Abstract
Objective
To determine whether initial presurgical evaluation of deep brain stimulation (DBS) candidacy with video telemedicine (VTEL) can reliably predict surgical candidacy (patients who will eventually undergo DBS surgery) and decrease resource utilization when compared to an in-person evaluation.
Methods
In this retrospective, cohort analysis, all out-of-state referrals to the San Francisco Veterans Affairs from 2008 to 2013 for DBS therapy were reviewed and their surgical outcomes were assessed until 2017. Patients were designated as good, borderline, or poor surgical candidates after initial evaluation, and their rates of undergoing DBS were recorded. An assessment of patient travel costs was performed.
Results
There were 60 out-of-state DBS referrals identified out of the 148 initial presurgical DBS evaluations completed for surgical treatment of dystonia, essential tremor, or Parkinson disease; 24 patients underwent in-person consultation and 36 patients underwent evaluation via VTEL. There was no difference between the rates of undergoing surgical treatment with DBS based on surgical candidacy for patients in the in-person and VTEL cohorts. Patients who underwent initial presurgical screening via VTEL saved time and money.
Conclusions
VTEL can be used to facilitate presurgical screening for DBS and saves costs.
Deep brain stimulation (DBS) is a safe, effective, and the standard-of-care treatment for some patients with certain movement disorders, including Parkinson disease (PD), essential tremor (ET), and various forms of dystonia.1–4 Evaluation for this therapy is most commonly indicated when significant motor impairment persists despite optimized medical treatment. Many patients stand to benefit from an evaluation by neurologists and neurosurgeons subspecialized in movement disorders, but racial, socioeconomic, and gender-based disparities in access to care remain.5–7 By 2040, the number of patients with PD is expected to double worldwide to over 12 million, further taxing health care systems.8,9
Many experts have proposed the use of video telemedicine (VTEL) to improve access to neurologic care for patients in resource-limited regions.5–7 Outpatient telemedicine is increasingly used to complement in-person visits, decreasing time between follow-up visits, and facilitating interactions between primary physicians and tertiary referral centers.10 Patients with PD have high rates of satisfaction with telemedicine.11 Telemedicine has been used to make adjustments to DBS settings.12 However, little data exist regarding the use of this technology to assess DBS candidacy. Herein, this retrospective study sought to determine whether evaluation of DBS candidacy with VTEL can reliably predict surgical candidacy and decrease resource utilization when compared to in-person consultation. Our hypothesis is that rates of eventual surgery would be similar between those initially screened via in-person and VTEL (within groups determined to be good, borderline, and poor surgical candidates), the time to surgery would be similar, and the patients in the VTEL cohort would save time and money.
Methods
This is a retrospective, cohort study of all out-of-state DBS referrals to the San Francisco Veterans Affairs: Parkinson's Disease Research, Education and Clinical Centers (SFVA PADRECC) consortium from 2008 to 2013 and each of the patient's eventual surgical status until July 2017. We compared those seen in-person to those evaluated remotely via VTEL for their initial DBS candidacy consultation. The primary outcome was rate of surgical completion of DBS following consultation. Secondary outcomes analyzed included resource utilization (e.g., patient-level travel costs, miles traveled, and hours spent traveling) and time from initial evaluation to surgery for those patients who underwent surgery.
The United States Department of Veterans Affairs (VA) has used telemedicine to provide care to over 2 million veterans since 1968.11 Today, VA medical centers use live VTEL visits to connect 700 community-based outpatient clinics to regional medical centers with subspecialty expertise.13 There are 6 PD centers of excellence located in San Francisco, Seattle, Portland, Los Angeles, Houston, and Richmond, which help support their affiliated, but geographically remote, clinics across the United States. The PADRECC consortium has improved access to movement disorders specialists for veterans. When a local general neurologist or movement disorder specialists affiliated with the VA thinks a patient may benefit from DBS or the patient would like to learn more about this therapeutic option, they may refer patients to their associated PADRECC. The SFVA PADRECC routinely evaluates candidates for DBS surgery from its referral area within the national PADRECC network, an area that reaches far outside of California, up to 2,500 miles away (figure 1). Increasingly over the last decade, out-of-state patients are initially evaluated remotely by VTEL, given this distance.
Figure 1. Home addresses of patients referred for deep brain stimulation.
This outlines the home address of each patient who was referred out-of-state for evaluation for DBS. Each color represents the local Veterans Affairs medical center that referred patients to San Francisco Veterans Affairs. These cities are part of the San Francisco Parkinson's Disease Research, Education and Clinical Centers catchment area. DBS = deep brain stimulation.
Patients with PD, ET, and dystonia were referred to the SFVA PADRECC from their local neurologist or movement disorder specialist. Pertinent medical records were screened prior to scheduling an appointment for an initial evaluation. The VA was an early pioneer of the electronic health record (EHR) with the development of Veterans Health Information Systems and Technology Architecture, which allowed for providers to access comprehensive medical records from any other VA in which the patient received care. Whether the patient was seen via VTEL was determined by pragmatic reasons, most commonly whether their referring center had a “Memorandum of Understanding” with the SFVA that would allow patients to be seen out of state. The clinical status of the patient did not influence whether the patient was seen in-person or via VTEL for the initial evaluation. In-person consultations required patients to travel to the San Francisco VA for their visits. For VTEL consultations, patients traveled to their local VA community clinic for a remote, live telemedicine visit with an SFVA movement disorder specialist using Cisco hardware and Jabber software (Cisco Systems, Inc., San Jose, CA).
Telemedicine and in-person evaluations were conducted in a similar manner. A complete history and neurologic examination were obtained to elucidate chief PD-related complaints, medication optimization, presence of motor complications, and potential contraindications to DBS surgery. In the VTEL group, neurologic examination used the modified Unified Parkinson's Disease Rating Scale-III validated for telemedicine use.14 Then, at the time of the clinical encounter, the movement disorders specialist determined whether the patient was a good, borderline, or poor surgical candidate based on criteria outlined in table 1. The same movement disorders specialists evaluated patients in the in-person and VTEL cohorts. Good candidates met all criteria outlined in table 1 at the time of initial consultation, and poor candidates had one or more absolute contraindications listed in table 1. Borderline candidates shared the same criteria as the poor surgical candidates, but the benefits of surgery were thought to potentially outweigh the risks or the contraindications were relative and amendable to improvement with clinical care over time. These visits also served to educate the patient and family about DBS candidacy, expectations, goals, and the plan of care. If needed, recommendations regarding medication adjustments and further diagnostic workup were made to the patient and primary neurologist. Patients seen via VTEL or in-person consultation who were determined to be good or borderline candidates for DBS surgery would then travel at a later date to San Francisco for an in-person evaluation and final presurgical workup. If the workup was reassuring, they would undergo DBS surgery (figure 2). Following the initial screening assessment, the additional presurgical workup that was subsequently completed at SFVA included an MRI brain with stereotactic protocol, neuropsychological evaluation (performed if this could not be completed locally), detection, and assessment of undiagnosed medical issues not previously identified by local provider (such as atrial fibrillation or undiagnosed depression), and verification of social support and realistic expectations.
Table 1.
Criteria for DBS candidacy

Figure 2. Flow diagram of patient evaluation for deep brain stimulations.
Patients were seen initially via traditional in-person consultation or via VTEL. Based on the surgical candidacy determined at that consultations, patients would undergo additional subsequent evaluations as outline prior to potentially undergoing deep brain stimulation at SFVA. SFVA = San Francisco Veterans Affairs; VTEL = video telemedicine.
Resource utilization was determined using the patients' home zip codes to estimate hours, miles, and dollars spent traveling to and from appointments at the SFVA or their local VA where the VTEL visit was conducted. For patients traveling to San Francisco for an in-person evaluation, the cost of travel for each patient was estimated by summating the cost of driving to the airport using Internal Revenue Service medical travel mileage rate ($0.19/mile), the cost of an average roundtrip flight from the nearest airport, and estimated $50.00 taxi ride to and from San Francisco International Airport to SFVA medical center.15 The most commonly used hotel near SFVA medical center charges an average rate of $150/night. Most patients spend at minimum 2 nights stay to travel; therefore, $300.00 in hotel costs were added to the total. For patients who had VTEL evaluation, these costs were calculated for travel by car to their local VA clinic. The cost, time, and miles traveled were calculated assuming the patient was traveling without an attendant.
Standard protocol approvals, registrations, and patient consents
Study activities were approved by the institutional review board at University of California San Francisco and the SFVA (14-12907). The ethical review board determined written consent was not required for this retrospective analysis.
Statistical analysis
The rate of surgical intervention following an in-person or VTEL consultation were compared within a subgroup of patient surgical candidacy (e.g., good, borderline, or poor) using Fisher exact test. Given the nature of this analysis as a retrospective cohort pilot study, a sample size calculation was not completed. Missing data were excluded from analysis. No patients were lost to follow up. For those who underwent DBS surgery, the time between initial evaluation and surgery was calculated, and statistical analysis was run using Mann-Whitney U test. Time, miles, and money saved were analyzed using Mann-Whitney U test. A p-value of less than 0.05 was used to indicate statistical significance. All statistical tests were analyzed using SAS version 9.3 (Cary, NC).
Data availability
Further anonymized data can be made available to qualified investigators on request to the corresponding author pursuant to a data use agreement approval from the SFVA IRB.
Results
Over the 6-year period, 148 DBS candidacy evaluations were completed at SFVA PADRECC. Sixty patients were from states other than California. Ninety percent of the out-of-state referrals came from VA medical centers in 5 cities (Denver, Minneapolis, Indianapolis, Ann Arbor, and Detroit) in the San Francisco PADRECC's catchment area (figure 1). Sixty-two percent of these patients lived in rural areas. All referrals were from neurologists, 42% of which were from movement disorders subspecialists. Of these out-of-state referrals, 24 patients (40%) underwent initial evaluation in-person at SFVA, and 36 (60%) via VTEL. Baseline demographic characteristics were similar between the 2 groups, except patients seen via VTEL were older than those evaluated in-person (64.9 ± 8.2 vs 60.2 ± 8.1 years old, p = 0.03) (table 2).
Table 2.
Baseline characteristics
In terms of candidacy determination, 100% of patients deemed to be good candidates eventually underwent DBS surgery, whether initially evaluated in-person (16/16) or via VTEL (19/19) (table 3). For patients deemed to be borderline candidates, 66.7% (2/3) underwent surgery in the in-person cohort compared to 100% (6/6) in the VTEL group (p = 0.33). Finally, 40% (2/5) of those determined to be poor candidates at in-person initial evaluations eventually underwent DBS surgery locally, while only 9% (1/11) of those judged poor candidates via VTEL did (p = 0.21).
Table 3.
Comparison of the rate of surgery

Patients who underwent initial presurgical screening via VTEL waited longer for their surgery; those who underwent presurgical screening via in-person evaluation received surgery after waiting a median of 1.5 months (interquartile range [IQR] 1–34) compared to 4 months (IQR 1–19) in the VTEL cohort (p = 0.01).
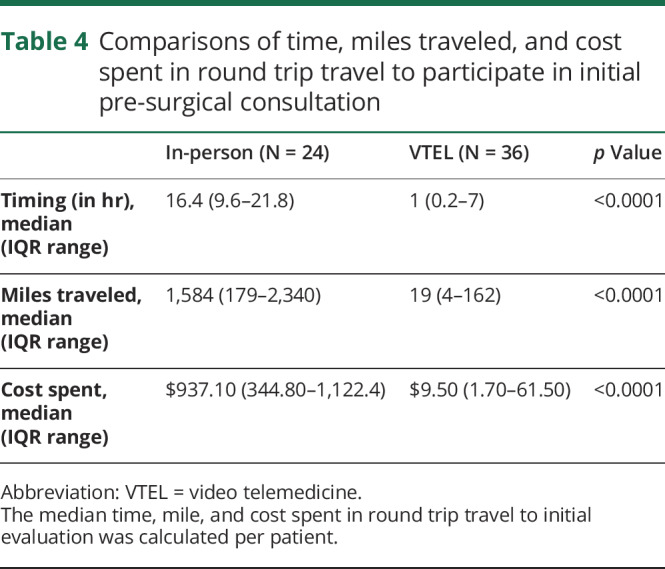
Those in the in-person cohort spent more money on travel ($937.10, IQR $344.80–$1,122.40) than those evaluated by VTEL ($9.50, IQR, $1.70–$61.50, p = 0.0001) (table 4). Likewise, those who presented to an in-person visit traveled a longer distance (median 1,548 miles [IRQ 179–2,340] for the in-person cohort vs 19 miles [IQR 4–162] in the VTEL cohort, p < 0.0001). Similarly, the in-person cohort traveled more hours than the VTEL cohort (16.4 hours [IQR, 9.6–21.8] roundtrip vs 1.0 hour [IQR, 0.2–7] respectively; p = 0.0001). In total, the group of 36 patients assessed by VTEL saved an estimated 116,617 miles, 568 hours, and $33,234 in round trip travel during their initial evaluation.
Table 4.
Comparisons of time, miles traveled, and cost spent in round trip travel to participate in initial pre-surgical consultation
Discussion
Telemedicine applications in PD care and neurology, in general, have proved feasible with reasonably high rates of patient satisfaction.16,17 This study investigated telemedicine use in evaluation of DBS surgical candidacy in the United States. There is little literature systematically assessing accuracy and cost savings of using telemedicine during the initial screening of surgical treatment candidacy in general. One case series of the Ontario Telemedicine Network showed that telemedicine is feasible option in providing care to patients with DBS, though a majority (80%) of these patients had telemedicine visits only after surgery.12
This study supports the use of telemedicine as a feasible method for pre-surgical evaluation of DBS candidacy.
This study supports the use of telemedicine as a feasible method for presurgical evaluation of DBS candidacy. All of the patients seen via VTEL who were deemed to be good surgical candidates also underwent in-person evaluation at SFVA prior to their surgery. Of note, all of patients judged to be “good” candidates via VTEL evaluation eventually underwent subsequent “gold-standard” in-person evaluation at SFVA and the group designations were concordant. All of these patients eventually underwent DBS surgery at the SFVA.
However, the largest limitation of this retrospective study was that patients were not randomized to 1 of the 2 methods of evaluation. Patients considered to be “poor” DBS candidates via VTEL were not routinely confirmed as such via the “gold-standard” in-person consultation. A prospective longitudinal study comparing surgical candidacy via VTEL and later using a gold-standard in-person evaluation would be ideal, but costly. Of the 11 patients judged to be “poor” candidates via VTEL, 1 patient was eventually seen in person. For this patient, during the initial telemedicine examination, the specialist disagreed with the primary neurologist's diagnosis of ET, because the patient appeared Parkinsonian, and suggested a trial of PD medications. However, when re-evaluated in-person, there was agreement with the initial diagnosis of ET, and he underwent DBS, after a substantial delay. Most determinations of “poor” and “borderline” candidacy were due to lack of medication optimization or a mismatch of patient goals and DBS expectations; both issues which can be discovered by telemedicine assessment and discussion. Most often, the referring provider did not trial a sufficient levodopa dose and were instructed to make medication adjustments and schedule a follow-up VTEL visit. However, neurologists must be aware of the rare potential pitfall of misdiagnosing patients using VTEL. Having a low threshold for a second, in-person evaluation can mitigate this pitfall. The study was not powered to assess differences between the 2 groups regarding clinical effect on specific rating scales or symptom control nor to detect differences between outcomes based diagnostic indication for DBS.
In terms of resource utilization, telemedicine screening of DBS candidacy provided cost savings in travel time and expenses.
In terms of resource utilization, telemedicine screening of DBS candidacy provided cost savings in travel time and expenses. Cost assessment relied on assumptions of cost, time, and miles saved. As noted, these costs were estimated assuming each patient traveled alone. Therefore, this study likely underestimates the savings incurred by the patient, as many patients travel with a care partner or other attendant, and many patients had longer hotel stays. In addition, lost work productivity was not assessed. Furthermore, this study did not investigate potential savings to the health care system. Also, these cost savings may not be realized in other health care systems or smaller countries with higher number of neurologist and specialists per capita. More research, ideally with cost-effectiveness analysis and in other health care systems, is needed to determine whether telemedicine screening of surgical candidacy leads to more efficient use of resources, both qualitatively and quantitatively.
Other benefits of telemedicine included allowing for education about DBS, discussion of patient goals, and management of patient and family expectations. When the referring provider is present at telemedicine visit, it allowed for real-time recommendations and building professional relationships that could improve referral efficiency over time. Patients who underwent initial screening via VTEL did wait a longer time for their surgery, but surgeries were still performed well within the standard-of-care timeframe for most DBS centers. The time delay was often due to coordinating travel after addressing medication trials and optimizing comorbid medical conditions.
The unique patient population and health care setting of this study (all male veterans in the VA health system) may restrict the generalizability of its findings. Early VA investment in robust telemedicine infrastructure and EHR system has allowed for comprehensive review of medical and psychiatric evaluations, including records from allied health professionals such as speech language pathologists, neuropsychologists, and social workers, prior to the initial in-person or VTEL visit. In 2017, the VA serviced 727,000 veterans using VTEL.18 Telemedicine practice at the VA is also relatively unencumbered by the restrictive legal and reimbursement environment present in other settings. Therefore, it is not surprising that this tool has yet to reach its full potential in most settings. For example, despite a ten-fold increase in the number of VTEL visits among rural Medicare beneficiaries from 2004 to 2013, this still only amounted to 1% of visits in this population, compared to an estimated 12% of veterans.18,19
As patient demand increases, technology becomes more user-friendly and inexpensive, and parity laws ease the restrictive reimbursement environment, telemedicine is poised to have an important effect beyond large integrated health systems. We believe this effect can be greatest and most immediate in improving access to highly specialized care. As an example, this study supports the use of telemedicine in improving access to the multidisciplinary subspecialty care needed to screen patients for DBS surgical candidacy, while decreasing the costs and travel burden experienced by patients.
Appendix. Authors

Study funding
No targeted funding reported.
Disclosure
N. Witek reports no disclosures. S.L. Health is a Medtronic educational consultant. B. Ouyang reports no disclosures. C.M. Tanner receives grants from The Michael J. Fox Foundation, the Parkinson's Foundation, the Department of Defense, BioElectron, Roche/Genentech, Biogen Idec, and the National Institutes of Health, compensation for serving on Data Monitoring Committees from Biotie Therapeutics, Voyager Therapeutics, and Intec Pharma, and personal fees for consulting from Neurocrine Biosciences, Adamas Therapeutics, Biogen Idec, 23andMe, Alexza, Acadia, Grey Matter, and CNS Ratings. N.B. Galifianakis receives grant support from National Institutes of Health (NINR and NIA), and the Boston Scientific Corp. Deep brain stimulation was performed using Medtronic devices. He receives no monitory support from Medtronic. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
TAKE-HOME POINTS
→ VTEL can be used to screen patients with movement disorders for DBS.
→ VTEL saves patients time and money in travel compared to traditional in-person visits.
→ VTEL can improve access to subspecialty care.
References
- 1.Weaver FM, Follett K, Stern M, et al. . Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA 2009;301:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deuschl G, Schade-Brittinger C, Krack P, et al. . A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med 2006;355:896–908. [DOI] [PubMed] [Google Scholar]
- 3.Deuschl G, Schupbach M, Knudsen K, et al. . Stimulation of the subthalamic nucleus at an earlier disease stage of Parkinson's disease: concept and standards of the EARLYSTIM-study. Parkinsonism Relat Disord 2013;19:56–61. [DOI] [PubMed] [Google Scholar]
- 4.Hu W, Klassen BT, Stead M. Surgery for movement disorders. J Neurosurg Sci 2011;55:305–317. [PubMed] [Google Scholar]
- 5.Dorsey ER, Venkataraman V, Grana MJ, et al. . Randomized controlled clinical trial of “virtual house calls” for Parkinson disease. JAMA Neurol 2013;70:565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willis AW, Schootman M, Evanoff BA, Perlmutter JS, Racette BA. Neurologist care in Parkinson disease: a utilization, outcomes, and survival study. Neurology 2011;77:851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willis AW, Schootman M, Kung N, Wang XY, Perlmutter JS, Racette BA. Disparities in deep brain stimulation surgery among insured elders with Parkinson disease. Neurology 2014;82:163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorsey ER, Sherer T, Okun MS, Bloem BR. The emerging evidence of the Parkinson pandemic. J Parkinsons Dis 2018;8:S3–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorsey ER, Bloem BR. The parkinson pandemic-A call to action. JAMA Neurol 2018;75:9–10. [DOI] [PubMed] [Google Scholar]
- 10.Chirra M, Marsili L, Wattley L, et al. . Telemedicine in neurological disorders: opportunities and challenges. Telemed J E Health 2019;25:541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilkinson JR, Spindler M, Wood SM, et al. . High patient satisfaction with telehealth in Parkinson disease: a randomized controlled study. Neurol Clin Pract 2016;6:241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jitkritsadakul O, Rajalingam R, Toenjes C, Munhoz RP, Fasano A. Tele-health for patients with deep brain stimulation: the experience of the Ontario Telemedicine Network. Mov Disord 2018;33:491–492. [DOI] [PubMed] [Google Scholar]
- 13.VA Telehealth Services: real-time clinic based video telehealth. 2015; Available at: telehealth.va.gov/real-time/. Accessed October 16, 2018.
- 14.Abdolahi A, Scoglio N, Killoran A, Dorsey ER, Biglan KM. Potential reliability and validity of a modified version of the Unified Parkinson's Disease Rating Scale that could be administered remotely. Parkinsonism Relat Disord 2013;19:218–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.IRS: standard mileage rates. 2018. Available at: irs.gov/tax-professionals/standard-mileage-rates. Accessed June 2018.
- 16.Dorsey ER, Topol EJ. State of telehealth. N Engl J Med 2016;375:154–161. [DOI] [PubMed] [Google Scholar]
- 17.Beck CA, Beran DB, Biglan KM, et al. . National randomized controlled trial of virtual house calls for Parkinson disease. Neurology 2017;89:1152–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Care VHAOoC. VA Telehealth Services Fact Sheet-FY 2017. US Department of Veterans Affairs; 2017. Available at: newengland.va.gov/nhvision/VATelehealthServicesFactsheet.pdf. Accessed October 17, 2018. [Google Scholar]
- 19.Mehrotra A, Jena AB, Busch AB, Souza J, Uscher-Pines L, Landon BE. Utilization of telemedicine among rural medicare beneficiaries. JAMA 2016;315:2015–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Further anonymized data can be made available to qualified investigators on request to the corresponding author pursuant to a data use agreement approval from the SFVA IRB.





