Summary
Tomato (Solanum lycopersicum L.) plants are cold‐sensitive, and the fruit are susceptible to postharvest chilling injury when stored at low temperature. However, the mechanisms underlying cold stress responses in tomato are poorly understood. We demonstrate that SlGRAS4, encoding a transcription factor induced by low temperature, promotes chilling tolerance in tomato leaves and fruit. Combined genome‐wide ChIP‐seq and RNA‐seq approaches identified among cold stress‐associated genes those being direct targets of SlGRAS4 and protein studies revealed that SlGRAS4 forms a homodimer to self‐activate its own promoter. SlGRAS4 can also directly bind tomato SlCBF promoters to activate their transcription without inducing any growth retardation. The study identifies the SlGRAS4‐regulon as a new cold response pathway conferring cold stress tolerance in tomato independently of the ICE1‐CBF pathway. This provides new track for breeding strategies aiming to improve chilling tolerance of cultivated tomatoes and to preserve sensory qualities of tomato fruit often deteriorated by storage at low temperatures.
Keywords: chilling injury, GRAS, CBF, antioxidant capacity, tomato (Solanum lycopersicum L.)
Introduction
Tomato (Solanum lycopersicum L.) is known as a cold‐sensitive crop which greatly limits the geographical areas where this important crop plant can be cultivated and shorten the period of its growing seasons. Chilling injury also represents a major issue with regard to the loss of sensory quality during postharvest storage and transportation of tomato fruit. Storage at low temperature is the most common method to limit postharvest losses and deterioration, but many fruit species including tomato are sensitive to temperatures below 12 °C as they develop chilling injury resulting in a number of physiological disorders such as uneven ripening, pitting and most importantly flavour deterioration (Zhang et al., 2016). In addition, low temperature has been reported to impact cell membrane conformation and structure, resulting in attenuated vegetative growth and reduced crop yield and quality (Sevillano et al., 2009). Plants from temperate regions such as Arabidopsis (Arabidopsis thaliana L.), wheat (Triticum aestivum L.) and Brassica napus L. exhibit freezing tolerance when they are pre‐exposed to temperatures in the range of 0 to 12 °C, a phenomenon called cold acclimation. By contrast, many tropical plants such as maize (Zea mays L.), rice (Oryza sativa L.) and tomato suffer from chilling injury when exposed to cold acclimation temperatures (Zhang et al., 2004). An important step towards deciphering the mechanisms underlying cold acclimation has been the discovery that the C‐repeat (CRT) binding factor (CBF), a transcriptional regulator, plays important roles in this process in Arabidopsis. Subsequently, the so‐called ‘CBF regulon’ has been further clarified, revealing that CBF proteins can directly bind to the CRT element in the promoter regions of COLD‐RESPONSIVE (COR) genes to activate their expression under cold stress, thus contributing to freezing tolerance via enhancement of cryoprotective substances production (Gilmour et al., 1998; Jaglo‐Ottosen et al., 1998; Kasuga et al., 1999; Liu et al., 1998; Stockinger et al., 1997; Thomashow, 2001; Yamaguchi‐Shinozaki and Shinozaki, 1994). The expression of CBF is also rapidly induced by multiple transcription factors under cold stress, including inducer of CBF expression 1 (ICE1) (Chinnusamy et al., 2003; Kim et al., 2015), calmodulin‐binding transcription activator 3 (CAMTA3) (Doherty et al., 2009; Kidokoro et al., 2017), brassinazole‐resistant 1/brassinosteroid‐insensitive 1‐EMS‐suppressor 1 (BZR1/BES1) (Li et al., 2017b), CESTA (Eremina et al., 2016) and circadian clock‐associated 1/late elongated hypocotyl (CCA1/LHY) (Dong et al., 2011). On the other hand, the expression of CBF is repressed by MYB15 (Agarwal et al., 2006; Kim et al., 2017), phytochrome‐interacting factors (PIFs) (Jiang et al., 2017), ethylene insensitive 3 (EIN3) (Shi et al., 2012) and suppressor of overexpression of constans 1 (SOC1) (Seo et al., 2009), as reviewed recently by Shi et al. (2018). ICE1 is regarded as the most important regulator of CBF expression and was shown to undergo multiple post‐translational modifications that are essential for its functionality including a ubiquitination process mediated by the high expression of responsive gene 1 (HOS1) (Dong et al., 2006) and a sumoylation mediated by SIZ1 (Miura et al., 2007). It was reported that OPEN STOMATA 1 (OST1) suppresses HOS1‐mediated ICE1 degradation under cold stress, through its phosphorylation which enhances its stability and potentiates its transcriptional activity (Ding et al., 2015). On the other hand, it was shown that MPK3 and MPK6 interact with and phosphorylate ICE1 to promote its degradation, thus attenuating the freezing tolerance (Li et al., 2017a; Zhao et al., 2017).
The CBF pathway associated with cold response is highly conserved in flowering plants, and not limited to those displaying cold acclimation, such as Brassica napus L. and barley (Hordeum vulgare L.), but also operates in plants unable to acclimate to cold stress, such as rice and tomato (Choi et al., 2002; Dubouzet et al., 2003; Jaglo et al., 2001; Zhang et al., 2004). In tomato, there are three CBF homologues, and overexpression of LeCBF1 in Arabidopsis stimulates the expression of CBF‐target genes and increases freezing tolerance, indicating that tomato CBF1 encodes a functional homologue of the Arabidopsis CBF proteins, supporting the idea that tomato has a complete CBF cold response pathway although the tomato CBF family members have been reported to display less diversified function than Arabidopsis CBFs (Zhang et al., 2004). Of particular note, it was reported that at least 28% of the cold‐responsive genes were not regulated by CBFs in Arabidopsis, suggesting the existence of additional, but yet unveiled, low‐temperature regulons (Fowler and Thomashow, 2002). More recently, it was shown that SlICE1‐overexpressing tomato plants exhibit higher antioxidant activity and enhanced chilling tolerance associated with increased SlCBF1 expression (Miura et al., 2012a, 2012b). Also, SlICE1a, an ICE1‐like transcription factor, was reported to bind to the MYC‐recognition elements on the promoters of SlCBF1 and SlCBF3, and to confer cold tolerance in transgenic tobacco (Feng et al., 2013). Overall, these data support the notion that responses to low temperature rely on the intervention of diverse types of transcription factors, most of which remain unknown in the case of tomato, a species of major economic importance but highly sensitive to chilling injury.
The GRAS gene family encodes plant‐specific transcription factors reported to play critical roles in plant growth and development, and remarkably, several GRAS genes are highly inducible by different abiotic stresses (Huang et al., 2015, 2017; Lee et al., 2008). Some DELLA proteins, belonging to the GRAS sub‐family, are involved in abiotic stress resistance via increasing the expression level of genes encoding enzymes that detoxify reactive oxygen species (ROS), thus reducing ROS levels, delaying cell death and promoting tolerance (Achard et al., 2008a). We previously reported that overexpression of SlGRAS40 in tomato enhances drought and salt resistance by regulating auxin and gibberellin homeostasis (Liu et al., 2017). Interestingly, among the 53 GRAS genes present in the tomato genome, only SlGRAS4 (Solyc01g100200) exhibits substantial expression increase under low‐temperature stress (Huang et al., 2015), yet, it remains to be elucidated whether this GRAS gene is involved in responses to low‐temperature stress. In the present study, we show that SlGRAS4 promotes cold tolerance in tomato mainly through direct regulation of many genes participating in the adaptation to low temperature as well as in inducing the expression of SlCBF genes. The outcome of the study uncovers a novel cold response mechanism in which SlGRAS4 promotes chilling injury resistance in tomato via multiple biological pathways and at least partly through the CBF pathway.
Results
SlGRAS4 expression is induced by low temperature
We previously identified 53 GRAS members in the tomato genome (Huang et al., 2015), but only SlGRAS4 was significantly induced by low‐temperature stress, raising the hypothesis of its potential role in tomato responses to cold treatment. To gain insight on the putative involvement of SlGRAS4 in cold stress responses, we first investigated its expression pattern at the transcript level in tomato leaves and fruit under low‐temperature treatment. In tomato leaves, SlGRAS4 transcripts undergo rapid and massive accumulation (more than 100‐fold increase) starting 1 h after placing the plants at 4 °C (Figure S1). The same cold treatment applied to mature green fruit also induced transcript accumulation but significantly later (24 h) and at much lower amplitude (five fold increase) than in leaves (Figure S1). This suggests that SlGRAS4 may act via different modes in tomato leaves and fruit subjected to cold stress.
SlGRAS4 plays a positive role in controlling cold tolerance in tomato plants
To address the functional significance of SlGRAS4, tomato plants (Solanum lycopersicum L. cv. Micro‐Tom) overexpressing (OE) and down‐regulated (RNAi) lines were generated. More than 10 independent lines were obtained for each construct among which three phenotypically representative lines were selected for subsequent physiological and molecular characterization. Transcript levels assessed by q‐RT‐PCR were 61–75 times higher in OE leaves than in WT, and the increase in transcript levels was between 31 to 42 times higher in OE mature green fruit compared to WT fruit at the same stage. In RNAi lines, SlGRAS4 transcript levels represented 33%–41% the amount in WT leaves and 55%–62% that in WT mature green fruit.
The behaviour of down‐regulated and overexpressing lines in response to cold stress was assessed using 45‐day‐old plants placed at 4 °C for 4 days. In contrast to WT plants that displayed severe wilting symptoms, OE lines exhibited remarkable cold tolerance, with only very few leaves showing slight wilting (Figure S2). When subjected to the same cold treatment, RNAi plants observed similar wilting damage than WT plants. Reducing the duration of cold treatment to 1 day revealed higher sensitivity to low temperature of under‐expressing lines, with WT plants displaying only slight wilting symptoms, whereas RNAi plants exhibiting more severe damages (Figure S2). The damages induced by low‐temperature treatment were further investigated by assessing malondialdehyde (MDA) content. It is known that under cold stress, the production of reactive oxygen species causes cellular oxidative damage, and the accumulation of MDA, as product of ROS attacking the lipids, reflects the embodiment of the membrane oxidative damage. Remarkably, cold‐treated leaves of OE lines accumulated lower amount of MDA than WT (Figure S2), indicating that overexpression of SlGRAS4 results in lower oxidative damage. On the contrary, suppression of SlGRAS4 reduces the plant capacity to control the negative effects induced by low‐temperature stress as indicated by the higher accumulation of MDA content in RNAi leaves (Figure S2). These results support the idea that SlGRAS4 promotes chilling tolerance in tomato plants at least partly via controlling oxidative damage.
SlGRAS4 positively regulates chilling injury resistance in tomato fruit
Tomato fruit are known as sensitive to cold‐induced physiological disorder which depreciates their sensory and commercial qualities. This, together with the increased expression of SlGRAS4, under low temperature in tomato fruit (Figure S1) prompted the investigation of the potential involvement of SlGRAS4 in fruit tolerance to chilling injury. WT and transgenic fruit picked at mature green stage were stored at 5 °C for 14 days and then brought back to 25 °C for 14 days. When subjected to this chilling treatment, WT fruit exhibited damage symptoms that can be visually observed including pitted skin and uneven ripening (Figure 1a). More severe injury symptoms were observed in RNAi fruit, with all fruit being severely damaged with pitted skin and impaired ripening as indicated by the presence of yellow and light orange colour (Figure 1a). By contrast, no obvious injury symptoms were detected in the OE fruit subjected to the same cold treatment, and once moved to room temperature (25 °C), the fruit ripen normally with no sign of pitting on the skin (Figure 1a). Consistently, fruit overexpressing SlGRAS4 showed much lower CI index than WT and RNAi ones (Figure 1b). Assessing the ‘a’ colour parameter by a colorimeter indicated that OE fruit were more towards the red colour than WT after CI treatment, whereas RNAi fruit were more towards the green (Figure 1c). In lines with the displayed colour differences, β‐carotene content in OE fruit was higher than in WT and RNAi fruit, while total chlorophyll content was higher in RNAi fruit (Figure S3). Fruit firmness of cold‐treated OE fruit was lower than WT fruit, whereas RNAi fruit showed higher firmness and contained more pectin and cellulose in pericarp tissue (Figure S3). Water loss in OE fruit was also lower than in WT and RNAi fruit after cold treatment (Figure S3). These data are indicative of a marked slowdown of the ripening process in SlGRAS4 down‐regulated fruit.
Figure 1.
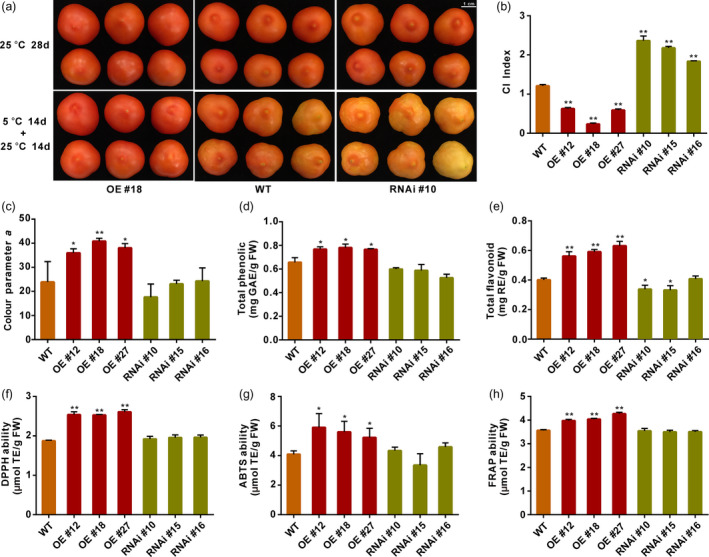
SlGRAS4 positively regulates chilling injury tolerance of tomato fruit. (a) Chilling injury symptoms in WT and transgenic tomato fruit following cold treatment. Transgenic and WT fruit harvested at mature green stage were either stored at 25 °C for 28 days (upper panel), or treated 14 days at 5 °C and then replaced at 25 °C for 14 days. (b–e) Chilling injury (CI) index (b), colour parameter a (c), total phenolic content (d), and total flavonoid content (e) of WT and transgenic tomato fruit subjected to cold stress as described above. Total phenolic content and total flavonoid were expressed as mg GAE/g FW and mg RE/g FW, respectively. (f–h) DPPH ability (f), ABTS ability (g) and FRAP ability (h) of WT and transgenic fruit subjected to cold stress as described above. The antioxidant capacities were expressed as µmol TE/g FW. In (b) and (d) to (h), data are the mean values of three independent replicates and error bars show the s.d. In (c), three independent repeats were performed showing similar results, and the data showed here are the mean values of one replicate and error bars show s.d. (n = 6). In all cases, asterisks indicate significant differences between wild‐type and transgenic lines (two‐tailed Student's t‐test, *P < 0.05, **P < 0.01).
No significant differences in total sugar content and titratable acidity were observed between WT and transgenic fruit after chilling injury‐inducing treatment (Figure S3). By contrast, assessing phenolics, known to contribute to the antioxidant capacity, revealed higher total phenolics and total flavonoid content in OE compared to WT cold‐treated fruit, and total flavonoid content exhibited lower level in RNAi fruit (Figure 1d,e). We then performed DPPH (1,1‐Diphenyl‐2‐picrylhydrazyl) and ABTS (2,2′‐azino‐bis(3‐ethylbenzthiazoline‐6)‐sulphonic acid) assays to monitor free radical scavenging capacity and FRAP (ferric ion reducing antioxidant power) assay to assess total antioxidant capacity (Figure 1f–h). The DPPH, ABTS and FRAP abilities were significantly higher in OE fruit than in WT under chilling injury condition, suggesting the overexpression of SlGRAS4 increases antioxidant capacity in a broad way.
SlGRAS4‐target genes identified by combined ChIP‐seq and RNA‐seq
To gain insight on the mechanisms by which SlGRAS4 confers enhanced chilling injury tolerance, we investigated the putative SlGRAS4‐binding sites at the genome‐wide level by a ChIP‐seq approach. Up to 5245 peaks were detected (Data S1) and the analysis of their genome‐wide distribution revealed that 18% of the SlGRAS4 binding sites were enriched in the gene promoter regions, 1 kb upstream of the coding regions (Figure 2a). Enriched GO categories (Figure 2b) of the putative SlGRAS4‐binding genes (Data S1) suggested that SlGRAS4 participates in multiple processes and de novo motif prediction performed with the SlGRAS4‐binding regions identified revealed four putative DNA‐binding motifs (Table S1). However, combining ChIP‐seq and RNA‐seq data revealed that motif 4 is by far the most abundant in the promoter of DEGs which guided the study towards the role of this specific motif in regulating the expression of selected DEGs (Figure 2c, Appendix S1). Further analysis performed by yeast‐one hybrid and dual‐luciferase assays revealed that motif 4 is efficient in directing SlGRAS4‐mediated gene expression, although the transcriptional activity varies from strong to mild depending on the target promoter (Figure 3b,c).
Figure 2.
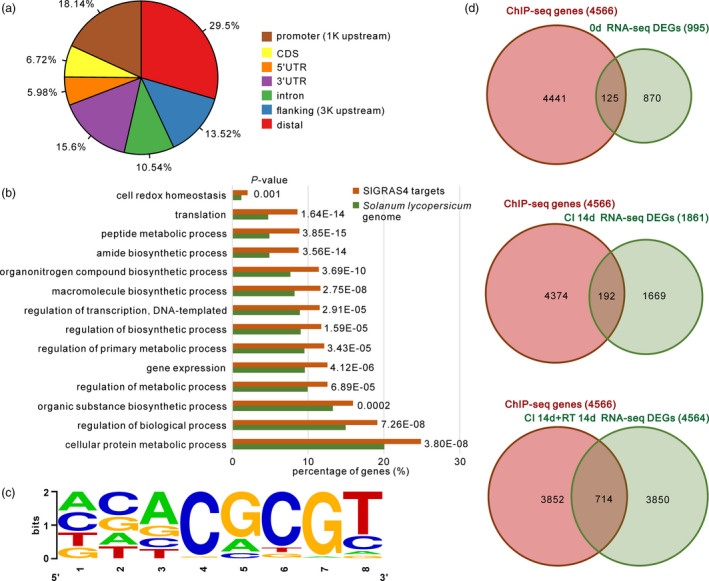
SlGRAS4 target genes identified by combined genome‐wide ChIP‐seq and RNA‐seq approaches. (a) Genome‐wide distribution analysis of the SlGRAS4‐binding peaks. (b) Gene ontology (GO) categorization of SlGRAS4‐binding genes. Enriched GO categories of SlGRAS4 compared with all Solanum lycopersicum L. genes are shown. A false discovery rate (FDR) cut‐off was implemented on the basis of a P‐value <0.001. Numbers indicate P‐values. (c) The putative DNA‐binding motif of SlGRAS4 was determined based on the occurrence with the highest frequency in the promoter region of the target genes identified by ChIP‐seq. (d) Venn diagram showing the overlapping genes between the SlGRAS4‐binding targets revealed by ChIP‐seq (red circle) and the differentially expressed genes (DEGs, green circle) identified by RNA‐seq in SlGRAS4‐overexpressing and down‐regulated lines. DEGs refer to those showing differential expression either after 14 days of cold treatment or 14 days of cold treatment followed by 14 days at room temperature when the chilling injury symptoms are observed. The complete lists of overlapping genes are given in Data [Link], [Link], [Link].
Figure 3.
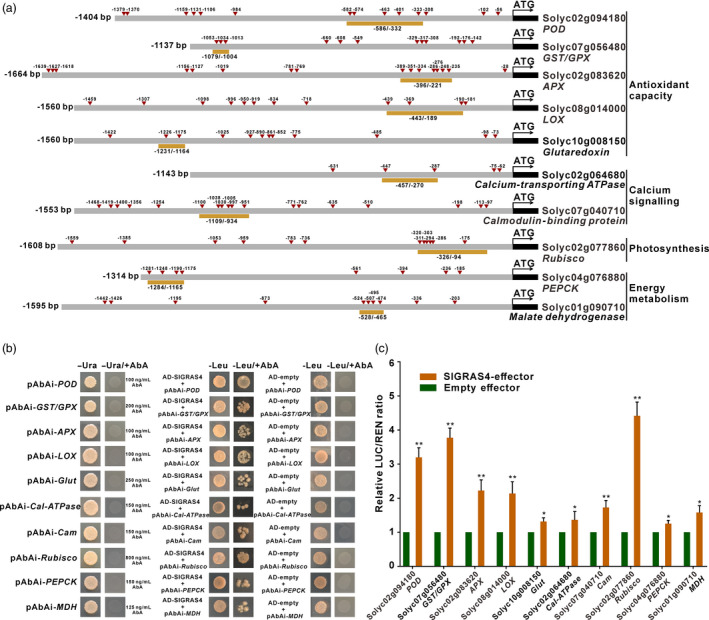
SlGRAS4 directly binds to and activates promoters of several target genes participate in multiple biological processes. (a) The promoter structure of SlGRAS4 target genes. The red triangles indicate the position of SlGRAS4‐binding sites corresponding to motif 4 present in the promoter of target genes. The displayed length of each promoter was amplified and cloned into reporter vectors for dual‐luciferase assays. The regions underlined in orange indicate promoter fragments used for yeast‐one hybrid assays. (b) Interaction of SlGRAS4 with fragments of target gene promoters assessed by yeast‐one hybrid assays. (c) The transcription activation ability of SlGRAS4 tested on the promoters of target genes by dual‐luciferase assays. The LUC/REN ratio of empty effector plus the promoter reporter was used as calibrator (set as 1). Data are the mean values of five independent replicates, and error bars show the s.d. Asterisks indicate significant differences between SlGRAS4‐effector group and control empty vector (two‐tailed Student's t‐test, *P < 0.05, **P < 0.01). In all cases, POD indicates peroxidase, GST/GPX indicates glutathione S‐transferase/peroxidase, APX indicates L‐ascorbate peroxidase, LOX indicates lipoxygenase, Glut indicates glutaredoxin, Cal‐ATPase indicates calcium‐transporting ATPase, Cam indicates calmodulin‐binding protein, Rubisco indicates ribulose bisphosphate carboxylase, PEPCK indicates phosphoenolpyruvate carboxykinase, and MDH indicates malate dehydrogenase.
Global transcriptomic profiling performed by RNA‐seq on OE, WT and RNAi fruit samples at mature green stage identified differentially expressed genes in these fruit with reference to WT fruit subjected to the same treatment. The samples analysed included untreated fruit the day of harvest (0d), cold‐treated for 14 days after harvest (CI 14d), and cold‐treated for 14 days and then placed at room temperature (25 °C) for 14 days (CI 14d + RT 14d). The complete lists of differentially expressed genes screened by pairwise comparison at different time points are given in Data [Link], [Link], [Link]. In the absence of cold treatment (0d), up to 995 DEGs were found when cumulating RNAi and OE lines compared to their expression level in WT. Using the WT reference samples, 1861 DEGs were found in cold‐treated group (CI 14d) and 4564 DEGs in cold‐treated followed by storage at room temperature CI 14d + RT 14d (Figure 2d, Data [Link], [Link], [Link]). Of particular note, the most important changes in gene expression (highest number of DEGs) were observed when the fruit were put back at room temperature following a storage at low temperature, regardless of the nature of the sample taken into consideration. Crossing the genome‐wide transcriptomic data with the ChIP‐seq data revealed that 125, 192 and 714 genes are both differentially expressed and direct binding targets of SlGRAS4 in 0d, CI 14d, and CI 14d + RT 14d samples, respectively (Figure 2d, Data [Link], [Link], [Link]).
SlGRAS4 regulates the promoter activity of genes participating in multiple biological processes
Genes that belong to both the ChIP‐seq and DEG groups were regarded as best candidates to be direct targets of SlGRAS4 and therefore to contribute to the cold tolerance mechanism (Figure 2d, Data [Link], [Link], [Link]). Several among these overlapping genes were selected to further confirm their regulation by SlGRAS4, and these included genes known to be involved in antioxidant capacity like peroxidase (Solyc02g094180), glutathione S‐transferase/peroxidase (Solyc07g056480), L‐ascorbate peroxidase (Solyc02g083620), lipoxygenase (Solyc08g014000) and glutaredoxin (Solyc10g008150). As well as calcium‐transporting ATPase (Solyc02g064680) and calmodulin‐binding protein (Solyc07g040710) are related to calcium signalling. Ribulose bisphosphate carboxylase (Solyc02g077860) participating in photosynthesis, and phosphoenolpyruvate carboxykinase (Solyc04g076880) and malate dehydrogenase (Solyc01g090710) involved in energy metabolism were also investigated. Interestingly, multiple putative SlGRAS4‐binding sequence motifs (Figure 2c) were identified by in silico search in the promoter region of all these target genes (Figure 3a), and the ability of SlGRAS4 to directly bind to their promoters was demonstrated by yeast‐one hybrid assay (Figure 3b). Furthermore, dual‐luciferase assay revealed that SlGRAS4 can directly activate these promoters (Figure 3c), supporting the conclusion that SlGRAS4 regulates the transcription of genes involved in multiple biological processes including antioxidant capacity, calcium signalling, photosynthetic activity and energy metabolism pathways. However, whether or not these genes and the related processes contribute to the chilling injury resistance mediated by SlGRAS4 remains to be elucidated.
SlGRAS4 increases antioxidant capacity
Because SlGRAS4 is shown here to activate the promoter of genes involved in antioxidant capacity, and given that SlGRAS4 expression is significantly induced in WT tomato leaves sprayed with H2O2 (Figure 4a), we therefore performed oxidative stress test by spraying 45‐day‐old WT and transgenic plants with 100 μm MV (methyl viologen) once a day for 3 days, and then illuminating the plants for 4 days. In response to the intense oxidative stress applied, WT and RNAi plants exhibited more severe withering than OE plants (Figure 4b,c) and total chlorophyll content in leaves was significantly lower than in OE (Figure 4d), indicating that SlGRAS4 expression attenuates oxidative stress damages. Moreover, SlGRAS4 overexpression also enhanced oxidative stress tolerance during seed germination and seedling growth (Figure 4e–g). Together, these data support the idea that SlGRAS4 promotes oxidative stress tolerance in tomato plants.
Figure 4.
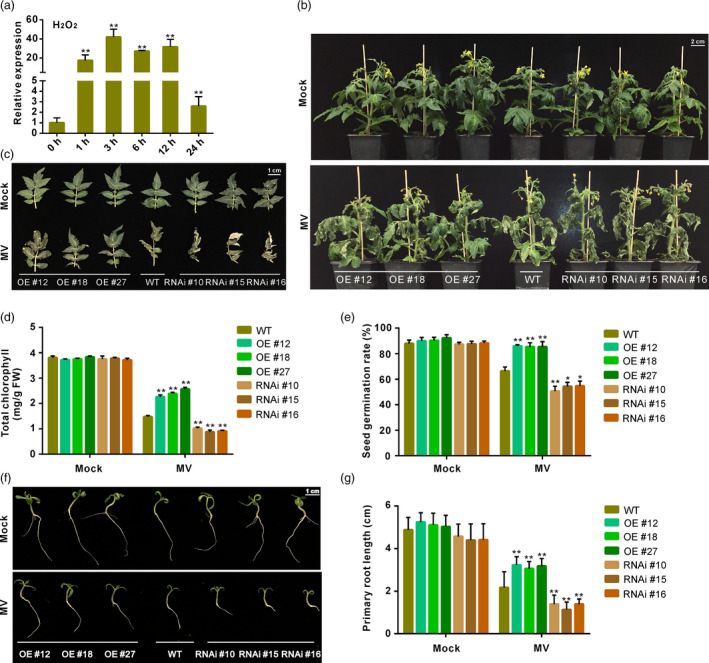
SlGRAS4 overexpression enhances antioxidant capacity. (a) The response of SlGRAS4 to oxidative stress in WT leaves following spraying the whole plants with 100 mm H2O2 for 0, 1, 3, 6, 12h and 24 h. (b–c) Representative plants (WT, OE and RNAi lines) after treatment for 7 days with 100 μm MV (methyl viologen) compared to untreated plants (mocks). (d) Total chlorophyll content of WT and transgenic leaves treated or not (mock) with 100 μm MV for 7 days. (e) Seed germination rate of WT and transgenic lines treated or not (mock) with 10 μm MV for 7 days. (f) Phenotypes of WT and transgenic seedling treated or not (mock) with 10 μm MV for 14 days. (g) Primary root length of WT and transgenic seedling treated or not (mock) with 10 μm MV for 14 days. In (a), SlGRAS4 transcript levels determined by q‐RT‐PCR were represented as the values relative to at time 0 h of the treatment, and the transcript level at 0 h was set as 1. Data are the mean values of three independent replicates, and error bars show the s.d. Asterisks indicate significant differences relative to the transcript level at 0 h (two‐tailed Student's t‐test, **P < 0.01). In (d) and (e), data are the mean values of three independent replicates and error bars show the s.d. In (g), three independent replicates were performed showed similar results, and the data showed here are the mean values of one replicate and error bars show the s.d. (n = 14). Asterisks indicate significant differences between wild‐type and transgenic lines (two‐tailed Student's t‐test, *P < 0.05, **P < 0.01).
SlGRAS4 forms a protein homodimer that directly binds and activates its own promoter
Several SlGRAS4‐binding motifs (Figure 2c) were found in the SlGRAS4 promoter (Figure 5a), consistent with the identification of SlGRAS4 among the target genes revealed by ChIP‐seq assay (Figure 5b, Data S1). The ability of SlGRAS4 to bind its own promoter was validated by both yeast‐one hybrid and dual‐luciferase assays (Figure 5c,d). In addition, yeast transcriptional activity test, using either full‐length or truncated SlGRAS4 proteins, restricted to the transcriptional activation domain located corresponding to the N‐terminal region, indicating that SlGRAS4 works as transcriptional activator (Figure 5e). Subsequently, a deletion series of the N‐terminal part of the protein identified the transactivation domain in a region encompassing amino acid residues 150–200 (Figure 5e). The truncated SlGRAS4 ΔN5 (200–667) and SlGRAS4 ΔN6 (286–667) proteins lacking the transcriptional activation domain were thereafter used in protein–protein interaction assays by yeast‐two hybrid approach, to demonstrate the ability of SlGRAS4 proteins to self‐dimerize (Figure 5f). The ability for homo‐dimerization was further confirmed by bimolecular fluorescence complementation (BiFC) assay (Figure 5g). Taken together, these data suggest that the expression of SlGRAS4 is at least partly under self‐regulation.
Figure 5.
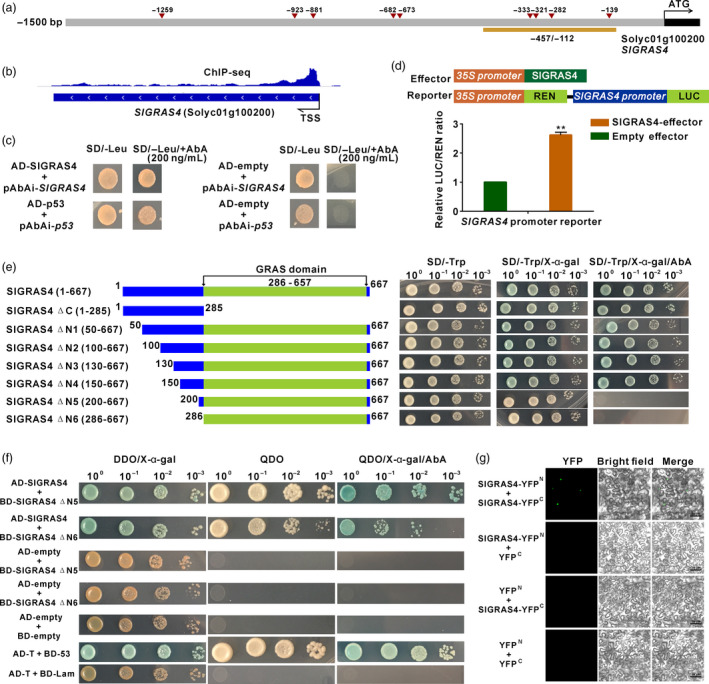
SlGRAS4 directly binds to and activates its own promoter and forms a homodimer by protein–protein interaction. (a) The promoter structure of SlGRAS4. The red triangles indicate the position of SlGRAS4‐binding sites corresponding to motif 4 present in its own promoter, the 1500 bp length of promoter was amplified and cloned into reporter vector used for dual‐luciferase assay, and the regions underlined in orange indicate promoter fragment used for yeast‐one hybrid assay. (b) Integrative Genomics Viewer (IGV) image of the SlGRAS4 gene in ChIP‐seq reads. (c) Interaction of SlGRAS4 with its own promoter fragment by yeast‐one hybrid assay. (d) Dual‐luciferase assay to test the transcription activation ability of SlGRAS4 on its own promoter. The LUC/REN ratio of empty effector plus SlGRAS4 promoter reporter was used as the calibrator (set as 1). Data are the mean values of five independent replicates, and error bars show the s.d. Asterisks indicate significant differences between SlGRAS4‐effector group and empty group (two‐tailed Student's t‐test, **P < 0.01). (e) Determining the transactivation domain of SlGRAS4 by deletion series and transcriptional activation test in yeast. ΔC and ΔN indicate deletion of the C and N terminus of SlGRAS4 protein, respectively, and numbers in bracket indicate the amino acid composition of the native and truncated SlGRAS4 proteins. (f) SlGRAS4 homo‐dimerization tested by protein–protein interaction in yeast. DDO indicates SD/‐Leu/‐Trp medium, and QDO indicates SD/‐Ade/‐His/‐Leu/‐Trp medium. AD‐T + BD‐53 is the positive control, and AD‐T + BD‐Lam is the negative control. (g) SlGRAS4 forms a homodimer as assessed by protein–protein interaction in Nicotiana benthamiana L. leaves.
SlGRAS4 can directly bind and activate the promoters of SlCBF1, SlCBF2 and SlCBF3
Multiple SlGRAS4‐binding motifs (Figure 2c) present in the promoters of SlCBF1, SlCBF2 and SlCBF3 genes (Figure 6a) and the ChIP‐seq data indicated that SlGRAS4 has the ability to bind the SlCBF1 and SlCBF3 promoters (Figure 6b, Data S1). The ability of SlGRAS4 to bind and activate the promoters of SlCBF1, SlCBF2 and SlCBF3 was further confirmed by yeast‐one hybrid and dual‐luciferase assays (Figure 6c–e). And the expression levels of SlCBF1, SlCBF2 and SlCBF3 in SlGRAS4‐OE fruit were higher than that in WT during chilling treatment (Figure 6f), suggesting that SlGRAS4 contributes to the higher expression of SlCBF1, SlCBF2 and SlCBF3 observed in SlGRAS4‐OE fruit subjected to cold stress. On the other hand, there were no continuous high expression levels of SlCBFs in SlGRAS4‐OE fruit under cold stress and exhibited the similar expression patterns to low temperature to that of the wild type, and three SlCBFs were also seriously decreased in OE fruit after treated 1 day (Figure 6f). These results indicate that other CBF regulators also participate in regulating chilling tolerance in tomato fruit. This raises the hypothesis that SlGRAS4 may enhance chilling injury tolerance in tomato, through both SlCBF‐dependent and SlCBF‐independent pathways.
Figure 6.
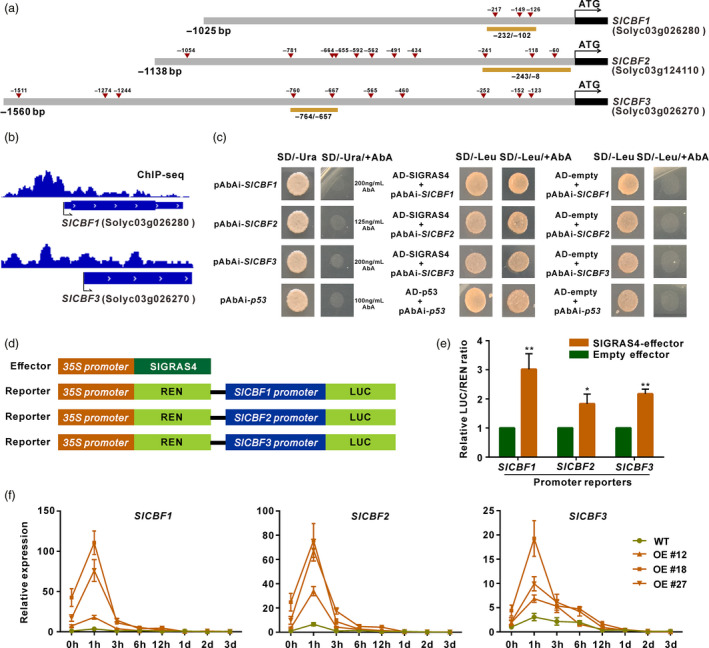
SlCBF1, SlCBF2 and SlCBF3 are under direct regulation of SlGRAS4 expression. (a) The promoter structure of SlCBF1, SlCBF2 and SlCBF3. The red triangles indicate the position of SlGRAS4‐binding sites corresponding to motif 4 present in the promoter of SlCBF1, SlCBF2 and SlCBF3. The displayed length of promoters was amplified and cloned into reporter vectors used for dual‐luciferase assays, and the regions underlined in orange indicate promoter fragments used for yeast‐one hybrid assays. (b) Integrative Genomics Viewer (IGV) image of the SlCBF1 and SlCBF3 genes as revealed by SlGRAS4 ChIP‐seq reads. (c) Interaction of SlGRAS4 with SlCBF1, SlCBF2 and SlCBF3 promoter fragments assessed by yeast‐one hybrid assays. (d) Effector and reporters constructs used for dual‐luciferase assays. (e) The transcription activation ability of SlGRAS4 tested on SlCBF1, SlCBF2 and SlCBF3 promoters by dual‐luciferase assays. The LUC/REN ratio of empty effector plus the promoter reporter was used as calibrator (set as 1). (f) Expression pattern of SlCBF1, SlCBF2 and SlCBF3 in WT and SlGRAS4‐OE mature green fruit in the absence (0 h) or presence of chilling stress treatment. In (e), data are the mean values of five independent replicates and error bars show the s.d. Asterisks indicate significant differences between SlGRAS4‐effector group and empty group (two‐tailed Student's t‐test, *P < 0.05, **P < 0.01). In (f), the transcript levels of SlCBF1, SlCBF2 and SlCBF3 in WT and transgenic fruit at different treatment points were relative to WT 0 h, and data are the mean values of three independent replicates and error bars show the s.d.
Discussion
Uncovering the mechanisms and factors underlying responses to cold stress is instrumental to the future design of efficient strategies to improving cold tolerance of important crop species. The present study shows that overexpression of SlGRAS4 in tomato confers chilling tolerance in both leaves and fruit resulting in minimal cellular damage compared to WT (Figures 1 and S2). Several cold stress‐associated genes involved in antioxidant activity, calcium signalling, photosynthesis and energy metabolism are directly regulated by SlGRAS4 (Figure 3). And the expression of these genes is also induced by chilling injury in wild‐type fruit in fact (Figure S4), suggesting that SlGRAS4 increases chilling tolerance in tomato fruit through these pathways. Indeed, the promoters of several genes encoding antioxidant enzymes exhibited high activation intensity mediated by SlGRAS4 (Figure 3c), consistent with the enhanced antioxidant capacity exhibited by SlGRAS4‐overexpressing lines (Figure 4). These data suggest that SlGRAS4 confers chilling tolerance in tomato at least partially by increasing antioxidant capacity. However, it has been reported that heterologous expression of the Arabidopsis CBF1 in tomato enhanced chilling tolerance via increasing antioxidant enzyme activities (Hsieh et al., 2002; Singh et al., 2011; Zhang et al., 2011), and considering the up‐regulation of CBF genes in SlGRAS4‐overexpression lines (Figure 6f), it cannot be ruled out that CBFs also contribute to mediating the increased antioxidant capacity in SlGRAS4‐OE plants.
The outcome of our study supports a working model (Figure 7) where two pathways operate in tomato during chilling tolerance, one mediated by ‘CBF‐regulon’ and a second one based on the ‘SlGRAS4‐regulon’. In the proposed model, the SlGRAS4 pathway intersects the ICE1/CBF pathway down‐stream of the ICE1 step, given the absence of interaction between SlGRAS4 and ICE1 proteins and considering that ICE1 expression is not affected in SlGRAS4 OE and RNAi lines. Although SlCBFs can be regulated by either SlGRAS4 or ICE1, the expression of SlCBFs in tomato is not strictly dependent on SlGRAS4 as indicated by their high expression levels in SlGRAS4 down‐regulated lines under cold stress (Figure S2). In a recent study, Wang et al. (2019) revealed the crosstalk of SlPIF4 and SlDELLA modulating SlCBF transcript and hormone homeostasis in cold response in tomato, the high expression level of SlCBFs in SlGRAS4‐RNAi leaves under low temperature, presumably via a SlGRAS4‐independent pathway. Strikingly, SlGRAS4‐down‐regulated plants exhibit higher sensitivity to cold stress than WT in despite of the strong expression of SlCBF1. These data support the notion that ‘SlGRAS4‐regulon’ plays an important role in promoting cold tolerance in tomato plants and seems to operate, at least partially, independently of the SlCBF pathway.
Figure 7.
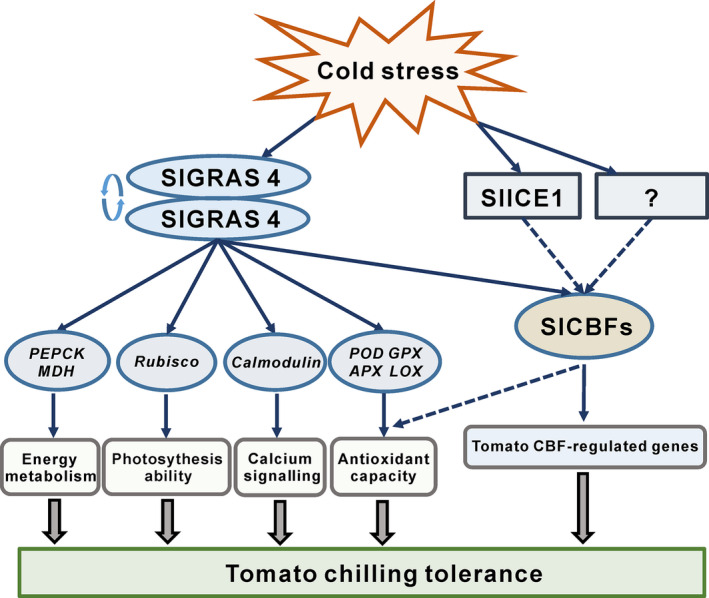
Proposed model for SlGRAS4‐dependent regulation of chilling injury tolerance in tomato. Our study uncovers a SlGRAS4‐regulated pathway underlying chilling injury tolerance. Deciphering the mechanism of this new pathway revealed that SlGRAS4 forms a homodimer that activates its own expression. SlGRAS4 is able to bind and activate the promoters of target genes involved in antioxidant capacity, calcium signalling, photosynthesis ability and energy metabolism. In this way, SlGRAS4 seems to operate independently of the ‘ICE1/CBF’ regulon. SlGRAS4 can also directly regulate SlCBF1, SlCBF2 and SlCBF3 expression by binding to their promoters. Therefore, the up‐regulation of SlCBFs may contribute to the higher chilling tolerance of SlGRAS4‐overexpressing lines. We provide a mechanism that SlGRAS4 positively regulates chilling injury resistance in tomato mainly by increasing antioxidant capacity and at least partially by CBF pathway.
On the other hand, the transcriptional levels of SlCBFs are increased rapidly in wild‐type fruit under chilling treatment and seriously decreased after 12 h (Figure 6f), whereas an significant induction of SlGRAS4 is observed until treated for 1 day (Figure S1), suggesting the responses of SlCBFs to low temperature in wild‐type fruit may not dependent on SlGRAS4. Furthermore, there are no persistent high levels of SlCBFs in SlGRAS4‐OE fruit under chilling stress; meanwhile, their expression is also seriously decreased in OE fruit after treated 12 h (Figure 6f). These data also suggest that other CBF regulators participate in regulating chilling tolerance in tomato fruit. SlGRAS4 may enhance chilling tolerance through both SlCBF‐dependent and SlCBF‐independent pathways in tomato.
It is worth noting that CBF overexpression in Arabidopsis, potato and B. napus L. resulted in a ‘stunted’ growth phenotype (Gilmour et al., 2000; Jaglo et al., 2001; Liu et al., 1998; Pino et al., 2008) and that overexpression of AtCBF1 in tomato also exhibited growth retardation with reduced fruit and seed number (Zhang et al., 2004). By contrast, SlGRAS4 overexpression have no detrimental effect on tomato growth which display normal plant height, leaf size, fruit set and fruit size, and seed numbers. Moreover, unlike the situation resulting from the overexpression CBF1 in Arabidopsis which results in the inactivation of the GA signalling pathway and the associated growth defects (Achard et al., 2008b), the expression level of genes involved in GA metabolism is not affected in SlGRAS4‐OE lines. These data argue for the existence of a new cold stress pathway in tomato and sustain the idea that the newly uncovered ‘SlGRAS4‐regulon’ plays a more prominent role than the ‘CBF‐pathway’ in conferring cold stress tolerance to tomato fruit and plants. The SlGRAS4 pathway provides new targets for novel breeding strategies aiming to enhance tomato tolerance to low temperature and to improve sensory qualities of tomato fruit that are often deteriorated by storage in temperature below 15°C.
Experimental procedures
Plant material growth conditions and generation of transgenic tomato lines
To generate SlGRAS4 overexpression (OE) plants, the ORF of SlGRAS4 without the stop codon was cloned into modified plant binary vector K303 under the CaMV 35S promoter (Liu et al., 2017). The SlGRAS4 RNA‐interference (RNAi) construct was generated by cloning a 320‐bp sequence fragment amplified by PCR into modified plant binary vector pCambia 1301 under the CaMV 35S promoter. Agrobacterium tumefaciens strain GV3101 was used to transform wild‐type tomato plants (Solanum lycopersicum L. cv. Micro‐Tom) following standard methods. Positive transgenic lines were screened by kanamycin (100 mg/L) selection and then confirmed by PCR, and the relative expression level was confirmed by q‐RT‐PCR using homozygous lines from T2 or T3 generations. All plants were grown in greenhouse in controlled conditions (18‐h light/6‐h dark cycles, 25 °C day/18 °C night, and 60% relative humidity).
Low‐temperature stress treatment
To assess the impact of low‐temperature stress on gene expression, 30‐day‐old wild‐type plants treated at 4 °C condition and leaves (5th from cotyledon) were harvested after 1‐, 3‐, 6‐, 12‐, and 24‐h cold treatment, and untreated leaves were used as control. For each sample, leaves from six different plants were mixed and the treatments were performed in three independent experiments at different times. Wild‐type and transgenic tomato fruit at mature green stage were harvested and washed by distilled water, and then treated at 4 °C for 1, 3, 6, 12 h, 1, 2 and 3 days, and untreated fruit were used as control. For each sample, six fruits were mixed and all treatments were performed three times in three independent replicates. All samples were frozen in liquid nitrogen and stored at −80 °C for RNA extraction and q‐RT‐PCR.
To assess low‐temperature tolerance of tomato plants, 45‐day‐old WT and transgenic plants grown under normal conditions were transferred to 4 °C condition. For physiological assessments, OE plants were treated for 4 days and RNAi plants for 1 day before collecting leaves, with leaves from plants not subjected to cold treatment used as control. At least 10 OE plants and 10 RNAi plants each corresponding to independent transformation events were used for low‐temperature stress treatment, and three independent repeats were performed.
For fruit chilling injury test, fruit at mature green stage were harvested and washed by distilled water, and divided into two lots, one placed at 5 °C for 14 days, and then restored to 25 °C condition for 14 days, the other group used as control was placed 25 °C for 28 days. Cold‐treated fruit were sampled at each time point and frozen in liquid nitrogen and then stored at −80 °C for further experiments. At least thirty fruits from ten plants of each transgenic line were used for chilling injury treatment, and three independent repeats from different plants were performed. The CI index (chilling injury index) was used to determine the chilling injury tolerance for fruit as subjective evaluation in this study (Cruz‐Mendívil et al., 2015). Briefly, CI index = (ILU + ILW)/2, ILU indicates injury as the level of uneven ripening (a five‐point scale based on the ripening stage for each criterion (0 = red, 1 = orange, 2 = yellow, 3 = breaker and 4 = green)), and ILW indicates injury as the level of pitting (a five‐point scale based on the percentage of tissue affected for each criterion (0 = no injury, 1 ≤ 10%, 2 = 11%–25%, 3 = 26%–40% and 4 ≥ 40%)). Fruit firmness was performed by GY‐4 digital fruit sclerometer (Aiwoshi, China). Colour parameter was measured by a colorimeter (Lovibond, Germany).
Oxidative stress treatment
To determine SlGRAS4 transcript accumulation in response to oxidative stress, 30‐day‐old wild‐type plants were sprayed with 100 mm hydrogen peroxide, and leaves (5th from cotyledons) were harvested after 1‐, 3‐, 6‐, 12‐ and 24‐h treatment, while untreated leaves were used as control. For each sample, leaves from six plants were mixed and all treatments were performed three independent times. All samples were frozen in liquid nitrogen and stored at −80 °C until RNA extraction for q‐RT‐PCR.
For oxidative stress test, 45‐day‐old WT, OE and RNAi plants were sprayed with 100 μm MV (methyl viologen) once a day for three days, and placed thereafter in continuous illumination condition for 4 days until the leaves display symptoms of wilting and desiccation, and then, leaf samples (5th and 6th from cotyledon) were harvested for total chlorophyll measurement.
For oxidative stress test at germination stage, seeds of WT, OE and RNAi lines were sterilized and sown on ½ × MS alone and ½ × MS containing 10 μm MV, and incubated under 18‐h light (25 °C)/6‐h dark (18 °C) cycle conditions. Seed germination rate was assessed after 7 days, and the lengths of primary roots were measured after 14 days. The treatment was performed three independent times, and at least 30 seeds were used for each treatment.
Physiological measurement
Total chlorophyll content and MDA content were measured according to the method described in our previous study (Liu et al., 2017). Total phenolic content and total flavonoid content were performed as described by Jian et al. (2019), total phenolic was expressed as mg GAE (Gallic acid)/g FW and total flavonoid was expressed as mg RE (Rutin)/g FW. DPPH ability, FRAP ability and ABTS ability were performed according to the protocol described previously with minor modification (Zhang et al., 2014), and these antioxidant capacities were expressed as µmol TE (Trolox)/g FW.
ChIP‐seq assay
Mature green transgenic tomato fruit expressing a GFP‐tagged SlGRAS4 protein (SlGRAS4‐ORF fused with GFP under 35S promoter) were used for chromatin immunoprecipitation, and the ChIP assay was performed as described by Lü et al. (2018). Briefly, fruit tissues were fixed in 1x PBS with 1% formaldehyde for 15 min under vacuum and ground to fine power under liquid nitrogen. Subsequently, nuclei were isolated in nuclei extraction buffer, then sonicated the chromatin to 300‐ to 500‐bp fragments with Covaris M220 in TE buffer containing 0.2% SDS and protease inhibitors, and diluted with low‐salt wash buffer with 1% Triton X‐100. The chromatin samples were first pre‐cleared with empty Dynabeads protein A/G and incubated overnight with Dynabeads with anti‐GFP antibody (Millipore). The beads were then washed twice with low‐salt buffer, followed by two times high‐salt and LiCl washing buffer. The ChIPmentation method was used for ChIP‐seq library construction (ChIPmentation: fast, robust, low‐input ChIP‐seq for histones and transcription factors). Beads washed above were resuspended in 30 μL of the tagmentation reaction buffer (10 mm MgCl2, 25 mm Tris pH 8.0, 10% DMF) containing 1 μL Tagment DNA Enzyme from the Nextera DNA Sample Prep Kit (Illumina) and incubated at 37 °C for 10 min in a thermocycler. The tagmented beads were then washed with low‐salt, high‐salt and TE washing buffers. At last, the samples were eluted in elution buffer for reverse cross‐linking overnight. After purification, the final DNA was used for PCR and sequencing. Raw reads were mapped to tomato genome (http://solgenomics.net/) using Bowtie2. And the ChIP‐seq datasets were supplied to MACS2 for peak calling. Peaks were then associated to genes if they were located within the gene body or the region 1 kb upstream of the TSS. Transcription factor binding motifs were predicted with HOMER.
RNA‐seq assay
Overexpression line OE #18 and down‐expression line RNAi #10 were used for RNA‐seq assay, with wild type used as control. Total RNA was extracted (RNeasy kit, QIAGEN, Germany) from mature green fruit (0d), mature green fruit treated 14 days at 5 °C (CI 14d) and mature green fruit treated 14 days at 5 °C then replaced at 25 °C for 14 days (CI 14d + RT 14d). cDNA libraries (Illumina) were then constructed for sequencing on the BGI‐Seq 500 system (BGI Inc., China). For each sample, the summary of sequencing data is shown in Data [Link], [Link], [Link], respectively. HISAT was used to map clean reads to the reference genome of Solanum lycopersicum L. in the Tomato Sol Genomic Network database (http://solgenomics.net/), and Bowtie2 was used to map clean reads to reference gene. And the homogenized data were used to calculate gene expression levels with RSEM. For 0d samples, differentially expressed genes (DEGs) were detected with DEGseq with the following parameters: fold change ≥2.00 and adjusted P‐value (Q‐value) ≤0.001. For the samples of CI 14d and CI 14d + RT 14d, DEGs were detected with NOIseq with the following parameters: fold change ≥2.00 and probability ≥0.8. Sequence data of RNA‐seq from this article can be found in the Sequence Read Archive (SRA) database under accession number PRJNA594085.
Yeast‐one hybrid assay
The promoters of target genes containing SlGRAS4‐binding motif were amplified by PCR and cloned into pAbAi vector as the baits. Recombined bait–pAbAi plasmids were digested by BstBI, and the linearized plasmids were transformed into Y1HGold yeast strain according to Yeastmaker™ Yeast Transformation System 2 (Clontech). The positive Y1HGold [bait/AbAi] strains were confirmed by colony PCR using Matchmaker Insert Check PCR Mix 1 (Clontech) and then screened inhibitory concentration of aureobasidin A (AbA) to avoid self‐activation by spreading gradient concentration SD/‐Ura/AbA plates. The full length of SlGRAS4 ORF lacking the stop codon was cloned into pGADT7 vector to construct the prey, the recombined SlGRAS4‐pGADT7 plasmid was transformed into Y1HGold [bait/AbAi] strains and spread on SD/‐Leu/AbA plates, and transformed empty pGADT7 plasmid was used as control. Protein–DNA interaction was determined based on growth ability of the transformed yeast cells on SD/‐Leu/AbA medium following the manufacturer's protocol (Clontech).
Dual‐luciferase assay
The full‐length ORF of SlGRAS4 was cloned into pGreenII 62‐SK binary vector to generate an effector construct, and about 1.5‐kb‐length promoter fragment of target genes was amplified by PCR and cloned into pGreenII 0800‐LUC binary vector as reporter constructs. The recombined plasmids were co‐transformed with pSoup plasmid into Agrobacterium tumefaciens strain GV3101, transfected to tobacco (Nicotiana benthamiana L.) leaves for transient gene expression analysis. The transformed tobacco plants were incubated at 25 °C in dark for 16 h and then replaced at 25 °C in normal light cycles for 3 days, and then, the leaves were subjected to LUC assays using the Dual‐Luciferase® Reporter Assay System (Promega) according to the manufacturer's instructions.
Transactivation in yeast and yeast‐two hybrid assays
The full‐length ORF of SlGRAS4 (Appendix S2) and the truncated SlGRAS4 versions were cloned into pGBDT7 vector. The recombined SlGRAS4‐pGBDT7 plasmids named SlGRAS4 (1‐667), SlGRAS4 ΔC (1‐285), SlGRAS4 ΔN1 (50‐667), SlGRAS4 ΔN2 (100‐667), SlGRAS4 ΔN3 (130‐667), SlGRAS4 ΔN4 (150‐667), SlGRAS4 ΔN5 (200‐667) and SlGRAS4 ΔN6 (286‐667) were transformed into Y2HGold strain and spread on SD/‐Trp, SD/‐Trp/X‐α‐gal and SD/‐Trp/X‐α‐gal/AbA plates for transcriptional activation test. The SlGRAS4‐pGADT7 recombined plasmid as prey and the truncated SlGRAS4 ΔN5 (200‐667) and SlGRAS4 ΔN6 (286‐667) have no transcriptional activation activity as baits were co‐transformed into Y2HGold strain and spread on SD/‐Leu/‐Trp/X‐α‐gal (DDO/X‐α‐gal), SD/‐Ade/‐His/‐Leu/‐Trp (QDO), and SD/‐Ade/‐His/‐Leu/‐Trp/X‐α‐gal/AbA (QDO/X‐α‐gal/AbA) plates for protein–protein interaction test following the manufacturer's protocol (Clontech).
Bimolecular fluorescence complementation (BiFC) assay
The vectors of BiFC (pXY104 and pXY106) were described previously (Liu and Howell, 2010; Yu et al., 2008). The full‐length ORF of SlGRAS4 was cloned into pXY104 vector to generate a C‐terminal YFP fluorescent fusion protein and into pXY106 vector to generate a N‐terminal YFP fluorescent protein. The BiFC assay was performed as described by Luo et al. (2014). The recombined plasmids were transformed into Agrobacterium tumefaciens strain GV3101, and tobacco leaves were used for transient expression. The transformed tobacco plants were incubated at 25 °C in the dark for 16 h and then replaced at 25 °C in the normal light cycles for 3 days, and then, fluorescence was observed by confocal laser scanning microscope (Leica, Germany).
Gene expression analysis
Total RNA was extracted using an RNeasy kit (QIAGEN, Germany), and first‐strand cDNA was synthesized with PrimeScript™ RT Reagent Kit with gDNA Eraser (Perfect Real Time) (TAKARA, Japan). Quantitative real‐time PCR was performed using SYBR® Premix Ex Taq™ (Tli RNaseH Plus), and the PCR amplification cycles were set according to the instructions (TAKARA, Japan). Melting curve analysis was performed in the temperature ranging 60–95 °C to verify the specificity of the amplicon for each primer pairs. The 2−ΔΔCt method was used to calculate relative fold differences (Bio‐Rad), using SlActin as an internal reference gene. All the primers used for q‐RT‐PCR are listed in Table S2.
Conflict of interest
The authors have no conflicts of interest to declare.
Author contributions
Y.L. and Z.L. designed research. Y.L. and Y.S. performed most of the described experiments. N.Z. and S.Z. performed the ChIP assay. Y.L. and M.B. analysed data. Y.L. wrote the paper. M.B. and Z.L. revised the paper.
Supporting information
Figure S1 SlGRAS4 is significantly induced by low temperature.
Figure S2 SlGRAS4 promotes cold tolerance in tomato plants.
Figure S3 Other physiological phenotypes in WT and transgenic fruit after chilling injury treatment.
Figure S4 The response to chilling stress of SlGRAS4‐targeted genes in wild type fruit.
Table S1 The potential SlGRAS4‐binding motifs analysed based on ChIP‐seq results
Table S2 Primers used in this study
Appendix S1 Nucleotide sequences of promoters of SlGRAS4‐target genes
Appendix S2 SlGRAS4 nucleotide sequence and encoded amino acid sequence
Data S1 SlGRAS4‐binding peaks and SlGRAS4‐binding genes identified from ChIP‐seq analysis
Data S2 RNA‐seq data of 0d group and overlapping ChIP‐seq and RNA‐seq genes
Data S3 RNA‐seq data of CI 14d group and overlapping ChIP‐seq and RNA‐seq genes
Data S4 RNA‐seq data of CI 14d + RT 14d group and overlapping ChIP‐seq and RNA‐seq genes
Acknowledgements
This work was supported by the National Key Research and Development Program (2016YFD0400101), the National Natural Science Foundation of China (Nos. 31772370 and 31572175) and Hong Kong GRF 14108117 and AoE/M‐403/16.
Liu, Y. , Shi, Y. , Zhu, N. , Zhong, S. , Bouzayen, M. and Li, Z. (2020) SlGRAS4 mediates a novel regulatory pathway promoting chilling tolerance in tomato. Plant Biotechnol. J., 10.1111/pbi.13328
Contributor Information
Mondher Bouzayen, Email: bouzayen@ensat.fr.
Zhengguo Li, Email: zhengguoli@cqu.edu.cn.
References
- Achard, P. , Renou, J.P. , Berthomé, R. , Harberd, N.P. and Genschik, P. (2008a) Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Curr. Biol. 18, 656–660. [DOI] [PubMed] [Google Scholar]
- Achard, P. , Gong, F. , Cheminant, S. , Alioua, M. , Hedden, P. and Genschik, P. (2008b) The cold‐inducible CBF1 factor‐dependent signaling pathway modulates the accumulation of the growth‐repressing DELLA proteins via its effect on gibberellin metabolism. Plant Cell, 20, 2117–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal, M. , Hao, Y. , Kapoor, A. , Dong, C.H. , Fujii, H. , Zheng, X. and Zhu, J.K. (2006) A R2R3 type MYB transcription factor is involved in the cold regulation of CBF genes and in acquired freezing tolerance. J. Biol. Chem. 281, 37636–37645. [DOI] [PubMed] [Google Scholar]
- Chinnusamy, V. , Ohta, M. , Kanrar, S. , Lee, B.H. , Hong, X. , Agarwal, M. and Zhu, J.K. (2003) ICE1: a regulator of cold‐induced transcriptome and freezing tolerance in Arabidopsis . Genes Dev. 17, 1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, D.W. , Rodriguez, E.M. and Close, T.J. (2002) Barley Cbf3 gene identification, expression pattern, and map location. Plant Physiol. 129, 1781–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz‐Mendívil, A. , López‐Valenzuela, J.A. , Calderón‐Vázquez, C.L. , Vega‐García, M.O. , Reyes‐Moreno, C. and Valdez‐Ortiz, A. (2015) Transcriptional changes associated with chilling tolerance and susceptibility in ‘Micro‐Tom’ tomato fruit using RNA‐Seq. Postharvest Biol. Technol. 99, 141–151. [Google Scholar]
- Ding, Y. , Li, H. , Zhang, X. , Xie, Q. , Gong, Z. and Yang, S. (2015) OST1 kinase modulates freezing tolerance by enhancing ICE1 stability in Arabidopsis . Dev. Cell, 32, 278–289. [DOI] [PubMed] [Google Scholar]
- Doherty, C.J. , Van Buskirk, H.A. , Myers, S.J. and Thomashow, M.F. (2009) Roles for Arabidopsis CAMTA transcription factors in cold‐regulated gene expression and freezing tolerance. Plant Cell, 21, 972–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, C.H. , Agarwal, M. , Zhang, Y. , Xie, Q. and Zhu, J.K. (2006) The negative regulator of plant cold responses, HOS1, is a RING E3 ligase that mediates the ubiquitination and degradation of ICE1. Proc. Natl. Acad. Sci. USA, 103, 8281–8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, M.A. , Farré, E.M. and Thomashow, M.F. (2011) CIRCADIAN CLOCK‐ASSOCIATED 1 and LATE ELONGATED HYPOCOTYL regulate expression of the C‐REPEAT BINDING FACTOR (CBF) pathway in Arabidopsis . Proc. Natl. Acad. Sci. USA, 108, 7241–7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubouzet, J.G. , Sakuma, Y. , Ito, Y. , Kasuga, M. , Dubouzet, E.G. , Miura, S. , Seki, M. et al. (2003) OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought‐, high‐salt‐ and cold‐responsive gene expression. Plant J. 33, 751–763. [DOI] [PubMed] [Google Scholar]
- Eremina, M. , Unterholzner, S.J. , Rathnayake, A.I. , Castellanos, M. , Khan, M. , Kugler, K.G. , May, S.T. et al. (2016) Brassinosteroids participate in the control of basal and acquired freezing tolerance of plants. Proc. Natl. Acad. Sci. USA, 113, E5982–E5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, H.L. , Ma, N.N. , Meng, X. , Zhang, S. , Wang, J.R. , Chai, S. and Meng, Q.W. (2013) A novel tomato MYC‐type ICE1‐like transcription factor, SlICE1a, confers cold, osmotic and salt tolerance in transgenic tobacco. Plant Physiol. Biochem. 73, 309–320. [DOI] [PubMed] [Google Scholar]
- Fowler, S. and Thomashow, M.F. (2002) Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. Plant Cell, 14, 1675–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour, S.J. , Zarka, D.G. , Stockinger, E.J. , Salazar, M.P. , Houghton, J.M. and Thomashow, M.F. (1998) Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold‐induced COR gene expression. Plant J. 16, 433–442. [DOI] [PubMed] [Google Scholar]
- Gilmour, S.J. , Sebolt, A.M. , Salazar, M.P. , Everard, J.D. and Thomashow, M.F. (2000) Overexpression of the Arabidopsis CBF3 transcriptional activator mimics multiple biochemical changes associated with cold acclimation. Plant Physiol. 124, 1854–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh, T.H. , Lee, J.T. , Yang, P.T. , Chiu, L.H. , Charng, Y.Y. , Wang, Y.C. and Chan, M.T. (2002) Heterology expression of the Arabidopsis C‐repeat/dehydration response element binding factor 1 gene confers elevated tolerance to chilling and oxidative stresses in transgenic tomato. Plant Physiol. 129, 1086–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, W. , Xian, Z. , Kang, X. , Tang, N. and Li, Z. (2015) Genome‐wide identification, phylogeny and expression analysis of GRAS gene family in tomato. BMC Plant Biol. 15, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, W. , Peng, S. , Xian, Z. , Lin, D. , Hu, G. , Yang, L. , Ren, M. et al. (2017) Overexpression of a tomato miR171 target gene SlGRAS24 impacts multiple agronomical traits via regulating gibberellin and auxin homeostasis. Plant Biotechnol. J. 15, 472–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaglo, K.R. , Kleff, S. , Amundsen, K.L. , Zhang, X. , Haake, V. , Zhang, J.Z. , Deits, T. et al. (2001) Components of the Arabidopsis C‐repeat/dehydration‐responsive element binding factor cold‐response pathway are conserved in Brassica napus and other plant species. Plant Physiol. 127, 910–917. [PMC free article] [PubMed] [Google Scholar]
- Jaglo‐Ottosen, K.R. , Gilmour, S.J. , Zarka, D.G. , Schabenberger, O. and Thomashow, M.F. (1998) Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science, 280, 104–106. [DOI] [PubMed] [Google Scholar]
- Jian, W. , Cao, H. , Yuan, S. , Liu, Y. , Lu, J. , Lu, W. , Li, N. et al. (2019) SlMYB75, an MYB‐type transcription factor, promotes anthocyanin accumulation and enhances volatile aroma production in tomato fruits. Hortic. Res. 6, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, B. , Shi, Y. , Zhang, X. , Xin, X. , Qi, L. , Guo, H. , Li, J. et al. (2017) PIF3 is a negative regulator of the CBF pathway and freezing tolerance in Arabidopsis . Proc. Natl. Acad. Sci. USA, 114, E6695–E6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga, M. , Liu, Q. , Miura, S. , Yamaguchi‐Shinozaki, K. and Shinozaki, K. (1999) Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress‐inducible transcription factor. Nat. Biotechnol. 17, 287–291. [DOI] [PubMed] [Google Scholar]
- Kidokoro, S. , Yoneda, K. , Takasaki, H. , Takahashi, F. , Shinozaki, K. and Yamaguchi‐Shinozaki, K. (2017) Different cold‐signaling pathways function in the responses to rapid and gradual decreases in temperature. Plant Cell, 29, 760–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y.S. , Lee, M. , Lee, J.H. , Lee, H.J. and Park, C.M. (2015) The unified ICE‐CBF pathway provides a transcriptional feedback control of freezing tolerance during cold acclimation in Arabidopsis . Plant Mol. Biol. 89, 187–201. [DOI] [PubMed] [Google Scholar]
- Kim, S.H. , Kim, H.S. , Bahk, S. , An, J. , Yoo, Y. , Kim, J.Y. and Chung, W.S. (2017) Phosphorylation of the transcriptional repressor MYB15 by mitogen‐activated protein kinase 6 is required for freezing tolerance in Arabidopsis . Nucleic Acids Res. 45, 6613–6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, M.H. , Kim, B. , Song, S.K. , Heo, J.O. , Yu, N.I. , Lee, S.A. , Kim, M. et al. (2008) Large‐scale analysis of the GRAS gene family in Arabidopsis thaliana . Plant Mol. Biol. 67, 659–670. [DOI] [PubMed] [Google Scholar]
- Li, H. , Ding, Y. , Shi, Y. , Zhang, X. , Zhang, S. , Gong, Z. and Yang, S. (2017a) MPK3‐ and MPK6‐mediated ICE1 phosphorylation negatively regulates ICE1 stability and freezing tolerance in Arabidopsis . Dev. Cell, 43, 630–642. [DOI] [PubMed] [Google Scholar]
- Li, H. , Ye, K. , Shi, Y. , Cheng, J. , Zhang, X. and Yang, S. (2017b) BZR1 positively regulates freezing tolerance via CBF‐dependent and CBF‐independent pathways in Arabidopsis. Mol. Plant, 10, 545–559. [DOI] [PubMed] [Google Scholar]
- Liu, J.X. and Howell, S.H. (2010) bZIP28 and NF‐Y transcription factors are activated by ER stress and assemble into a transcriptional complex to regulate stress response genes in Arabidopsis . Plant Cell, 22, 782–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Q. , Kasuga, M. , Sakuma, Y. , Abe, H. , Miura, S. , Yamaguchi‐Shinozaki, K. and Shinozaki, K. (1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought‐ and low‐temperature‐responsive gene expression, respectively, in Arabidopsis. Plant Cell, 10, 1391–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Huang, W. , Xian, Z. , Hu, N. , Lin, D. , Ren, H. , Chen, J. et al. (2017) Overexpression of SlGRAS40 in tomato enhances tolerance to abiotic stresses and influences auxin and gibberellin signaling. Front. Plant Sci. 8, 1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lü, P. , Yu, S. , Zhu, N. , Chen, Y.R. , Zhou, B. , Pan, Y. , Tzeng, D. et al. (2018) Genome encode analyses reveal the basis of convergent evolution of fleshy fruit ripening. Nat. Plants, 4, 784–791. [DOI] [PubMed] [Google Scholar]
- Luo, Q. , Lian, H.L. , He, S.B. , Li, L. , Jia, K.P. and Yang, H.Q. (2014) COP1 and phyB physically interact with PIL1 to regulate its stability and photomorphogenic development in Arabidopsis . Plant Cell, 26, 2441–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura, K. , Jin, J.B. , Lee, J. , Yoo, C.Y. , Stirm, V. , Miura, T. , Ashworth, E.N. et al. (2007) SIZ1‐mediated sumoylation of ICE1 controls CBF3/DREB1A expression and freezing tolerance in Arabidopsis . Plant Cell, 19, 1403–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura, K. , Sato, A. , Shiba, H. , Kanga, S.W. , Kamada, H. and Ezura, H. (2012a) Accumulation of antioxidants and antioxidant activity in tomato, Solanum lycopersicum, are enhanced by the transcription factor SlICE1. Plant Biotechnol. 29, 261–269. [Google Scholar]
- Miura, K. , Shiba, H. , Ohta, M. , Kang, S.W. , Sato, A. , Yuasa, T. , Iwaya‐Inoue, M. et al. (2012b) SlICE1 encoding a MYC‐type transcription factor controls cold tolerance in tomato, Solanum lycopersicum . Plant Biotechnol. 29, 253–260. [Google Scholar]
- Pino, M.T. , Skinner, J.S. , Jeknić, Z. , Hayes, P.M. , Soeldner, A.H. , Thomashow, M.F. and Chen, T.H. (2008) Ectopic AtCBF1 over‐expression enhances freezing tolerance and induces cold acclimation‐associated physiological modifications in potato. Plant Cell Environ. 31, 393–406. [DOI] [PubMed] [Google Scholar]
- Seo, E. , Lee, H. , Jeon, J. , Park, H. , Kim, J. , Noh, Y.S. and Lee, I. (2009) Crosstalk between cold response and flowering in Arabidopsis is mediated through the flowering‐time gene SOC1 and its upstream negative regulator FLC . Plant Cell, 21, 3185–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevillano, L. , Sanchez‐Ballesta, M.T. , Romojaro, F. and Flores, F.B. (2009) Physiological, hormonal and molecular mechanisms regulating chilling injury in horticultural species. Postharvest technologies applied to reduce its impact. J. Sci. Food Agric. 89, 555–573. [Google Scholar]
- Shi, Y. , Tian, S. , Hou, L. , Huang, X. , Zhang, X. , Guo, H. and Yang, S. (2012) Ethylene signaling negatively regulates freezing tolerance by repressing expression of CBF and type‐A ARR genes in Arabidopsis. Plant Cell, 24, 2578–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Y. , Ding, Y. and Yang, S. (2018) Molecular regulation of CBF signaling in cold acclimation. Trends Plant Sci. 23, 623–637. [DOI] [PubMed] [Google Scholar]
- Singh, S. , Rathore, M. , Goyary, D. , Singh, R.K. , Anandhan, S. , Sharma, D.K. and Ahmed, Z. (2011) Induced ectopic expression of At‐CBF1 in marker‐free transgenic tomatoes confers enhanced chilling tolerance. Plant Cell Rep. 30, 1019–1028. [DOI] [PubMed] [Google Scholar]
- Stockinger, E.J. , Gilmour, S.J. and Thomashow, M.F. (1997) Arabidopsis thaliana CBF1 encodes an AP2 domain‐containing transcriptional activator that binds to the C‐repeat/DRE, a cis‐acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc. Natl. Acad. Sci. USA, 94, 1035–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow, M.F. (2001) So what's new in the field of plant cold acclimation? Lots! Plant Physiol. 125, 89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, F. , Chen, X. , Dong, S. , Jiang, X. , Wang, L. , Yu, J. and Zhou, Y. (2019) Crosstalk of PIF4 and DELLA modulates CBF transcript and hormone homeostasis in cold response in tomato. Plant Biotechnol. J. 10.1111/pbi.13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi‐Shinozaki, K. and Shinozaki, K. (1994) A novel cis‐acting element in an Arabidopsis gene is involved in responsiveness to drought, low‐temperature, or high‐salt stress. Plant Cell, 6, 251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, X. , Li, L. , Li, L. , Guo, M. , Chory, J. and Yin, Y. (2008) Modulation of brassinosteroid‐regulated gene expression by jumonji domain‐containing proteins ELF6 and REF6 in Arabidopsis . Proc. Natl. Acad. Sci. USA, 105, 7618–7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Fowler, S.G. , Cheng, H. , Lou, Y. , Rhee, S.Y. , Stockinger, E.J. and Thomashow, M.F. (2004) Freezing‐sensitive tomato has a functional CBF cold response pathway, but a CBF regulon that differs from that of freezing‐tolerant Arabidopsis . Plant J. 39, 905–919. [DOI] [PubMed] [Google Scholar]
- Zhang, Y.J. , Yang, J.S. , Guo, S.J. , Meng, J.J. , Zhang, Y.L. , Wan, S.B. , He, Q.W. et al. (2011) Over‐expression of the Arabidopsis CBF1 gene improves resistance of tomato leaves to low temperature under low irradiance. Plant Biol. 13, 362–367. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Sun, Y. , Xi, W. , Shen, Y. , Qiao, L. , Zhong, L. , Ye, X. et al. (2014) Phenolic compositions and antioxidant capacities of Chinese wild mandarin (Citrus reticulata Blanco) fruits. Food Chem. 145, 674–680. [DOI] [PubMed] [Google Scholar]
- Zhang, B. , Tieman, D.M. , Jiao, C. , Xu, Y. , Chen, K. , Fei, Z. , Giovannoni, J.J. et al. (2016) Chilling‐induced tomato flavor loss is associated with altered volatile synthesis and transient changes in DNA methylation. Proc. Natl. Acad. Sci. USA, 113, 12580–12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, C. , Wang, P. , Si, T. , Hsu, C.C. , Wang, L. , Zayed, O. , Yu, Z. et al. (2017) MAP kinase cascades regulate the cold response by modulating ICE1 protein stability. Dev. Cell, 43, 618–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 SlGRAS4 is significantly induced by low temperature.
Figure S2 SlGRAS4 promotes cold tolerance in tomato plants.
Figure S3 Other physiological phenotypes in WT and transgenic fruit after chilling injury treatment.
Figure S4 The response to chilling stress of SlGRAS4‐targeted genes in wild type fruit.
Table S1 The potential SlGRAS4‐binding motifs analysed based on ChIP‐seq results
Table S2 Primers used in this study
Appendix S1 Nucleotide sequences of promoters of SlGRAS4‐target genes
Appendix S2 SlGRAS4 nucleotide sequence and encoded amino acid sequence
Data S1 SlGRAS4‐binding peaks and SlGRAS4‐binding genes identified from ChIP‐seq analysis
Data S2 RNA‐seq data of 0d group and overlapping ChIP‐seq and RNA‐seq genes
Data S3 RNA‐seq data of CI 14d group and overlapping ChIP‐seq and RNA‐seq genes
Data S4 RNA‐seq data of CI 14d + RT 14d group and overlapping ChIP‐seq and RNA‐seq genes


