Abstract
Axonal damage leads to the release of neurofilament light chain (NFL), which enters the CSF or blood. In this work, an assay kit for plasma NFL utilizing immunomagnetic reduction (IMR) was developed. Antibodies against NFL were immobilized on magnetic nanoparticles to develop an IMR NFL kit. The preclinical properties, such as the standard curve, limit of detection (LoD), and dynamic range, were characterized. Thirty-one normal controls (NC), fifty-two patients with Parkinson’s disease (PD) or PD dementia (PDD) and thirty-one patients with Alzheimer’s disease (AD) were enrolled in the study evaluating the plasma NFL assay using an IMR kit. T-tests and receiver operating characteristic (ROC) curve analysis were performed to investigate the capability for discrimination among the clinical groups according to plasma NFL levels. The LoD of the NFL assay using the IMR kit was found to be 0.18 fg/ml. The dynamic range of the NFL assay reached 1000 pg/ml. The NC group showed a plasma NFL level of 7.70 ± 4.00 pg/ml, which is significantly lower than that of the PD/PDD (15.85 ± 7.82 pg/ml, p < 0.001) and AD (19.24 ± 8.99 pg/ml, p < 0.001) groups. A significant difference in plasma NFL levels was determined between the PD and AD groups (p < 0.01). Through ROC curve analysis, the cut-off value of the plasma NFL concentration for differentiating NCs from dementia patients (AD and PD/PDD) was found to be 12.71 pg/ml, with a clinical sensitivity and specificity of 73.5% and 90.3%, respectively. The AUC was 0.868. Furthermore, the cut-off value of the plasma NFL concentration for discriminating AD from PD/PDD was found to be 18.02 pg/ml, with a clinical sensitivity and specificity of 61.3% and 65.4%, respectively. The AUC was 0.630. An ultrasensitive assay for measuring plasma NFL utilizing IMR technology was developed. Clear differences in plasma NFL concentrations were observed among NCs and PD and AD patients. These results imply that the determination of plasma NFL is promising not only for screening dementia but also for differential diagnosis.
Introduction
Neurofilament proteins are major constituents of the neuronal cytoskeleton. There are three subunits of neurofilament proteins: the light (NFL), the medium (NFM) and the heavy (NFH) chains. NFL is a putative biomarker of subcortical large-caliber axonal damage. Elevation of NFL levels in body fluid is relevant to not only brain atrophy but also brain diseases [1]. For example, for subjects carrying the MAPT, GRN, or C90rf72 genotypes, the concentration of NFL in cerebrospinal fluid (CSF) was increased after the onset of frontotemporal dementia (FTD) [2]. Thus, the level of NFL in CSF is a promising biomarker for diagnosing semantic dementia [3]. In addition to FTD, CSF NFL was validated as a screening biomarker for inflammatory disease [4], Creutzfeldt-Jakob disease (CJD) [5], acute neuronal ischemia [6], Alzheimer’s disease (AD) [1,7], Parkinson’s disease (PD) [8], multiple sclerosis (MS) [9] and traumatic brain injury [10,11].
CSF NFL has been validated to be useful for differentiating various types of neurodegenerative diseases. For example, CSF NFL levels were higher in patients with FTD than in early-onset Alzheimer’s disease patients [12]. CSF NFL was proposed as a biomarker to discriminate CJD from AD, FTD or DLB and to differentiate atypical cases from typical patients for CJD and FTD [13]. Therefore, CSF NFL may have an impact on clinical applications for improving diagnostic accuracy for dementia.
Lumbar puncture is necessary for sampling CSF. There are many uncomfortable side effects of lumbar puncture, so it is not easy to perform the CSF NFL assay in clinical practice. Instead of CSF, blood may serve as a promising alternative. However, the concentrations of NFL in blood are extremely low and are hardly detectable using traditional assays. With the development of ultrasensitive assay technologies [14,15], it has become possible to precisely assay NFL in human plasma. Previously reported results show a significant correlation between plasma and CSF NFL concentrations (ρ > 0.5, p < 0.001) [16–18]. This demonstrates the possibility of evaluating dementia by measuring plasma NFL rather than CSF NFL.
Several independent studies have revealed that the levels of plasma NFL are significantly elevated in patients with mild cognitive impairment (MCI) or AD compared to those in normal controls (NC) [18–20]. The AUC (area under receiver operating characteristic curve) value that discriminates MCI/AD from NC according to plasma NFL is higher than 0.8. Increased NFL levels in plasma were associated with reduced Mini-Mental State Examination (MMSE) (r < -0.3, p < 0.001) [18–20], hippocampal volume, and thickness in cortex regions in AD [18]. Higher levels of plasma NFL at baseline predicted accelerated declines in these measurements [17,18]. However, plasma NFL levels are not able to predict conversion to AD in MCI [18,19].
A recently published paper shows the feasibility of differentiating PD from NC according to plasma NFL (AUC > 0.7) [21]. PD patients at advanced Hoehn-Yohr stages had increased levels of plasma NFL (p < 0.001). Detailed analysis shows that the plasma NFL concentration is modestly correlated with the UPDRS part III (r = 0.42, p < 0.001). A longitudinal study revealed that plasma NFL was able to predict declines in movement and cognition in PD (p < 0.05) [21]. Furthermore, the level of plasma NFL could discriminate atypical Parkinsonism syndrome from typical PD (AUC > 0.8) [16,21].
These published results demonstrate the possible clinical impacts of the diagnosis of AD or PD using plasma NFL. However, to be approved as a regular medical device for clinical use, the preclinical performance of reagents used for assaying NFL must be characterized according to guidelines issued by Clinical and Laboratory Standards Institute (CLSI). Such characterizations have been truthfully reported for the NFL reagents used in published papers. In this work, the preclinical performance of an NFL reagent used with immunomagnetic reduction is explored. All measurements were conducted according to CLSI guidelines EP5- A3, EP7-A2, EP17-A2, and C28-A2.
Materials and methods
Synthesis of NFL reagent
In the IMR assay, the reagent consisted of antibody-functionalized Fe3O4 magnetic nanoparticles dispersed in phosphate-buffered saline (PBS) (1x). To achieve the specific association between magnetic nanoparticles and NFL, antibodies (sc20011, Santa Cruz) against NFL were covalently bound to dextran, which acted as an interface between the antibody and the Fe3O4 cores of the nanoparticles. A detailed description of the binding of the antibody to dextran is given in [22]. The mean diameter of the magnetic nanoparticles was 53 nm, as measured by laser dynamic scattering. The concentration of the NFL reagent was 10 mg Fe/ml. The reagent was stored at 4 °C.
IMR measurement
A mixture of 60 μl of NFL reagent and 60 μl of sample was used for the IMR measurement. A superconducting quantum interference device-based alternative-current magnetic susceptometer (XacPro-S, MagQu) was used to detect the IMR signal of a sample [23–26]. During detection of the IMR signal of a sample, two control solutions were used. One was blank, i.e., PBS solution, and the other contained 50 pg/ml NFL (Ab224840, Abcam) in PBS solution.
Exploration of preclinical performance via assay of NFL
All experiments were designed and conducted according to the global standards EP5- A3, EP7-A2, EP17-A2, and C28-A2 described by the Clinical & Laboratory Standards Institute (CLSI). Thus, the standard curve, detection limit, assay linearity, dilution recovery range, assay reproducibility, spike recovery, reagent stability, and interference tests were investigated.
Enrollment of subjects
Subjects were enrolled at National Taiwan University Hospital and Kaohsiung Chang Gung Memorial Hospital with the approval of the ethics committees of both hospitals. Every participant provided written informed consent. Enrolled subjects were categorized into groups of normal controls (NC) and patients with Parkinson’s disease (PD), or PD dementia (PDD), and Alzheimer’s disease (AD) according to the inclusion and exclusion criteria listed in Table 1. PD, PDD and AD were combined as patient group. All experimental protocols with human samples were approved by the ethics committees at both hospitals.
Table 1. Exclusion and inclusion criteria used for recruiting normal controls (NC) and subjects with Alzheimer’s disease (AD) and Parkinson’s disease in this study.
| Group | Inclusion criteria | Exclusion criteria |
|---|---|---|
| NC | 1. Education: at least primary school 2. Age > 50 years 3. Body weight ≥ 40 kg 4. CDR* = 0 5. MMSE++ ≥ 26 |
1. Subjects with cranial metallic implants, cardiac pacemakers or claustrophobia. 2. Previous diagnosis of MCI or dementia 3. Significant history of depression 4. Geriatric Depression Scale > 8 |
| PD/PDD | 1. Subjects must have symptoms of bradykinesia and at least one of the following: muscular rigidity, resting tremor (4–6 Hz), or postural instability unrelated to primary visual, cerebellar, vestibular or proprioceptive dysfunction [27]. 2. Three or more of the following symptoms: unilateral onset, resting tremor, progressive disorder, persistent asymmetry most affecting the side of onset, excellent response to levodopa, severe levodopa-induced chorea, levodopa response for over 5 years, and clinical course of over 10 years. 3. MOCA# score greater than 26 for PD with normal cognition 4. MOCA score less than 21 for PD with dementia |
1. Significant history of depression 2. History of repeated strokes with stepwise progression, repeated head injury, antipsychotic or dopamine-depleting drugs, definite encephalitis and/or oculogyric crises on no drug treatment, negative response to large doses of levodopa (if malabsorption was excluded), strictly unilateral features after 3 years, other neurological features (supranuclear gaze palsy, cerebellar signs, early severe autonomic involvement, Babinski sign, early severe dementia with disturbances of language, memory or praxis), exposure to a known neurotoxin, or presence of cerebral tumor or communicating hydrocephalus according to neuroimaging. |
| AD | 1. Subjects must meet the 2011 NIA-AA diagnostic guidelines for probable AD dementia [28]. 2. Subjects must have MMSE scores between 10 and 22 and CDR = 0.5 or 1. |
1. Subjects with cranial metallic implants, cardiac pacemakers or claustrophobia. 2. Significant history of depression 3. Geriatric Depression Scale > 8 |
*CDR: Clinical Dementia Ranking
++MMSE: Mini-Mental State Examination
#MOCA: Montreal Cognitive Assessment
Preparation of plasma samples
The blood draw was performed using a 9 ml or 6 ml K3 EDTA lavender-top tube, followed by centrifugation at 1500–2500 g at room temperature for 15 minutes. Five hundred microliters of plasma was aliquoted into each 0.5-ml cryotube and stored at -20 °C or -80 °C. The plasma was required to be frozen no later than 3 hours after the blood draw. Each frozen plasma aliquot was placed in wet ice and then brought to room temperature prior to the IMR measurement.
Statistical methods
The continuous variables for each measurement are presented as the means ± standard deviations. The data of NFL concentrations were checked for normality using the Kolmogorov-Smirnov test and the result showed suggested a violation in the normality assumption (p = 0.033; data not shown). Therefore, the comparison of NFL concentrations between groups (i.e., normal control vs. disease groups; PD/PDD vs. AD groups) was made using the Mann-Whitney U-test. To clarify the discrimination between two of PD/PDD, AD, patient and NC, receiver operating characteristic (ROC) curve analysis was conducted. The optimal cutoff of NFL concentrations was determined by the Youden index. The confidence interval of area under the curve, sensitivity and specificity was calculated using the DeLong’s nonparametric method. At last, the relationship between spiked NFL and measure NFL (both were log10-transformed) was assessed using the Pearson’s correlation. A two-sided P value less than 0.5 was considered statistically significant. Analyses were done using GraphPad Prism 5 and MedCalc version 13.
Results
Hook effect on the assay
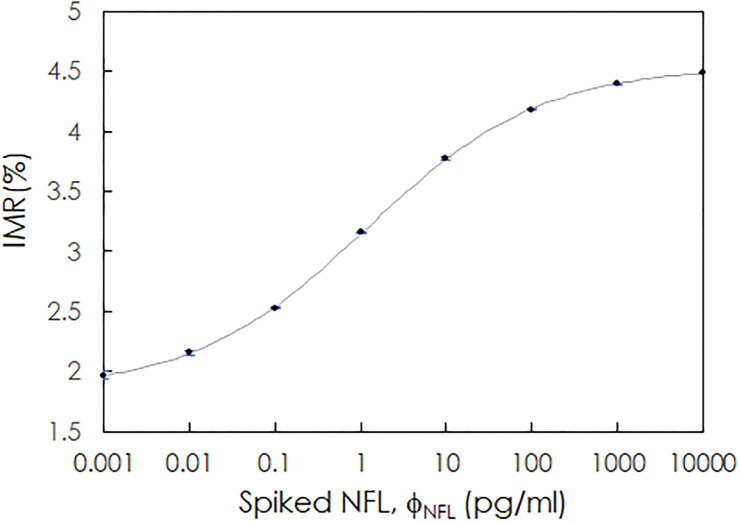
The NFL concentration-dependent IMR signal was investigated. Phosphate-buffered saline (PBS) solutions spiked with NFL (Ab224840, Abcam) at various concentrations were used for the IMR measurements. The concentrations of the spiked NFL samples, denoted as ϕNFL, ranged from 0.001 to 10000 pg/ml. The measured IMR signal, denoted as IMR(%), was 1.967 for 0.001 pg/ml NFL and increased to 4.487 for 10000 pg/ml NFL. The relationship between IMR(%) and ϕNFL is plotted in Fig 1. The data points were well fitted to the logistic function
| (1) |
where A, B, ϕo and γ were fitting parameters and were found to be 1.840, 4.538, 1.166 and 0.428, respectively. ΦNFL is the spiked NFL concentration in PBS. The fitted logistic curve is plotted as a gray line in Fig 1. The data point at 10000 pg/ml NFL still lies on the fitted line. This implies that the Hook effect does not occur at 10000 pg/ml NFL.
Fig 1. Relationship between the IMR signal, IMR(%), and the spiked NFL concentrations.
The gray solid line is the fitted curve determined with Eq (1).
Assay detection limit
The limit of blank (LoB) and the limit of detection (LoD) were investigated according to the guidelines for evaluating the detection capacity of clinical laboratory measurement procedures described in CLSI EP17-A2. The LoB was obtained by determining the appropriate percentile (p) value of the ranked measured concentrations for the blank samples, which was p = 0.95 in this case:
| (2) |
where NB = 60 (NB is the number of trials) in the current case. Eq (2) becomes
| (3) |
The LoB was calculated by performing linear interpolation using the 57th- and 58th-ranked measured concentrations. Table 2 lists the 60 ranked measured concentrations for the PBS samples (blank samples) without NFL. The 57.5th-ranked measured concentration was 0.000 fg/ml. Therefore, the LoB used for assaying NFL with the IMR NFL reagent was 0.000 fg/ml.
Table 2. Ranked list of the 60 measured NFL concentrations for PBS samples not spiked with NFL using the IMR NFL reagent.
| Rank | Measured concentration (fg/ml) | Rank | Measured concentration (fg/ml) |
|---|---|---|---|
| 1 | -0.03 | 31 | 0.00 |
| 2 | -0.03 | 32 | 0.00 |
| 3 | -0.02 | 33 | 0.00 |
| 4 | -0.02 | 34 | 0.00 |
| 5 | -0.02 | 35 | 0.00 |
| 6 | -0.02 | 36 | 0.00 |
| 7 | -0.02 | 37 | 0.00 |
| 8 | -0.02 | 38 | 0.00 |
| 9 | -0.02 | 39 | 0.00 |
| 10 | -0.02 | 40 | 0.00 |
| 11 | -0.02 | 41 | 0.00 |
| 12 | -0.01 | 42 | 0.00 |
| 13 | -0.01 | 43 | 0.00 |
| 14 | -0.01 | 44 | 0.00 |
| 15 | -0.01 | 45 | 0.00 |
| 16 | -0.01 | 46 | 0.00 |
| 17 | -0.01 | 47 | 0.00 |
| 18 | -0.01 | 48 | 0.00 |
| 19 | -0.01 | 49 | 0.00 |
| 20 | -0.01 | 50 | 0.00 |
| 21 | -0.01 | 51 | 0.00 |
| 22 | -0.01 | 52 | 0.00 |
| 23 | -0.01 | 53 | 0.00 |
| 24 | -0.01 | 54 | 0.00 |
| 25 | -0.01 | 55 | 0.00 |
| 26 | -0.01 | 56 | 0.00 |
| 27 | 0.00 | 57 | 0.00 |
| 28 | 0.00 | 58 | 0.00 |
| 29 | 0.00 | 59 | 0.00 |
| 30 | 0.00 | 60 | 0.00 |
The limit of detection (LoD) was calculated with the equation
| (4) |
where σS denotes the standard deviation of the measured NFL concentrations of the NFL solutions spiked with a fixed NFL concentration (e.g., 1.0 fg/ml in the current case) in PBS. Table 3 lists the measured NFL concentrations of the 60 NFL solutions. The mean value of the 60 measured concentrations was 1.05 fg/ml. The σS of the 60 measured concentrations was 0.11 fg/ml. The LoD for assaying NFL using IMR was 0.18 fg/ml according to Eq (4).
Table 3. Ranked list of the 60 measured NFL concentrations for PBS samples spiked with 1.0 fg/ml NFL using the IMR NFL reagent.
| Rank | Measured concentration (fg/ml) | Rank | Measured concentration (fg/ml) |
|---|---|---|---|
| 1 | 0.86 | 31 | 1.06 |
| 2 | 0.90 | 32 | 1.06 |
| 3 | 0.91 | 33 | 1.06 |
| 4 | 0.92 | 34 | 1.06 |
| 5 | 0.92 | 35 | 1.07 |
| 6 | 0.93 | 36 | 1.08 |
| 7 | 0.93 | 37 | 1.08 |
| 8 | 0.93 | 38 | 1.08 |
| 9 | 0.93 | 39 | 1.09 |
| 10 | 0.94 | 40 | 1.09 |
| 11 | 0.94 | 41 | 1.09 |
| 12 | 0.94 | 42 | 1.09 |
| 13 | 0.95 | 43 | 1.09 |
| 14 | 0.95 | 44 | 1.09 |
| 15 | 0.96 | 45 | 1.10 |
| 16 | 0.96 | 46 | 1.10 |
| 17 | 0.96 | 47 | 1.10 |
| 18 | 0.97 | 48 | 1.11 |
| 19 | 0.99 | 49 | 1.11 |
| 20 | 0.99 | 50 | 1.12 |
| 21 | 1.00 | 51 | 1.13 |
| 22 | 1.01 | 52 | 1.14 |
| 23 | 1.01 | 53 | 1.14 |
| 24 | 1.03 | 54 | 1.17 |
| 25 | 1.05 | 55 | 1.20 |
| 26 | 1.05 | 56 | 1.20 |
| 27 | 1.05 | 57 | 1.21 |
| 28 | 1.05 | 58 | 1.31 |
| 29 | 1.05 | 59 | 1.38 |
| 30 | 1.05 | 60 | 1.41 |
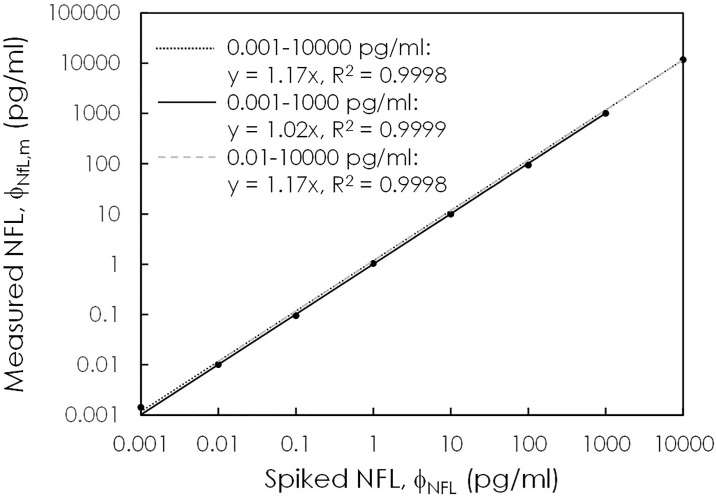
Assay linearity
The linear range of the NFL assay is defined according to the NFL concentrations that reflect the proportionality between the measured NFL concentration ϕNFL,m and the spiked NFL concentration ϕNFL. The proportional coefficient should be between 0.9 and 1.1, and the coefficient of determination R2 for the ϕNFL,m-ϕNFL curve should be higher than 0.95. To investigate the assay linearity, the detected IMR (%) values in Fig 1 were converted to the measured NFL concentrations ϕNFL,m via Eq (1). Fig 2 plots the relationship between ϕNFL,m and ϕNFL. It was found that ϕNFL,m is proportional to ϕNFL, as expressed by
| (5) |
Fig 2. Relationship between the measured NFL concentrations using IMR and the spiked NFL concentrations.
The solid/dotted/dashed lines show the proportionalities between the measured NFL concentrations and the spiked NFL concentrations according to various ranges of spiked NFL concentrations.
The proportional coefficient s depends on the selected ranges of NFL concentrations. For example, for the selected NFL concentration range from 0.001 to 10000 pg/ml, s and R2 are equal to 1.17 and 0.9998, respectively, according to the plotted gray dotted line in Fig 2. In this case, the proportional coefficient is higher than 1.1, which does not meet the requirement of the linear range. The data of the highest concentration to the lowest concentration are removed to find s in Eq (5), i.e., 0.001 to 1000 pg/ml or 0.01 to 10000 pg/ml. The results show that s is 1.02 and R2 is 0.9999 once the concentration range is selected to be from 0.001 to 1000 pg/ml, according to the plotted solid line in Fig 2. Therefore, the linear range for the NFL assay using the IMR NFL reagent ranges from 0.001 to 1000 pg/ml.
Dilution recovery range
The dilution recovery is defined as
| (6) |
The acceptable dilution recovery range is from 90% to 110%. In this work, an NFL solution with a measured concentration of 101.87 pg/ml was diluted by a factor of between 2 and 100 with PBS solution. The expected and measured NFL concentrations after dilution are listed in Table 4. The dilution recoveries of the 2X- to 100X-diluted samples ranged from 1001.1% to 105.2%, which are within the acceptable dilution recovery range. Hence, the sample used for the IMR NFL assay could be diluted up to 100 times.
Table 4. Dilution factors, expected concentrations, measured concentrations, and dilution recovery of diluted samples used in the tests of the dilution recovery range for assaying NFL using the IMR NFL reagent.
| Dilution factor | Expected concentration (pg/ml) | Measured concentration (pg/ml) | Dilution recovery |
|---|---|---|---|
| 2X | 50.93 | 52.63 | 103.3% |
| 5X | 20.37 | 20.43 | 100.3% |
| 10X | 10.19 | 10.56 | 103.7% |
| 20X | 5.09 | 5.36 | 105.2% |
| 50X | 2.04 | 2.11 | 103.5% |
| 100X | 1.02 | 1.03 | 101.1% |
Spiked recovery
A sample with a higher NFL concentration was spiked into another sample with a lower NFL concentration. The NFL concentration of the mixture was assayed with IMR NFL reagent. The spiked recovery was calculated via
| (7) |
Two plasma samples with original concentrations of 5.39 (plasma sample no. PRA) and 101.09 pg/ml (plasma sample no. PRF) were mixed at various volume ratios, as shown in Table 5. The original concentrations were assayed using IMR NFL reagent. The results in Table 5 reveal that the spiked recovery ranged from 93.4% to 99.1% for the IMR NFL reagent.
Table 5. Measured NFL concentration using the IMR NFL reagent and the spiked recovery of plasma samples.
| Plasma sample No. | Volume ratio (PRA:PRF) | Original concentration (pg/ml) | Expected concentration (pg/ml) | Measured concentration (pg/ml) | Spiked recovery |
|---|---|---|---|---|---|
| PRA | - | 5.39 | - | - | - |
| PRB | 80%:20% | - | 24.53 | 22.91 | 93.4% |
| PRC | 60%:40% | - | 43.67 | 41.41 | 94.8% |
| PRD | 40%:60% | - | 62.81 | 62.27 | 99.1% |
| PRE | 20%:80% | - | 81.95 | 80.22 | 97.0% |
| PRF | - | 101.09 | - | - | - |
Assay reproducibility
The reproducibility of the NFL assay with IMR was investigated according to CLSI EP5-A3: Approved Guidelines for Evaluation of the Precision Performance of Quantitative Measurement Methods. The NFL solutions were measured precisely in one run using IMR NFL reagent. Two sequential measurements of two duplicate measurements each were regarded as two runs, referred to as Run1 and Run2. Two PBS solutions spiked with different NFL concentrations were used for the tests of reproducibility. The measured NFL concentrations are listed in Table 6 for NFL-PBS sample 1 and in Table 7 for NFL-PBS sample 2. The results show that NFL-PBS sample 1 had a concentration of 1.00 pg/ml, and NFL-PBS sample 2 had a concentration of 100.17 pg/ml. By following the statistical method described in the CLSI EP5-A3 guidelines, the within-lab precision and standard deviations of repeatability for NFL-PBS samples 1 and 2 were calculated and are listed in Table 8. The imprecision (%CV) of the NFL assay using the IMR NFL reagent was less than 10%.
Table 6. Measured NFL concentrations (listed in the Result11, Result12, Result21 and Result22 columns) in NFL-PBS sample 1 used for the analysis of the precision and reproducibility of the IMR NFL reagent.
| Run 1 | Run 2 | ΔMean | Mean (pg/ml) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | Date | Result11 (pg/ml) | Result12 (pg/ml) | Mean1 (pg/ml) | ΔResult1 | Day | Date | Result21 (pg/ml) | Result22 (pg/ml) | Mean2 | ΔResult2 | ||
| 1 | 2019/7/24 | 1.04 | 1.00 | 1.02 | 0.002 | 2 | 2019/7/29 | 0.995 | 1.097 | 1.046 | 0.010 | 0.001 | 1.03 |
| 3 | 2019/7/31 | 1.011 | 1.008 | 1.01 | 0.000 | 4 | 2019/8/1 | 1.018 | 1.002 | 1.010 | 0.000 | 0.000 | 1.01 |
| 5 | 2019/8/2 | 0.964 | 1.010 | 0.99 | 0.002 | 6 | 2019/8/6 | 1.000 | 1.020 | 1.010 | 0.000 | 0.001 | 1.00 |
| 7 | 2019/8/7 | 1.018 | 0.992 | 1.01 | 0.001 | 8 | 2019/8/13 | 0.995 | 0.995 | 0.995 | 0.000 | 0.000 | 1.00 |
| 9 | 2019/8/14 | 1.012 | 0.990 | 1.00 | 0.000 | 10 | 2019/8/21 | 0.950 | 0.980 | 0.965 | 0.001 | 0.001 | 0.98 |
| 11 | 2019/8/27 | 1.015 | 1.020 | 1.02 | 0.000 | 12 | 2019/8/30 | 1.010 | 1.010 | 1.010 | 0.000 | 0.000 | 1.01 |
| 13 | 2019/9/3 | 1.010 | 0.972 | 0.99 | 0.001 | 14 | 2019/9/4 | 0.985 | 1.010 | 0.998 | 0.001 | 0.000 | 0.99 |
| 15 | 2019/9/5 | 0.995 | 1.005 | 1.00 | 0.000 | 16 | 2019/9/9 | 0.995 | 1.025 | 1.010 | 0.001 | 0.000 | 1.01 |
| 17 | 2019/9/10 | 1.005 | 1.000 | 1.00 | 0.000 | 18 | 2019/9/12 | 0.970 | 0.975 | 0.973 | 0.000 | 0.001 | 0.99 |
| 19 | 2019/9/17 | 1.005 | 1.010 | 1.01 | 0.000 | 20 | 2019/9/18 | 0.980 | 0.990 | 0.985 | 0.000 | 0.001 | 1.00 |
| Sum | 0.007 | 0.013 | 0.004 | 10.02 | |||||||||
Mean1 = (Result11+Result12)/2 ΔResult1 = (Result11-Result12)^2
Mean2 = (Result21+Result22)/2 ΔResult2 = (Result21-Result22)^2
ΔMean = (Mean1-Mean2)^2 Mean = (Mean1+Mean2)/2
Table 7. Measured NFL concentrations (listed in the Result11, Result12, Result21 and Result22 columns) in NFL-PBS sample 2 used for the analysis of the precision and reproducibility of the IMR NFL reagent.
| Run1 | Run2 | ΔMean | Mean (pg/ml) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | Date | Result11 (pg/ml) | Result12 (pg/ml) | Mean1 (pg/ml) | ΔResult1 | Day | Date | Result21 (pg/ml) | Result22 (pg/ml) | Mean2 | ΔResult2 | ||
| 1 | 2019/7/24 | 99.51 | 100.31 | 99.91 | 0.640 | 2 | 2019/7/29 | 100.735 | 99.625 | 100.180 | 1.232 | 0.073 | 100.05 |
| 3 | 2019/7/31 | 100.245 | 100.085 | 100.17 | 0.026 | 4 | 2019/8/1 | 99.780 | 99.375 | 99.578 | 0.164 | 0.345 | 99.87 |
| 5 | 2019/8/2 | 99.820 | 99.655 | 99.74 | 0.027 | 6 | 2019/8/6 | 99.865 | 99.710 | 99.788 | 0.024 | 0.002 | 99.76 |
| 7 | 2019/8/7 | 99.975 | 99.975 | 99.98 | 0.000 | 8 | 2019/8/13 | 100.835 | 99.630 | 100.233 | 1.452 | 0.066 | 100.10 |
| 9 | 2019/8/14 | 99.945 | 100.075 | 100.01 | 0.017 | 10 | 2019/8/21 | 100.195 | 99.830 | 100.013 | 0.133 | 0.000 | 100.01 |
| 11 | 2019/8/27 | 100.315 | 100.465 | 100.39 | 0.023 | 12 | 2019/8/30 | 99.615 | 100.390 | 100.003 | 0.601 | 0.150 | 100.20 |
| 13 | 2019/9/3 | 99.915 | 100.035 | 99.98 | 0.014 | 14 | 2019/9/4 | 104.440 | 100.350 | 102.395 | 16.728 | 5.856 | 101.19 |
| 15 | 2019/9/5 | 100.440 | 99.770 | 100.11 | 0.449 | 16 | 2019/9/9 | 100.500 | 100.110 | 100.305 | 0.152 | 0.040 | 100.21 |
| 17 | 2019/9/10 | 99.905 | 99.925 | 99.92 | 0.000 | 18 | 2019/9/12 | 99.570 | 100.485 | 100.028 | 0.837 | 0.013 | 99.97 |
| 19 | 2019/9/17 | 100.250 | 101.895 | 101.07 | 2.706 | 20 | 2019/9/18 | 99.760 | 99.405 | 99.583 | 0.126 | 2.220 | 100.33 |
| Sum | 3.902 | 21.449 | 8.766 | 1001.68 | |||||||||
Mean1 = (Result11+Result12)/2 ΔResult1 = (Result11-Result12)^2
Mean2 = (Result21+Result22)/2 ΔResult2 = (Result21-Result22)^2
ΔMean = (Mean1-Mean2)^2 Mean = (Mean1+Mean2)/2
Table 8. Standard deviations of the repeatability and within-lab precision for the assay of NFL concentrations in PBS using the IMR NFL reagent.
The samples used show mean measured NFL concentrations of 1.00 pg/ml and 100.17 pg/ml. The coefficient of variation is the ratio of the standard deviation and the mean of the measured NFL concentrations.
| Material | Mean of measured NFL concentrations | Standard deviation (Coefficient of variation) | |
|---|---|---|---|
| Repeatability | Within-lab precision | ||
| NFL-PBS sample 1 | 1.00 pg/ml | 0.027 pg/ml (2.67%) | 0.019 pg/ml (1.89%) |
| NFL-PBS sample 2 | 100.17 pg/ml | 0.528 pg/ml (0.53%) | 1.313 pg/ml (1.31%) |
Interference test
Possible false signals during the measurement of NFL concentrations due to molecules referred to as interfering materials in the tested sample were investigated. Table 9 lists all the interfering materials tested in this work. Sample No. 1 was pure PBS solution spiked only with 100 pg/ml NFL without any interfering material. The measured NFL concentration of Sample No. 1 (= 99.35 pg/ml) was used as a reference. The recovery rate of a given sample (Sample No. 2–17) was the ratio of the measured NFL concentration of the given sample to that of the reference sample. The recovery rate ranged from 93.4% to 106.4%, which satisfied the requirement for nonsignificant interference in the NFL assay. The results show that the molecules listed in Table 9, including NF heavy and medium peptides, did not significantly interfere with the NFL assay using the IMR NFL reagent.
Table 9. Materials and their concentrations used for interference tests for the NFL assay utilizing the NFL reagent with IMR.
The concentration of NFL in each sample was 100 pg/ml. The matrix was PBS solution. The measured NFL concentrations of each sample are listed. Using the NFL concentration of the pure NFL-PBS sample (sample No. 1) as a reference, the recovery rates of the NFL concentrations of the other samples were calculated and are listed in the rightmost column.
| Sample No. | Interfering material | Concentration | Measured NFL concentration (pg/ml) | Recovery rate |
|---|---|---|---|---|
| 1 | None | - | 99.35 | - |
| 2 | Conjugated bilirubin | 600 μg/ml | 96.16 | 96.8% |
| 3 | Hemoglobin | 10000 μg/ml | 105.46 | 106.2% |
| 4 | Intralipid | 30000 μg/ml | 97.03 | 97.7% |
| 5 | Albumin | 60000 μg/ml | 92.77 | 93.4% |
| 6 | Rheumatoid factor | 500 IU/ml | 96.30 | 96.9% |
| 7 | Uric acid | 200 μg/ml | 100.92 | 101.6% |
| 8 | Neurofilament, Heavy peptide | 100 pg/ml | 101.29 | 102.0% |
| 9 | Neurofilament, Medium peptide | 100 pg/ml | 105.69 | 106.4% |
| 10 | Acetylsalicylic acid | 500 μg/ml | 97.06 | 97.7% |
| 11 | Ascorbic acid | 300 μg/ml | 97.35 | 98.0% |
| 12 | Ampicillin sodium | 1000 μg/ml | 101.21 | 101.9% |
| 13 | Quetiapine fumarate | 100 μg/ml | 100.87 | 101.5% |
| 14 | Galantamine hydrobromide | 90 ng/ml | 98.23 | 98.9% |
| 15 | Rivastigmine hydrogen tartrate | 100 ng/ml | 96.36 | 97.0% |
| 16 | Donepezil hydrochloride | 1000 ng/ml | 100.44 | 101.1% |
| 17 | Memantine hydrochloride | 150 ng/ml | 102.75 | 103.4% |
Plasma NFL concentrations in patients with dementia
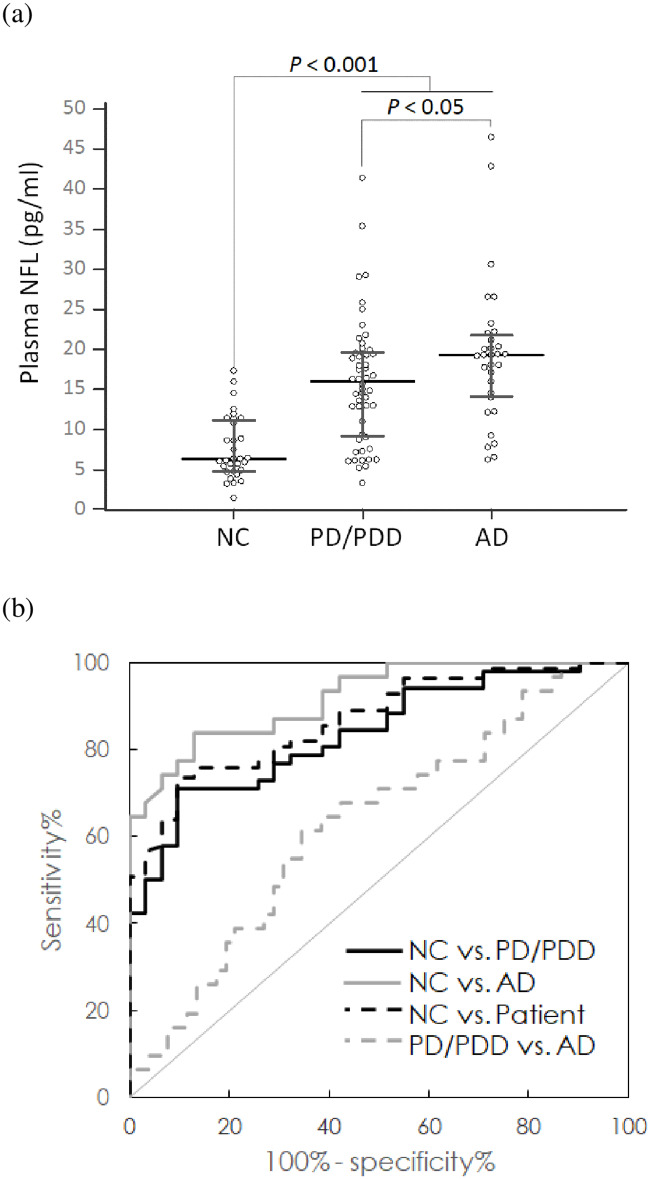
In this work, thirty-one normal controls (NC), fifty-two patients with Parkinson’s disease (PD) or PD dementia (PDD), and thirty-one patients with Alzheimer’s disease (AD) were enrolled at National Taiwan University Hospital and Kaohsiung Chang Gung Memorial Hospital. The demographic information of the subjects is listed in Table 10. NCs had a mean level of 7.70 ± 4.00 pg/ml for plasma NFL. Patient group showed 66.8 ± 10.3 pg/ml for plasma NFL level, which is significantly higher than NCs (p < 0.001), as shown in Fig 3(A). A significant difference in the plasma NFL concentrations between AD (19.24 ± 8.99 pg/ml) and PD/PDD (15.85 ± 7.82 pg/ml) (p < 0.05) was found.
Table 10. Brief demographic information for the enrolled subjects.
| Group (n) | NC (31) | Patient (83)* | PD/PDD (52) | AD (31) |
|---|---|---|---|---|
| Female% | 80.0 | 56.5 | 61.7 | 48.0 |
| Age (years) | 60.3 ± 7.2 | 66.8 ± 10.3 | 62.6 ± 9.5 | 74.6 ± 9.6 |
| MMSE | 28.0 ± 1.9 | 23.2 ± 5.3 | 24.8 ± 4.6 | 20.2 ± 5.3 |
| H-Y stage | - | - | 1.96 ± 0.98 | - |
| NFL (pg/ml) | 7.70 ± 4.00 | 17.11 ± 8.39 | 15.85 ± 7.82 | 19.24 ± 8.99 |
*: Patient includes PD/PDD and AD NC: normal control PD: Parkinson’s disease
PDD: Parkinson’s disease dementia AD: Alzheimer’s disease
MMSE: Mini-Mental State Examination
H-Y stage: Hoehn and Yahr stage
Fig 3. (A) Measured NFL concentrations in human plasma using IMR and (B) ROC curves used for differentiating various diagnostic groups (NC: Normal control, PD: Parkinson’s disease, PDD: Parkinson’s disease dementia, AD: Alzheimer’s disease, dementia: PD/PDD+AD).
The horizontal line represents median and the range of error bar represents interquartile range.
By using the ultrasensitive and highly specific IMR NFL assay, the clear discrimination of the concentrations of plasma NFL in NCs (7.70 ± 4.00 pg/ml) and PD/PDD patients (15.85 ± 7.82 pg/ml) was achieved (p < 0.001). Through the analysis of the receiver operating characteristic (ROC) curve plotted with the black solid line in Fig 3(B), the cut-off value of the plasma NFL concentration used to discriminate PD/PDD patients from NCs was found to be 12.71 pg/ml, which resulted in values of 0.712 and 0.903 for clinical sensitivity and specificity, respectively. The corresponding area under the curve (AUC) was 0.838, as shown in Table 11. These results reveal the feasibility of differentiating PD/PDD patients from normal controls.
Table 11. Results of the ROC curve analysis using plasma NFL concentrations as a discriminative index.
| ROC curve analysis | Cut-off value of the plasma NFL concentration (pg/ml) | Sensitivity (95% CI) | Specificity (95% CI) | AUC (95% CI) |
|---|---|---|---|---|
| Group | ||||
| NC vs. PD/PDD | 12.71 | 0.712 (0.569–0.829) | 0.903 (0.743–0.980) | 0.838 (0.754–0.921) |
| NC vs. AD | 12.04 | 0.838 (0.663–0.946) | 0.871 (0.702–0.964) | 0.919 (0.855–0.984) |
| NC vs. Patient* | 12.71 | 0.735 (0.627–0.826) | 0.903 (0.743–0.980) | 0.868 (0.803–0.933) |
| PD/PDD vs. AD | 18.02 | 0.613 (0.422–0.782) | 0.654 (0.509–0.780) | 0.630 (0.507–0.753) |
ROC: receiver operating characteristic AUC: area under the curve
*: including PD/PDD and AD NC: normal control PD: Parkinson’s disease
PDD: Parkinson’s disease dementia AD: Alzheimer’s disease
In addition to PD/PDD patients, AD patients (19.24 ± 8.99 pg/ml) showed higher levels of plasma NFL than NCs (p < 0.001). The ROC curve for differentiating AD patients from NCs using plasma NFL concentrations is plotted with the gray solid line in Fig 3(B). The cut-off value (= 12.04 pg/ml), sensitivity (= 0.838), specificity (= 0.871) and AUC (= 0.919) values are listed in Table 12. Notably, the accuracy of differentiating AD patients from NCs was higher than 85%. By combining PD/PDD and AD into a patient group, discrimination between patient and NCs using plasma NFL concentrations was investigated. The ROC curve is plotted as the black dashed line in Fig 3(B). The cut-off value of the plasma NFL concentration used to discriminate patient from NCs is 12.71 pg/ml, the sensitivity is 0.735, the specificity is 0.903 and the AUC is 0.868. These results demonstrate the feasibility of identifying PD/PDD or AD using plasma NFL concentrations. This was also shown by other clinical studies listed in Table 12 [1,16,20,21,29]. However, differentiation between AD and PD/PDD patients using plasma NFL concentrations was not as accurate because the AUC is 0.630. This suggests that the plasma NFL concentration is not suitable for differential diagnosis between AD and PD/PDD. Alternatively, plasma NFL concentrations could be a promising biomarker for screening for neurodegenerative diseases.
Table 12. Clinical sensitivity and specificity of the under the curve (AUC) value for discriminating PD/PDD or AD patients from NCs according to NFL concentrations in blood.
| Control (n) | Patient (n) | Sample | Sensitivity | Specificity | AUC | Ref. |
|---|---|---|---|---|---|---|
| NC (31) | PD/PDD (52) | Plasma | 0.712 | 0.903 | 0.838 | This work |
| NC (26) | PD (20) | Plasma | 0.82 | 0.92 | - | 16 |
| NC (40) | PD/PDD (116) | Plasma | 0.532 | 0.905 | 0.754 | 21 |
| NC (52) | PD/PDD (139) | Serum | 0.61 | 0.68 | 0.64 | 29 |
| NC (31) | AD (31) | Plasma | 0.838 | 0.871 | 0.919 | This work |
| NC (193) | MCI (197) + AD (180) | Plasma | - | - | 0.87 | 1 |
| NC (41) | aMCI (25) + ADD (33) | Plasma | 0.84 | 0.78 | 0.92 | 20 |
NC: normal control PD: Parkinson’s disease
PDD: Parkinson’s disease dementia AD: Alzheimer’s disease
aMCI: amnesic mild cognitive impairment
Discussion
In this work, the reagent for assaying NFL while utilizing the IMR platform showed ultrasensitive detection at a sub-fg/ml concentration. According to published papers, several assay platforms, such as enzyme-linked immunosorbent assay (ELISA) [30–33], single-molecule assay (SIMOA) [18,34] or electrochemiluminescence (ECL) [35], have been utilized to detect NFL. The reported limits of detection (LoDs) in representative works are listed in Table 13. Depending on the antibodies used, a given assay platform might show various LoDs. Roughly speaking, ECL is more sensitive than ELISA but less sensitive than SIMOA. All three assay platforms show sensitivity at a level of pg/ml or higher in terms of the LoDs for assaying NFL. However, IMR shows a LoD of sub-fg/ml for assaying NFL. This evidence indicates that the IMR assay is much more sensitive than either ELISA, SIMOA or ECL by at least 1000-fold. Notably, the results of interference tests shown in Table 9 reveal the high specificity of assaying NFL with IMR. Thus, the IMR NFL assay is not only ultrasensitive but also specific.
Table 13. Limits of detection (LoDs) reported for various assay platforms, such as enzyme-linked immunosorbent assay (ELISA), electrochemiluminescence (ECL) and single-molecule assay (SIMOA).
| Assay platform | LoD | Ref. |
|---|---|---|
| ELISA | 15.6 pg/ml | 30 |
| ELISA | 5 pg/ml | 31 |
| ELISA | 125 pg/ml | 32 |
| ELISA | 250 pg/ml | 33 |
| SIMOA | 0.97 pg/ml | 34 |
| SIMOA | 2.2 pg/ml | 18 |
| ECL | 15.6 pg/ml | 35 |
| IMR | 0.18 fg/ml | This work |
The reasons for achieving ultrasensitive and highly specific IMR assays have been discussed in previous works [36,37]. There are three key factors involved in achieving high sensitivity and specificity for the IMR assay. One is the utilization of antibody-functionalized magnetic nanoparticles homogeneously suspended in liquid. The surfaces of these magnetic nanoparticles serve as substrates of antibodies to catch target antigens. The total area of the association between antibodies and antigens in the IMR assay is obviously larger than that ELISA or ECL.
Another key factor is the use of the ultrasensitive sensor to detect the tiny reductions in magnetic signals due to the associations between antibodies and antigens. The adopted sensor is a high-temperature superconducting quantum interference device (SQUID) magnetometer. The noise level of the SQUID magnetometer is on the order of approximately fT/√Hz. The magnetic signal generated by core Fe3O4 magnetic nanoparticles that are 35 nm in diameter is approximately 1 pT/√Hz [36]. This means that, in principle, the change in the magnetic signal due to a single magnetic nanoparticle can be detected. Notably, a sample with an ultralow concentration has an extremely low number of target antigens. After mixing the sample with IMR reagent, only a few magnetic nanoparticles can bind with the target antigen. The reduction in the magnetic signal of the reagent would be very small. As described, the SQUID magnetometer is sensitive enough to detect the tiny reduction in the magnetic signal.
The other factor is the so-called “spin-wash” mechanism involved in IMR [37]. Once a biomolecule binds with the antibody on a magnetic nanoparticle, the bound biomolecule experiences a centrifugal force because the nanoparticle is rotating. The specific biomolecule shows a stronger binding force with the antibody than the nonspecific biomolecule. By controlling the rotating frequency of the nanoparticles adequately, the strength of the centrifugal force can be made stronger than that of nonspecific binding but weaker than that of specific binding. Hence, nonspecific binding is significantly suppressed. Remarkably, the specificity of the antibody against NFL also plays a role in the “spin-wash” mechanism. Therefore, due to these three key factors, IMR shows merits of high sensitivity and high specificity in assaying NFL.
Conclusion
An assay kit for the measurement of NFL utilizing immunomagnetic reduction was developed. The preclinical characterizations performed according to CLSI guidelines revealed that the IMR NFL assay is ultrasensitive, highly specific and reliable. By applying the IMR NFL kit for assaying plasma NFL in NCs and PD/PDD and AD patients, an accuracy higher than 80% in discriminating PD/PDD or AD patients from NCs was shown. However, the concentrations of plasma NFL are not suitable for differentiating PD/PDD from AD.
Supporting information
(XLSX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
MagQu Co., Ltd provided funding for this study in the form of salaries for Huei-Chun Liu, Chin-Yi Lin and Shieh-Yueh Yang. MagQu Co., Ltd did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘Author contributions’ section.
References
- 1.Zetterberg H, Skillback T, Mattsson N, Trojanowski JQ, Portelius E, Shaw LM, et al. , Alzheimer’s Disease Neuroimaging Initiative. Association of cerebrospinal fluid neurofilament light concentration with Alzheimer disease progression. JAMA Neurol. 2016;73:60–67. 10.1001/jamaneurol.2015.3037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meeter LH, Dopper EG, Jiskoot LC, Sanchez-Valle R, Graff C, Benussi L et al. Neurofilament light chain: a biomarker for genetic frontotemporal dementia. Ann Clin Trans Neurol. 2016;3:623–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meeter LH, Steketee RME, Salkovic D, Vos ME, Grossman M, McMillan CT, et al. Clinical value of cerebrospinal fluid neurofilament light chain in semantic dementia. J Neurol Neurosurg Psychiatry. 2019;90:997–1004. 10.1136/jnnp-2018-319784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christensen JR, Börnsen L, Khademi M, Olsson T, Jensen PE, Sørensen PS, et al. CSF inflammation and axonal damage are increased and correlate in progressive multiple sclerosis. Mult Scler J. 2013;19:877–884. [DOI] [PubMed] [Google Scholar]
- 5.van Eijk JJJ, van Everbroeck B, Abdo WF, Kremer BPH, Verbeek MM. CSF neurofilament proteins levels are elevated in sporadic Creutzfeldt-Jakob disease. J Alz Dis. 2010;21:569–576. [DOI] [PubMed] [Google Scholar]
- 6.Merisson E, Mattsson N, Zetterberg H, Blennow K, Pikwer A, Mehmedagic I, et al. Total-tau and neurofilament light in CSF reflect spinal cord ischaemia after endovascular aortic repair. Neurochem Int. 2016;93:1–5. 10.1016/j.neuint.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 7.Mattsson N, Andreasson U, Zetterberg H, Blennow K. Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2017;74:557–566. 10.1001/jamaneurol.2016.6117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall S, Öhrfelt A, Constantinescu R, Andreasson U, Surova Y, Bostrom F, et al. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch Neurol. 2012;69:1445–1452. 10.1001/archneurol.2012.1654 [DOI] [PubMed] [Google Scholar]
- 9.Teunissen CE, Dijkstra C, Polman C. Biological markers in CSF and blood for axonal degeneration in multiple sclerosis. Lancet Neurol. 2005;4:32–41. 10.1016/S1474-4422(04)00964-0 [DOI] [PubMed] [Google Scholar]
- 10.Shahim P, Gren M, Liman V, Andreasson U, Norgren N, Tegner Y et al. Serum neurofilament light protein predicts clinical outcome in traumatic brain injury. Sci Rep. 2016;6:36791-1-9. 10.1038/srep36791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14:577–589. 10.1038/s41582-018-0058-z [DOI] [PubMed] [Google Scholar]
- 12.de Jong D, Jansen RWMM, Pijnenburg YAL, van Geel WJA, Borm GF, Kremer HPH, et al. CSF neurofilament proteins in the differential diagnosis of dementia. J Neurol Neurosurg Psychiatry. 2007;78:936–938. 10.1136/jnnp.2006.107326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abu-Rumeileh S, Capellari S, Stanzani-Maserati M, Polischi B, Martinelli P, Caroppo P et al. The CSF neurofilament light signature in rapidly progressive neurodegenerative dementias. Alz Res Ther. 2018;10:3-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zetterberg H, Wilson D, Andreasson U, Minthon L, Blennow K, Randall J, et al. Plasma tau levels in Alzheimer’s disease. Alz Res Ther. 2013;5:9-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang CC, Yang SY, Chieh JJ, Horng HE, Hong CY, Yang HC, et al. Biofunctionalized magnetic nanoparticles for specifically detecting biomarkers of Alzheimer’s disease in vitro. ACS Chem Neurosci. 2011;2:500–5. 10.1021/cn200028j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansson O, Janelidze S, Hall S, Magdalinou N, Lees AJ, Andreasson U et al. Blood-based NfL: A biomarker for differential diagnosis of parkinsonian disorder. Neurol. 2017;88:930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mielke MM, Syrjanen JA, Blennow K, Zetterberg H, Vemuri P, Skoog I et al. Plasma and CSF neurofilament light: Relation to longitudinal neuroimaging and cognitive measures. Neurol. 2019;93:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mattsson N, Andreasson U, Zetterberg H, Blennow K, Alzheimer’s Disease Neuroimaging Initiative. Association of plasma nurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2017;74:557–566. 10.1001/jamaneurol.2016.6117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin YS, Lee WJ, Wang SJ, Fuh JL. Levels of plasma neurofilament light chain and cognitive function in patients with Alzheimer or Parkinson disease. Sci Rep. 2018;8:17368-1-8. 10.1038/s41598-018-35766-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewczuk P, Ermann P, Andreasson U, Schultheis C, Podhorna J, Spitzer P et al. Plasma neurofilament light as a potential biomarker of neurodegeneration in Alzheimer’s disease. Alz Res Ther. 2018;10:71-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin CH, Li CH, Yang KC, Lin FJ, Wu CC, Chieh JJ, et al. Blood NfL: A biomarker for disease severity and progression in Parkinson disease. Neurol. 2019;93:e1–8. [DOI] [PubMed] [Google Scholar]
- 22.Yang SY, Jian ZF, Horng HE, Hong CY, Yang HC, Wu CC, et al. Dual immobilization and magnetic manipulation of magnetic nanoparticles. J Magn Magn Mater. 2008;320:2688–2691. [Google Scholar]
- 23.Hong CY, Chen WH, Jian ZF, Yang SY, Horng HE, Yang LC, Yang HC. Wash-free immunomagnetic detection for serum through magnetic susceptibility reduction. Appl Phys Lett. 2007;90:74105-1-3. [Google Scholar]
- 24.Chieh JJ, Yang SY, Jian ZF, Wang WC, Horng HE, Yang HC, et al. Hyper-high-sensitivity wash-free magnetoreduction assay on bio-molecules using high-Tc superconducting quantum interference devices. J Appl Phys. 2008;103:14703-1-6. [Google Scholar]
- 25.Yang SY, Wang WC, Lan CB, Chen CH, Chieh JJ, Horng HE et al. Magnetically enhanced high-specificity virus detection using bio-activated magnetic nanoparticles with antibodies as labeling markers. J Virol Methods. 2010;64:14–18. [DOI] [PubMed] [Google Scholar]
- 26.Yang CC, Yang SY, Chen HH, Weng WL, Horng HE, Chieh JJ, et al. Effect of molecule-particle binding on the reduction in the mixed-frequency ac magnetic susceptibility of magnetic bio-reagents. J Appl Phys. 2012;112:24704-1-4. [Google Scholar]
- 27.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. 10.1136/jnnp.55.3.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alz Dement. 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oosterveld LP, Verberk IMW, Majbour NK, El-Agnaf OM, Weinstein HC, Berendse HW et al. CSF or serum neurofilament light added to α-synuclein panel discriminates Parkinson’s from controls. Movement Disorders. 2019; 10.1002/mds.27897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosengren LE, Karlsson JE, Karlsson JO, Persson LI, Wikkelso C. Patients with amyotrophic lateral sclerosis and other neurodegenerative diseases have increased levels of neurofilament protein in CSF. J Neurochem. 1996;67:2013–2018. 10.1046/j.1471-4159.1996.67052013.x [DOI] [PubMed] [Google Scholar]
- 31.van Geel WJA, Rosengren LE, Verbeek MM. An enzyme immunoassay to quantify neurofilament light chain in cerebrospinal fluid. J Immunol Methods. 2005;296:179–185. 10.1016/j.jim.2004.11.015 [DOI] [PubMed] [Google Scholar]
- 32.Gaiottino J, Norgren N, Dobson R, Topping J, Nissim A, Malaspina A et al. Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLOS ONE 2013;8:e75091-1-9. 10.1371/journal.pone.0075091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waldö ML, Santillo AF, Passant U, Zetterberg H, Rosengren L, Nilsson C, et al. Cerebrospinal fluid neurofilament light chain protein levels in subtypes of frontotemporal dementia. BMC Neurol. 2013;13:54-1-8. 10.1186/1471-2377-13-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rohrer JD, Woollacott IOC, Dick KM, Brotherhood E, Gordon E, Fellows A et al. Serum neurofilament light chain protein is a measure of disease intensity in frontotemporal dementia. Neurol. 2016;87:1329–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuhle J, Barro C, Andreasson U, Derfuss T, Lindberg R, Sandelius Å, ET AL. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med. 2016;54:1655–1661. 10.1515/cclm-2015-1195 [DOI] [PubMed] [Google Scholar]
- 36.Yang SY, Wang WC, Lan CB, Chen CH, Chieh JJ, Horng HE et al. Magnetically enhanced high-specificity virus detection using bio-activated magnetic nanoparticles with antibodies as labeling markers. J Virol Method. 2010;164:14–18. [DOI] [PubMed] [Google Scholar]
- 37.Yang CC, Huang KW, Yang SY, Chen HH, Chen TC, Ho CS et al. Development for high-accuracy in vitro assay of vascular endothelial growth factor using nanomagnetically labeled immunoassay. J Nanomater. 2013;2013:695276-1-7. [Google Scholar]