Abstract
In this article, the State of the Art lecture “Platelet CLEC‐2 and Lung Development” presented at the ISTH congress 2019 is reviewed. During embryonic development, blood cells are often considered as porters of nutrition and oxygen but not as active influencers of cell differentiation. However, recent studies revealed that platelets actively facilitate cell differentiation by releasing biological substances during development. C‐type lectin‐like receptor 2 (CLEC‐2) has been identified as a receptor for the platelet‐activating snake venom rhodocytin. An endogenous ligand of CLEC‐2 is the membrane protein podoplanin (PDPN), which is expressed on the surface of certain types of tumor cells and lymphatic endothelial cells (LECs). Deletion of CLEC‐2 from platelets in mice results in death just after birth due to lung malformation and blood/lymphatic vessel separation. During development, lymphatic vessels are derived from cardinal veins. At this stage, platelets are activated by binding of CLEC‐2 to LEC PDPN and release trandforming growth factor‐β (TGF‐β). This cytokine inhibits LEC migration and proliferation, facilitating blood/lymphatic vessel separation. TGF‐β released upon platelet‐expressed CLEC‐2/LEC PDPN also facilitates differentiation of lung mesothelial cells into alveolar duct myofibroblasts (adMYFs) in the developing lung. AdMYFs generate elastic fibers inside the lung, so that the lung can be properly inflated. Thus, platelets act as an ultimate natural drug delivery system that enables biological substances to be specifically delivered to the target at high concentrations by receptor/ligand interactions during development.
Keywords: CLEC‐2, lung development, lymphatic endothelial cells, platelets, podoplanin
Essentials.
C‐type lectin‐like receptor‐2 (CLEC‐2) is a podoplanin (PDPN) receptor on platelets.
CLEC‐2 null mice die due to lung malformation and show blood/lymphatic misconnection.
Binding of CLEC‐2 to PDPN on lymphatic endothelial cells facilitates TGF‐β release.
TGF‐β differentiates myofibroblasts in the developing lung and separates blood/lymphatics.
1. INTRODUCTION
During embryonic development, organogenesis starts at the end of gastrulation and continues until birth. The cells in the ectoderm, endoderm, and mesoderm undergo differentiation by cell signaling. Cell differentiation during organ development depends on exposure to bioactive substances released from adjacent cells (juxtacrine signaling) or neighboring cells (paracrine signaling). Blood and blood cells are considered porters of nutrition and oxygen but not active influencers of cell differentiation.
Among blood cells, platelets are the small anucleate cells that play a pivotal role not only in thrombosis and hemostasis but also in inflammation, wound healing, host defense, and tumor progression. Recent studies show that platelets also play a pivotal role in organogenesis including lymphangiogenesis and lung development. 1 , 2 , 3 , 4 The role of platelets in organogenesis has been revealed by the analysis of mice deficient in the platelet activation receptor C‐type lectin‐like receptor 2 (CLEC‐2), which has been identified as a receptor for a platelet‐activating snake venom, rhodocytin. 1 , 2 , 3 , 4 , 5 An endogenous ligand for CLEC‐2 has been identified as a membrane protein: podoplanin (PDPN). 6 , 7 PDPN is expressed on the surface of certain types of tumor cells and activates platelets by binding to CLEC‐2, which facilitates hematogenous tumor metastasis. 8 , 9 , 10 , 11 , 12 PDPN is also expressed in normal lymphatic endothelial cells (LECs), 12 but PDPN in these cells cannot have access to CLEC‐2 in platelets under normal conditions. However, platelet‐expressed CLEC‐2 can interact with LEC PDPN during embryonic development, which has been revealed to be a necessary step for lymphangiogenesis and lung development. In this review, we introduce these molecules, CLEC‐2 and PDPN, and review their role in organogenesis.
2. CLEC‐2 AND PDPN
2.1. CLEC‐2
Snake venom contains a vast number of toxins that interact with human platelet membrane proteins and coagulation factors. 13 Identification of receptors for these toxins has made an enormous contribution to our understanding of thrombosis and hemostasis. Similar to collagen, rhodocytin induces powerful platelet activation depending on protein tyrosine kinases. 14 , 15 However, rhodocytin can induce platelet aggregation in mice lacking the collagen receptor glycoprotein (GP) VI/Fc receptor γ‐chain (FcRγ), suggesting that GPVI/FcRγ is not a receptor for rhodocytin. 14 , 16 , 17 Furthermore, some groups, including ours, proposed that rhodocytin interacts with integrin α2β1 or GPIb and induces platelet aggregation based on biochemical analysis using antibodies or recombinant proteins. 14 , 16 However, Bergmeier et al 17 showed that rhodocytin induces platelet aggregation in mice deficient in either integrin α2 or the ligand‐binding site of GPIb. Their study demonstrated that rhodocytin may bind to integrin α2β1 or GPIb but does not induce platelet activation via these receptors. Additionally, they determined that there must be an activation receptor for rhodocytin other than α2β1 and GPIb. Finally, pulldown assays using platelet lysate and rhodocytin‐coated CNBr beads and subsequent tandem mass spectrometry analysis identified CLEC‐2 as a receptor for rhodocytin. 5 CLEC‐2 protein is highly expressed in platelets and megakaryocytes and at lower levels in liver Kupffer cells and liver sinusoidal endothelial cells in humans. 18 , 19 , 20 , 21 In mice, CLEC‐2 is also expressed in neutrophils, dendritic cells, and macrophages at low levels. 22 , 23 , 24 , 25 , 26
Signal transduction pathways mediated through CLEC‐2 were investigated using genetically modified mice deficient in signaling molecules in platelets and immunoprecipitation/western blotting. CLEC‐2 contains a single YxxL (x represents any amino acid) motif called hemi‐ITAM (immunoreceptor tyrosine‐based activation motif). 27 Upon CLEC‐2 clustering by rhodocytin, a tyrosine residue in hemi‐ITAM is phosphorylated by the Src family kinase. This is followed by binding of spleen tyrosine kinase (Syk) to the phosphorylated hemi‐ITAM with its SH2 domain. 5 , 28 The binding activates Syk, leading to phosphorylation of the downstream adaptor proteins, LAT and SLP76, and phosphorylation/activation of the downstream tyrosine kinase Btk and phospholipase Cγ2 (PLCγ2). 5 , 28 PLCγ2 activation generates inositol trisphosphate and diacylglycerol, resulting in Ca2+ mobilization and protein kinase C (PKC) activation. Intracellular Ca2+ mobilization and PKC activate integrin αIIbβ3, which leads to fibrinogen binding and platelet aggregation (Figure 1). At the same time, activated platelets release extracellular matrix and growth factors from the α granules. The resulting growth factors aid wound healing and include a platelet‐derived growth factor, fibroblastic growth factor, and transforming growth factor‐β (TGF‐β). They also release platelet‐activating small molecules in dense granules including ADP, serotonin, and Ca2+, which further activate platelets nearby. 29
Figure 1.
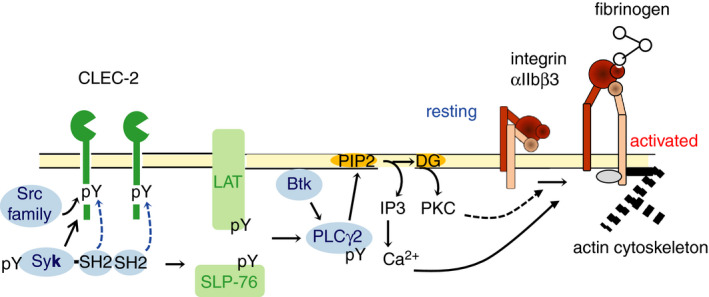
Signal transduction pathway mediated through C‐type lectin‐like receptor 2 (CLEC‐2). Upon CLEC‐2 clustering by rhodocytin, a tyrosine residue in hemi‐ITAM (immunoreceptor tyrosine‐based activation motif) is phosphorylated by the Src family kinase, followed by binding of spleen tyrosine kinase (Syk) to the phosphorylated hemi‐ITAM with its SH2 domain. This binding results in activation of Syk, leading to phosphorylation of the downstream adaptor proteins, LAT and SLP76, and phosphorylation/activation of the downstream tyrosine kinase Btk and phospholipase Cγ2 (PLCγ2). PLCγ2 activation generates of inositol trisphosphate (IP3) and diacylglycerol, resulting in subsequent Ca2+ mobilization and protein kinase C (PKC) activation. Intracellular Ca2+ mobilization and PKC activate integrin αIIbβ3, which leads to fibrinogen binding and platelet aggregation. pY, phosphorylated tyrosine
2.2. PDPN
It has long been recognized that PDPN expressed on the surface of certain types of cancer cells facilitates hematogenous metastasis by activating platelets in the bloodstream. 9 Platelet aggregates surrounding cancer cells protect them from shear stress or immune cells in the blood stream and provide them with the scaffolding to invade outside of the vessels. 30 Growth factors or angiogenic factors released from activated platelets facilitate cancer growth or angiogenesis in cancer. 30 Targeting PDPN binding to platelets would be a good strategy to inhibit cancer metastasis. Thus, PDPN receptors on the surface of platelets have been intensively studied. We noticed that podoplanin‐expressing CHO cells induced platelet aggregation with a long lag phase before eliciting platelet aggregation similar to rhodocytin and identified CLEC‐2 as a receptor for PDPN. 6 PDPN is a type I transmembrane sialomucin‐like glycoprotein with short cytoplasmic tail. 11 In addition to cancer cells, it is expressed on the surface of several normal tissues including type I lung alveolar cells, kidney podocytes, fibroblastic reticular cells in the lymph nodes, and LECs. 11 Because PDPN in these cells cannot interact with platelet‐expressed CLEC‐2 and PDPN is not expressed in vascular endothelial cells, the role of CLEC‐2/PDPN interaction in normal conditions remained unknown.
3. ROLE OF PLATELET CLEC‐2/LEC PDPN IN LYMPHATIC VESSEL DEVELOPMENT
CLEC‐2 and PDPN can interact with each other during embryonic development when the primitive lymphatic vessels (lymph sacs) are derived from the cardinal vein. Mice deficient in either PDPN or signaling molecules downstream of CLEC‐2 including Syk, SLP‐76, PLCγ2, and Btk/Tec show abnormalities in lymphatic vessels. 31 , 32 , 33 , 34 We hypothesized that the interaction between platelet CLEC‐2 and LEC PDPN plays a role in lymphatic vessel development. Both CLEC‐2 null mice (Clec1b‐/‐) and platelet/megakaryocyte‐specific CLEC‐2‐deficient mice (Clec1b flox/flox; PF4‐Cre) (CLEC‐2 Δplt) show impaired blood/lymphatic vessel separation and blood‐filled lymphatic vessels 1 , 2 , 3 (Figure 2). This suggests that platelet CLEC‐2 is required for blood/lymphatic vessel separation during embryonic development. We proposed the following mechanism, but other hypotheses have also been proposed. 35 , 36 , 37 At the separation zone of lymph sacs and cardinal veins, TGF‐β family members, among which we proposed TGF‐β and bone morphogenetic protein‐9, are released from activated platelets by the platelet CLEC‐2/LEC PDPN interaction. These cytokines inhibit migration and proliferation of LECs, facilitating blood–lymphatic vessel separation. 2 The second hypothesis is that clustering of PDPN leads to the inhibition of migration in LECs by PDPN‐mediated signaling through ezrin/radixin/moesin family proteins. 36 The third hypothesis is that at the site of the lymphovenous (LV) junction, platelet CLEC‐2/LV valve PDPN interaction stimulates the formation of platelet thrombi, which prevents the backflow of blood. 35 This theory is widely accepted in the field of hematology. However, these mechanisms are not mutually exclusive, and all mechanisms may be spatiotemporally regulated.
Figure 2.
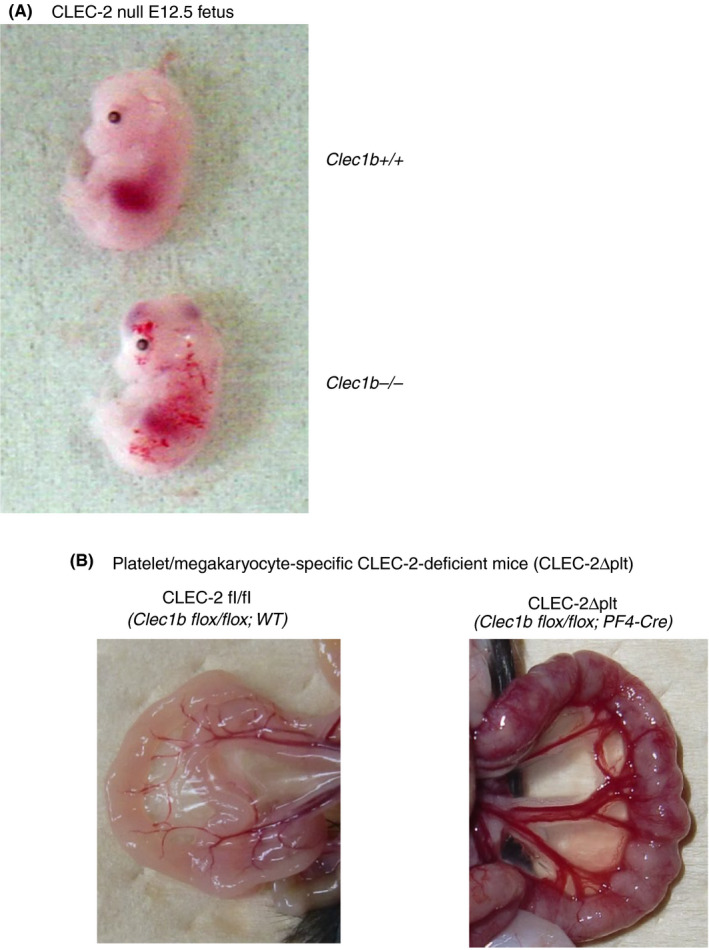
C‐type lectin‐like receptor 2 (CLEC‐2)–deficient mice show blood/lymphatic vessel misconnection. (A) CLEC‐2 null mice (Clec1b‐/‐) (lower E12.5 fetus), but not wild‐type mice (Clec1b+/+) (upper E12.5 fetus), showed blood‐filled lymphatic vessel due to blood/lymphatic vessel misconnection. (B) The small intestine from platelet/megakaryocyte‐specific conditional CLEC‐2–deficient mice (CLEC‐2Δplt, Clec1b flox/flox; PF4‐Cre) (right panel) and control mice (CLEC‐2 fl/fl, Clec1b flox/flox; WT) (left panel). Clec1b is a gene symbol of Clec‐2. Lymphatic vessels on the surface of the small intestine are filled with blood and showed red reticular markings in the CLEC‐2Δplt mouse. In this mouse, blood‐filled lymphatic vessels are also observed between the mesenteric artery and the vein
4. ROLE OF PLATELET CLEC‐2/PDPN IN LUNG DEVELOPMENT
4.1. The lung malformation in CLEC‐2 null mice
CLEC‐2 null mice die just after birth. 1 , 38 CLEC‐2 null neonates showed cyanosis, although respiratory movement was observed. 38 Therefore, we hypothesized that CLEC‐2 null mice resulted in death by respiratory failure due to lung malformation. We placed the lung from CLEC‐2 null fetuses or wild‐type fetuses at embryonic day (E) 18.5 inside syringes and pulled the plungers to give negative pressure. We found that the lung from CLEC‐2 null fetuses were poorly inflated compared with that from wild‐type fetuses (unpublished observation, Tsukiji N. et al). We hypothesized that CLEC‐2 null mice die of respiratory failure due to lung malformation and analyzed lung phenotypes of CLEC‐2 null mice. In E16.5, there is no difference in the appearance of the lung between wild‐type and Clec1b‐deficient mice. However, after E17.5, the edge of the wild‐type lung was sharp and translucent, but CLEC‐2‐null lungs showed a lumpy and unclear alveolar structure (Figure 3A). Hematoxylin‐eosin (HE) (Figure 3B) and Elastica van Gieson staining (Figure 3C) of the lung revealed that CLEC‐2 null lungs showed narrow alveolar space, thick alveolar septa, and decreased elastic fibers after E17.5 suggesting that insufficient inflation of the lung of CLEC‐2 null mice is due to loss of elastic fibers. 38
Figure 3.
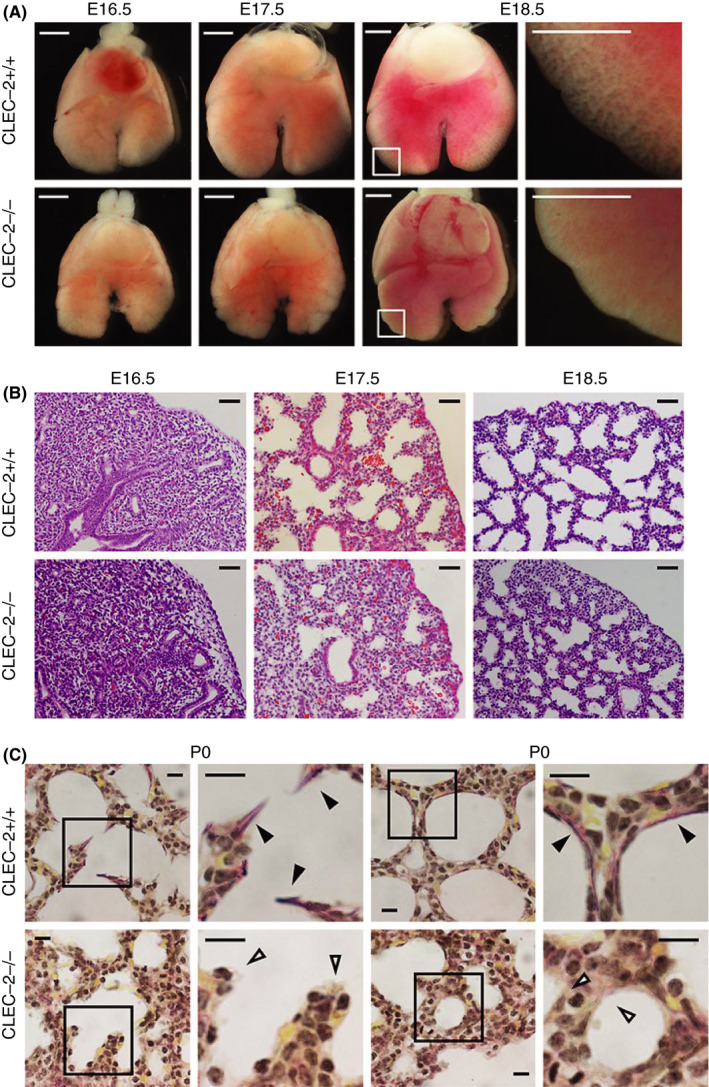
Morphological and histological abnormalities in C‐type lectin‐like receptor 2 (CLEC‐2)–deficient lung. (A) Ventral views of E16.5/E17.5/E18.5 fetal lungs; magnified views of framed areas in images are shown on the right. (B) Hematoxylin and eosin staining of E16.5/E17.5/E18.5 lung sections. (C) Elastica van Gieson staining of P0 lung sections. Magnified views of framed areas in images are shown on the right. Black and open arrowheads: mature elastic fibers (dark purple) and alveolar septa without elastic fibers, respectively. Scale bars: 1 mm (A); 25 μm (B); 10 μm (C). Modified from Tsukiji et al, 38 Figure 1
In developing lungs, elastic fibers are produced by alveolar duct myofibroblasts (adMYFs). Immunohistological analysis by staining α‐smooth muscle actin (α‐SMA) as a marker of adMYFs showed that they were greatly reduced inside the lungs of CLEC‐2‐null mice at E17.5, but there was no difference observed at E16.5 (Figure 4A). No abnormality was found in type I/II lung alveolar cells, vascular/lymphatic endothelial cells, and surfactant secretion in CLEC‐2–deficient mice. Moreover, lung edema was not found in Clec1b‐deficient mice. 38 These findings suggest that decreased elastic fibers are due to decreased adMYFs in the CLEC‐2‐null lung.
Figure 4.
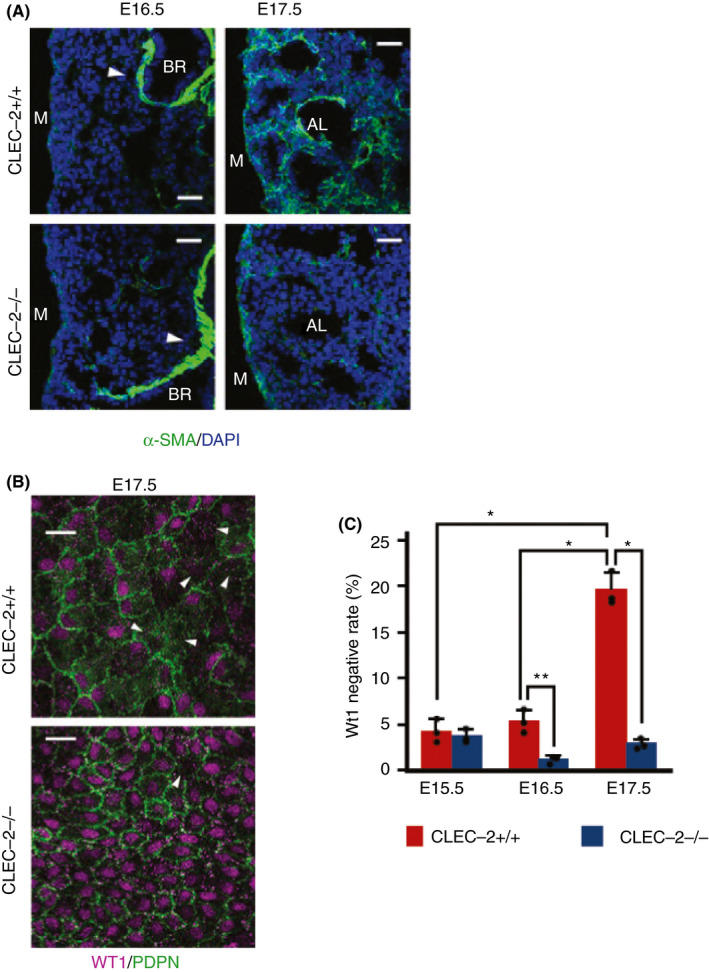
C‐type lectin‐like receptor 2 (CLEC‐2) deficiency results in absence of alveolar duct myofibroblasts (adMYFs), lung mesothelial cell hyperproliferation, and abnormal differentiation. (A) Immunostaining of α‐smooth muscle actin (α‐SMA) (green) and DAPI (blue) in E16.5/E17.5 distal lung. Arrowheads: bronchial smooth muscle cells, which are not adMYFs. (B) Whole‐mount immunohistochemistry of Wt1 (magenta) and Pdpn (green) in E17.5 lung mesothelial cells (luMCs). Arrowheads: Wt1‐negative luMCs. (C) Quantification of Wt1‐negative rate in luMCs at E15.5, E16.5, and E17.5; mean ± SD, n = 5‐3 each. *P = .0001; **P = .0074, Tukey's test. Scale bars: 25 μm (A, B). Modified from Tsukiji et al, 38 Figures 2 and 3
It is known that adMYFs are mainly derived from lung mesothelial cells (luMCs) . 39 , 40 In developing lungs, luMCs specifically express Wilms tumor 1 (WT‐1), but this disappears with time. WT‐1 is a transcription factor, which regulates cell proliferation and is essential for normal development of the urogenital system. It was initially isolated as the gene responsible for Wilms tumor in the kidney. LuMCs migrate into the lung, differentiate into adMYFs, and generate elastic fibers. To investigate luMCs on the surface of the lung, we performed whole‐mount immunofluorescent microscopy of podoplanin and WT‐1. LuMCs express podoplanin on the cell membrane and WT‐1 in its nucleus. At E17.5, a significantly higher percentage of luMCs were WT‐1 positive, and significantly more luMCs stayed on the surface of the lung in CLEC‐2‐null mice (Figure 4B, C). This difference was also found at E16.5 before the number of adMYFs decreased (Figure 4C). These findings suggest that CLEC‐2 facilitate differentiation of luMCs into adMYFs through WT‐1 downregulation. 38 AdMYFs generate elastic fibers inside of the lung, and the lung can be properly inflated. However, this process is impaired in the absence of CLEC‐2, which causes lung malformation.
4.2. CLEC‐2 expressed in platelets is required for lung development
In mice, CLEC‐2 is also expressed in neutrophils or dendritic cells at low levels. 22 , 23 , 24 , 25 We generated CLEC‐2 Δplt mice and found that these mice are viable. 2 These findings in themselves indicate that CLEC‐2 in cells other than platelets regulate lung development. However, neonatal lethality in these mice was slightly but significantly higher than normal mice (Figure 5A), and the lung was mildly lumpy (Figure 5B). 38 We found that small amounts of CLEC‐2 (2% compared with normal platelets) remains on the surface of platelets from CLEC‐2 Δplt mice (Figure 5C). We assume that mild phenotype in CLEC‐2 Δplt mice is due to the remaining CLEC‐2 molecule on the surface of platelets from CLEC‐2 Δplt. We then depleted the remaining CLEC‐2 by injection of the anti‐CLEC‐2 antibody, 2A2B10, into mice pregnant with the CLEC‐2 Δplt fetus. 2A2B10 is not a blocking antibody but a “depletion” antibody. It activates platelets and depletes CLEC‐2 from platelets mainly by internalization and degradation. 10 2A2B10 injection did not result in CLEC‐2 depletion in cells other than platelets, including natural killer cells, B cells, peripheral neutrophils, and macrophages. 10 This procedure resulted in the complete loss of CLEC‐2 molecules on CLEC‐2 Δplt fetal platelets and greatly increased neonatal lethality (Figure 5D) and decreased adMYFs inside the lung (Figure 5E). 38 These findings suggest that platelet CLEC‐2 is required for lung development.
Figure 5.
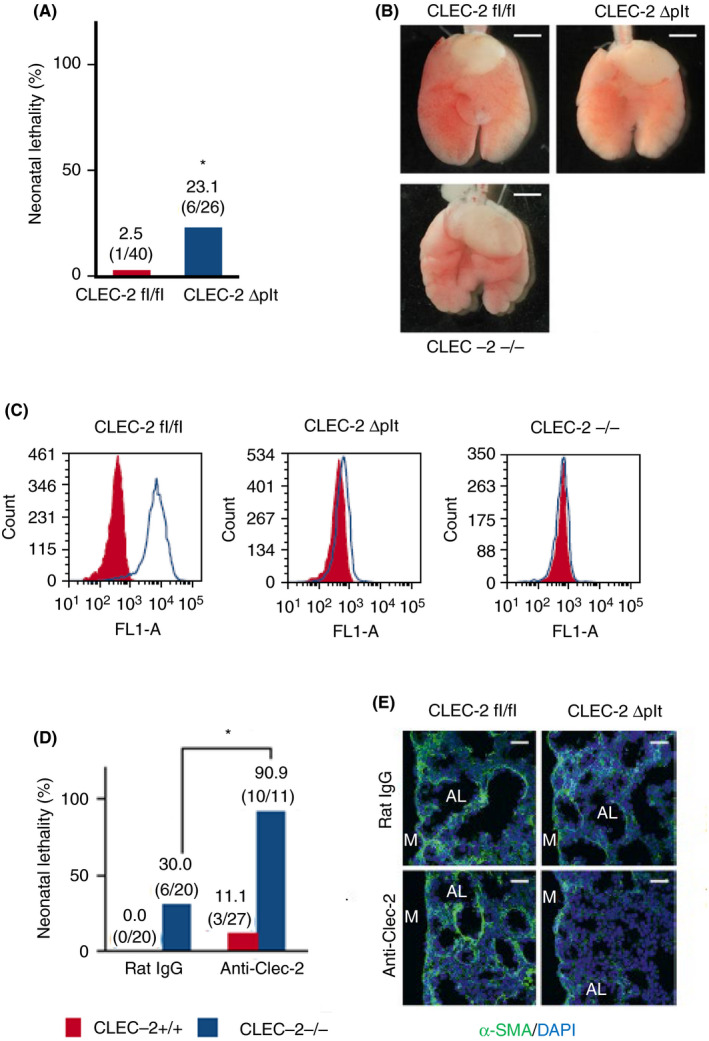
Platelets are required for lung development. (A) Neonatal lethality of C‐type lectin‐like receptor 2 (CLEC‐2) fl/fl and CLEC‐2 Δplt neonates. *P = .00127, Fisher's exact test. (B) Ventral views of CLEC‐2fl/fl (wild‐type), CLEC‐2Δplt, and CLEC‐2‐/‐ fetal lungs at E17.5. (C) CLEC‐2 expression on platelets in E17.5 CLEC‐2fl/fl (wild‐type), CLEC‐2 Δplt, and CLEC‐2‐/‐ fetuses. Red‐filled and open histograms indicate isotype control and CLEC‐2 antibody, respectively. (D) Neonatal lethality in CLEC‐2fl/fl and CLEC‐2 Δplt neonates treated with rat IgG (control), anti‐CLEC‐2. *P = .0021, Fisher's exact test. (E) Immunostaining of α‐smooth muscle actin ( α‐SMA (green) and DAPI (blue) in E17.5 distal lung. AL, alveolar sac; M, lung mesothelium. Scale bars: 1 mm (B), 25 μm (E). Modified from Tsukiji et al, 38 Figures 4 and 5
4.3. Mechanism of adMYF differentiation by platelet CLEC‐2
To elucidate a counterpart of CLEC‐2 necessary for lung development, we generated PDPN null mice (Pdpn‐/‐) and found a phenotype closely resembling the CLEC‐2 null mice: high neonatal lethality, lumpy lung surface, decreased adMYFs, and increased luMCs on the surface of the lung. 38 These findings suggest that binding between CLEC‐2 and PDPN is required for lung development.
The next question is: Which is required for lung development—activation signals downstream of CLEC‐2 or those downstream of PDPN? Previous studies show that mice deficient in cytoplasmic domain of PDPN are viable 25 and that mice deficient in cytoplasmic Tyr in hemi‐ITAM motif die just after birth. 41 These reports suggest that activation signals downstream of CLEC‐2 are required for lung development. To confirm this hypothesis, we generated mice deficient in Syk, which is a necessary signaling molecule downstream of CLEC‐2. Syk‐deficient mice showed the phenotype closely resembling CLEC‐2‐deficient mice, 38 further supporting this hypothesis.
Because platelet activation induces release of granule contents, we hypothesized that granule contents released from activated platelets facilitate lung development. To test this, we placed small pieces of lung specimen at E16.5 on microporous membrane in a Boyden chamber and activated platelet supernatants (APSs) were added to the lower chamber. This procedure increased α‐SMA expression in lung interstitium, suggesting that luMCs differentiate into adMYFs. 38 TGF‐β is secreted by several cell types in a latent form in which it is complexed with 2 other polypeptides, latent TGF‐ β binding protein and latency‐associated peptide. Proteinases, integrins, or reactive oxygen species are known to activate the latent form of TGF‐β. Additionally, acidic conditions also resulted in increased activation of TGF‐β. Acid‐treated (TGF‐β activated) APS more effectively increased αSMA expression, suggesting that TGF‐β is an important component to facilitate adMYF differentiation. This is supported by significantly lower TGF‐β1 concentration in the lungs of CLEC‐2 null fetuses. 38 We then depleted TGF‐β by the injection of anti‐TGF‐β blocking antibody into mice pregnant with CLEC‐2 Δplt fetus. This procedure produced CLEC‐2 null‐like severe phenotypes in CLEC‐2 Δplt fetus including increased neonatal lethality and decreased adMYFs inside the lung. 38 These findings suggest that TGF‐β released after CLEC‐2 engagement is necessary for lung development.
4.4. PDPN in LECs is necessary for lung development
Three types of cells express PDPN in the lung: type I lung alveolar cells, lymphatic endothelial cells, and lung mesothelial cells. We generated 3 kinds of conditional PDPN‐deficient mice: type I lung alveolar cell‐specific, LEC‐specific, and luMC‐specific PDPN‐deficient mice, using transgenic mice are shown in Table 1. We found that only LEC‐specific PDPN‐deficient mice showed CLEC‐2 null mice–like phenotype, including high neonatal lethality and an absence of adMYFs. 38 These findings suggest that PDPN in lymphatic endothelial cells are required for lung development.
Table 1.
Transgenic mice used to make the conditional PDPN‐deficient mice
| Cell types from which PDPN specifically deleted | Used transgenic mice |
|---|---|
| Type I lung alveolar cells | Shh‐Cre transgenic mice |
| LECs | Tie2‐Cre transgenic mice |
| luMCs | WT‐1‐Cre transgenic mice |
Abbreviations: LECs, lymphatic endothelial cells; luMCs, lung mesothelial cells; PDPN, podoplanin;Shh, sonic hedgehog; WT‐1, Wilms tumor 1.
In summary, we proposed how platelets regulate lung development as follows: platelets are activated by binding between platelet CLEC‐2 and LEC PDPN, and then release TGF‐β. This cytokine facilitates differentiation of luMCs into adMYFs, which generate elastic fibers to confer elasticity to the lung (Figure 6). 42
Figure 6.
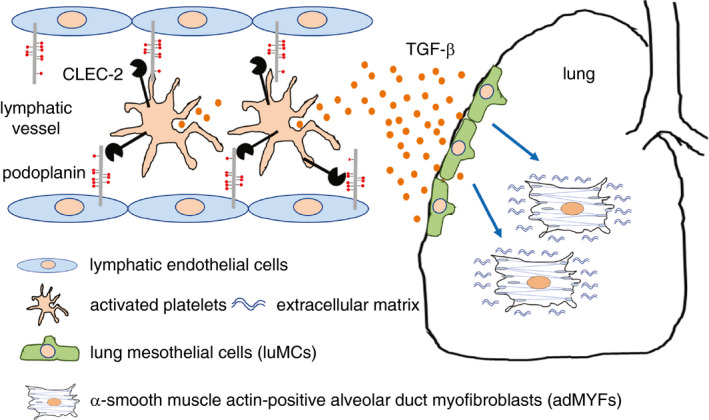
Suggested mechanism of lung development regulated by platelet C‐type lectin‐like receptor 2 (CLEC‐2). During development, platelets are activated by binding between CLEC‐2 and lymphatic endothelial cell podoplanin (PDPN). Transforming growth factor‐β (TGF‐β) is released from activated platelets and facilitates differentiation of lung mesothelial cells luMCs into alveolar duct myofibroblasts (adMYFs). AdMYFs generate elastic fibers so the lung can be properly inflated (cited from P.475 of Illustrated State‐of‐the‐Art Capsules of the ISTH 2019 congress in Melbourne, Australia 42 )
5. UNANSWERED QUESTIONS
We demonstrated a role of platelets in organogenesis, but several issues remain unsolved. Very low expression of CLEC‐2 (only 2% expression compared with wild‐type platelets) is almost enough for lung development, but not enough for blood/lymphatic vessel separation. 1 , 38 In the case of lung development, a kind of amplification mechanism may exist. TGF‐β released from activated platelets acts on other cells, which release substances stimulating adMYF differentiation together with TGF‐β. Alternatively, release of TGF‐β may occur in lymphatic vessels in the vicinity of luMCs and very low levels of TGF‐β from activated platelets expressing low levels of CLEC‐2 may be enough for luMC differentiation into adMYFs.
Platelet activation during embryonic development appears to induce marked thrombus formation in embryos. However, platelets can induce secretion without platelet aggregation. Ollivier et al 43 showed that collagen at a low dose (0.25 μg/mL) selectively triggers a platelet secretory phenotype characterized by the release of dense‐ and α granule‐derived soluble factors without causing substantial platelet changes that usually accompany thrombus formation. Similarly, PDPN may function in a local concentration‐dependent manner on LECs by inducing secretion without causing complete platelet activation and thrombus formation. Moreover, Margraf et al showed physiologically low platelet numbers and hyporeactivity during the early stages of fetal development, 44 further indicating the potential nonhemostatic role of platelets in organogenesis.
Although we proved that association between PDPN in LECs and CLEC‐2 in platelets are necessary for lung development, the sites in which platelets might directly contact LECs to promote lung development remain unclear. This interaction is possible when lymph sacs are sprouted from the cardinal vein at E12.5 to 13.5 in mice. 45 However, the lung phenotype in CLEC‐2 null fetuses was observed at later stages, after E17.5. 38 Lung‐specific lymphatic vessel development may be important. At E14.5, lymphatic vessels with lumen are observed in proximal and distal bronchovascular bundles, but no significant connections between these. At E18.5, a continuous lymphatic network is established. 46 , 47 Thus, during the connection process, transient anastomosis between veins and lymphatic vessels may occur, which leads to contact with LECs and platelet activation. Alternatively, since the lung lymphatic system is abundant in the pleura, 46 the LEC PDPN/platelet CLEC‐2 interaction and TGF‐β release may occur specifically in the pleural lymphatics (ie, in the vicinity of luMCs) allowing effective delivery of TGF‐β to luMCs. Further investigation is required for full elucidation of this aspect.
6. A SPECULATIVE CONSIDERATION ABOUT “DELIVERED SIGNALING” BY PLATELETS DURING EMBRYONIC DEVELOPMENT AND ABOUT EVOLUTION OF PLATELETS AND THE LUNG
Why are platelets used for embryonic development? We speculate that platelets act as ultimate natural drug delivery systems, enabling biological substances to specifically act on the target in high concentrations. In the case of systemic drug administration such as intravenous injection or oral administration, high doses are required for sufficient efficacy. Unexpected effects on nontargets can be a problem with using high doses of drugs. On the other hand, platelets realize selective and local release of biological substances via specific interaction between platelet receptors and their ligands on the surface of target cells.
Platelets contain various kinds of biological substances in their granules and release them upon activation. Platelets also immediately generate them in the cytoplasm and release them in the extracellular space upon activation (eg, thromboxane A2 and sphingosine‐1‐phosphate). Do platelets nonselectively release all the granule contents upon platelet activation by any kind of platelet agonists? Growing bodies of evidence show that distinct granule contents can be released differentially in response to individual platelet agonists. For example, proteinase‐activated receptor (PAR) 1 (thrombin receptor), ADP, and GPVI stimulation favors proangiogenic (eg, vascular endothelial growth factor), whereas PAR4 and thromboxane A2 promotes antiangiogenic factor (eg, endostatin) release. 48 , 49 It has been proposed that platelets differentially package pro‐ and antiangiogenic proteins in distinct α‐granules that undergo differential release upon platelet activation. 50 Although evidence against these hypotheses has been reported, 51 it may be possible that a specific association between platelet receptors and the corresponding ligands on the surface of cells differentially release necessary bioactive substances for the cells.
During development, platelets release biological substances in close vicinity to the targets. Therefore, it is possible to deliver sufficient concentrations of biological substances to the target. This is similar to juxtacrine/paracrine signaling, which are well‐known methods facilitating embryonic development. We call these platelet‐mediated signals during embryonic development “delivered signaling.”
Although mammals, amphibians, and reptiles have lungs with alveolar cells and capillary beds, the latter 2 have simple septa (called faveoli, ediculi, or traveculi) but not grapelike clusters (alveoli) or lobular units. 52 , 53 Moreover, only mammals have a dense lymphatic plexus in the subpleural region and interlobular space, which is extremely close to lung mesothelial cells. 54 , 55 Platelets of mammals are small anucleate cells, whereas those of amphibians and reptiles are relatively large nucleate cells called thrombocytes. It is tempting to speculate that a well‐developed lymphatic plexus in the interlobular space and small‐sized platelets in mammals allows podoplanin‐CLEC‐2 interactions, leading to dense and complex alveolus formation. In other words, by using small anucleate platelets and lymphatic plexus for embryonic development, mammals are able to develop new organ structures, alveoli, and lobular units. Further investigation is required to address this issue.
RELATIONSHIP DISCLOSURE
The authors have no conflicts of interest to report.
ACKNOWLEDGMENTS
This study was supported in part by KAKENHI (16K19572) and the Funding Program for Next Generation World‐leading Researchers (LS052).
Suzuki‐Inoue K, Tsukiji N. Platelet CLEC‐2 and lung development. Res Pract Thromb Haemost. 2020;4:481–490. 10.1002/rth2.12338
Handling Editor: Alisa Wolberg
REFERENCES
- 1. Suzuki‐Inoue K, Inoue O, Ding G, Nishimura S, Hokamura K, Eto K, et al. Essential in vivo roles of the C‐type lectin receptor CLEC‐2: embryonic/neonatal lethality of CLEC‐2‐deficient mice by blood/lymphatic misconnections and impaired thrombus formation of CLEC‐2‐deficient platelets. J Biol Chem. 2010;285:24494–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Osada M, Inoue O, Ding G, Shirai T, Ichise H, Hirayama K, et al. Platelet activation receptor clec‐2 regulates blood/lymphatic vessel separation by inhibiting proliferation, migration, and tube formation of lymphatic endothelial cells. J Biol Chem. 2012;287:22241–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bertozzi CC, Schmaier AA, Mericko P, Hess PR, Zou Z, Chen M, et al. Platelets regulate lymphatic vascular development through CLEC‐2‐SLP‐76 signaling. Blood. 2010;116:661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tsukiji N, Osada M, Sasaki T, Shirai T, Satoh K, Inoue O, et al. Cobalt hematoporphyrin inhibits CLEC‐2‐podoplanin interaction, tumor metastasis, and arterial/venous thrombosis in mice. Blood Adv. 2018;2:2214–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Suzuki‐Inoue K, Fuller GL, Garcia A, Eble JA, Pohlmann S, Inoue O, et al. A novel Syk‐dependent mechanism of platelet activation by the C‐type lectin receptor CLEC‐2. Blood. 2006;107:542–9. [DOI] [PubMed] [Google Scholar]
- 6. Suzuki‐Inoue K, Kato Y, Inoue O, Kaneko MK, Mishima K, Yatomi Y, et al. Involvement of the snake toxin receptor CLEC‐2, in podoplanin‐mediated platelet activation, by cancer cells. J Biol Chem. 2007;282:25993–6001. [DOI] [PubMed] [Google Scholar]
- 7. Christou C, Pearce A, Watson A, Mistry A, Pollitt A, Fenton‐May A, et al. Renal cells activate the platelet receptor CLEC‐2 through podoplanin. Biochem J. 2008;411:133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kato Y, Kaneko MK, Kunita A, Ito H, Kameyama A, Ogasawara S, et al. Molecular analysis of the pathophysiological binding of the platelet aggregation‐inducing factor podoplanin to the C‐type lectin‐like receptor CLEC‐2. Cancer Sci. 2008;99:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kato Y, Fujita N, Kunita A, Sato S, Kaneko M, Osawa M, et al. Molecular identification of Aggrus/T1alpha as a platelet aggregation‐inducing factor expressed in colorectal tumors. J Biol Chem. 2003;278:51599–605. [DOI] [PubMed] [Google Scholar]
- 10. Shirai T, Inoue O, Tamura S, Tsukiji N, Sasaki T, Endo H, et al. C‐type lectin‐like receptor 2 promotes hematogenous tumor metastasis and prothrombotic state in tumor‐bearing mice. J Thromb Haemost. 2017;15:513–25. [DOI] [PubMed] [Google Scholar]
- 11. Takemoto A, Miyata K, Fujita N. Platelet‐activating factor podoplanin: from discovery to drug development. Cancer Metastasis Rev. 2017;36:225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Suzuki‐Inoue K, Osada M, Ozaki Y. Physiologic and pathophysiologic roles of interaction between C‐type lectin‐like receptor 2 and podoplanin: partners from in utero to adulthood. J Thromb Haemost. 2017;15:219–29. [DOI] [PubMed] [Google Scholar]
- 13. Morita T. Structures and functions of snake venom CLPs (C‐type lectin‐like proteins) with anticoagulant‐, procoagulant‐, and platelet‐modulating activities. Toxicon. 2005;45:1099–114. [DOI] [PubMed] [Google Scholar]
- 14. Suzuki‐Inoue K, Ozaki Y, Kainoh M, Shin Y, Wu Y, Yatomi Y, et al. Rhodocytin induces platelet aggregation by interacting with glycoprotein Ia/IIa (GPIa/IIa, Integrin alpha 2beta 1). Involvement of GPIa/IIa‐associated src and protein tyrosine phosphorylation. J Biol Chem. 2001;276:1643–52. [DOI] [PubMed] [Google Scholar]
- 15. Inoue K, Ozaki Y, Satoh K, Wu YI, Yatomi Y, Shin Y, et al. Signal transduction pathways mediated by glycoprotein Ia/IIa in human platelets: comparison with those of glycoprotein VI. Biochem Biophys Res Commun. 1999;256:114–20. [DOI] [PubMed] [Google Scholar]
- 16. Navdaev A, Clemetson JM, Polgar J, Kehrel BE, Glauner M, Magnenat E, et al. Aggretin, a heterodimeric C‐type lectin from Calloselasma rhodostoma (malayan pit viper), stimulates platelets by binding to alpha 2beta 1 integrin and glycoprotein Ib, activating Syk and phospholipase Cgamma 2, but does not involve the glycoprotein VI/Fc receptor gamma chain collagen receptor. J Biol Chem. 2001;276:20882–9. [DOI] [PubMed] [Google Scholar]
- 17. Bergmeier W, Bouvard D, Eble JA, Mokhtari‐Nejad R, Schulte V, Zirngibl H, et al. Rhodocytin (aggretin) activates platelets lacking alpha(2)beta(1) integrin, glycoprotein VI, and the ligand‐binding domain of glycoprotein Ibalpha. J Biol Chem. 2001;276:25121–6. [DOI] [PubMed] [Google Scholar]
- 18. Chaipan C, Soilleux EJ, Simpson P, Hofmann H, Gramberg T, Marzi A, et al. DC‐SIGN and CLEC‐2 mediate human immunodeficiency virus type 1 capture by platelets. J Virol. 2006;80:8951–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tang T, Li LI, Tang J, Li Y, Lin WY, Martin F, et al. A mouse knockout library for secreted and transmembrane proteins. Nat Biotechnol. 2010;28:749–55. [DOI] [PubMed] [Google Scholar]
- 20. Nakamura‐Ishizu A, Takubo K, Kobayashi H, Suzuki‐Inoue K, Suda T. CLEC‐2 in megakaryocytes is critical for maintenance of hematopoietic stem cells in the bone marrow. J Exp Med. 2015;212:2133–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tamura S, Suzuki‐Inoue K, Tsukiji N, Shirai T, Sasaki T, Osada M, et al. Podoplanin‐positive periarteriolar stromal cells promote megakaryocyte growth and proplatelet formation in mice by CLEC‐2. Blood. 2016;127:1701–10. [DOI] [PubMed] [Google Scholar]
- 22. Kerrigan AM, Dennehy KM, Mourão‐Sá D, Faro‐Trindade I, Willment JA, Taylor PR, et al. CLEC‐2 is a phagocytic activation receptor expressed on murine peripheral blood neutrophils. J Immunol. 2009;182:4150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Acton SE, Farrugia AJ, Astarita JL, Mourão‐Sá D, Jenkins RP, Nye E, et al. Dendritic cells control fibroblastic reticular network tension and lymph node expansion. Nature. 2014;514:498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Acton S, Astarita J, Malhotra D, Lukacs‐Kornek V, Franz B, Hess P, et al. Podoplanin‐rich stromal networks induce dendritic cell motility via activation of the C‐type lectin receptor CLEC‐2. Immunity. 2012;37:276–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Astarita JL, Cremasco V, Fu J, Darnell MC, Peck JR, Nieves‐Bonilla JM, et al. The CLEC‐2‐podoplanin axis controls the contractility of fibroblastic reticular cells and lymph node microarchitecture. Nat Immunol. 2015;16:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chang CH, Chung CH, Hsu CC, Huang TY, Huang TF. A novel mechanism of cytokine release in phagocytes induced by aggretin, a snake venom C‐type lectin protein, through CLEC‐2 ligation. J Thromb Haemost. 2010;8:2563–70. [DOI] [PubMed] [Google Scholar]
- 27. Colonna M, Samaridis J, Angman L. Molecular characterization of two novel C‐type lectin‐like receptors, one of which is selectively expressed in human dendritic cells. Eur J Immunol. 2000;30:697–704. [DOI] [PubMed] [Google Scholar]
- 28. Fuller GL, Williams JA, Tomlinson MG, Eble JA, Hanna SL, Pohlmann S, et al. The C‐type lectin receptors CLEC‐2 and Dectin‐1, but not DC‐SIGN, signal via a novel YXXL‐dependent signaling cascade. J Biol Chem. 2007;282:12397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Flaumenhaft R. Platelet secretion In: Michelson AD, editor. Platelets. 3rd ed Amsterdam, the Netherlands: Elsevier, 2012; p. 343–66. [Google Scholar]
- 30. Tsuruo T, Fujita N. Platelet aggregation in the formation of tumor metastasis. Proc Jpn Acad Ser B Phys Biol Sci. 2008;84:189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Abtahian F, Guerriero A, Sebzda E, Lu MM, Zhou R, Mocsai A, et al. Regulation of blood and lymphatic vascular separation by signaling proteins SLP‐76 and Syk. Science. 2003;299:247–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schacht V, Ramirez MI, Hong YK, Hirakawa S, Feng D, Harvey N, et al. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 2003;22:3546–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Manne BK, Badolia R, Dangelmaier C, Eble JA, Ellmeier W, Kahn M, et al. Distinct pathways regulate Syk protein activation downstream of immune tyrosine activation motif (ITAM) and hemITAM receptors in platelets. J Biol Chem. 2015;290:11557–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ichise H, Ichise T, Ohtani O, Yoshida N. Phospholipase Cgamma2 is necessary for separation of blood and lymphatic vasculature in mice. Development. 2009;136:191–5. [DOI] [PubMed] [Google Scholar]
- 35. Hess PR, Rawnsley DR, Jakus Z, Yang Y, Sweet DT, Fu J, et al. Platelets mediate lymphovenous hemostasis to maintain blood‐lymphatic separation throughout life. J Clin Invest. 2014;124:273–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pollitt AY, Poulter NS, Gitz E, Navarro‐Nuñez L, Wang Y‐J, Hughes CE, et al. Syk and Src family kinases regulate C‐type lectin receptor 2 (CLEC‐2)‐mediated clustering of podoplanin and platelet adhesion to lymphatic endothelial cells. J Biol Chem. 2014;289:35695–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang Y, Daubel N, Stritt S, Makinen T. Transient loss of venous integrity during developmental vascular remodeling leads to red blood cell extravasation and clearance by lymphatic vessels. Development. 2018;145(3):dev156745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tsukiji N, Inoue O, Morimoto M, Tatsumi N, Nagatomo H, Ueta K, et al. Platelets play an essential role in murine lung development through Clec‐2/podoplanin interaction. Blood. 2018;132:1167–79. [DOI] [PubMed] [Google Scholar]
- 39. Que J, Wilm B, Hasegawa H, Wang F, Bader D, Hogan BL. Mesothelium contributes to vascular smooth muscle and mesenchyme during lung development. Proc Natl Acad Sci USA. 2008;105:16626–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cano E, Carmona R, Munoz‐Chapuli R. Wt1‐expressing progenitors contribute to multiple tissues in the developing lung. Am J Physiol Lung Cell Mol Physiol. 2013;305:L322–32. [DOI] [PubMed] [Google Scholar]
- 41. Haining EJ, Cherpokova D, Wolf K, Becker IC, Beck S, Eble JA, et al. CLEC‐2 contributes to hemostasis independently of classical hemITAM signaling in mice. Blood. 2017;130:2224–8. [DOI] [PubMed] [Google Scholar]
- 42. Ward CM, Andrews RK. Illustrated State‐of‐the‐Art Capsules of the ISTH 2019 Congress in Melbourne, Australia. Res Pract Thromb Haemost. 2019;3:431–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ollivier V, Syvannarath V, Gros A, Butt A, Loyau S, Jandrot‐Perrus M, et al. Collagen can selectively trigger a platelet secretory phenotype via glycoprotein VI. PLoS ONE. 2014;9:e104712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Margraf A, Nussbaum C, Rohwedder I, Klapproth S, Kurz ARM, Florian A, et al. Maturation of platelet function during murine fetal development in vivo. Arterioscler Thromb Vasc Biol. 2017;37:1076–86. [DOI] [PubMed] [Google Scholar]
- 45. Uhrin P, Zaujec J, Bauer M, Breuss J, Alitalo K, Stockinger H, et al. Podoplanin‐induced platelet aggregation mediates separation of blood and lymphatic vessels. Vasc Pharm. 2006;45:190. [Google Scholar]
- 46. Wong BW, Zecchin A, Garcia‐Caballero M, Carmeliet P. Emerging concepts in organ‐specific lymphatic vessels and metabolic regulation of lymphatic development. Dev Cell. 2018;45:289–301. [DOI] [PubMed] [Google Scholar]
- 47. Kulkarni RM, Herman A, Ikegami M, Greenberg JM, Akeson AL. Lymphatic ontogeny and effect of hypoplasia in developing lung. Mech Dev. 2011;128:29–40. [DOI] [PubMed] [Google Scholar]
- 48. Chatterjee M, Huang Z, Zhang W, Jiang L, Hultenby K, Zhu L, et al. Distinct platelet packaging, release, and surface expression of proangiogenic and antiangiogenic factors on different platelet stimuli. Blood. 2011;117:3907–11. [DOI] [PubMed] [Google Scholar]
- 49. Battinelli EM, Markens BA, Italiano JEJr. Release of angiogenesis regulatory proteins from platelet alpha granules: modulation of physiologic and pathologic angiogenesis. Blood. 2011;118:1359–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Battinelli EM, Thon JN, Okazaki R, Peters CG, Vijey P, Wilkie AR, et al. Megakaryocytes package contents into separate alpha‐granules that are differentially distributed in platelets. Blood Adv. 2019;3:3092–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. van Holten TC, Bleijerveld OB, Wijten P, de Groot PG, Heck AJR, Barendrecht AD, et al. Quantitative proteomics analysis reveals similar release profiles following specific PAR‐1 or PAR‐4 stimulation of platelets. Cardiovasc Res. 2014;103:140–6. [DOI] [PubMed] [Google Scholar]
- 52. Hsia CC, Schmitz A, Lambertz M, Perry SF, Maina JN. Evolution of air breathing: oxygen homeostasis and the transitions from water to land and sky. Compr Physiol. 2013;3:849–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Maina JN. Structure, function and evolution of the gas exchangers: comparative perspectives. J Anat. 2002;201:281–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Weber E, Sozio F, Borghini A, Sestini P, Renzoni E. Pulmonary lymphatic vessel morphology: a review. Ann Anat. 2018;218:110–7. [DOI] [PubMed] [Google Scholar]
- 55. Yamamoto M, Wilting J, Abe H, Murakami G, Rodriguez‐Vazquez JF, Abe SI. Development of the pulmonary pleura with special reference to the lung surface morphology: a study using human fetuses. Anat Cell Biol. 2018;51:150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


