Dear editor,
With the global climate change, drought has become one of the most serious environmental stresses that affect crop yield (Fahad et al., 2017). As one of the most important food and energy crops, cassava (Manihot esculenta) feeds about 750 million people in the world, especially in Africa (Yan et al., 2018). It is widely known that cassava is highly tolerant to drought and poor nutritional environment (De Souza et al., 2017). However, the key regulators of drought response in cassava remain elusive. In our previous study, we have identified Whirly (MeWHY) transcriptional factors and revealed their roles in modulating plant disease resistance against cassava bacteria blight (CBB) through interacting with MeWRKY75 (Liu et al., 2018). Herein, we further found that MeWHYs could physically interact with MeCIPK23 (Hu et al., 2015; Yan et al., 2018), as revealed by yeast two‐hybrid, biomolecular fluorescence complementation (BiFC), luciferase (LUC) complementation and pull‐down assays (Figure 1a‐d).
Figure 1.
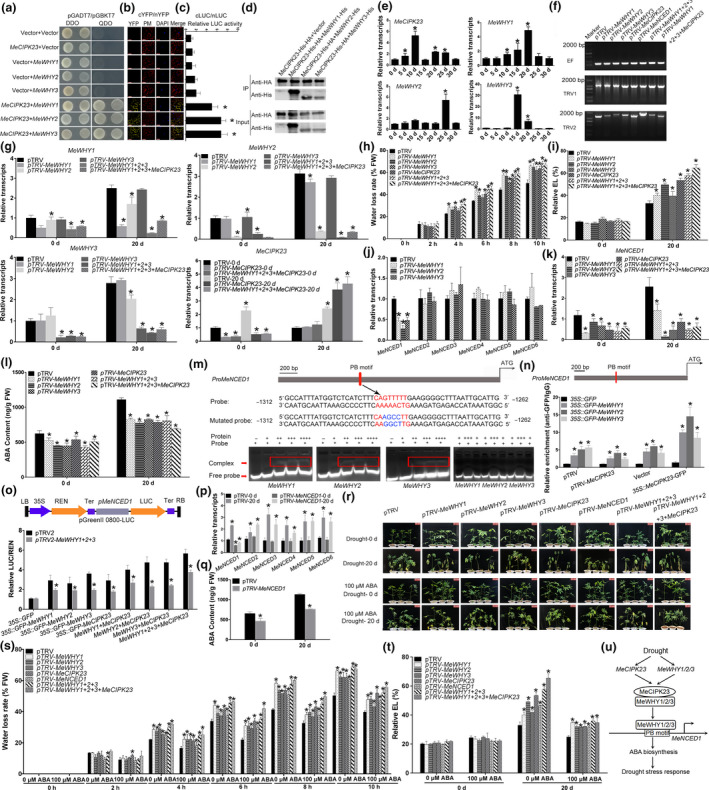
The interaction between MeCIPK23 and MeWHYs is essential for the activation of abscisic acid biosynthesis and drought stress resistance in cassava. (a)‐(d) Yeast two‐hybrid (a), BiFC (b), LUC complementation assay (c) and pull down (d) showing the physical interaction between MeCIPK23 and MeWHYs. DAPI‐stained cell nuclei and yellow fluorescent in BiFC were visualized using a confocal laser‐scanning microscope. Bar = 25 μm. (e) The transcript levels of MeCIPK23 and MeWHYs in cassava leaves in response to drought stress. For the assay, plant leaves under control conditions (well‐watered) and drought stress conditions (with‐holding water) for indicated days were harvested. (f) RT‐PCR showing the expression of TRV1 and TRV2 in the VIGS plants. The reference gene MeEF1a and the viral transcripts TRV1/TRV2 were examined. (g) The transcript levels of corresponding genes in the gene‐silenced plant leaves. (h)‐(i) Water loss rate (h) and EL (i) in the gene‐silenced plant leaves in response to drought stress. (j) The transcript levels of MeNCEDs in the gene‐silenced plant leaves under control conditions. (k)‐(l) The transcript levels of MeNCED1 (k) and the endogenous ABA accumulation (l) in the gene‐silenced plant leaves in response to drought stress. (m) EMSA showing the direct binding of MeWHYs to the probes of MeNCED1 promoter. The sequences of control probe with PB motif and mutated probe with mutated PB motif are shown. The position of free probe and the protein‐probe complex are marked by arrow. (n) ChIP‐PCR showing the relative enrichment of MeWHYs in MeNCED1 promoter. The same buffer without GFP antibody (IgG) was used as the native control of the GFP antibody. (o) Dual LUC assay showing the effects of MeWHYs and MeCIPK23 on the activity of MeNCED1 promoter. (p) The transcript level of MeNCED1 in the gene‐silenced plants. (q) The endogenous ABA accumulation in the gene‐silenced plants in response to drought stress. (r) Exogenous ABA restores the drought stress sensitivity of MeCIPK23‐MeWHYs‐MeNCED1 silencing plants. The pictures of different plants during drought stress conditions. Bars = 10 cm. (s)‐(t) Water loss rate (s) and EL (t) in the leaves in response to drought stress. (u) A proposed module of MeCIPK23‐MeWHYs‐MeNCED1 in drought stress response in cassava. In this study, cassava leaves were harvested for the assays. VIGS and gene overexpression in cassava leaves were performed through Agrobacterium tumefacien‐mediated transformation as we previously described (Liu et al., 2018; Zeng et al., 2019). All experiments were performed with at least three biological repeats. Statistical test was performed by SPSS. Kolmogorov–Smirnov test and Levene’s tests were used to check the normality of the data distribution and the variance homogeneity of the data, respectively. Asterisk symbols (*) suggested significant differences compared with control at P < 0.05.
WHY family proteins widely exist in plants and play multiple roles in modulating growth and development (Liu et al., 2018; Prikryl et al., 2008). In barley, WHY1 regulates drought stress‐induced leaf senescence through modulation of the expression of drought stress‐related genes and senescence‐related genes (Janack et al., 2016). Previous study has identified a total of 25 MeCIPKs and shown that the transcripts of some MeCIPKs including MeCIPK23 could be significantly regulated by drought stress and exogenous abscisic acid (ABA) treatment (Hu et al., 2015). In addition, OsCIPK23 positively regulates plant drought stress resistance (Hu et al., 2015). Based on previous studies (Hu et al., 2015; Janack et al., 2016) and the protein interaction between MeWHYs and MeCIPK23, their transcriptional levels in response to drought stress and their in vivo roles in plant drought stress resistance were investigated. The expression of MeCIPK23 and MeWHYs were significantly and largely up‐regulated upon drought stress treatment at least at one time point (Figure 1e). The common induced transcripts of MeWHYs and MeCIPK23 by drought stress in cassava indicated their possible involvement in plant drought stress response. Thereafter, we obtained MeWHYs‐ and MeCIPK23‐silenced cassava plants to silence single, triple or tetrad gene(s) via virus‐induced gene silencing (VIGS) (Zeng et al., 2019; Figure 1f), and confirmed the decreased transcriptional levels of the corresponding genes but not other homologous genes in the silenced lines under both normal and drought stress conditions (Figure 1g). Compared with mock, gene‐silenced plants exhibited obvious drought stress sensitivity with more wilted leaves, higher water loss rate and higher electric leakage (EL) upon drought stress treatment for 20 day, and the effects are more obvious in triple‐ and tetrad‐silenced plants (Figure 1h, i, r). More wilted leaves, higher water loss rate and higher EL under drought stress conditions reflected worse leaf phenotype, lower water‐holding capacity and severer plasma membrane damage, respectively, suggesting that MeWHY1‐, MeWHY2‐, MeWHY3‐ and MeCIPK23‐silenced plants displayed enhanced drought stress sensitivity in cassava.
Abscisic acid plays a crucial role in plant drought stress resistance (Cai et al., 2017). Therefore, we wondered whether MeWHYs and MeCIPK23 regulated ABA level. We firstly detected the expression of MeNCED genes, which encode the key enzymes controlling ABA biosynthesis (Cai et al., 2017). Because only the transcript of MeNCED1 among six MeNCEDs exhibited a dramatic decrease in MeWHYs‐silenced cassava leaves (Figure 1j), this gene was selected for further analysis. Moreover, MeWHYs‐ and MeCIPK23‐silenced cassava leaves had lower expression levels of MeNCED1 after drought stress treated for 20 days in comparison to mock (Figure 1k). Consistent with compromised MeNCED1 expression level, ABA content was also dramatically lower in MeWHYs‐ and MeCIPK23‐silenced cassava leaves (Figure 1l).
Interestingly, we found a WHY‐binding PB motif existing in the promoter region of MeNCED1 (Figure 1m), which has previously been suggested as the target of WHY proteins (Desveaux et al., 2005; Liu et al., 2018). Then, we analysed whether MeNCED1 was a direct target of MeWHYs. Firstly, electrophoretic mobility shift assay (EMSA) indicated that MeWHYs could bind to the promoter region (−1312 to −1262) with PB motif of MeNCED1, since a second band with lower gel shift rate appeared and increased with the addition of MeWHY proteins (Figure 1m). However, MeWHYs could not bind to the mutated probe with mutated PB motif (Figure 1m), confirming that MeWHYs could specifically bind to the PB motif. In addition, ChIP‐PCR suggested that the promoter region of MeNCED1 with PB motif was largely enriched by MeWHYs, and the enrichment levels were higher in MeCIPK23 overexpressing background but lower in MeCIPK23‐VIGS background, indicating that MeCIPK23 could positively regulate the ability of MeWHYs to bind to PB motif (Figure 1n). Moreover, three MeWHYs could significantly activate the activity of MeNCED1 promoter in dual LUC reporter system (Figure 1o). To sum up, these results suggested that MeNCED1 is a direct target of MeWHYs. Notably, MeCIPK23 overexpression could enhance the activity of MeNCED1 promoter and enhance the effects of MeWHYs on activating the activity of MeNCED1 promoter under mock conditions, but the effects of MeCIPK23 overexpression were significantly lower under MeWHY1/2/3‐VIGS background (Figure 1o), indicating that the interaction between MeCIPK23 and MeWHYs could direct regulate the activity of MeNCED1 promoter. Consistently, we further constructed MeNCED1‐silenced plants by VIGS and found that the expression of MeNCED1 but not other MeNCEDs (Figure 1p) and ABA content (Figure 1q) were attenuated in MeNCED1‐silenced cassava plants.
Exogenous application of 100 µm ABA enhanced plant drought stress resistance in wild‐type cassava plants (Figure 1r), and the drought stress sensitivity in MeWHYs‐ and MeCIPK23‐silenced cassava plants could be restored by exogenous ABA treatment, at least partially (Figure 1r). Consistent with this, drought‐induced increase of water loss rate and relative EL was dramatically compromised by ABA treatment in MeWHYs‐ and MeCIPK23‐silenced cassava leaves (Figure 1s‐t), indicating that ABA biosynthesis is directly involved in MeWHYs‐ and MeCIPK23‐ mediated drought stress resistance in cassava.
Taken together, we proposed a potential model for MeCIPK23‐MeWHYs‐mediated drought stress response in cassava (Figure 1u). Under drought stress conditions, the expression of MeCIPK23 and MeWHYs are up‐regulated. In addition, MeCIPK23 interacts with MeWHYs, which directly bind to the PB element in the promoter of MeNCED1 and activate its transcription. Then, the up‐regulated expression of MeNCED1 results in elevated ABA biosynthesis and enhanced drought stress response. Therefore, this study provides new insight into the drought‐resistance mechanism in cassava and potential strategies for further crop breeding and germplasm enhancement.
Conflict of interest
The authors declare no conflicts of interest.
Authors’ contributions
Shi H conceived and directed this study, and revised the manuscript; Yan Y, Liu W and Wei Y performed the experiments, analysed the data, wrote and revised the manuscript.
Acknowledgements
We thank Dr. Chris R. Somerville, Dr. Yanru Hu and Dr. Jie Zhou for sharing their vector plasmids. This research was supported by National Key R & D Program of China (No. 2018YFD1000500), National Natural Science Foundation of China (No. 31960527 and No. 31760067), the start‐up funding and the scientific research foundation of Hainan University (No. kyqd1531) and the Innovation Project of Postgraduates of Hainan Province (No. Hyb2019‐17).
Yan, Y. , Liu, W. , Wei, Y. and Shi, H. (2020) MeCIPK23 interacts with Whirly transcription factors to activate abscisic acid biosynthesis and regulate drought resistance in cassava. Plant Biotechnol. J. 10.1111/pbi.13321
References
- Cai, S. , Chen, G. , Wang, Y. , Huang, Y. , Marchant, D.B. , Wang, Y. , Yang, Q. et al. (2017) Evolutionary conservation of ABA signaling for stomatal closure. Plant Physiol. 174, 732–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza, A.P. , Massenburg, L.N. , Jaiswal, D. , Cheng, S. , Shekar, R. and Long, S.P. (2017) Rooting for cassava: insights into photosynthesis and associated physiology as a route to improve yield potential. New Phytol., 213, 50–65. [DOI] [PubMed] [Google Scholar]
- Desveaux, D. , Maréchal, A. and Brisson, N. (2005) Whirly transcription factors: defense gene regulation and beyond. Trends Plant Sci. 10, 95–102. [DOI] [PubMed] [Google Scholar]
- Fahad, S. , Bajwa, A.A. , Nazir, U. , Anjum, S.A. , Farooq, A. , Zohaib, A. , Sadia, S. et al. (2017) Crop production under drought and heat stress: plant responses and management options. Front. Plant Sci. 8, 1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, W. , Xia, Z. , Yan, Y. , Ding, Z. , Tie, W. , Wang, L. , Zou, M. et al. (2015) Genome‐wide gene phylogeny of CIPK family in cassava and expression analysis of partial drought‐induced genes. Front. Plant Sci. 6, 914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janack, B. , Sosoi, P. , Krupinska, K. and Humbeck, K. (2016) Knockdown of WHIRLY1 affects drought stress‐induced leaf senescence and histone modifications of the senescence‐associated gene HvS40. Plants, 5, E37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, W. , Yan, Y. , Zeng, H. , Li, X. , Wei, Y. , Liu, G. , He, C. et al. (2018) Functional characterization of WHY‐WRKY75 transcriptional module in plant response to cassava bacterial blight. Tree Physiol. 38, 1,502–1,512. [DOI] [PubMed] [Google Scholar]
- Prikryl, J. , Watkins, K.P. , Friso, G. , van Wijk, K.J. and Barkan, A. (2008) A member of the Whirly family is a multifunctional RNA‐ and DNA‐binding protein that is essential for chloroplast biogenesis. Nucleic Acids Res. 36, 5,152–5,165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, Y. , He, X. , Hu, W. , Liu, G. , Wang, P. , He, C. and Shi, H. (2018) Comprehensive expression profile of MeCIPKs in response to pathogen and functional analysis reveal novel role of MeCIPK23 and MeCBL1/9 in plant defense response. Plant Cell Rep. 37, 887–900. [DOI] [PubMed] [Google Scholar]
- Zeng, H. , Xie, Y. , Liu, G. , Wei, Y. , Hu, W. and Shi, H. (2019) Agrobacterium‐mediated gene transient overexpression and tobacco rattle virus (TRV)‐based gene silencing in cassava. Int. J. Mol. Sci. 20, 3976. [DOI] [PMC free article] [PubMed] [Google Scholar]


