Abstract
Introduction:
Bladder cancer is the most common malignancy of the urinary tract, and recurrence following transurethral resection poses the biggest challenge. Intravesical Bacillus Calmette–Guerin (BCG) maintenance with the Southwest Oncology Group (SWOG) protocol remains the gold standard but with poor patient compliance.
Materials and Methods:
The present study aims to compare the SWOG maintenance protocol with a monthly maintenance protocol comprising 12 monthly doses of intravesical BCG. Patients are included in the study only if induction BCG is completed and cystoscopy at 3 months is negative. All patients receive 80 mg BCG in each dose with strict cystoscopic surveillance every 3 months.
Results:
Patient demographics and tumor characteristics were similar in the two groups. There were no statistically significant differences in outcome in terms of recurrence, progression, and adverse reactions in both the groups. Although a larger number of patients in the SWOG maintenance group were lost to follow-up, the difference was not statistically significant proportions.
Conclusion:
From this study, we can conclude that monthly maintenance BCG for 1 year is comparable in terms of outcome with SWOG protocol maintenance BCG. A greater percentage of patients in the monthly maintenance protocol completed the treatment as planned.
Keywords: BCG immunotherapy, maintenance schedule, progression, recurrence
INTRODUCTION
Urinary bladder cancer is the most common urinary tract malignancy and the ninth most common cancer worldwide. In women, it is the 17th most common malignancy, whereas in men, it is the seventh most common malignancy.[1] Nearly 70% of urothelial cancers at presentation are nonmuscle invasive and are treated by transurethral resection (TUR). Without further treatment, a majority of nonmuscle-invasive bladder cancers (NMIBCs) undergo recurrence and progression. Recurrence occurs in 50%–70% of cases of low-grade Ta lesions and in over 80% of high-grade T1 lesions. Progression occurs in 5% of low-grade Ta and up to 50% of high-grade T1 lesions over 3 years.[2]
A variety of intravesical adjuvant immunotherapy and chemotherapy agents have been used to prevent progression and decrease recurrence in NMIBC. Five meta-analyses have confirmed that BCG after TURBT is superior to TURBT alone or TURBT + chemotherapyfor preventing the recurrence of NMIBC. Three RCTs of intermediate-and high-risktumours have compared BCG with epirubicin and interferon, MitomycinC, or epirubicin alone and have confirmed the superiority of BCG for prevention of tumour recurrence.[3] Intravesical BCG acts by direct bindings to fibronectin in the bladder wall, leading to direct stimulation of cell-mediated immunological response and antiangiogenic state. Although BCG has been in use for over 40 years, the dosage and instillation regimen remains largely empirical and arbitrary. In multiple studies, maintenance regimens have been found to be more effective than induction only regimens. The maintenance protocol advocated by Lamm[4] is considered the present gold standard and includes an intense maintenance protocol of 3 weekly doses of BCG at the 3rd and 6th months following transurethral resection of bladder tumor (TURBT) and then 6 monthly up to 3 years. Despite good results, patient compliance is poor and only 16% of patients complete the entire treatment as planned. Several studies report good results with 1-year monthly maintenance regimens. The EAU guidelines recommend BCG immunotherapy maintenance for one year in intermediate risk patients and three years in high risk patients.[3]
The present study aims to compare the tolerability and efficacy in preventing recurrence and progression with an induction course of one instillation every week for 6 weeks and a single monthly instillation as maintenance for 1 year and in those who receive the present gold standard maintenance therapy according to the SWOG protocol with a minimum of 1-year follow-up.
MATERIALS AND METHODS
The study was conducted from October 2016 to December 2018 in a tertiary hospital in Eastern India. The study was conducted after ethical approval from the Institutional Ethics committee. It was a prospective randomized controlled study which included all patients having completely resected high-grade Ta/T1 NMIBC (high-risk group) and those having multiple/recurrent low-grade NMIBC (intermediate-risk group) and was previously BCG naive. Patients who were elder than 85 years, immunocompromised, incontinent, had a history of BCG sepsis, history of prior intravesical chemotherapy or immunotherapy, or had muscle-invasive bladder cancer were excluded from the study. All patients fulfilling inclusion and exclusion criteria were included in the study after completing induction phase of BCG and having a negative check cystoscopy at 3 months from initiation of induction phase.
The primary study end point was recurrence of disease. The sample size calculation was done with a level of significance set at 5% and power of 80%. Using statistical software, the sample size was calculated as 35 individuals in standard group and 38 in new treatment group. The patients were randomized into two groups using a computer-generated program after completion of induction phase of intravesical BCG. All patients recruited to the study received 80 mg intravesical BCG for 6 consecutive weeks as induction phase of intravesical BCG, 4 weeks after TURBT in sterile urine and in the absence of hematuria. Six weeks after induction phase, a check cystoscopy was done, and patients with no tumor were randomized into the two study groups.
The two study groups received differing maintenance regimens of intravesical BCG. Group A patients received BCG following the SWOG protocol of 80 mg BCG for 3 consecutive weeks at 3 and 6 months and 6 monthly thereafter for a period of 3 years. Group B patients received a monthly dosage of intravesical BCG for 12 doses. For this study, injection BCG (Moscow strain) containing 40 mg each vial (1-8 × 108) CFU were used; the dose was prepared by dissolving two vials of BCG with 50 ml of saline. The cold chain procedure was maintained strictly. The prepared solution was administered in the urinary bladder per urethral under gravity drainage. Patients were asked to retain urine for 2 h. Patients were kept under cystoscopic surveillance every 3 monthly for 2 years and 6 monthly thereafter. At each visit, any BCG toxicity following previous BCG dose was documented according to the Cleveland Clinic approach.[5] A very strict schedule was followed for BCG maintenance with telephonic reminders to patients within 48 h of missing a scheduled dose. Prior counseling before recruitment to the study was mandatory, and patients explained the need to complete maintenance on time and to be on regular cystoscopic surveillance for early detection of any recurrence.
The objectives of the study were to compare the efficacy of the two maintenance protocols in preventing recurrence and progression, to compare patient compliance and tolerability of BCG in both the groups. A minimum duration of follow-up of 1 year, i.e., three maintenance cycles in Group A and all 12 monthly cycles in Group B, was selected based on prior subset analysis studies by Decobert et al.[6] All data for statistical analyses were entered into a Microsoft Excel spreadsheet and then analyzed by SPSS (version 24.0; SPSS Inc., Chicago, IL, USA) and GraphPad Prism version 5 (Graph Pad Software Inc., San Diego, California).
RESULTS
A total of 90 patients were included in this study and randomized into two groups. Group A and Group B had 49 and 41 patients, respectively. Eight patients of Group A were lost to follow-up or discontinued therapy, whereas one patient died of acute myocardial infarction (nondisease-related mortality). Three patients of Group B were lost to follow-up. Thus, in the final analysis, Group A included 40 patients and Group B included 38 patients. Group A had 7 patients 17.5% (n = 40) and Group B had 7 patients 18.4% (n = 38) in intermediate-risk group [Figure 1].
Figure 1.
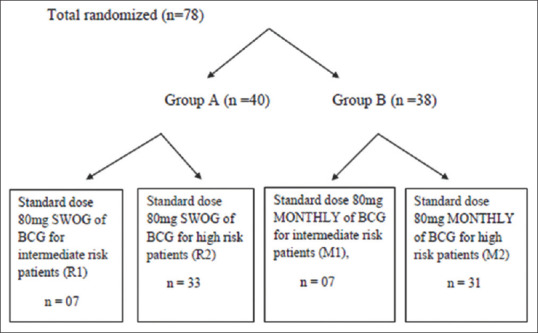
Randomization of patients
Table 1 shows the patient demographics and tumor characteristics in the two groups. The median age of patients in both the groups was 55 years. The mean age of patients in Group A was 52.7 ± 12.75 years (standard deviation [SD]) and that in Group B was 55.58 ± 12.12 years which was not statistically significant (P = 0.3106). Majority of the patients in both the groups were over 40 years of age. About 87.5% of the patients in Group A and 84.2% of the patients in Group B were males which was not statistically significant (P = 0.6765).
Table 1.
Patient demographics and tumor characteristics in the two groups
| Group A | Group B | χ2 | P | |
|---|---|---|---|---|
| Patient demographics | ||||
| Age group (years) | ||||
| <30 | 3 | 1 | 2.0914 | 0.8364 |
| 31-40 | 4 | 4 | ||
| 41-50 | 9 | 7 | ||
| 51-60 | 13 | 11 | ||
| 61-70 | 10 | 13 | ||
| >70 | 1 | 2 | ||
| Sex | ||||
| Male | 35 | 32 | 0.1741 | 0.6765 |
| Female | 5 | 6 | ||
| Tumor characteristics | ||||
| Type of bladder tumor | ||||
| Primary | 33 | 30 | 0.1583 | 0.6906 |
| Recurrent | 7 | 8 | ||
| T staging | ||||
| Ta | 7 | 8 | 0.1583 | 0.6906 |
| T1 | 33 | 30 | ||
| Histopathological grade of tumor | ||||
| Low grade | 21 | 23 | 0.5106 | 0.4749 |
| High grade | 19 | 15 | ||
| Risk stratification | ||||
| Intermediate risk | 7 | 7 | 0.0112 | 0.9156 |
| High risk | 33 | 31 |
Table 2 shows the outcome in terms of recurrence, progression, and adverse reactions to BCG. There was a recurrence of tumor in one patient in Group A and none in Group B in the intermediate-risk group. In the high-risk group, there was a recurrence in 15.2% of the patients in Group A and 16.1% of the patients in Group B. There was a disease progression to muscle-invasive disease in one patient in either group. Recurrence and progression difference among the two groups was not statistically significant. In Group A, 10 (25.0%) patients had Grade 1 toxicity dose of BCG. In Group B, 11 (28.9%) patients had Grade 1 toxicity dose of BCG. This was not statistically significant (P = 0.6944). Thus, the patient outcomes in the two groups were comparable and not statistically significant.
Table 2.
Results in terms of recurrence, progression, and Bacillus Calmette-Guerin toxicity in the two groups
| Group A | Group B | χ2 | P | |
|---|---|---|---|---|
| Recurrence | ||||
| Intermediate-risk group | 1 out of 7 | 0 out of 7 | 1.077 | 0.2993 |
| High-risk group (%) | 5.33 (15.2) | 5.31 (16.1) | 0.0116 | 0.9142 |
| Progression | 1.40 | 1.38 | 0.0014 | 0.9706 |
| BCG toxicity | ||||
| Grade 1 (%) | 10.40 (25) | 11.38 (28.9) | 0.1543 | 0.6944 |
| Grade 2 | 0 | 0 | ||
| Grade 3 | 0 | 0 |
BCG: Bacillus Calmette-Guerin
Figure 2 shows the Kaplan–Meier plotting of recurrence-free survival in the two groups with increasing follow-up duration. The results are not statistically significant (P = 0.875).
Figure 2.
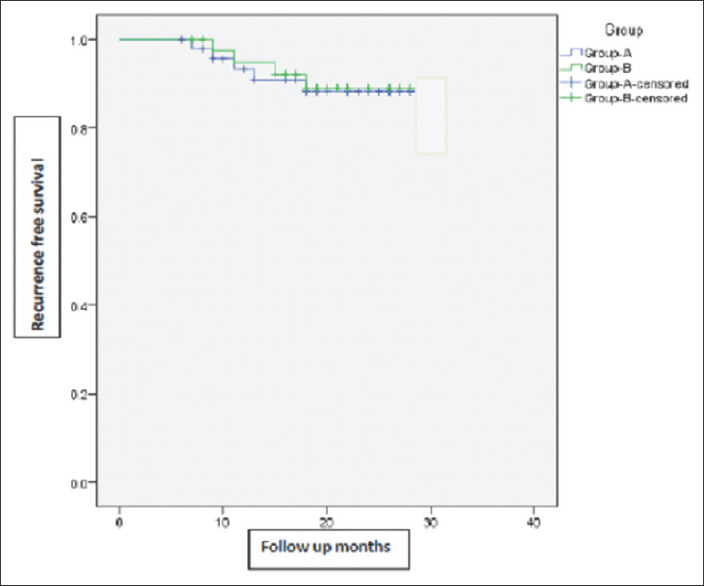
Kaplan–Meier chart showing recurrence-free survival in the two groups with increasing follow-up duration
In Group A, 9 patients out of the initially allotted 49 were lost to follow-up (18.4%). In Group B, 3 patients out of the initially allotted 41 were lost to follow-up (7.3%). On telephonic reminder, a majority of these patients could be contacted and were further counseled to complete BCG maintenance. Table 3 shows the reason for discontinuation and the duration of follow-up after which the patients discontinued maintenance BCG.
Table 3.
Details of patients who discontinued treatment
| Group | Follow-up months | Doses | Reason | Symptoms |
|---|---|---|---|---|
| A | 8 | 9 | No need to continue | Doing well |
| A | 6 | 9 | Could not be contacted | |
| A | 9 | 9 | Died due to AMI | - |
| A | 13 | 12 | Workday loss | Doing well |
| A | 10 | 9 | Discomfort due to BCG | Doing well |
| A | 7 | 9 | Could not be contacted | |
| A | 10 | 12 | No need to continue | Doing well |
| A | 13 | 12 | Discomfort due to BCG | Doing well |
| A | 19 | 15 | No reason given | Hematuria |
| B | 8 | 9 | Discomfort due to BCG | Doing well |
| B | 7 | 8 | Could not be contacted | |
| B | 8 | 8 | Discomfort due to BCG | Doing well |
BCG: Bacillus Calmette-Guerin, AMI: Acute myocardial infarction
DISCUSSION
The optimal schedule as well as the dose of BCG is unclear even though it is being used since 1976. A variety of maintenance regimens of BCG have been studied. Initial studies were designed to establish whether maintenance regimens were at all more beneficial than induction only regimens. Sylvester[7] described a study of 397 patients where maintenance in the form of single-dose 3 monthly BCG failed to fare significantly better than induction only regimen.
Sylvester et al.[8] in a meta-analysis of seven randomized controlled trials showed that 36.7% of patients who received a single postoperative instillation of epirubicin, mitomycin C, thiotepa, or pirarubicin had recurrence compared to 48.4% who did not receive any adjuvant treatment, a decrease of 39% in the odds of recurrence (odds ratio [OR]: 0.61, P < 0.0001). It also showed that for patients with multiple tumors, a single instillation is not sufficient. Thus, a need for prolonged adjuvant treatment emerged. Of the various drugs considered for adjuvant treatment for reducing recurrence in NMIBC, BCG was one alternative as it had shown antitumor activities in several different cancers including urothelial carcinoma.[9] The initial studied regimen included percutaneous dosing which was later modified to intravesical dosing.[9]
Han and Pan[10] performed a meta-analysis which demonstrated a statistically significant difference in the odds ratio (OR = 0.61, P < 0.0001) for tumor recurrence between the BCG and non-BCG-treated groups. Sylvester et al.[11] demonstrated in a meta-analysis that intravesical BCG was not only effective in reducing recurrence but also significantly reduces the risk of progression in NMIBC patients receiving BCG maintenance. The European Organization for Research and Treatment of Cancer meta-analysis suggested that there are no large differences in efficacy between various BCG strains.[11]
In the landmark SWOG trial by Lamm et al.,[12] they showed that a maintenance regimen consisting of 3 weekly doses of intravesical BCG at the 3rd and 6th months followed by 6 monthly till 3 years from the start of induction was significantly better to decrease recurrence and progression in NMIBC. Although this study firmly established the role of BCG maintenance, only 16% of the patients completed the full treatment as initially planned. Two-thirds of the patients who stopped treatment stopped within the first 6 months. As the treatment group still fared better significantly despite failing to complete treatment, the maximum benefit might have been achieved earlier. The reasons for stopping treatment were not mentioned in the study.
Decobert et al. evaluated the outcome of number of maintenance cycles of BCG received on recurrence. Using subset analysis, they showed that lower recurrence and progression rates associated with more cycles administered. Based on their results, they presented the conclusion that a minimum of three cycles of BCG maintenance are required to reduce recurrence rates significantly.[6] This was the basis of selecting 1-year follow-up with three maintenance cycles in our patients.
Okamura et al.[13] studied the role of monthly 80 mg BCG maintenance 6–8 doses in 75 patients with NMIBC. At 5-year follow-up, the recurrence-free survival rate was 83% in the maintenance group compared to 51.9% in the nonmaintenance group. They concluded that the monthly regimen was effective in decreasing recurrence. Yoo et al.[14] in another study of 126 patients demonstrated the efficacy of monthly maintenance regimen for 1 year. The 2-year cumulative recurrence-free survival was 52.6% in the nonmaintenance group versus 77.3% in the maintenance group. Progression at 2 years was seen in 8.9% of the patients in the maintenance group and 19.5% of the patients in the nonmaintenance group. Side effects were seen 40.2% of the patients receiving maintenance. Farah et al.[15] in their study on monthly maintenance BCG showed that 70% of patients completed the treatment as planned. At a median follow-up of 24.2 months, 26.7% of the patients had recurrence. At a median follow-up of 33 months, 8.3% of the patients had disease progression. There were no cancer-specific deaths. In a previous study by Badalament et al.,[16] the authors concluded that monthly maintenance BCG for 2 years did not prevent, decrease, or delay recurrence or progression in comparison to patients who received only induction BCG.
In our study, patient demographics and tumor characteristics were comparable in both the groups. The mean follow-up was 24 months in both the groups ranging from 15 to 31 months. In case of the high-risk group of patients, recurrence is 15.2% in Group A and 16.1% in Group B which is statistically insignificant in both the groups (P > 0.05). One patient in each group had disease progression (P > 0.05). Regarding toxicity, no patients suffered from Grade 2 or 3 toxicity. Toxicity in both the groups was also statistically insignificant. There was no need to stop BCG due to toxicity in any patient. Thus, we see in our study that in the monthly maintenance group (Group B), at 2-year medial follow-up, 83.9% of the patients are recurrence free. This recurrence rate is in line with other studies by Okamura et al.[13] and Yoo et al.[14]
In the SWOG maintenance group (Group A), 15% of the patients had recurrence at a 2-year mean follow-up. This has varied in various studies from as low as 11% to as high as 45%. Both the groups of patients tolerated BCG well with no need to stop BCG due to adverse reactions. Nearly 18.4% of the patients in Group A were lost to follow-up, whereas only 7.3% of the patients were lost to follow-up in Group B. This is in line with few studies with monthly maintenance which have reported nearly all patients completing full treatment as planned, whereas others have reported as high as 30% dropout rates. In comparison, only 16% of the patients completed the full treatment in the SWOG study.[12] We hypothesize from the reason given by our patients that the greater number of patients completing treatment as planned in the monthly maintenance group is due to continuing treatment at short regular intervals. In the SWOG maintenance group, most dropouts occurred after first maintenance when patients remained asymptomatic after the first maintenance for 3 months and discontinued further treatment.
The limitations of our study were small number of patients, short follow up duration and the study being from a single centre. Thus, to summarize, there were no statistically significant differences in outcome in terms of recurrence, progression, and adverse reactions in both the groups. Although a larger proportion of patients in the SWOG maintenance group were lost to follow-up, it did not reach statistically significant proportions.
CONCLUSION
From this study, we can conclude that monthly maintenance BCG for 1 year is comparable in terms of outcome with SWOG protocol maintenance BCG. A greater percentage of patients in the monthly maintenance protocol completed the treatment as planned. Although patient compliance was numerically better in the monthly maintenance group, it did not reach statistically significant levels.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder Cancer Incidence and Mortality: A Global Overview and recent trends. EurUrol. 2017;71:96–108. doi: 10.1016/j.eururo.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Van Rhijn BWG, Burger M, Lotan Y, Solsona E, Stief CG, Sylvester RJ, et al. Recurrence and Progression of Disease in Non–Muscle-Invasive Bladder Cancer: From Epidemiology to Treatment Strategy. EurUrol. 2009;56:430–42. doi: 10.1016/j.eururo.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 3.Babjuk M, Burger M, Comperat E, Gontero P, Mostafid AH, Polau J, et al. EAU Guidelines Non Muscle Invasive Bladder Cancer presented at the EAU Annual Congress Barcelona. 2019 [Google Scholar]
- 4.Lamm DL. Efficacy and safety of bacillus Calmette-Guerin immunotherapy in superficial bladder cancer. Clin Infect Dis. 2000;31(Supplement 3):86–90. [Google Scholar]
- 5.Stephen Jones J. In: Non Muscle invasive Bladder cancer Campbell Walsh Urology. 11th ed. Wein AJ, editor. Elsevier Publication; 2016. pp. 2214–5. [Google Scholar]
- 6.Decobert M, LaRue H, Harel F, Meyer F, Fradet Y, Lacombe L. Maintenance Bacillus Calmette-Guérin in high-risk nonmuscle-invasive bladder cancer: How much is enough? Cancer. 2008;113:710–6. doi: 10.1002/cncr.23627. [DOI] [PubMed] [Google Scholar]
- 7.Sylvester RJ. Maintenance BCG therapy – the search for optimum treatment schedule continues. EurUrol. 2015;68:263–4. doi: 10.1016/j.eururo.2015.03.029. [DOI] [PubMed] [Google Scholar]
- 8.Sylvester RJ, Oosterlinck W, van der Meijden AP. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: A meta-analysis of published results of randomized clinical trials. J Urol. 2004;171:2186–90. doi: 10.1097/01.ju.0000125486.92260.b2. [DOI] [PubMed] [Google Scholar]
- 9.Morales A, Eidinger D, Bruce AW. Intracavitary bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116:180–3. doi: 10.1016/s0022-5347(17)58737-6. [DOI] [PubMed] [Google Scholar]
- 10.Han RF, Pan JG. Can intravesical Bacillus Calmette-Guérin reduce recurrence in patients with superficial bladder cancer.A meta-analysis of randomized trials? Urology. 2006;67:1216–23. doi: 10.1016/j.urology.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 11.Sylvester RJ, van der Meijden AP, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: A meta-analysis of the published results of randomized clinical trials. J Urol. 2002;168:1964–70. doi: 10.1016/S0022-5347(05)64273-5. [DOI] [PubMed] [Google Scholar]
- 12.Lamm DL, Blumenstein BA, Crissman JD, Montie JE, Gottesman JE, Lowe BA, et al. Maintenance Bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: A randomized Southwest Oncology Group Study. J Urol. 2000;163:1124–9. [PubMed] [Google Scholar]
- 13.Okamura T, Akita H, Ando R, Ikegami Y, Naiki T, Kawai N, et al. Single monthly Bacillus Calmette-Guérin intravesical instillation is effective maintenance therapy to prevent recurrence in Japanese patients with non-muscle-invasive bladder cancer. Int J Clin Oncol. 2012;17:477–81. doi: 10.1007/s10147-011-0314-3. [DOI] [PubMed] [Google Scholar]
- 14.Yoo KH, Lim TJ, Chang SG. Monthly intravesical Bacillus Calmette-Guérin maintenance therapy for non-muscle-invasive bladder cancer: 10-year experience in a single institute. Exp Ther Med. 2012;3:221–5. doi: 10.3892/etm.2011.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farah NB, Ghanem R, Amr M. Treatment efficacy and tolerability of intravesical Bacillus Calmette.Guerin (BCG).RIVM strain: Induction and maintenance protocol in high grade and recurrent low grade non.muscle invasive bladder cancer (NMIBC) BMC Urol. 2014;14:11. doi: 10.1186/1471-2490-14-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Badalament RA, Herr HW, Wong GY, Gnecco C, Pinsky CM, Whitmore WF, Jr, et al. A prospective randomized trial of maintenance versus nonmaintenance intravesical Bacillus Calmette-Guérin therapy of superficial bladder cancer. J Clin Oncol. 1987;5:441–9. doi: 10.1200/JCO.1987.5.3.441. [DOI] [PubMed] [Google Scholar]


