Background:
The lack of a standardized system for describing cell therapies acts as a barrier to advancement in clinical and basic research and practice. The aim of this study was to establish an international expert consensus on strategies to improve standardization and transparency when describing cell therapies. The secondary aim was to develop a consensus among experts on the contents of a standardized tool for describing cell therapies.
Methods:
The need for expert consensus on strategies to improve cell therapy communication was confirmed at the American Academy of Orthopaedic Surgeons/National Institutes of Health Optimizing Clinical Use of Biologics Symposium in 2018. A working group of 6 experts convened an international consensus process involving clinicians and basic scientists using validated Delphi methodology. This iterative process was used to define statements on communication of cell therapies and develop a standardized tool for describing cell therapies.
Results:
Thirty-four experts completed 3 rounds survey with use of the Delphi process. After 3 rounds, 27 statements relating to existing nomenclature, solutions to improve communication, ideal characteristics of a framework, mandatory elements of a new framework, and future work to facilitate application reached consensus with >80% agreement and <5% disagreement. Consensus was reached on the contents of a tool for improving standardization and transparency when describing cell therapies. This tool, dubbed “DOSES,” is based on the reporting of 5 core items: donor (i.e., autologous, allogeneic, xenogeneic), origin of tissue, separation from other cell types/preparation method, exhibited cell characteristics associated with behavior, and the site of delivery.
Conclusions:
This study has established expert consensus on the communication of cell therapies. The DOSES tool has been developed to improve standardization and transparency in describing cell therapies.
Clinical Relevance:
The DOSES tool for describing cell therapies can be utilized by researchers, clinicians, regulators, and industry professionals to improve standardization and transparency when describing cell therapies. The use of this tool may allow clinicians and patients to better understand the characteristics of current and future cell preparations.
Over the past decade, there has been an exponential growth in the use of cell therapies to treat musculoskeletal disease1. Cell therapy involves the delivery of viable cells into a patient to positively influence therapeutic outcomes2. Cells delivered can be autologous, allogeneic, or xenogeneic and can range from terminally differentiated adult cells and adult multipotent populations to pluripotent populations isolated from embryos or generated from adult cells as induced pluripotent cells. Therapies that claim to contain adult multipotent populations, including cultured mesenchymal stromal cells (MSCs) and unpurified bone-marrow-derived preparations, are the most widely researched, with over 800 clinical trials listed on ClinicalTrials.gov (Fig. 1). To date, results of these studies have generally been disappointing3, and the widespread use of these treatments is not currently supported by rigorous evidence. Nonetheless, as of May 2017, 716 clinics in the United States alone were engaged in direct-to-consumer marketing of “stem cell”-based interventions, the vast majority of which are promoted for musculoskeletal injuries4.
Fig. 1.
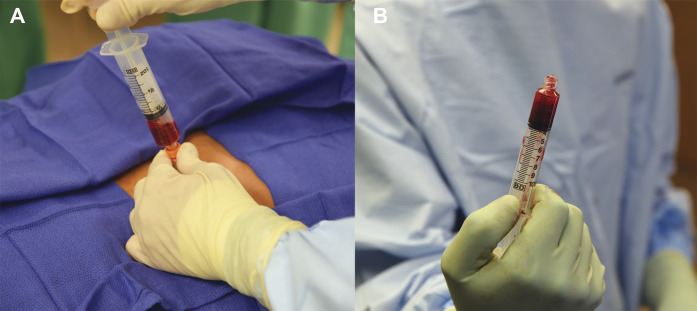
Figs. 1-A and 1-B Bone marrow is harvested from sites such as the iliac crest. In orthopaedics, bone marrow is often concentrated by centrifugation prior to injection into joints, tissue, or the blood system. The popularity of “stem cell”-containing products, such as bone marrow aspirate concentrate, has been driven by ease of use and direct-to-consumer marketing.
The lack of a standardized and transparent system for describing cell therapies has impeded scientific advancement and afforded an opportunity for clinics to potentially exploit ambiguity in definitions and descriptions to provide treatments that may not be evidence-based5-8. Although checklists have been generated that set out to encourage the comprehensive reporting of methodology and biologic characteristics in clinical studies9, there remains no standardized system for describing cell therapies. At present, a myriad of terms is being used to describe cell populations without a clear description of their characteristics or origins10.
The term “stem cells” is itself being used inappropriately, generating confusion among clinicians, researchers, and patients11. MSCs have been defined by these minimal criteria proposed by the International Society for Cellular Therapy (ISCT): (1) MSCs must be plastic-adherent in standard culture conditions; (2) MSCs must express CD105, CD73, and CD90 and lack expression of CD45, CD34, CD14 or CD11b, CD79alpha or CD19, and human leukocyte antigen-DR surface molecules; and (3) MSCs must differentiate to osteoblasts, adipocytes, and chondroblasts in vitro12. The term MSC is frequently being applied to populations without these demonstrated attributes.
Misleading or ambiguous terminology can result in mistaken assumptions regarding cell origins and characteristics, making interpretation of studies difficult9. A lack of standards for conveying the characteristics of cell therapies is being increasingly exploited with misinformation of unproven treatments13. Therefore, a more transparent and standardized system for accurately describing cell therapies used to treat musculoskeletal conditions is mandatory. The purpose of this study was to establish an international expert consensus on strategies to improve transparency and effectiveness of cell therapy communication using Delphi methods. A secondary purpose was to develop consensus among experts on the contents of a standardized tool for describing cell therapies.
Materials and Methods
The need for expert consensus on strategies to improve communication for cell therapies was confirmed at the American Academy of Orthopaedic Surgeons/National Institutes of Health Optimizing Clinical Use of Biologics Symposium in 201814. A working group of 6 individuals (I.R.M, J.C., M.R.S., A.J.K., D.B.F.S., and R.F.L) facilitated the development of consensus with use of modified Delphi techniques9. Details of the consensus are presented in Figure 2. In the absence of exact criteria listed in the literature for the selection of Delphi participants, experts were selected in a nomination process by all 6 members of the working group15. Although the majority of Delphi studies have utilized between 15 and 20 respondents15, a larger group of 30 to 40 experts was sought to increase representation in this broad field. For inclusion, all nominated individuals had to fulfill the following criteria: (1) clinician, clinician scientist, or basic scientist; (2) active leadership or senior involvement in studies relating to cell therapies; and (3) affiliation with an academic institution or research institute. All 36 individuals identified in the first round of nominations met the criteria for eligibility. All members of the working group were satisfied that the group was representative of the wider international community of academics working in cell therapies. All 36 experts identified were invited by e-mail to take part in a Delphi project relating to the communication of cell therapies. There were 2 non-respondents following a single additional reminder of invitation. Of the 34 experts who agreed to take part, 19 (56%) were from North America, 11 (32%) were from Europe, 2 (6%) from South America, and 2 (6%) from Asia; 10 (29%) were basic scientists and 24 (71%) were orthopaedic clinicians or clinician scientists.
Fig. 2.
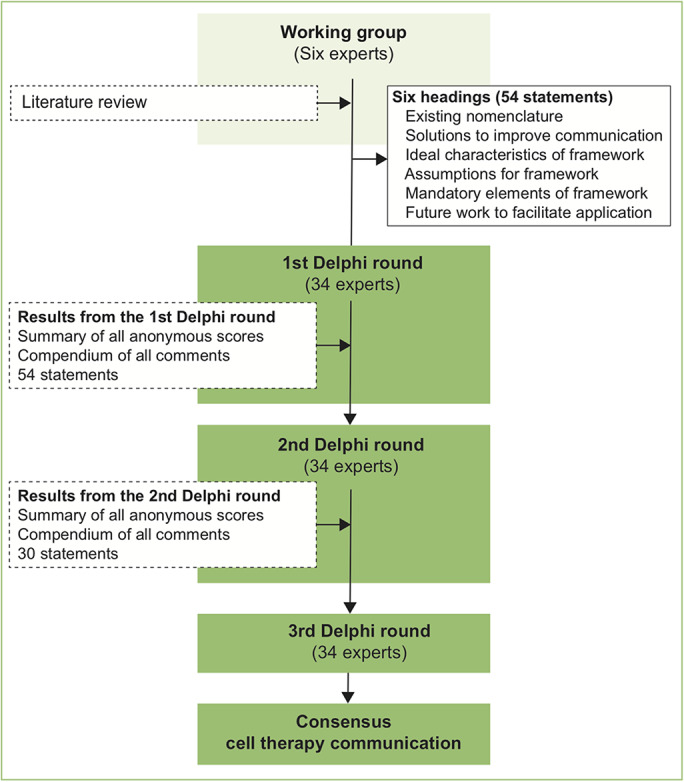
Flowchart showing the consensus process.
In order to generate the items for rating within the first-round survey, the working group reviewed factors relating to deficiencies of current cell therapy nomenclature. Discussions were based on a review of papers identified in recently published systematic reviews that evaluated cell therapies for musculoskeletal pathology, with a focus on items that may guide the development of a tool to improve transparency in the description of cell therapies. Draft statements were then generated. An online survey was created allowing experts to rate agreement on a Likert scale16: “strongly agree,” “agree,” “neither agree nor disagree,” “disagree,” or “strongly disagree.” A free-text comments section was included to enable suggestions of modifications or additional items. These inputs were integrated and amended consensus statements were prepared. In the second round, participants were asked to review the anonymized results from round 1 and score all items within the second survey. As with round 1, a free-text comments section was included to allow for suggestions of modifications or additional items. Questionnaires were reanalyzed and the cycle was repeated. The process was continued until a consensus was reached for all items as defined below, or for a maximum of 3 rounds.
Levels of agreement required for inclusion within subsequent Delphi rounds and within the final consensus survey were defined a priori. Following round 1, items with >70% agreement and <20% disagreement were retained for round 2. Items not meeting these criteria were discarded or modified per the suggestions of responders. Responses were analyzed with stricter cutoff criteria in round 2, with items retained only if >70% agreement was reached and <10% of experts disagreed. Items surveyed in the third round were included in the final consensus if >80% of respondents agreed and <5% disagreed.
Results
Delphi Process and Overall Consensus
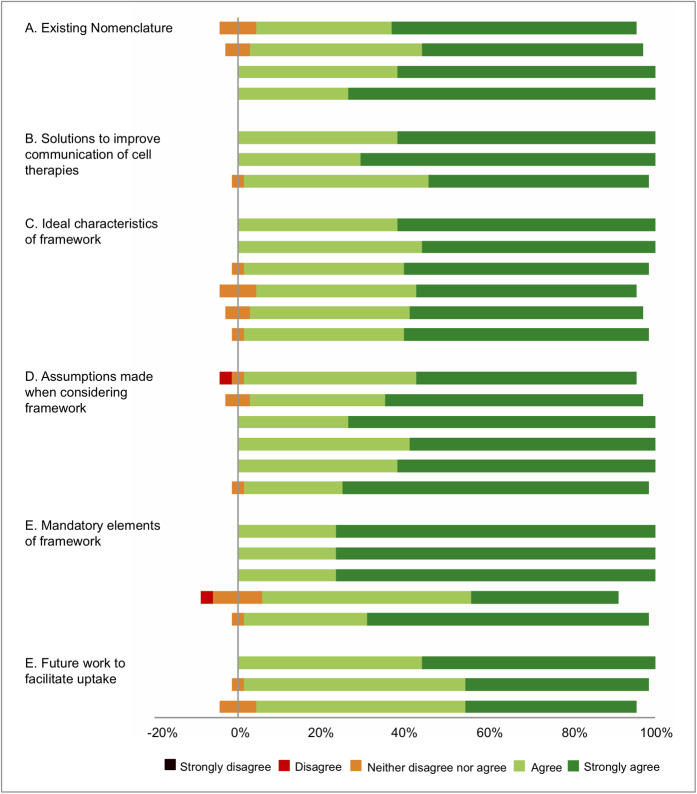
From group discussions and a review of existing related literature, the working group identified 54 statements for consideration by the expert group in the first round. Items were categorized under 6 headings: existing nomenclature, solutions to improve communication of cell therapies, ideal characteristics of a framework for communicating cell therapies, assumptions made when considering a new framework, mandatory elements of cell therapy for describing cell therapies, and future work to facilitate application. Thirty-four experts completed all 3 rounds of the survey. The results of each round of the survey are summarized in Table I. Consensus was achieved in 27 items relating to strategies for improving standardization and transparency when describing cell therapies (Table I). All 27 items (100%) included within the final survey achieved consensus, with >80% of experts in agreement and <5% in disagreement (Fig. 3, Table II). The levels of agreement for items not meeting criteria for consensus at each round are shown in Appendix Table E-I.
Fig. 3.
Stacked leaning bar chart representing breakdown of agreement levels in the third-round Delphi survey.
TABLE I.
Summary of Results at Completion of Each Survey Round in the Delphi Process
| Delphi Round | Responses | Total Items Included in Survey | Existing Items Reaching Consensus | New Items or Modifications Suggested |
| 1 | 34 | 54 | 89% | 14 |
| 2 | 34 | 30 | 97% | 6 |
| 3 | 34 | 27 | 100% | 0 |
TABLE II.
Levels of Agreement and Disagreement for the Items Included in the Round-3 Survey
| % Disagreement | % Agreement | |
| Existing nomenclature | ||
| There has been an increase in cell therapies used to treat musculoskeletal pathology. | 0 | 91 |
| The current use of ambiguous terms to describe cell therapies is limiting scientific progress. | 0 | 94 |
| Ambiguous terminology has a detrimental effect on consumer understanding of treatments. | 0 | 100 |
| The term “stem cells” to describe cell preparations in the absence of demonstrated multipotency and self-renewal creates substantial confusion for patients, physicians, and the public. | 0 | 100 |
| Solutions to improve communication of cell therapies | ||
| The scientific community has a responsibility to address deficiencies relating to inadequate cell therapy terminology. | 0 | 100 |
| Researchers, clinicians, and commercial entities should describe their product in a manner that is accurate and transparent. | 0 | 100 |
| The reporting of certain critical features relating to cell processing or characteristics (known as “core descriptors”) will improve transparency and understanding. | 0 | 97 |
| Ideal characteristics of a “core descriptors/attributes” framework | ||
| An ideal framework for describing cell therapies should encourage standardization in reporting. | 0 | 100 |
| An ideal framework for describing cells therapies should encourage transparency of cell characteristics. | 0 | 100 |
| A new framework for describing cells should incorporate available information that may critically influence cell behavior. | 0 | 97 |
| An ideal framework would accommodate forthcoming technologies and understanding that we do not currently have. | 0 | 91 |
| A new “core descriptors” framework should include sufficient items to enable appreciation of cell therapy attributes. | 0 | 94 |
| The number of items included in a new “core descriptors” framework should not be so onerous as to prevent uptake as a communication tool or to act as a barrier to research and development. | 0 | 97 |
| Assumptions made when considering a framework for communicating cell therapies | ||
| Cell therapies represent a complex mixture of cells, growth factors, and cytokines in variable compositions. | 3 | 94 |
| Donor factors may critically influence cell characteristics (e.g., age, sex, genomic and epigenetic factors). | 0 | 94 |
| The distinction between autologous, allogeneic, and xenogeneic sources of cells is important. | 0 | 100 |
| The tissue type of origin (e.g., bone, fat) may influence cell characteristics. | 0 | 100 |
| The cellular composition of preparations (including presence of non-regenerative cells) may influence therapeutic effect. | 0 | 100 |
| Methods of preparation may influence behavior, including (1) “minimal manipulation” processing techniques (i.e., mechanical disruption, centrifugation), (2) laboratory culture, and (3) purification through affinity-based separation (i.e., FACS, MACS). | 0 | 97 |
| Mandatory elements of a framework for communicating cell therapies. A framework requiring the reporting of “core descriptors/attributes” of cell preparations should include the following items: | ||
| A distinction between autologous, allogeneic, and xenogeneic donor source. | 0 | 100 |
| The tissue of origin (e.g., fat, bone marrow). | 0 | 100 |
| Methods of preparation including (1) “minimal manipulation” processing techniques (i.e., mechanical disruption, centrifugation), (2) laboratory culture, and (3) purification through affinity-based separation (i.e., FACS, MACS) should be reported. | 0 | 100 |
| Expression of confirmed cell surface markers should be stated (or indicated if not tested). | 3 | 85 |
| The method of delivery (i.e., intra-articular, intravenous) should be stated. | 0 | 97 |
| Future work to facilitate a comprehensive and prognostic classification system | ||
| Researchers, clinicians and commercial entities should report the items considered “core descriptors” when communicating regarding cell therapies. | 0 | 100 |
| Regulators, societies, and funding bodies should make the reporting of the above “core descriptors” mandatory when any product involving cell therapies is discussed. | 0 | 97 |
| Journals should make the reporting of “core descriptors” mandatory when authors describe a cell therapy. | 0 | 91 |
Consensus Findings
Five principal domains were identified within the consensus, with critical elements discussed below.
Existing Nomenclature
There has been an increase in cell therapies utilized to treat musculoskeletal pathology17,18. However, the current use of ambiguous terms to describe cell therapies is limiting scientific progress and consumer understanding14. The term “stem cells” is frequently used as a marketing tool, leading many patients to believe that the therapy exerts a therapeutic effect by replacing damaged or lost cells1,2,19,20. This term is frequently used to describe cell preparations in the absence of demonstrated multipotency and self-renewal, creating substantial confusion for patients, physicians, and the public21-23. The majority of experts agreed with the rigorous use of the ISCT standard for defining a cell population as an MSC (61% agreed and 18% disagreed). However, agreement did not reach the threshold of 80% set for the present study. The ISCT standard was, therefore, not included in this consensus statement, but it is by no means rejected as a valuable standard for rigor in nomenclature in the field. There was little disagreement (only 3%) on the point that future frameworks for describing cells should be compatible with existing systems of cell description.
Solutions to Improve Communication of Cell Therapies
The scientific community has a responsibility to address deficiencies relating to inadequate cell therapy terminology and communication. Researchers, clinicians, and commercial providers should describe their product accurately and transparently.
A majority of experts believed that scientific understanding was insufficient to enable the development of a hierarchical classification system, although this did not meet consensus criteria (71% agreed and 12% disagreed). However, there was consensus that a system for describing cells with certain critical features of cell processing or characteristics will improve transparency and understanding and would be worthwhile (97% agreed and 0% disagreed).
It was agreed by a majority of experts that a tool mandating the description of critical aspects of processing or characteristics should be applied to all cell types, accommodating future populations not yet discovered. As a result, providers of cell therapies that are reported “novel” and cannot be classified within the existing framework4,18 could instead utilize the system proposed above. Furthermore, it was clear that a descriptive tool would not seek to replace existing nomenclature or terminology, but rather encourage transparency and clarity when describing any given population in a system that could be universally applied.
Ideal Characteristics of a Framework
An ideal framework for describing cell therapies should encourage standardization and transparency and include information that may critically influence cell behavior. An ideal framework would accommodate forthcoming technologies and understanding. A new tool should include sufficient items to enable appreciation of cell therapy attributes but not be so onerous as to prevent uptake as a communication tool or to act as a barrier to research.
Assumptions Made When Considering a Framework for Communicating Cell Therapies
Cell therapies often represent a complex mixture of cells, growth factors, and cytokines in variable compositions10,24-26. The distinction between autologous, allogeneic, and xenogeneic sources of cells is important. The tissue type of origin and donor factors may critically influence cell characteristics. The cellular composition of preparations, including the presence of non-regenerative cells, may influence the therapeutic effect. Methods of preparation may influence behavior, including (1) “minimal manipulation” processing techniques (e.g., mechanical disruption or centrifugation), (2) laboratory culture, and (3) purification through affinity-based separation (i.e., fluorescence-assisted cell sorting [FACS] and magnetic-activated cell sorting [MACS]).
Mandatory Elements of a Framework for Communicating Cell Therapies (DOSES)
Consensus was reached on the inclusion of the following items within a cell therapy communication tool: (1) a distinction between autologous, allogeneic, and xenogeneic donor source should be made; (2) the tissue of origin (e.g., fat, bone marrow) should be stated; (3) methods of preparation, including “minimal manipulation” processing techniques (e.g., mechanical disruption or centrifugation), laboratory culture, and purification through affinity-based separation (i.e., FACS or MACS) should be reported; (4) expression of confirmed cell surface markers should be stated (or indicated if not tested); and (5) the method of delivery (i.e., intra-articular, intravenous) should be stated.
These 5 items formed the foundation for the cell-communication tool dubbed “DOSES” (Fig. 4). In practice, health providers or researchers would be encouraged to use the DOSES tool as the basis for a description of any cell therapy. This tool could be used when authors first introduce a cell population in a manuscript and when commercial entities introduce a product to providers and consumers. The DOSES tool is not intended to provide an exhaustive description of all cell attributes that may influence behavior, but rather to provide sufficient information to enable rapid indication of core attributes to facilitate efficient communication. As such, the tool should not be considered a replacement for the use of existing checklists for minimum reporting standards of methodology details, such as the CONSORT (Consolidated Standards of Reporting Trials)27 or MIBO (Minimum Information for Studies Evaluating Biologics in Orthopaedics)9 checklists.
Fig. 4.
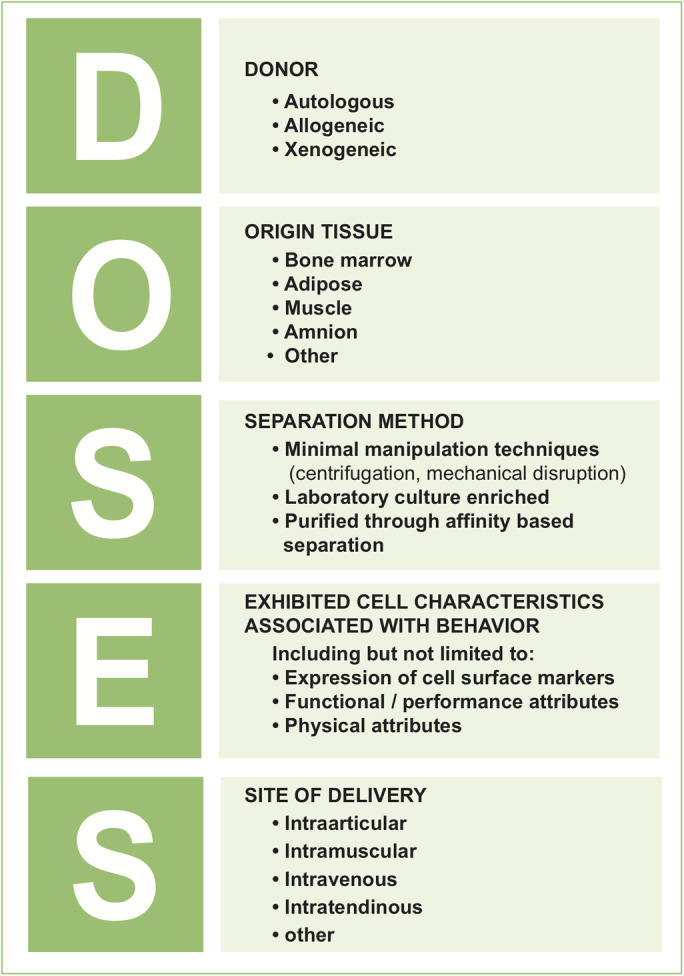
Summary of the “DOSES” cell-therapy communication tool.
Examples of use of the DOSES tool are: (1) bone marrow MSCs (DOSES: autologous, bone-marrow-derived, FACS-purified and culture-expanded cells, with 90% viability and expressing CD90 and CD146, intra-articular delivery); (2) bone marrow aspirate concentrate (DOSES: autologous, bone-marrow-derived, minimally manipulated through centrifugation, with 80% viability and unknown cell surface marker expression, intra-articular delivery).
There has been increasing skepticism regarding the cell surface marker phenotype of MSCs within the ISCT definition28. The DOSES tool encourages users to report all markers characterized without an artificial focus on existing panels that are increasingly controversial.
Future Work to Facilitate a Comprehensive and Prognostic Classification System
Consensus was reached that researchers, clinicians, and commercial entities should use this tool when communicating cell therapies. Furthermore, 97% of experts agreed that regulators, societies, and funding bodies should make the reporting of the above tool mandatory when any product involving cell therapies is discussed, and journals should mandate the disclosure of these critical factors when authors describe a cell therapy. Adherence to the use of the DOSES tool may best be achieved by expanding the discussion to include a larger community to enable consensus among relevant professional societies and standards organizations. We believe that the DOSES tool may be helpful for applications beyond the musculoskeletal system; however, further studies including representative expert panels from these fields would be of value.
Discussion
The most important finding of the present study was the consensus among experts that the current use of ambiguous terms to describe cell therapies is limiting scientific progress and that there is a need for tools to facilitate transparency in communication. Clinical research and practice are being undermined by ambiguous terminology that acts as a barrier to understanding the basic attributes of cell therapies. The present study has established consensus on the requirement for a descriptive tool to improve cell therapy communication. Through 3 Delphi rounds, 34 experts agreed on the inclusion of 5 distinct items within a descriptive communication tool. This tool will allow researchers, clinicians, funding bodies, and commercial entities to rapidly communicate critical aspects of a cell preparation in a standardized fashion. Although a stated advantage of this tool is the applicability to future cell types and technologies, the DOSES tool should undergo future reappraisal and, if necessary, modifications.
The Delphi methods utilized in this study offer several advantages over group-based methods29. Anonymity of responses reduces the effects of dominant individuals29. Online methods are as reliable as face-to-face panels30, improving rather than jeopardizing the quality of results. The high response rate across all 3 survey rounds in both Delphi studies demonstrates engagement with the process by all experts. The strict criteria for inclusion in the final statement (>80% experts agreeing and <5% experts disagreeing) were set more tightly than in most published Delphi studies31 to ensure that only items reaching high levels of agreement were included.
We recognize that this study had some limitations. Although Delphi panel methodology facilitates a more scientific approach to consensus than popular nominal group techniques32, it does not avoid the potential risk of bias in the selection of participants. It is possible that individual biases relating to the involvement with industry may have influenced certain responses. In selecting experts, the working group sought to minimize bias by including experts from different backgrounds, working in a range of clinical settings, with representation from all continents15,33. Although as few as 10 experts are considered adequate for content validation34, a larger group was chosen to increase representation in this broad field. The potential influence of any single individual was reduced by including more experts than most published Delphi studies and by setting the threshold levels of agreement for consensus high. Although experts were drawn from throughout Europe and Asia, the majority were based in North America. Efforts to establish if these standards are practical and generalizable to other populations may be merited.
In summary, the development of an international consensus on strategies to improve transparency and understanding when communicating about cell therapies has been presented. The DOSES tool can be utilized by researchers, clinicians, regulators, and industry professionals to improve standardization and transparency when describing cell therapies. In detailing key features of cells, the use of this tool may allow clinicians and patients to better understand the characteristics of current and future cell preparations.
Appendix
Supporting material provided by the author is posted with the online version of this article as a data supplement at jbjs.org (http://links.lww.com/JBJS/F192).
Footnotes
Arnold I. Caplan, PhD, Brian J. Cole, MD, MBA, Farshid Guilak, PhD, Scott A. Rodeo, MD, Christian Lattermann, MD, Mark A. Birch, PhD, Bruno Peault, PhD, Leela C. Biant, BSc(Hons), MBBS, AFRCSEd, FRCSEd(Tr&Orth), MSres, MFSTEd, Jorge Chahla, MD, PhD, Constance R. Chu, MD, Matthew J. Dalby, PhD, Allan B. Dietz, PhD, Jason L. Dragoo, MD, Lars Engebretsen, MD, PhD, Denis Evseenko, PhD, Alan Getgood, MD, FRCS(Tr&Orth), DipSEM, Andrew G. Geeslin, MD, Anthony P. Hollander, PhD, Johnny Huard, PhD, Elizaveta Kon, MD, Aaron J. Krych, MD, Robert F. LaPrade, MD, PhD, Nicola Maffulli, MD, MS, PhD, FRCS, FRCS(Orth), FFSEM, Bert R. Mandelbaum, MD, Rodrigo Mardones, MD, Iain R. Murray, BMedSci(Hons), MRCS, MFSEM, PhD, Frank A. Petrigliano, MD, Marc R. Safran, MD, Daniel B.F. Saris, MD, PhD, A. Hamish R.W. Simpson, MA(Cantab), FRCS(Tr&Orth), DM(Oxon), James H.C. Wang, PhD, Henning Madry, MD, Chris H. Jo, MD, PhD, and Norimasa Nakamura, MD, PhD
A commentary by Scott A. Rodeo, MD, is linked to the online version of this article at jbjs.org.
Disclosure: The authors indicated that no external funding was received for any aspect of this work. On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one or more of the authors checked “yes” to indicate that the author had a relevant financial relationship in the biomedical arena outside the submitted work and “yes” to indicate that the author had other relationships or activities that could be perceived to influence, or have the potential to influence, what was written in this work (http://links.lww.com/JBJS/F181).
Contributor Information
Collaborators: Arnold I. Caplan, Brian J. Cole, Farshid Guilak, Scott A. Rodeo, Christian Lattermann, Mark A. Birch, Bruno Peault, Leela C. Biant, Jorge Chahla, Constance R. Chu, Matthew J. Dalby, Allan B. Dietz, Jason L. Dragoo, Lars Engebretsen, Denis Evseenko, Alan Getgood, Andrew G. Geeslin, Anthony P. Hollander, Johnny Huard, Elizaveta Kon, Aaron J. Krych, Robert F. LaPrade, Nicola Maffulli, Bert R. Mandelbaum, Rodrigo Mardones, Iain R. Murray, Frank A. Petrigliano, Marc R. Safran, Daniel B.F. Saris, A. Hamish R.W. Simpson, James H.C. Wang, Henning Madry, Chris H. Jo, and Norimasa Nakamura
References
- 1.Piuzzi NS, Khlopas A, Sodhi N, Oak S, Sultan AA, Chughtai M, Mantripragada VP, Mont MA, Muschler GF. Cellular therapies in orthopedics: where are we? Surg Technol Int. 2017. December 22;31:359-64. [PubMed] [Google Scholar]
- 2.Caplan AI, Mason C, Reeve B. The 3Rs of cell therapy. Stem Cells Transl Med. 2017. January;6(1):17-21. Epub 2016 Aug 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boregowda SV, Booker CN, Phinney DG. Mesenchymal stem cells: the moniker fits the science. Stem Cells. 2018. January;36(1):7-10. Epub 2017 Oct 17. [DOI] [PubMed] [Google Scholar]
- 4.Turner L. The US direct-to-consumer marketplace for autologous stem cell interventions. Perspect Biol Med. 2018;61(1):7-24. [DOI] [PubMed] [Google Scholar]
- 5.LaPrade RF, Dragoo JL, Koh JL, Murray IR, Geeslin AG, Chu CR. AAOS Research Symposium updates and consensus: biologic treatment of orthopaedic injuries. J Am Acad Orthop Surg. 2016. July;24(7):e62-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piuzzi NS, Hussain ZB, Chahla J, Cinque ME, Moatshe G, Mantripragada VP, Muschler GF, LaPrade RF. Variability in the preparation, reporting, and use of bone marrow aspirate concentrate in musculoskeletal disorders: a systematic review of the clinical orthopaedic literature. J Bone Joint Surg Am. 2018. March 21;100(6):517-25 [DOI] [PubMed] [Google Scholar]
- 7.Chahla J, Piuzzi NS, Mitchell JJ, Dean CS, Pascual-Garrido C, LaPrade RF, Muschler GF. Intra-articular cellular therapy for osteoarthritis and focal cartilage defects of the knee: a systematic review of the literature and study quality analysis. J Bone Joint Surg Am. 2016. September 21;98(18):1511-21. [DOI] [PubMed] [Google Scholar]
- 8.Chahla J, Cinque ME, Piuzzi NS, Mannava S, Geeslin AG, Murray IR, Dornan GJ, Muschler GF, LaPrade RF. A call for standardization in platelet-rich plasma preparation protocols and composition reporting: a systematic review of the clinical orthopaedic literature. J Bone Joint Surg Am. 2017. October 18;99(20):1769-79. [DOI] [PubMed] [Google Scholar]
- 9.Murray IR, Geeslin AG, Goudie EB, Petrigliano FA, LaPrade RF. Minimum Information for Studies Evaluating Biologics in Orthopaedics (MIBO): platelet-rich plasma and mesenchymal stem cells. J Bone Joint Surg Am. 2017. May 17;99(10):809-19. [DOI] [PubMed] [Google Scholar]
- 10.Murray IR, Corselli M, Petrigliano FA, Soo C, Péault B. Recent insights into the identity of mesenchymal stem cells: Implications for orthopaedic applications. Bone Joint J. 2014. March;96-B(3):291-8. [DOI] [PubMed] [Google Scholar]
- 11.Caplan AI. Mesenchymal stem cells: time to change the name! Stem Cells Transl Med. 2017. June;6(6):1445-51. Epub 2017 Apr 28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-7. [DOI] [PubMed] [Google Scholar]
- 13.Piuzzi NS, Ng M, Chughtai M, Khlopas A, Ng K, Mont MA, Muschler GF. The stem-cell market for the treatment of knee osteoarthritis: a patient perspective. J Knee Surg. 2018. July;31(6):551-6. Epub 2017 Jul 24. [DOI] [PubMed] [Google Scholar]
- 14.Chu CR, Rodeo S, Bhutani N, Goodrich LR, Huard J, Irrgang J, LaPrade RF, Lattermann C, Lu Y, Mandelbaum B, Mao J, McIntyre L, Mishra A, Muschler GF, Piuzzi NS, Potter H, Spindler K, Tokish JM, Tuan R, Zaslav K, Maloney W. Optimizing clinical use of biologics in orthopedic surgery: consensus recommendations from the 2018 AAOS/NIH U-13 Conference. J Am Acad Orthop Surg. 2019. January 15;27(2):e50-e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu C, Sandford B. The Delphi technique: making sense of consensus. Pract Assess, Res Eval. 2007;12(10):1-8. [Google Scholar]
- 16.Likert R. A technique for the measurement of attitudes. In: Woodworth RS, editor. Archives of psychology. Vol 22, no 140. New York: The Science Press; 1932. [Google Scholar]
- 17.Knoepfler PS, Turner LG. The FDA and the US direct-to-consumer marketplace for stem cell interventions: a temporal analysis. Regen Med. 2018. January;13(1):19-27. Epub 2018 Jan 12. [DOI] [PubMed] [Google Scholar]
- 18.Turner L, Knoepfler P. Selling stem cells in the USA: assessing the direct-to-consumer industry. Cell Stem Cell. 2016. August 4;19(2):154-7. Epub 2016 Jun 30 [DOI] [PubMed] [Google Scholar]
- 19.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007. November;213(2):341-7, [DOI] [PubMed] [Google Scholar]
- 20.Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013. November 15;45:e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011. July 8;9(1):11-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008. April 10;2(4):313-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bianco P, Cao X, Frenette PS, Mao JJ, Robey PG, Simmons PJ, Wang CY. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013. January;19(1):35-42. Epub 2013 Jan 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaPrade RF, Geeslin AG, Murray IR, Musahl V, Zlotnicki JP, Petrigliano F, Mann BJ. Biologic Treatments for Sports Injuries II Think Tank-current concepts, future research, and barriers to advancement, part 1: biologics overview, ligament injury, tendinopathy. Am J Sports Med. 2016. December;44(12):3270-83. Epub 2016 Mar 29. [DOI] [PubMed] [Google Scholar]
- 25.Murray IR, LaPrade RF, Musahl V, Geeslin AG, Zlotnicki JP, Mann BJ, Petrigliano FA. Biologic Treatments for Sports Injuries II Think Tank-current concepts, future research, and barriers to advancement, part 2: rotator cuff. Orthop J Sports Med. 2016. March 31;4(3):2325967116636586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zlotnicki JP, Geeslin AG, Murray IR, Petrigliano FA, LaPrade RF, Mann BJ, Musahl V. Biologic Treatments for Sports Injuries II Think Tank-current concepts, future research, and barriers to advancement, part 3: articular cartilage. Orthop J Sports Med. 2016. April 15;4(4):2325967116642433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, Pitkin R, Rennie D, Schulz KF, Simel D, Stroup DF. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA. 1996. August 28;276(8):637-9. [DOI] [PubMed] [Google Scholar]
- 28.Lin CS, Xin ZC, Dai J, Lue TF. Commonly used mesenchymal stem cell markers and tracking labels: limitations and challenges. Histol Histopathol. 2013. September;28(9):1109-16. Epub 2013 Apr 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenhalgh T, Wong G, Jagosh J, Greenhalgh J, Manzano A, Westhorp G, Pawson R. Protocol—the RAMESES II study: developing guidance and reporting standards for realist evaluation. BMJ Open. 2015. August 3;5(8):e008567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Washington DL, Bernstein SJ, Kahan JP, Leape LL, Kamberg CJ, Shekelle PG. Reliability of clinical guideline development using mail-only versus in-person expert panels. Med Care. 2003. December;41(12):1374-81. [DOI] [PubMed] [Google Scholar]
- 31.Diamond IR, Grant RC, Feldman BM, Pencharz PB, Ling SC, Moore AM, Wales PW. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol. 2014. April;67(4):401-9. [DOI] [PubMed] [Google Scholar]
- 32.Hohmann E, Brand JC, Rossi MJ, Lubowitz JH. Expert opinion is necessary: Delphi panel methodology facilitates a scientific approach to consensus. Arthroscopy. 2018. February;34(2):349-51. [DOI] [PubMed] [Google Scholar]
- 33.Jones J, Hunter D. Consensus methods for medical and health services research. BMJ. 1995. August 5;311(7001):376-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lynn MR. Determination and quantification of content validity. Nurs Res. 1986. Nov-Dec;35(6):382-5. [PubMed] [Google Scholar]