Abstract
Introduction:
Nail toxicity is a relatively uncommon cutaneous adverse effect of chemotherapeutic agents. Rapidly dividing cells of the nail matrix are perturbed by the antimitotic activity of these agents. Although most of these changes are cosmetic and regress once the therapy is completed, a few of these adverse effects are challenging to manage and require temporary or permanent suspension of chemotherapeutic agents.
Materials and Methods:
A total of 205 patients with various malignancies and under chemotherapy in oncology ward of the hospital over a period of 3 months were screened for nail involvement postchemotherapy. Relevant details, protocol of chemotherapeutic agents were assessed. Nail examination was carried out in daylight and the changes were analyzed.
Results:
A total of 124 (60.4%) patients had nail changes due to chemotherapeutic agents. The most common change was diffuse hyperpigmentation in 101 (81.4%) patients commonly due to a combination of cyclophosphamide and adriamycin in 43 (42.5%) patients. Longitudinal melanonychia was seen in 36 (29%), Beau's lines in 31 (25%), onychomadesis in 17 (13.7%), Mees' lines in 15 (12%), paronychia in 12 (9.6%), subungual hyperkeratosis in 10 (8%), and Muehrcke's lines in 4 (3.2%) patients. All the patients who developed Muehrcke's lines were on a combination of cyclophosphamide/doxorubicin/5 FU. Exudative onycholysis was observed in 2 (1.6%) patients; both these patients were on paclitaxel therapy. A total 2 (1.6%) patients who developed exudative onycholysis were advised discontinuation and another substitute chemotherapy was advised. Therapy for 2 (1.6%) patients who developed acute paronychia due to gefitinib was temporarily suspended. Unfortunately, most of the patients were on multiple chemotherapeutic agents hence, we could not pinpoint one drug as a cause. Therefore, a combination of agents was implicated in most cases.
Conclusion:
Nail toxicities are common with chemotherapeutic agents, however less importance is given to nail involvement. Apart from being cosmetically significant, a few adverse effects may warrant modification of the chemotherapy.
KEY WORDS: Beau's lines, chemotherapeutic agents, Mees' lines, nail changes, nail matrix
Introduction
Nail toxicity is a relatively uncommon adverse effect of chemotherapeutic agents. A wide array of nail changes ranging from cosmetic disfigurement to those requiring alteration in chemotherapy has been reported. Continuously dividing nail matrix cells make the nail apparatus an easy target of antimitotic activity of chemotherapeutic agents.[1] The nail changes may involve multiple or all 20 nails which appear in temporal relation with the drug intake. In most cases, the nail changes are only cosmetically disturbing; however, at times, pain and associated discomfort can result in the inability to perform daily activities and may require alteration in chemotherapy. Effects are mostly transitory in nature and subside on withdrawal of the chemotherapeutic agents but occasionally these may persist.[2] Common nail changes reported in literature include leukonychia, Beau's lines, brittle thin nails, and nail hyperpigmentation which may be diffuse or horizontal.[3,4,5,6]
At present most of these nail toxicities are reported in the form of case reports, especially from our country. In the present study, we have compiled the entire spectrum of nail changes noted with chemotherapeutic agents.
Materials and Methods
This is a descriptive study conducted over a period of 3 months from June 2018 to August 2018 in a tertiary care hospital in western India. Ethics committee permission was obtained and patients were briefed about the nature of the study. Written informed consent was obtained from the patients who were willing to participate in the study. Patients who denied to be a part of the study, on concurrent radiotherapy or were terminally ill were excluded. All the patients admitted to the oncology department of the hospital or referred to dermatology center from oncology for any skin condition were included and examined for nail involvement. All patients who had nail changes postchemotherapy initiation were included.
The relevant demographic data, details of chemotherapy protocol, and details of nail changes were recorded. Nails were examined in daylight and photographs were taken. The data were recorded and analyzed.
Results
Out of the 205 patients screened, 124 (60.4%) had nail changes postchemotherapy. Of 124 patients with nail involvement, 65 (52.4%) were female and 59 (47.6%) were male. The mean age was 43 (range: 14–77 years). The most common nail change was diffuse hyperpigmentation in 101 (81.4%) patients [Figures 1 and 2]; a combination of chemotherapeutic agents associated with it are depicted in Table 1. Longitudinal melanonychia was seen in 36 (29%) patients on a combination of cyclophosphamide/adriamycin/vincristine, cyclophosphamide/adriamycin, and hydroxyurea, bleomycin, and cyclophosphamide. Various other nail adverse effects and their frequency of occurrence and associated chemotherapeutic agents are detailed in Table 1. Beau's lines were seen in 31 (25%) patients and drugs implicated were docetaxel, paclitaxel, and combination of epirubicin/vincristine/cyclophosphamide and fluorouracil/doxorubicin/cyclophosphamide. Onychomadesis was seenin 17 (13.7%) [Figure 3] on imatinib, paclitaxel, capecitabine, and cyclophosphamide/vincristine/procarbazine/prednisolone combination; Mees' lines in 15 (12%) patients [Figure 4], followed by acute paronychia in 12 (9.6%) patients, subungual hyperkeratosis in 5 (41.6%) while exudative onycholysis was seen in 2 (1.6%) patients on paclitaxel therapy [Figure 5]. The details of chemotherapeutic agents and their frequency of association with nail changes have been detailed in Table 1.
Figure 1.

Diffuse nail hyperpigmentation in a patient
Figure 2.
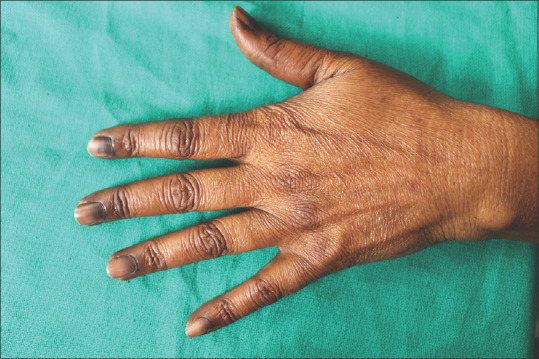
Diffuse hyperpigmentation of nails of all the fingers in a patient on a combination of adriamycin and cyclophosphamide
Table 1.
Chemotherapeutic agents associated with nail toxicity
| Nail Changes | Combinations used | Number (%) |
|---|---|---|
| Diffuse hyperpigmentation | -Cyclophosphamide/Adriamycin | 43/101 (42.5%) |
| -Cyclophosphamide/Adriamycin/Vincristine | 26/101 (25.7%) | |
| -Cyclophosphamide/Adriamycin/Docetaxel | 16/101 (15.8%) | |
| -Paclitaxel/Carboplatin | 7/101 (6.9%) | |
| -Hydroxyurea | 5/101 (4.9%) | |
| -Bleomycin | 4/101 (3.9%) | |
| Longitudinal Melanonychia | -Cyclophosphamide/Adriamycin/Vincristine | 13/36 (36.1%) |
| -Cyclophosphamide/Adriamycin | 9/36 (25%) | |
| -Hydroxyurea | 6/36 (16.6%) | |
| -Bleomycin | 4/36 (11.1%) | |
| -Cyclophosphamide | 4/36 (11.1%) | |
| Beau’s lines | -Docetaxel | 14/31 (45.1%) |
| -Paclitaxel | 9/31 (29%) | |
| -Epirubicin/vincristine/cyclophosphamide | 5/31 (16.1%) | |
| -Fluorouracil/doxorubicin/cyclophosphamide | 3/31 (9.6%) | |
| Onychomadesis | -Imatinib | 5/17 (29.4%) |
| -Paclitaxel | 4/17 (23.5%) | |
| -Capecitabine | 4/17 (23.5%) | |
| -Cyclophosphamide/vincristine/procarbazine/prednisolone | 4/17 (23.5%) | |
| Mees’ lines | -Cyclophosphamide/doxorubicin/vincristine/prednisolone | 6/15 (40%) |
| -Cytarabine/daunorubicin | 5/15 (33.3%) | |
| - Docetaxel | 4/15 (26.6%) | |
| Paronychia | -Docetaxel | 5/12 (41.6%) |
| -Cetuximab | 4/12 (33.3%) | |
| -Geftinib | 2/12 (16.6%) | |
| -Fluorouracil | 1/12 (8.3%) | |
| Subungal hyperkeratosis | -Docetaxel | 3/5 (60%) |
| - Fluorouracil | 2/5 (40%) | |
| Muehrcke’s lines | -Cyclophosphamide/doxorubicin/5 FU | 4/4 (100%) |
| Exudative Onycholysis | -Paclitaxel | 2/2 (100%) |
*Percentages are derived from the total number of patients with that particular side effect
Figure 3.
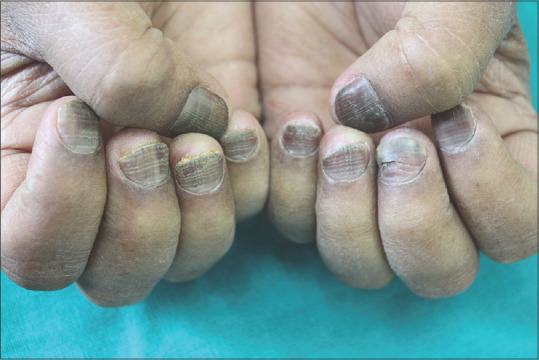
Onychomadesis of the nail of left middle finger in a patient on Imatinib
Figure 4.

Mees' lines in nails of all fingers in a patient on a combination of cytarabine and daunorubicin
Figure 5.
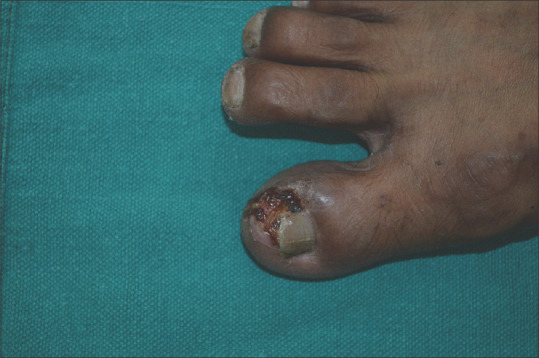
Acute paronychia of the nail of great toe in a patient on geftinib
Therapy for both the patients who developed exudative onycholysis was stopped as they developed purulent discharge with secondary infection. Two (1.6%) patients who developed acute paronychia [Figure 6] due to gefitinib were advised for substitution of the therapy and were sent back to treating oncologist.
Figure 6.
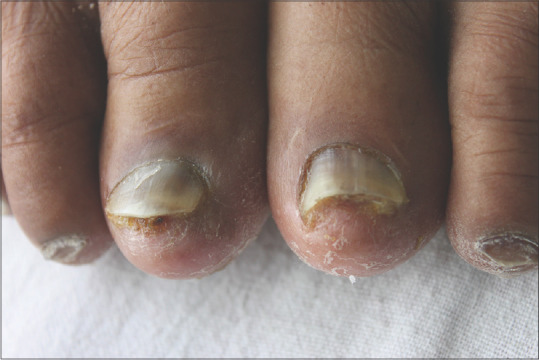
Paclitaxel induced exudative onycholysis of nails of the bilateral great toe in a patient
Discussion
Chemotherapeutic agents are associated with a wide array of cutaneous and appendageal toxicities. The involvement of nail in cancer therapy is reported in fragmented literature from all over the world. Although it is mostly of cosmetic concern, at times it may require alteration or modification of the therapy. Most of the nail toxicities are results of acute insult to the nail epithelium; however, symptoms vary depending upon the structure of the nail apparatus involved and severity of the insult. In the present study, diffuse hyperpigmentation of nail plate was the most common nail change observed in 101 (81.5%) patients followed by longitudinal melanonychia in 36 (29%) patients. Other adverse effects of chemotherapy on nail noted were Beau's lines in 31 (25%), onychomadesis in 17 (13.7%), Mees' lines in 15 (12.1%), paronychia in 12 (9.7%), subungual hyperkeratosis in 5 (41.6%) while exudative onycholysis was seen in 2 (1.6%) patients. At present there is lack of comparative data on frequency of these adverse effects involving nails, hence we could not compare our results with that of other studies.
Diffuse hyperpigmentation of nails is a common adverse effect of chemotherapeutic agents. Pavey et al. found nail hyperpigmentation as the most common adverse effect of chemotherapeutic agents, like the present study.[7] This pigmentation is proposed to be the outcome of matrix melanocyte activation, which usually affects several nails. Activation of a cluster of melanocytes will give rise to longitudinal melanonychia which may be a single longitudinal band while diffuse activation of melanocytes would result in diffuse nail pigmentation.[2] Nail hyperpigmentation is reported with chemotherapeutic agents like cyclophosphamide, docetaxel, doxorubicin, fluorouracil, imatinib, hydroxyurea, and bleomycin which is consistent with the findings of the present study.[8,9,10,11,12,13,14,15,16,17,18]
Beau's lines were seen in 31 (25%) patients in our study. Beau's line is the result of the temporary arrest of the nail plate production by an insult that affects nail matrix keratinocyte. It typically involves all nails and appears after a few weeks of chemotherapy.[19,20,21] In the present study, chemotherapeutic agents such as docetaxel, paclitaxel, combination of epirubicin/vincristine/cyclophosphamide, and cyclophosphamide/doxorubicin/fluorouracil were associated with Beau's lines which are again in agreement with earlier findings.[22,23,24]
Mees' lines and Muehrcke's lines were seen in 15 (12.1%) and 4 (3.2%) patients, respectively in our study while Paveyet al. observed Muechrcke's lines in 4 (12.1%) and Mees' lines in 3 (9%) patients in their study.[7] Mees' lines, also known as true leukonychia are transverse white, non-blanching parallel lines to lunula across the entire nail bed and have no palpable ridges while Muehrcke's lines (apparent leukonychia) are paired white lines caused by vascular congestion in the nail bed which fade on digital compression. The distance between the two lines is related to the interval between two cycles of chemotherapy.[25,26] We observed Mees' lines with the use of docetaxel and combination of cytarabinec/daunorubicin and cyclophosphamide/doxorubicin/vincristine/prednisolone. Similar combinations have been implicated as causative agents of Mees' lines in earlier studies also.[27,28,29] In our study, all 4 (3.2%) patients who developed Muehrcke's lines, were on a combination of cyclophosphamide/doxorubicin, and 5-flurouracil. These lines have been reported with a combination of vincristine/doxorubicin, dexamethasone earlier.[30]
Onychomadesis induced by chemotherapeutic agents was originally described by Kochupillai et al.[31] Since then only five cases have been reported in the literature. In the present study 17 (13.7%) patients developed onychomadesis. It is thought to be the result of arrested mitotic activity in the nail matrix resulting in nail separation and shedding.[32,33] In the present study, the drugs associated with development of onychomadesis were imatinib, paclitaxel, capecitabine, and combination of cyclophosphamide/vincristine/procarbazine/prednisolone. It has been reported earlier with capecitabine.[20]
Paronychia is a frequent but uncommonly reported adverse effect of epidermal growth factor inhibitors which is the result of aberrant vascular response affecting nail folds.[34,35] It is the result of inflammation of proximal/lateral nail folds, which result in erythema, edema, and tenderness of the nail folds. It usually develops soon after intake of the drug and involves one or several nails and is thought to be the result of the toxic effect of the drug on nail epithelium.[2,4] In the present study, patients on cetuximab, gefitinib, 5-fluorouracil, and docetaxel developed paronychia.[36] Therapy for two patients who developed acute paronychia due to gefitinib was suspended temporarily and patients were referred back to treating oncologists for change in management.
Exudative onycholysis was seen in 2 (1.6%) patients, both of them were on paclitaxel. Both the patients presented to us after the first cycle with complaints of painful toenails along with malodorous discharge. The pus from one case showed secondary bacterial growth. The therapy for both the patients was substituted by the oncologist. Paclitaxel has also been reported earlier to cause exudative onycholysis.[37,38,39] At present, there is paucity of data on nail involvement in chemotherapy in consolidated form along with lack of its impact on functional status of the patient.[40] The data on nail involvement are largely available in the form of case reports and the frequency of nail involvement with these agents remains unknown. This study is an attempt to put up the frequency of nail involvement and drugs responsible for these changes. Table 2 depicts the summary of few studies where the nail involvement in chemotherapy has been analyzed.
Table 2.
Key findings in other similar studies
| Study | Sample size | Key findings |
|---|---|---|
| Chen et al.[38] | Sample size 30 Nail involvement in 10 patients |
Muehrcke’s lines, beau’s lines, Mees’ lines trachonychia |
| Pavey et al.[7] | Sample size 53 33 developed nail changes |
Nail hyperpigmentation most common nail change in 26 (78.7%) followed by Muechrcke’s lines 4 (12.1%) and Mees’ lines in 3 (9%) |
| Present study | Sample size 205 Nail changes in 124 |
Diffuse hyperpigmentation in 101 (81.4%), 36 (29%) Melanonychia in 31 (25%) had Beau’s lines, 17 (13.7%), Onychomadesis. 15 (12%) had Mees lines. 12 (9.6%) had Paronychia, 05 (8%) Subungal hyperkeratosis, 04 (6.4%) developed Muehrcke’s lines while 02 (1.6%) had Exudative Onycholysis in |
Conclusion
Nail toxicity is a relatively uncommon adverse effect of the chemotherapeutic agent. Chemotherapeutic agents can involve any site of nail apparatus and have myriads of clinical presentation ranging from cosmetic disfigurement to acute toxicity in the form of paronychia and loss of nail. At times these nail involvements may require urgent suspension of the chemotherapy or alteration in the dosage. It is very important for dermatologists to be well aware of these toxicities and to guide the treating oncologist.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Hinds G, Thomas VD. Malignancy and cancer treatment-related hair and nail changes. Dermatol Clin. 2008;26:59–68. doi: 10.1016/j.det.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Piraccini BM, Tosti A. Drug-induced nail disorders. Drug Safety. 1999;21:187–201. doi: 10.2165/00002018-199921030-00004. [DOI] [PubMed] [Google Scholar]
- 3.Piraccini BM, Iorizzo M, Starace M, Tosti A. Drug induced nail diseases. Dermatol Clin. 2006;24:387–91. doi: 10.1016/j.det.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Piraccini BM, Iorizzo M. Drug reactions affecting the nail unit: Diagnosis and management. Dermatol Clin. 2007;25:215–21. doi: 10.1016/j.det.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Piraccini BM, Iorizzo M, Tosti A. Drug-induced nail abnormalities. Am J Clin Dermatol. 2003;4:31–7. doi: 10.2165/00128071-200304010-00004. [DOI] [PubMed] [Google Scholar]
- 6.Uyttendaele H, Geyer A, Scher RK. Drugs and nails. J Eur Acad Dermatol Venereol. 2004;18:124–5. doi: 10.1111/j.1468-3083.2004.00775.x. [DOI] [PubMed] [Google Scholar]
- 7.Pavey RA, Kambil SM, Bhat RM. Dermatological adverse reactions to cancer chemotherapy. Indian J Dermatol Venereol Leprol. 2015;81:434. doi: 10.4103/0378-6323.159950. [DOI] [PubMed] [Google Scholar]
- 8.Vassallo C, Brazzelli V, Ardigo M, Borroni G. Nail changes secondary to hematologic conditions. Haematologica. 2001;86:334–6. [PubMed] [Google Scholar]
- 9.Susser WS, Whitaker-Worth DL, Grant-Kels JM. Mucocutaneous reactions to chemotherapy. J Am Acad Dermatol. 1999;40:367–98. doi: 10.1016/s0190-9622(99)70488-3. [DOI] [PubMed] [Google Scholar]
- 10.Harrison BM, Wood CBS. Cyclophosphamide and pigmentation. Br Med J. 1972;2:548–9. doi: 10.1136/bmj.2.5809.352-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah P, Rao KR, Patel A. Cyclophosphamide-induced nail pigmentation. Lancet. 1975;306:548–9. doi: 10.1016/s0140-6736(75)90916-2. [DOI] [PubMed] [Google Scholar]
- 12.Shah PC, Rao KR, Patel AR. Cyclophosphamide induced nail pigmentation. Br J Dermatol. 1978;98:675–80. doi: 10.1111/j.1365-2133.1978.tb03587.x. [DOI] [PubMed] [Google Scholar]
- 13.Adam BA. Cyclophosphamide nail pigmentation. Sing Med J. 1981;22:35–7. [PubMed] [Google Scholar]
- 14.Srikanth M, Van Veen J, Raithatha A, Reilly JT. Cyclophosphamide-induced nail pigmentation. Br J Haematol. 2002;117:2. doi: 10.1046/j.1365-2141.2002.03449.x. [DOI] [PubMed] [Google Scholar]
- 15.Dave S, Thappa DM. Peculiar pattern of nail pigmentation following cyclophosphamide therapy. Dermatol Online J. 2003;9:14. [PubMed] [Google Scholar]
- 16.Priestman TJ, James K. Adriamycin and longitudinal pigmented banding of fingernails. Lancet. 1975;305:1337–8. doi: 10.1016/s0140-6736(75)92341-7. [DOI] [PubMed] [Google Scholar]
- 17.Curran CF. Onycholysis in doxorubicin-treated patients. Arch Dermatol. 1990;126:1244. [PubMed] [Google Scholar]
- 18.Prabhash K, Biswas G, Prasad N, Karant N, Sastry PS, Parikh PM. Imatinib-induced nail hyperpigmentation in chronic myeloid leukemia. Indian J Dermatol Venereol Leprol. 2006;72:63–4. doi: 10.4103/0378-6323.19727. [DOI] [PubMed] [Google Scholar]
- 19.Llombart-Cussac A, Pivot X, Spielmann M. Docetaxel chemotherapy induces transverse superficial loss of the nail plate. Arch Dermatol. 1997;133:1466–7. [PubMed] [Google Scholar]
- 20.Chen GY, Chang TW, Chen WC. Exudative hyponychial dermatitis associated with capecitabine and docetaxel combination chemotherapy for metastatic breast carcinoma: Report of three cases. Br J Dermatol. 2003;148:1071–3. doi: 10.1046/j.1365-2133.2003.05272.x. [DOI] [PubMed] [Google Scholar]
- 21.Valero V, Holmes FA, Walters RS, Theriault RL, Esparza L, Fraschini G, et al. Phase II trial of docetaxel: A new, highly effective antineoplastic agent in the management of patients with anthracycline-resistant metastatic breast cancer. J Clin Oncol. 1995;13:2886–94. doi: 10.1200/JCO.1995.13.12.2886. [DOI] [PubMed] [Google Scholar]
- 22.Jacob CI, Patten SF. Nail bed dyschromia secondary to docetaxel therapy. Arch Dermatol. 1998;134:1167–8. doi: 10.1001/archderm.134.9.1167. [DOI] [PubMed] [Google Scholar]
- 23.Pavithran K, Doval DC. Nail changes due to docetaxel. Br J Dermatol. 2002;146:709–10. doi: 10.1046/j.1365-2133.2002.46824.x. [DOI] [PubMed] [Google Scholar]
- 24.Mourad YA, Matta-Muallem M, Shamseddine A. Nail toxicity related to taxanes. Dermatol Online J. 2003;9:15. [PubMed] [Google Scholar]
- 25.Parakh A, Kochhar AM. Chemotherapy induced transverse leukonychia (Mees' lines) Indian Pediatr. 2007;44:865. [PubMed] [Google Scholar]
- 26.Mirfazaelian H, Namazi MR, Daneshbod Y. Mees' lines. ScientificWorldJournal. 2011;11:267–8. doi: 10.1100/tsw.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar S, Diwan S, Dekate M, Goyal S. Mees' lines following chemotherapy. Our Dermatol Online. 2013;4:382. [Google Scholar]
- 28.Shelley WB, Humphrey GB. Transverse leukonychia (Mees' lines) due to aunorubidn chemotherapy. Pediatr Dermatol. 1997;14:144–5. doi: 10.1111/j.1525-1470.1997.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 29.Alzahrani M, Al Jasser M. Nail changes during chemotherpy. N Engl J Med. 2018;379:1561. doi: 10.1056/NEJMicm1801702. [DOI] [PubMed] [Google Scholar]
- 30.Dasanu CA, Vaillant JG, Alexandrescu DT. Distinct patterns of chromonychia, Beau's lines, and melanoderma seen with vincristine, adriamycin, dexamethasone therapy for multiple myeloma. Dermatol Online J. 2006;12:10. [PubMed] [Google Scholar]
- 31.Kochupillai V, Prabhu M, Bhide NK. Cancer chemotherapy and nail loss (Onychomadesis)/Meningeal relapse in acute promyelocytic leukaemia. Acta Haematol. 1983;70:137. doi: 10.1159/000206708. [DOI] [PubMed] [Google Scholar]
- 32.Hussain S, Anderson DN, Salvatti ME, Adamson B, McManus M, Braverman AS. Onycholysis as a complication of systemic chemotherapy: Report of five cases associated with prolonged weekly paclitaxel therapy and review of the literature. Cancer. 2000;88:2367–71. doi: 10.1002/(sici)1097-0142(20000515)88:10<2367::aid-cncr22>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 33.Maino KL, Norwood C, Stashower ME. Onycholysis with the appearance of a “sunset” secondary to capecitabine. Cutis. 2003;72:234–6. [PubMed] [Google Scholar]
- 34.Boucher KW, Davidson K, Mirakhur B, Goldberg J, Heymann WR. Paronychia induced by cetuximab, an antiepidermal growth factor receptor antibody. J Am Acad Dermatol. 2002;47:632–3. doi: 10.1067/mjd.2002.124621. [DOI] [PubMed] [Google Scholar]
- 35.Nakano J, Nakamura M. Paronychia induced by gefitinib, an epidermal growth factor receptor tyrosine kinase inhibitor. J Dermatol. 2003;30:261–2. doi: 10.1111/j.1346-8138.2003.tb00387.x. [DOI] [PubMed] [Google Scholar]
- 36.Mortimer NJ, Mills J. Images in clinical medicine.Beau's lines. N Engl J Med. 2004;351:1778. doi: 10.1056/NEJMicm040187. [DOI] [PubMed] [Google Scholar]
- 37.Freites-Martínez A, Martinez-Sánchez D, Calderón-Komaromy A, Córdoba S, Burbujo J. Exudative onycholysis and acute bacterial paronychia related to BIBF-1120 and paclitaxel: Response to topical therapy. Invest Clin. 2014;55:55–60. [PubMed] [Google Scholar]
- 38.Chen W, Yu YS, Liu YH, Sheen JM, Hsiao CC. Nail changes associated with chemotherapy in children. J Eur Acad Dermatol Venereol. 2007;21:186–90. doi: 10.1111/j.1468-3083.2006.01887.x. [DOI] [PubMed] [Google Scholar]
- 39.Minisini AM, Tosti A, Sobrero AF, Mansutti M, Piraccini BM, Sacco C, et al. Taxane-induced nail changes: Incidence, clinical presentation and outcome. Ann Oncol. 2003;14:333–7. doi: 10.1093/annonc/mdg050. [DOI] [PubMed] [Google Scholar]
- 40.Winther D, Saunte DM, Knap M, Haahr V, Jensen AB. Nail changes due to docetaxel—a neglected side effect and nuisance for the patient. Support Care Cancer. 2007;15:1191–7. doi: 10.1007/s00520-007-0232-0. [DOI] [PubMed] [Google Scholar]


