk patients in general practice, and resulted in the detection of a newly diagnosed CMD in one-fifth of patients. The future yield of this program is expected to be higher given the considerable amount of additional risk factors found, such as prediabetes and elevated blood pressure and cholesterol levels, requiring active follow-up and presumably treatment in the (near) future.
Supplementary data
Supplementary data are available at EURPUB online.
Funding
This work was supported by ZonMW (The Netherlands Organization for Health Research and Development) under Grant No 50-51515-98-192; Lekker Lang Leven (a collaboration of the Dutch Diabetes Research Foundation, the Dutch Heart Foundation and the Dutch Kidney Foundation) under Grant No 2012.20.1595 and Innovatiefonds Zorgverzekeraars (Healthcare Insurance Innovation Fund) under Grant No 2582.
Conflicts of interest: None declared.
Key points
Early detection and treatment of CMD in high-risk patients is a promising preventive strategy and recommended by European guidelines
The Dutch CMD detection program adequately identifies high-risk patients in general practice and detects a new CMD diagnosis in one-fifth of patients
The future yield of this program is expected to be higher given the considerable amount of additional risk factors found, requiring active follow-up and presumably treatment in the future
Supplementary Material
References
- 1.World Health Organization. World health statistics 2018: monitoring health for the SDGs, sustainable development goals. 2018. Available at: https://www.who.int/gho/publications/world_health_statistics/2018/en/ (22 October 2019, date last accessed).
- 2.Volksgezondheid en Zorg. Netherlands: Available at: https://www.volksgezondheidenzorg.info/onderwerp/hart-en-vaatziekten/cijfers-context/ziektelast (22 October 2019, date last accessed).
- 3. Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937–52. [DOI] [PubMed] [Google Scholar]
- 4. Piepoli MF, Hoes AW, Agewall S, et al. European Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2016;37:2315–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volksgezondheid en Zorg. Netherlands. Available at: https://www.volksgezondheidenzorg.info/onderwerp/overgewicht/cijfers-context/trends#node-trend-overgewicht-volwassenen (22 October 2019, date last accessed).
- 6. Alssema M, Newson RS, Bakker SJL, et al. One risk assessment tool for cardiovascular disease, type 2 diabetes, and chronic kidney disease. Diabetes Care 2012;35:741–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Waard A-KM. Selective cardiometabolic prevention programmes across Europe; neither “one size fits all” nor “sine qua non”. In: A-KM De Waard, editor. Towards Successful Selective Prevention of Cardiometabolic Diseases in Primary Care Challenges across Europe. Utrecht: Ipskamp, 2018: 21–40. ISBN 978-94-028-1223-7. [Google Scholar]
- 8. Robson J, Dostal I, Sheikh A, et al. The NHS Health Check in England: an evaluation of the first 4 years. BMJ Open 2016;6:e008840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hooper C, Hardie-Boys N, White E, et al. More heart and diabetes checks evaluation –final report. Allen + Clarke, New Zealand, 2016. Available at: https://www.health.govt.nz/publication/more-heart-and-diabetes-checks-evaluation (22 October 2019, date last accessed).
- 10. Hollander M, Stol D, Badenbroek I, et al. De impasse van het cardiometabool preventieconsult (Impasse of Dutch cardiometabolic prevention). Huisarts Wet 2014;57:290–1. [Google Scholar]
- 11. Dekker J, Alssema M, Janssen P, et al. NHG-Standaard Het PreventieConsult module Cardiometabool NHG-Standaard (Guideline for cardiometabolic prevention by Dutch college of GPs). Huisarts Wet 2011;54:138–55. [Google Scholar]
- 12. Van Der Meer V, Nielen MMJ, Drenthen AJM, et al. Cardiometabolic prevention consultation in the Netherlands: screening uptake and detection of cardiometabolic risk factors and diseases—a pilot study. BMC Fam Pract 2013;14:29.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van de Kerkhof RM, Spigt MG, Knottnerus JA, et al. Development, implementation and yield of a cardiometabolic health check. Fam Pract 2011;29:174–81. [DOI] [PubMed] [Google Scholar]
- 14. Klomp M. PreventieConsult in praktijk: een pilot. Med Contact (Bussum) 2011;659–61. [Google Scholar]
- 15. Badenbroek IF, Stol DM, Nielen MM, et al. Design of the INTEGRATE study: effectiveness and cost-effectiveness of a cardiometabolic risk assessment and treatment program integrated in primary care. BMC Fam Pract 2014;15(1):1–10. Erratum in BMC Fam Pract 2016;17(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lindström J, Tuomilehto J. The Diabetes Risk Score: a practical tool to predict type 2 diabetes risk. Diabetes Care 2003;26:725–31. [DOI] [PubMed] [Google Scholar]
- 17. Conroy R, Pyörälä K, Fitzgerald A, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J 2003;24:987–1003. [DOI] [PubMed] [Google Scholar]
- 18. Rauh SP, Rutters F, van der Heijden A, et al. External validation of a tool predicting 7-year risk of developing cardiovascular disease, type 2 diabetes or chronic kidney disease. J Gen Intern Med 2018;33:182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. NHG-Standaard Cardiovasculair risicomanagement (eerste herziening) (Guideline for cardiovascular risk management by Dutch college of GPs). Huisarts Wet 2012;55:14–28. [Google Scholar]
- 20. Rutten G, De Grauw W, Nijpels G, et al. NHG-Standard Diabetes mellitus type 2 (derde herziening) (Guideline for diabetes type 2 by Dutch college of GPs). Huisarts Wet 2013;56:512–25. [Google Scholar]
- 21. Godefrooij M, Spigt M, van der Minne W, et al. Implementing cardiometabolic health checks in general practice: a qualitative process evaluation. BMC Fam Pract 2014;15:132.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Groenenberg I, Crone MR, Van Dijk S, et al. Response and participation of underserved populations after a three-step invitation strategy for a cardiometabolic health check chronic disease epidemiology. BMC Public Health 2015;15:854.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Forster AS, Dodhia H, Booth H, et al. Estimating the yield of NHS Health Checks in England: a population-based cohort study. J Public Health 2015;37:234–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dyakova M, Shantikumar S, Colquitt JL, et al. Systematic versus opportunistic risk assessment for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2016;29:CD010411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Putting Prevention First. Best Practice Guidance. London: Department of Health, NHS, 2009. [Google Scholar]
- 26. De Vegt F, Dekker JM, Jager A, et al. Relation of impaired fasting and postload glucose with incident type 2 diabetes in a Dutch population the Hoorn Study. JAMA 2001;285:2109–13. [DOI] [PubMed] [Google Scholar]
- 27. Den Engelsen C, Koekkoek PS, Godefrooij MB, et al. Screening for increased cardiometabolic risk in primary care: a systematic review. Br J Gen Pract 2014;64:e616–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nielen MMJ, van der Meer V, Schellevis FG. Evaluatie Pilot PreventieConsult Cardiometabool Risico (Pilot Study of a Dutch Prevention Program for Cardiometabolic Disease). Utrecht: NIVEL, 2010. [Google Scholar]
- 29. Vos HMM, Van Delft D, De Kleijn MJJ, et al. Selective prevention of cardiometabolic diseases in general practice: attitudes and working methods of male and female general practitioners before and after the introduction of the Prevention Consultation guideline in the Netherlands. J Eval Clin Pract 2014;20:478–85. [DOI] [PubMed] [Google Scholar]
- 30. Hiligsmann M, Wyers CE, Mayer S, et al. A systematic review of economic evaluations of screening programmes for cardiometabolic diseases. Eur J Public Health 2017;27:621–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.