Abstract
Seriously ill patients with coronavirus disease 2019 (COVID‐19) at risk for death exhibit elevated cytokine and chemokine levels and D‐dimer, and they often have comorbidities related to vascular dysfunctions. In preclinical studies, activated protein C (APC) provides negative feedback downregulation of excessive inflammation and thrombin generation, attenuates damage caused by ischemia‐reperfusion in many organs including lungs, and reduces death caused by bacterial pneumonia. APC exerts both anticoagulant activities and direct cell‐signaling activities. Preclinical studies show that its direct cell‐signaling actions mediate anti‐inflammatory and anti‐apoptotic actions, mortality reduction for pneumonia, and beneficial actions for ischemia‐reperfusion injury. The APC mutant 3K3A‐APC, which was engineered to have diminished anticoagulant activity while retaining cell‐signaling actions, was safe in phase 1 and phase 2 human trials. Because of its broad spectrum of homeostatic effects in preclinical studies, we speculate that 3K3A‐APC merits consideration for clinical trial studies in appropriately chosen, seriously ill patients with COVID‐19.
Keywords: activated protein C, coronavirus, COVID‐19, cytokine, D‐dimer, SARS‐CoV‐2
Essentials.
Seriously ill patients with coronavirus disease 2019 (COVID‐19) exhibit viral pneumonia, cytokine storm, disseminated intravascular coagulation, and comorbidities.
There are no approved therapies for severely ill patients with COVID‐19.
Activated protein C (APC) reduces inflammation and apoptosis, and it stabilizes endothelial and epithelial barriers.
The 3K3A‐APC mutant merits evaluation for seriously ill, appropriately chosen patients with COVID‐19.
Infection with the virus identified as severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) can lead to virus‐induced pneumonia that is designated coronavirus disease 2019 (COVID‐19) by the World Health Organization, and this infection has reached pandemic proportions. The virus is thought to invade a host by attachment to a receptor for the virus spike protein, namely, human angiotensin‐converting enzyme 2, which is expressed on epithelial cells throughout the body. However, almost nothing is known about the pathobiology leading to severe illness and death for patients with COVID‐19. Peer‐reviewed reports from China indicate that seriously ill patients with COVID‐19 at risk for death develop extensive elevations in plasma levels of cytokines and chemokines (interleukin [IL]‐2, IL‐6, IL‐7, IL‐10, granulocyte colony‐stimulating factor, interferon‐γ–induced protein 10, monocyte chemoattractant protein‐1, macrophage inflammatory protein 1A, and tumor necrosis factor‐α) and in markers of disseminated intravascular coagulation (D‐dimer and fibrinogen degradation products). 1 , 2 , 3 , 4 , 5 , 6 Comorbidities in patients with COVID‐19, including cardiovascular disease, diabetes, and hypertension, enhance risk for death. In one retrospective study of 201 patients admitted to the hospital with COVID‐19, 84 developed acute respiratory distress syndrome (ARDS), and 44 of those 84 (52%) died. For patients with ARDS who died, the values of IL‐6 and of D‐dimer were significantly elevated compared with patients with ARDS who survived (P < .001 and P = .001, respectively). In another report of 191 hospitalized patients, odds of in‐hospital death increased with older age, higher Sequential Organ Failure Assessment score, and D‐dimer plasma levels. 6 These initial COVID‐19 retrospective clinical data reports 1 , 2 , 3 , 4 , 5 , 6 support the concept that dysregulation of inflammation and distortion of the hemostatic balance contribute to COVID‐19 lethality.
While much is known about beneficial mechanisms for host defense involving the innate immune system and the coagulation systems, much remains unknown. Research into each subsystem of host defense for many years helped to define their molecular components and activities. However, studies of the more complex regulatory interrelationships between major host defense subsystems are more recent, and further studies are urgently needed. In only the past 7 years have the terms thromboinflammation and immunothrombosis been used. 7 , 8 , 9 These terms are useful to denote extensive interactions between the body’s innate and acquired immune systems and coagulation systems; notably, plasma coagulation proteases have a wide range of direct and indirect effects on many immune system cell types, which enable crosstalk between circulating proteases and host defense immune cell responses. 7 , 8 , 9 , 10 , 11 , 12 , 13 Healthy crosstalk between circulating proteases and cells is critical for providing the optimal benefits of protective inflammation reactions and protective coagulation reactions to combat invading pathogens, such as SARS‐CoV‐2. Healthy crosstalk can help stabilize and preserve endothelial and epithelial vascular functions. Excessive, inadequate, or inappropriate crosstalk between proteases and cells can lead to hyperinflammation and vascular dysfunction, that is, lead to processes that are likely to contribute to the pathobiology and mortality of COVID‐19 in seriously ill patients. The scheme in Figure 1 broadly summarizes the importance of regulation of the host defense subsystems, the immune system and inflammation and the coagulation systems.
Figure 1.
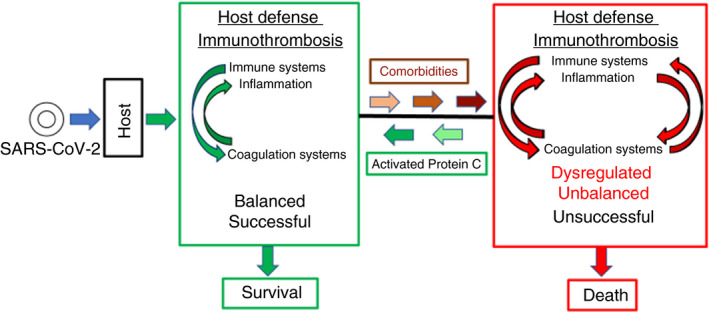
COVID‐19: balanced host defense versus unbalanced, dysregulated host defense. SARS‐CoV‐2 infection triggers host defense, which comprises multiple subsystems including, among others, the innate immune system and the blood coagulation systems, which reciprocally activate each other to attack and help neutralize the virus with integration from other host defense subsystems. The crosstalk and integration of molecular and cellular defense mechanisms for these 2 subsystems may be termed immunothrombosis or thromboinflammation. 7 , 8 , 9 Provided that the inflammatory reactions and the coagulation reactions are appropriately regulated by both positive and negative feedback loops, host defense is successful, resulting in rejection of the invader and survival of the host. However, when one or more of the host defense subsystems is less responsive or already chronically hyperactivated (eg, inflammatory condition, hypercoagulable state, etc) due to comorbidities such as cardiovascular diseases, hypertension, diabetes, and so on, then the normal regulatory processes of host defense subsystems are unbalanced. As observed in seriously ill patients with COVID‐19, biomarkers for dysregulated, excessively active immune system include markedly elevated cytokines and chemokines and, for excessive blood coagulation, D‐dimer reflecting fibrin formation and fibrinolysis. The net result of such comorbidities can be that the inflammatory reactions and the coagulation reactions are dysregulated and excessive, resulting ultimately in death. APC provides multiple cytoprotective activities and, in preclinical studies, APC’s cell‐signaling actions attenuate many types of injuries and reduces death from bacterial pneumonia. Thus, it is hypothesized that the recombinant signaling‐selective 3K3A‐APC protein will help counteract the dysregulation of immunothrombosis that can arise in the course of host defense against the SARS‐CoV‐2 virus and will increase the probability for survival of severely ill COVID‐19 patients. APC, activated protein C; COVID‐19, coronavirus disease 2019; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
Activated protein C (APC), the serine protease generated from plasma protein C by thrombin‐thrombomodulin complex, is remarkable for its ability to regulate multiple host defense subsystems, for example, both the innate immune system’s inflammation and the coagulation system. 10 , 11 , 12 Its multiple activities include proteolytic inactivation of procoagulant factors Va and VIIIa and initiation of cell signaling by cleavage of protease‐activated G‐protein–coupled receptors. The cell‐signaling actions of APC target many types of cells in many organs (eg, endothelial, epithelial, cerebral, and immune system cells). APC’s multiple beneficial in vivo activities in preclinical injury models and in vitro on cultured cells include anti‐inflammatory, anti‐apoptotic, cytoprotective, and endothelial and epithelial barrier stabilizing activities. anti‐inflammatory signaling pathways initiated by APC acting on its receptors include those of endothelial cells, monocytes, neutrophils, dendritic cells, and T cells, allowing APC to control and regulate immune cell physiology. 12 , 14 , 15 APC and several of its recombinant mutants have well‐understood mechanisms of action on many cell types and in well‐characterized rodent injury models where its anti‐inflammatory, anti‐apoptotic, cytoprotective, neuroprotective, and mortality reduction activities are apparent. 10 , 11 , 12 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21
Protein engineering provided a cell‐signaling–selective recombinant APC mutant, 3K3A‐APC (in which 3 Lys residues are replaced by 3 Ala residues), which retains multiple beneficial cell signaling actions but has greatly reduced (<10%) anticoagulant activity because these 3 mutations ablate APC’s interactions with its substrate, clotting factor Va. This triad of mutations does not affect APC’s interactions with its cell receptors, including binding to endothelial protein C receptor and its proteolytic agonism of the G‐protein–coupled receptors, protease‐activated receptors 1 and 3. 10 Thus, both wild‐type APC and 3K3A‐APC have primary anti‐inflammatory and cytoprotective cell‐signaling properties, making each a multiple‐action, multiple‐targeting therapeutic agent; however, loss of anticoagulant activity in the 3K3A mutant greatly reduces risk of bleeding when it is infused. Because APC and its signaling‐selective mutants have potent neuroprotective activities, 21 clinical trial studies of 3K3A‐APC for neuroprotection in ischemic stroke were undertaken. 22 , 23 Recombinant 3K3A‐APC mutant successfully completed phase 1 and phase 2 human trials for ischemic stroke, and it is poised for an upcoming phase 3 trial. 22 , 23
A successful clinical trial for recombinant wild‐type APC (Xigris, Eli Lilly) resulted in its US Food and Drug Administration approval in 2001 for treating adult severe sepsis, but Xigris was voluntarily withdrawn a decade later after one large adult severe sepsis trial showed no benefit for the drug (see Griffin et al 21 for details). Xigris was not effective in trials for less severe sepsis in adults or children. The APC severe adult sepsis trial was undertaken last century when only APC’s anticoagulant actions were known. Notably, APC’s direct initiation of cell signaling was discovered only in this century, paving the path for preclinical studies showing that APC’s cell signaling is responsible for reducing death due to pneumonia and sepsis. 16 , 17 , 18 , 21 Bolus dosing of 3K3A‐APC for ischemic stroke 21 , 22 , 23 is based on a mechanism of action due to cell signaling, whereas the prolonged 4‐day infusion of very‐low‐dose Xigris used for adult severe sepsis was based on an anticoagulant mechanism of action. We hypothesize that APC’s mechanism of action, which may benefit patients with COVID‐19, is based on its multiple cell‐signaling actions. 21
Particularly relevant to patients with COVID‐19, APC and its mutants attenuated pulmonary injury, reduced death caused by pneumonia in murine models, and enhanced barrier function of cultured human alveolar epithelial cells. 16 , 17 , 18 , 19 , 20 Furthermore, in animal studies, APC and 3K3A‐APC reduced organ dysfunction and host death caused by ischemia‐reperfusion injury of brain, heart, kidney, and lung. 10 , 11 , 12 , 15 , 20 , 21 In one study, after a pulmonary ischemic insult, when an APC bolus was given to rats during reperfusion, it reduced lung damage, improved lung function, and reduced cytokines. 20 Taken together, these preclinical studies imply that APC’s cell‐signaling actions may reduce SARS‐CoV‐2–induced damage in severely ill patients with COVID‐19 involving pneumonia, lung inflammation, pulmonary hypoxemia, and some other organ pathologies. Thus, we hypothesize that 3K3A‐APC, which has been demonstrated to be safe in humans when given as repeated boluses over 2 days, 22 , 23 would be a beneficial therapy of appropriately selected patients with COVID‐19 at risk of serious illness and death because it would reduce uncontrolled inflammation and endothelial and epithelial dysfunction and would attenuate ischemia‐reperfusion injury in lungs and/or other organs.
1. RELATIONSHIP DISCLOSURE
JHG is a consultant for ZZ Biotech LLC, and Scripps Research Institute has intellectual property related to activated protein C. PL has received a National Institutes of Health grant support to study 3K3A‐APC in patients with acute ischemic stroke.
AUTHOR CONTRIBUTIONS
JHG and PL are responsible for writing the manuscript, and each gives final approval for the submitted paper.
Funding information
Support was provided, in part, by grants to JHG (R01 HL133728 and R01 HL142975) and to PL (U01 NS088312) from the National Institutes of Health.
Handling Editor: Dr Alisa Wolberg.
References
- 1. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu YI, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang D, Hu BO, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang L, He W, Yu X, Hu D, Bao M, Liu H, et al. Coronavirus disease 2019 in elderly patients: Characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80:639-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13:34–45. [DOI] [PubMed] [Google Scholar]
- 8. Jackson SP, Darbousset R, Schoenwaelder SM. Thromboinflammation: challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood. 2019;133:906–18. [DOI] [PubMed] [Google Scholar]
- 9. Karbach S, Lagrange J, Wenzel P. Thromboinflammation and vascular dysfunction. Hamostaseologie. 2019;39:180–7. [DOI] [PubMed] [Google Scholar]
- 10. Griffin JH, Zlokovic BV, Mosnier LO. Activated protein C: biased for translation. Blood. 2015;125:2898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Isermann B. Homeostatic effects of coagulation protease-dependent signaling and protease activated receptors. J Thromb Haemost. 2017;15:1273–84. [DOI] [PubMed] [Google Scholar]
- 12. McDonnell CJ, Soule EE, Walsh PT, O’Donnell JS, Preston RJS. The immunoregulatory activities of activated protein C in inflammatory disease. Semin Thromb Hemost. 2018;44:167–75. [DOI] [PubMed] [Google Scholar]
- 13. Zelaya H, Rothmeier AS, Ruf W. Tissue factor at the crossroad of coagulation and cell signaling. J Thromb Haemost. 2018;16:1941–52. [DOI] [PubMed] [Google Scholar]
- 14. Healy LD, Rigg RA, Griffin JH, McCarty OJT. Regulation of immune cell signaling by activated protein C. J Leukoc Biol. 2018;102:1197-1203.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nazir S, Gadi I, Al-Dabet MM, Elwakiel A, Kohli S, Ghosh S, et al. Cytoprotective activated protein C averts Nlrp3 inflammasome-induced ischemia-reperfusion injury via mTORC1 inhibition. Blood. 2017;130:2664–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kerschen EJ, Fernandez JA, Cooley BC, Yang XV, Sood R, Mosnier LO, et al. Endotoxemia and sepsis mortality reduction by non-anticoagulant activated protein C. J Exp Med. 2007;204:2439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kerschen E, Hernandez I, Zogg M, Jia S, Hessner MJ, Fernandez JA, et al. Activated protein C targets CD8+ dendritic cells to reduce the mortality of endotoxemia in mice. J Clin Invest. 2010;120:3167–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bir N, Lafargue M, Howard M, Goolaerts A, Roux J, Carles M, et al. Cytoprotective-selective activated protein C attenuates Pseudomonas aeruginosa-induced lung injury in mice. Am J Respir Cell Mol Biol. 2011;45:632–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Puig F, Fuster G, Adda M, Blanch L, Farre R, Navajas D, et al. Barrier-protective effects of activated protein C in human alveolar epithelial cells. PLoS ONE. 2013;8:e56965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lan CC, Peng CK, Huang SF, Huang KL, Wu CP. Activated protein C attenuates ischemia-reperfusion-induced acute lung injury. Exp Lung Res. 2015;41:241–50. [DOI] [PubMed] [Google Scholar]
- 21. Griffin JH, Zlokovic BV, Mosnier LO. Activated protein C, protease activated receptor 1, and neuroprotection. Blood. 2018;132:159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lyden P, Levy H, Weymer S, Pryor K, Kramer W, Griffin JH, et al. Phase 1 safety, tolerability and pharmacokinetics of 3K3A‐APC in healthy adult volunteers. Curr Pharm Des. 2013;19:7479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lyden P, Pryor KE, Coffey CS, Cudkowicz M, Conwit R, Jadhav A, et al. Final results of the RHAPSODY trial: a multi-center, phase 2 trial using a continual reassessment method to determine the safety and tolerability of 3K3A‐APC, a recombinant variant of human activated protein C, in combination with tissue plasminogen activator, mechanical thrombectomy or both in moderate to severe acute ischemic stroke. Ann Neurol. 2019;85:125–36. [DOI] [PMC free article] [PubMed] [Google Scholar]


