Introduction
As the most important natural fibre crop in the world, upland cotton (Gossypium hirsutum L.) accounts for >95% of the world’s cotton production. However, most of the current upland cotton cultivars are susceptible to Verticillium wilt, a major fungal disease termed as the ‘cancer of cotton’, which exists in cotton‐producing areas worldwide and causes huge economic losses every year (Li et al., 2019). Currently, selecting and breeding cultivars with broad‐spectrum resistance to Verticillium wilt are considered to be one of the most effective approaches for controlling this disease. In the past two decades, there were many studies on the genetic engineering of cotton Verticillium wilt resistance using resistance genes cloned from cotton or other plant species, and even functional genes from the pathogen like V. dahliae and Xanthomonas oryzae pv. oryzae (Wang et al., 2004; Zhang et al., 2018). However, most of these studies have focused on gene function analysis using transgenic Arabidopsis, tomato or tobacco, and only a few genes have been shown to increase the resistance of transgenic cotton to Verticillium wilt (Jun et al., 2015; Li et al., 2019).
For more than 20 years, our group has studied the Gastrodia antifungal protein (GAFP) family and their functions in Verticillium wilt resistance of transgenic cotton (Wang et al., 2016). We found that expression of GAFPs in cotton could significantly enhance Verticillium wilt resistance and GAFP4 is an excellent target gene for genetic engineering to control Verticillium wilt. Previously, we utilized the cauliflower mosaic virus (CaMV) 35S constitutive promoter to drive the GAFP genes, while the persistent and stable expression of exogenous genes in all plant tissues raises concerns about potential cost of possible metabolic disorders and the excessive consumption of intracellular substances and energy, and further the expression of multiple exogenous genes under the control of the same promoter may also cause gene silencing or co‐inhibition (Zheng et al., 2007). Therefore, it will be desirable to use tissue‐specific or stage‐specific promoter for particular application of genetic engineering. Generally, the fungal pathogen Verticillium dahliae infects the plant via the roots and then spreads through the vascular tissue. The infection finally blocks water uptake and causes leaf yellowing, necrosis, defoliation and more severely the death of whole plant (Zhou et al., 2017). Therefore, it will be desirable to express the GAFP4 gene under the control of a vascular‐specific promoter, which may enhance the level of resistance to Verticillium wilt without penalty on plant growth and development.
Previously, we found that the promoter of Glycine decarboxylase (GDC) P‐protein subunit (gdcsP) from the C3–C4 intermediate plant Flaveria anomala has vascular bundle‐sheath‐specific expression pattern (Chu, 1996). Furthermore, it also has been shown to have vascular‐specific expression in monocotyledonous rice (Chen et al., 2001). Using bioinformatics tools, we analysed the nearly 5 kb regions of gdcsP promoter. We found 4 elements required for vascular expression and 16 root motifs that required for strong root‐specific expression. Moreover, the gdcsP promoter also contains 8 methyl jasmonate (Me‐JA) and 3 salicylic acid (SA)‐responsive elements, and few elicitor‐induced, defence‐related WRKY binding site (W‐box) and the MYB binding motif (Figure 1a). Therefore, the gdcsP promoter could respond to both pathogen infection and wounding (Nishiuchi et al., 2004), which would more effectively inhibit the fungal pathogen at a very early stage with the gdcsP promoter, as the pathogen V. dahliae normally infects cotton roots through wounding. Therefore, the gdcsP promoter should be an ideal promoter for genetic engineering of cotton Verticillium wilt resistance. To examine whether the gdcsP promoter is active in different dicotyledonous plants, we used it to drive the expression of β‐glucuronidase (GUS) reporter gene (gdcsPPro::GUS) in Arabidopsis and upland cotton variety R15. In Arabidopsis, the gdcsP promoter could drive vascular‐specific GUS expression in different parts of the plant at a level similar to that of the CaMV 35S promoter (Figure 1b‐d and l). In transgenic cotton seedlings, strong GUS activity was observed in the vascular bundles of the root, stem, leaf, petiole and even young bolls (Figure 1e‐k). More importantly, the activity of gdcsP promoter could keep at high level throughout the entire cotton life cycle (Figure 1m). Furthermore, the gdcsP promoter could also be induced by SA and Me‐JA (Figure 1n‐o), suggesting that it would be a very potential promoter used for manipulation of biotic stress resistance.
Figure 1.
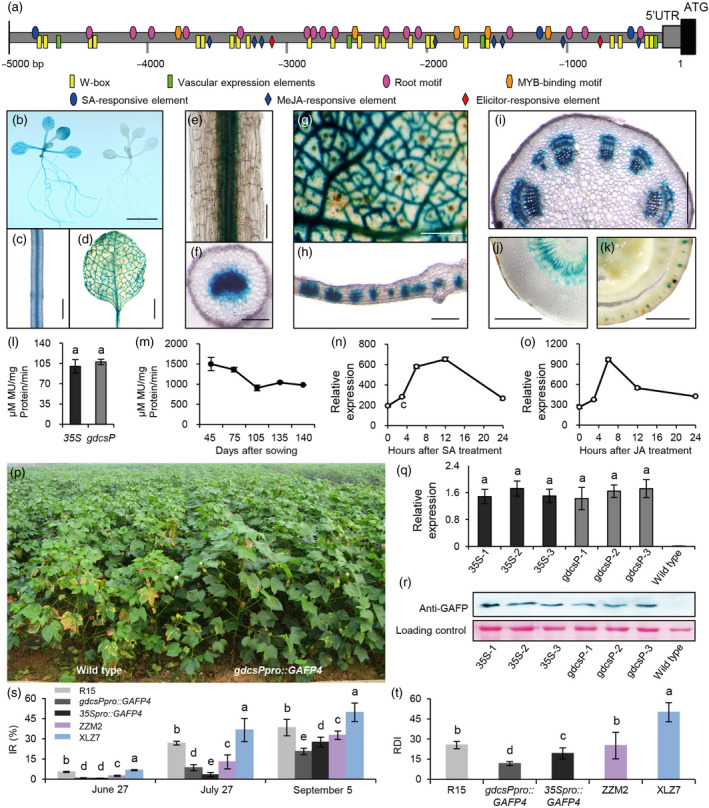
Vascular‐specific expression of the gdcsP promoter and its application in GAFP4 transgenic cotton. (a) A map of cis‐acting regulatory elements in gdcsP promoter from analysis using bioinformatics tools Sogo (https://sogo.dna.affrc.go.jp) and PlantCARE (http://bioinformatics. psb.ugent.be/webtools/plantcare). (b‐d) Expression of the gdcsP promoter in Arabidopsis seedlings. (b): whole plant of transgenic (left) and control (right), c: root; d: leaf. (e‐k) Expression of the gdcsP promoter in transgenic cotton. e‐f: root; g: young leaf; h: mature leaf; i: young stem; j: petiole; k: young boll. (l) Comparison of the activity of CaMV 35S promoter with the gdcsP promoter in transgenic Arabidopsis. For each promoter, four transgenic lines with similar GUS expression level and six individual plants per line are used. (m) Activity of the gdcsP promoter at different growth stages of transgenic cotton. Three transgenic lines with similar GUS expression level and six individual plants per line are used. (n‐o) Induction of the gdcsP promoter by phytohormones. n: 50 μm SA; o: 50 μM Me‐JA. Each treatment group included 5 individual seedlings. (p) The evaluation of gdcsPPro::GAFP4 transgenic cotton in the Verticillium wilt nursery in Anyang. (q‐r) The molecular evidence of transgenic cotton. Three lines with similar expression of GAFP4 (q) and protein content (r) were selected from different promoter transgenic plants for further field test in Langfang. (s‐t) The evaluation of GAFP4 transgenic cotton in the Verticillium wilt nursery in Langfang. The resistant and susceptible control is ZhongZhiMian‐2 (ZZM‐2) and XinLuZao‐7 (XLZ‐7), respectively. Each transgenic line has 3 random repetitions, and each repetition has more than 30 individual plants. The disease surveys were conducted at seedling stage, flowering stage and bolling stage, respectively, when plants were growing for about 8, 12 and 16 weeks after germination. The disease index (DI) was calculated according to the following formulas: DI = [(Σdisease score × amount of infected plants)/total checked plants × 4] × 100. Relative DI (RDI) = DI × K (correction factor, K = 50.0/DI‐sc, sc: susceptible control). Different letters indicate significant difference according to Duncan’s multiple range tests (P < 0.05).
We further constructed the gdcsPPro::GAFP4 vector and transformed it into upland cotton variety R15. In Anyang (35°12′N, 113°37′E), Henan province, the transgenic plants had significantly higher level of resistance compared with the non‐transgenic controls in the Verticillium wilt disease nursery (Figure 1p). To compare the efficiency of gdcsP promoter with CaMV 35S promoter, three homozygous gdcsPPro::GAFP4 transgenic lines, together with three 35SPro::GAFP4 transgenic lines with similar expression level of GAFP4 and the content of antifungal protein (Figure 1q‐r), were selected for further field evaluation in Verticillium wilt disease nursery in Langfang (39°56′N, 116°20′E), Hebei Province. From the result of surveys, we observed that all GAFP4 transgenic lines performed better than the non‐transgenic control lines in this large scale evaluation. The gdcsPPro::GAFP4 transgenic lines have lower infection rate (IR) and relative disease index (RDI) than the 35SPro::GAFP4 transgenic lines in the last survey, which is even better than the resistance control ZhongZhiMian‐2 (ZZM‐2) (Figure 1s‐t). These results demonstrate that vascular‐specific expression of the GAFP4 is more effective than constitutive expression in conferring Verticillium wilt disease resistance. From the result of GUS staining, the strong and specific expression of gdcsPpro in vascular tissue was observed (Figure 1e‐k). Therefore, with the similar expression level of GAFP, the antifungal protein in the gdcsPpro::GAFP4 transgenic plants would be more concentrated in the vascular tissue, leading to more effective disease resistance compared with the transgenic plants with constitutive expression of GAFP4. Therefore, the strong vascular‐specific activity of the gdcsP promoter makes it an effective tool for genetic engineering of Verticillium wilt resistance in cotton.
Since the gdcsP promoter is active in both monocot and dicot plants, this promoter also has great potential in the genetic engineering of other disease species. For example, as it can express in young bolls (Figure 1k), the gdcsP promoter could be used for engineering resistance to cotton boll diseases such as bollworm and phytophthora boll rot, etc. Similar to the fungal pathogen V. dahliae, plant viruses need to infect the vascular tissue to spread in the entire plant, and most piercing‐sucking insects also sucked the nutrients by inserting their mouthparts into the vascular tissue. Therefore, the vascular‐specific promoters such as the gdcsP promoter could have great potential and broad application in genetic engineering of virus‐ and insect‐resistant plants.
Conflict of interests
The authors have no conflict of interests.
Author contributions
Y.W. and C.C. designed experiments; S.W. H.Z., and J.L. performed cotton transformations; S.W., Ga.J., Gu.J., X.Z., F.L., C.L., J.T. and Y.W. performed field trials; Y.W., C.C. and C.L. performed data analysis and wrote the paper.
Acknowledgements
This work was supported by the grants from the Ministry of Agriculture of China (2008ZX08005‐004 and 2011ZX08005‐004‐008) and Chinese Academy of Agricultural Sciences (Y2018YJ10).
Wang, Y. , Liang, C. , Wu, S. , Jian, G. , Zhang, X. , Zhang, H. , Tang, J. , Li, J. , Jiao, G. , Li, F. and Chu, C. (2019) Vascular‐specific expression of Gastrodia antifungal protein gene significantly enhanced cotton Verticillium wilt resistance. Plant Biotechnol. J., 10.1111/pbi.13308
References
- Chen, S. , Qu, N. , Cao, S. , Bauwe, H. , Chen, S. , Tian, W. and Chu, C. (2001) Expression of the upstream regulation sequence of the glycine decarboxylase P‐ protein subunit in the C3–C4 intermediate plant Flaveria anomala in transgenic rice. Chin. Sci. Bull. 46, 939–942. [Google Scholar]
- Chu, C. (1996) Molecular structure and expression patterns of glycine decarboxylase genes from Flaveria pringlei (C3) and Flaveria anomala (C3–C4). Dissertation, Martin‐Luther Unvertsität, Halle‐Wettenberg, 1–100.
- Jun, Z. , Zhang, Z. , Gao, Y. , Zhou, L. , Fang, L. , Chen, X. , Ning, Z. et al. (2015) Overexpression of GbRLK, a putative receptor‐like kinase gene, improved cotton tolerance to Verticillium wilt. Sci. Rep. 5, 15048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z. , Chen, B. , Li, X. , Wang, J. , Zhang, Y. , Wang, X. , Yan, Y. et al. (2019) A newly identified cluster of glutathione S‐transferase genes provides Verticillium wilt resistance in cotton. Plant J. 98, 213–227. [DOI] [PubMed] [Google Scholar]
- Nishiuchi, T. , Shinshi, H. and Suzuki, K. (2004) Rapid and transient activation of transcription of the ERF3 gene by wounding in tobacco leaves: possible involvement of NtWRKYs and autorepression. J. Biol. Chem. 279, 55355–55361. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Chen, D. , Wang, D. , Huang, Q. , Yao, Z. , Liu, F. , Wei, X. et al. (2004) Over-expression of Gastrodia anti-fungal protein enhances Verticillium wilt resistance in coloured cotton. Plant Breeding. 123, 454–459. [Google Scholar]
- Wang, Y. , Liang, C. , Wu, S. , Zhang, X. , Tang, J. , Jian, G. , Jiao, G. et al. (2016) Significant improvement of cotton Verticillium wilt resistance by manipulating the expression of Gastrodia antifungal proteins. Mol. Plant. 9, 1436–1439. [DOI] [PubMed] [Google Scholar]
- Zhang, L. , Wang, M. , Li, N. , Wang, H. , Qiu, P. , Pei, L. , Xu, Z. et al. (2018) Long non‐coding RNAs involve in resistance to Verticillium dahliae, a fungal disease in cotton. Plant Biotechnol. J. 16, 1172–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, X. , Deng, W. , Luo, K. , Duan, H. , Chen, Y. , McAvoy, R. , Song, S. et al. (2007) The cauliflower mosaic virus (CaMV) 35S promoter sequence alters the level and patterns of activity of adjacent tissue‐ and organ‐specific gene promoters. Plant Cell Rep. 26, 1195–1203. [DOI] [PubMed] [Google Scholar]
- Zhou, T. , Zhao, Y. and Guo, H. (2017) Secretory proteins are delivered to the septin‐organized penetration interface during root infection by Verticillium dahliae . PLoS Pathog. 13, e1006275. [DOI] [PMC free article] [PubMed] [Google Scholar]


