Abstract
Background
Hemophilia gene therapy is a rapidly evolving therapeutic approach in which a number of programs are approaching clinical development completion.
Objective
The aim of this study was to evaluate knowledge and perceptions of a variety of health care practitioners and scientists about gene therapy for hemophilia.
Methods
This survey study was conducted February 1 to 18, 2019. Survey participants were members of the ISTH, European Hemophilia Consortium, European Hematology Association, or European Association for Hemophilia and Allied Disorders with valid email contacts. The online survey consisted of 36 questions covering demographic information, perceptions and knowledge of gene therapy for hemophilia, and educational preferences. Survey results were summarized using descriptive statistics.
Results
Of the 5117 survey recipients, 201 responded from 55 countries (4% response rate). Most respondents (66%) were physicians, and 59% were physicians directly involved in the care of people with hemophilia. Among physician respondents directly involved in hemophilia care, 35% lacked the ability to explain the science of adeno‐associated viral gene therapy for hemophilia, and 40% indicated limited ability or lack of comfort answering patient questions about gene therapy for hemophilia based on clinical trial results to date. Overall, 75% of survey respondents answered 10 single‐answer knowledge questions correctly, 13% incorrectly, and 12% were unsure of the correct answers.
Conclusions
This survey highlighted knowledge gaps and educational needs related to gene therapy for hemophilia and, along with other inputs, has informed the development of “Gene Therapy in Hemophilia: An ISTH Education Initiative.”
Keywords: gene therapy, hemophilia, knowledge gaps, physicians, respondents, survey
Essentials.
Hemophilia gene therapy is a rapidly evolving therapeutic approach with several phase 3 trials ongoing.
This study explored knowledge and perceptions about hemophilia gene therapy among the health care team and scientists.
The results highlighted knowledge gaps and educational needs related to gene therapy for hemophilia.
These results informed an educational initiative to meet these needs for the hemophilia professional community.
1. INTRODUCTION
Replacement therapy with plasma‐derived clotting factors or recombinant clotting factor concentrates has long been the mainstay for the management of people with hemophilia; however, a limitation of this approach is the relatively short half‐lives of the factor VIII (FVIII) and factor IX (FIX) replacement products.1 As a result, regular prophylaxis with the goal of preventing bleeding episodes by maintaining trough factor levels above ~1% is associated with a high treatment burden (3‐4 infusions per week for hemophilia A; 2‐3 infusions per week for hemophilia B).2 Recent advances have expanded the therapeutic options for people with hemophilia, including extended half‐life (EHL) products and nonreplacement factor therapies. These recent advances expand the treatment options for people with hemophilia, yet regular prophylaxis still imposes a significant treatment burden for both patients and health systems.
In contrast, gene therapy represents a potentially curative approach for people with hemophilia. As a monogenic disease, hemophilia is a logical target for gene therapy strategies, and minimal increases in clotting factor levels may result in a change from a severe to mild phenotype, translating into reduced need for replacement therapy, reduced breakthrough bleeds, and improved quality of life. Strategies evaluated for FVIII and FIX gene transfer have included nonviral vectors, retroviral vectors, adenoviral vectors, lentiviral vectors, and adeno‐associated viral (AAV) vectors. Gene editing and cell‐based therapies are additional investigational gene therapy strategies for hemophilia. Early phase clinical trials of AAV mediated liver‐directed gene therapy for people with hemophilia A and people with hemophilia B have yielded promising results.3, 4, 5, 6, 7 This is a rapidly evolving therapeutic approach, in which several programs are approaching clinical development completion. There is limited information about the knowledge, skills, and expertise of members of the hemophilia comprehensive care team related to gene therapy, an entirely new therapeutic paradigm. Such gene therapy knowledge will enable health care providers to understand and be better prepared to integrate this novel therapy into their practice, and to be able to educate both their peers and patients on the benefits, limitations, and potential risks of gene therapy. This study was conducted to evaluate knowledge and perceptions of a variety of health care practitioners and scientists on gene therapy for hemophilia.
2. MATERIALS AND METHODS
This was an international survey study conducted February 1 to 18, 2019. Survey participants were all members of the ISTH, the European Haemophilia Consortium (EHC), the European Hematology Association, and the European Association for Haemophilia and Allied Disorders (EAHAD) with valid email contacts. The survey questionnaire was composed of 36 questions covering demographic information (9), perceptions about gene therapy for hemophilia (8), knowledge of gene therapy (10 single correct answer; 4 subjective), and educational preferences (5). A variety of question formats were included: single‐answer check box, single answer from a drop‐down menu, “select all that apply” or multiple responses allowed, 5‐point Likert‐type scale questions with descriptors of the point rating, open text response, and ranking. The ranking question format used a weighted approach in which overall rank was assigned by calculated score. Items ranked first by individual respondents are given higher value or weight than lower‐ranked items; the sum of all weighted values is the score for a given item. Knowledge‐based questions were presented only to those respondents who self‐reported at least some understanding of the science of AAV‐mediated liver‐directed gene therapy for hemophilia, as determined by response to a 5‐point Likert‐type scale question. Each knowledge‐based question included the answer option, “I’m not sure.” The questionnaire was accessed by participants online using the SurveyGizmo (https://www.surveygizmo.com/) platform. Survey results were summarized using descriptive statistics. Participation in the survey was voluntary and without compensation, and responses were confidential and deidentified for summarization and analysis.
3. RESULTS
3.1. Demographics
Of the 5117 survey recipients, 201 responded from 55 countries (4% response rate). Most respondents (66%) were physicians, and the majority (59%) were physicians directly involved in the care of people with hemophilia (Table 1). More than half of the participants practiced in academic medical centers, and 58% of respondents had ≥15 years of experience in their current profession.
Table 1.
Demographic characteristics of hemophilia gene therapy survey respondents
| Variable |
Percentage of respondents (n = 194) |
|---|---|
| Professional category | |
| Physician | 66 |
| PhD/Researcher | 13 |
| Registered nurse, advanced practice registered nurse, or physician assistant | 7 |
| Technician, scientist, or technologist | 5 |
| Health business/medical administration professional | 4 |
| Other (including pharmacist; patient advocate; NGO; educator; health economist) | 4 |
| Student/Resident | 1 |
| Primary clinical focus | |
| Bleeding disorders including hemophilia | 39 |
| Thrombosis and hemophilia | 28 |
| Hematology | 11 |
| Hematology/Oncology | 8 |
| Thrombosis/Clotting | 7 |
| Other nonclinical (bleeding disorders, FVIII, gene therapy, industry, product development, thrombosis and bleeding disorders, transfusion) | 5 |
| Internal medicine | 1% |
| Other clinical (clinical chemistry) | 1% |
| Role in the care of people with hemophilia a | |
| Physician involved in direct patient care | 59 |
| Investigator in clinical trials of people with hemophilia | 38 |
| Patient education | 27 |
| Perform assays/assessments in a specialized coagulation laboratory | 22 |
| Basic research related to hemophilia but not related to patient care | 20 |
| Other (including nurse coordinator, nurse clinician, industry, administration, health care education, health economics and outcomes research, patient advocacy) | 17 |
| Practice setting | |
| Academic medical center | 54 |
| Industry | 13 |
| Hemophilia treatment center (independent of an academic medical center) | 11 |
| Government hospital | 9 |
| Other (including nonprofit organization; patient organization; government research center) | 7 |
| Hemostasis center | 3 |
| Community‐based hospital | 2 |
| Outpatient clinic/ambulatory setting | 1 |
Abbreviations: FVIII, factor VIII; NGO, nongovernmental organization.
Respondents could select all applicable responses.
3.2. Perceptions and knowledge related to hemophilia gene therapy
For 3 self‐reported ability questions, an average of 58% of physicians directly involved in the care of people with hemophilia rated their abilities as 4 or 5 on a 5‐point Likert‐type scale, with 1 being the lowest and 5 being the highest ability, compared with 46% of respondents not directly involved in the care of people with hemophilia. When asked to rate their ability to explain the science behind AAV‐mediated gene therapy for hemophilia to a colleague, 65% of physicians directly involved in the care of people with hemophilia rated their abilities as 4 or 5 (Table 2). Among physician respondents involved in hemophilia care, 26% lacked the ability to explain AAV gene therapy, with an additional 9% lacking a clear understanding or had never heard of gene therapy. In addition, 40% of physicians involved in the care of people with hemophilia indicated limited ability or lack of comfort answering patient questions about gene therapy for hemophilia based on clinical trial results to date, and 17% indicated limited or no understanding of how gene therapy may affect an individual’s current treatment for hemophilia.
Table 2.
Self‐reported ability
| Self‐reported ability | Physicians directly involved in care of people with hemophilia (%) | Other respondents (%) |
|---|---|---|
| Ability to explain the science of AAV‐mediated liver‐directed gene therapy for hemophilia to a colleague (n = 177) | ||
| 1—I’ve never heard of gene therapy | 3 | 3 |
| 2—I do not have a clear understanding of how gene therapy works | 6 | 6 |
| 3—I’ve learned about gene therapy, but still don’t think that I could explain it well to someone else | 26 | 41 |
| 4—I know enough about gene therapy to feel comfortable educating colleagues, patients, and caregivers | 54 | 40 |
| 5—I consider myself an expert | 11 | 10 |
| Ability to answer patient questions about gene therapy for hemophilia based on clinical trial results to date (n = 143) | ||
| 1—I would not be able to answer questions about clinical trials of gene therapy for hemophilia | 2 | 7 |
| 2—I have read about some of the studies, but would not feel very comfortable answering questions | 11 | 24 |
| 3—I could answer a few basic questions about the studies | 27 | 28 |
| 4—I feel comfortable answering questions about clinical trial results in gene therapy | 50 | 30 |
| 5—I consider myself an expert | 10 | 11 |
| Ability to describe how gene therapy may impact an individual’s current treatment for hemophilia (n = 143) | ||
| 1—I am not able to describe how gene therapy may impact an individual’s current treatment | 1 | 9 |
| 2—I have a limited understanding of how gene therapy may impact current treatment practice for patients | 16 | 22 |
| 3—I am able to explain how gene therapy may impact current treatment practice for patients | 35 | 21 |
| 4—I am very comfortable with my ability to explain to my patients and colleagues how gene therapy may impact current treatment practice | 38 | 35 |
| 5—I consider myself an expert | 10 | 13 |
Abbreviation: AAV, adeno‐associated viral.
Survey participants ranked 15 potential concerns or barriers related to gene therapy for hemophilia. Among physicians directly involved in the care of people with hemophilia, the top 3 concerns based on weighted ranking scores were long‐term safety and monitoring, durability of expression/response, and challenges associated with use in specific populations (ie, children, those with inhibitors, neutralizing antibodies to AAV capsid proteins, etc) (Table 3).
Table 3.
Potential concerns or barriers related to gene therapy for hemophilia (weighted ranking)
|
Physicians directly involved in care of people with hemophilia (n = 81) Rank [score] |
Concern or barrier |
Other respondents (n = 49) Rank [score] |
|---|---|---|
| 1 [832] | Long‐term safety and monitoring | 2 [454] |
| 2 [815] | Durability of expression/response | 1 [516] |
| 3 [723] | Challenges associated with use in specific populations (ie, children; those with inhibitors, neutralizing antibodies to AAV capsid, etc) | 3 [417] a |
| 4 [674] | Cost/reimbursement | 3 [417] a |
| 5 [666] | Elevation in liver enzymes; immune response to AAV capsid proteins | 7 [371] |
| 6 [622] | Insertional mutagenesis | 5 [407] |
| 7 [597] | Patient eligibility for clinical trials | 11 [333] |
| 8 [571] | Limited scientific evidence will be available with which to make decisions about this treatment approach | 10 [341] |
| 9 [556] | Current availability of safe and effective therapies | 9 [347] |
| 10 [547] | Patient access to treatment | 6 [386] |
| 11 [530] | Unknown unknowns | 8 [352] |
| 12 [510] | Patient acceptance of gene therapy relative to their current treatment approach | 15 [255] |
| 13 [506] | Risk for development of hepatocellular carcinoma | 12 [328] |
| 14 [474] | Determining if it will be more advantageous for patients to wait for other emerging options | 13 [318] |
| 15 [449] | Vector shedding | 14 [274] |
Abbreviation: AAV, adeno‐associated viral.
Identical weighted scores.
Survey respondents who rated their ability to explain the science of AAV gene therapy for hemophilia to a colleague as 3, 4, or 5 on a 5‐point Likert‐type scale were presented knowledge‐based questions related to gene therapy for hemophilia. Of these 161 respondents, 124 (77%) completed the knowledge‐based questions. This subset of overall survey respondents was composed of a greater percentage of physicians directly involved in the care of people with hemophilia compared to the overall group of survey respondents (64% vs 59%, respectively). Overall, 75% of respondents answered 10 single‐answer knowledge questions correctly. Of physician respondents directly involved in the care of people with hemophilia, 78% answered the 10 questions correctly, 12% incorrectly, and 10% were unsure of the correct answers (Table 4). Among those respondents who self‐reported as “expert” in their ability to explain the science of AAV gene therapy for hemophilia, 90% answered these 10 questions correctly, 6% incorrectly, and 4% were unsure of correct answers. Responses to 4 subjective knowledge questions are included in Table S1.
Table 4.
Answers to knowledge‐based question about gene therapy for hemophilia
| Question [correct answer] | Answered correctly (%) | Answered incorrectly (%) | Answered “I’m not sure” (%) |
|---|---|---|---|
| Which of the following was a methodological challenge associated with early gene transfer studies for hemophilia A (relative to hemophilia B)? [size of the factor VIII protein (and therefore packaged transgene)] | 84 | 12 | 4 |
| 60 | 31 | 9 | |
| Recent publications of success with gene therapy in people with hemophilia A and B have incorporated which of the following approaches? [adeno‐associated viral vector gene transfer] | 76 | 21 | 3 |
| 82 | 16 | 2 | |
| Recent publications have described successful gene therapy in hemophilia A and B. In these studies, was gene transfer accomplished in vivo or ex vivo? [in vivo] | 81 | 13 | 6 |
| 91 | 5 | 4 | |
| Gene therapy for people with hemophilia has used a viral vector transfer approach that results in [Episomal persistence in the nucleus of target cells] | 53 | 32 | 15 |
| 44 | 27 | 29 | |
| Successful gene therapy for people with hemophilia has affected which cell populations? [somatic cells] | 87 | 4 | 9 |
| 73 | 5 | 22 | |
| In early‐phase clinical trials using AAV‐FVIII gene transfer in men with severe hemophilia A, what was the protein produced by this method? [B‐domain deleted human FVIII] | 67 | 16 | 17 |
| 64 | 14 | 22 | |
| In some studies of AAV‐mediated FIX gene transfer in people with hemophilia B, a codon‐optimized FIX Padua (FIX‐R338L) transgene is being used. What is the rationale for this approach? [The high specific activity of this FIX variant means a lower dose can be used to achieve therapeutic expression and minimize risk of AAV capsid immune response] | 85 | 2 | 13 |
| 76 | 8 | 16 | |
| Which statement describes safety outcomes from recent investigation of AAV5‐gene therapy with expression of wild‐type FIX in adults with hemophilia B? [The procedure has been generally well tolerated] | 67 | 10 | 23 |
| 61 | 12 | 27 | |
| Which of the following has been a common adverse event noted in clinical trials of gene therapy for people with hemophilia? [Transient elevation of alanine aminotransferase (ALT) levels] | 87 | 10 | 3 |
| 77 | 12 | 11 | |
| What is the anticipated treatment frequency with gene therapy for patients with hemophilia? [One‐time infusion, with long‐term follow‐up] | 89 | 5 | 6 |
| 78 | 13 | 9 |
n = 79 physicians directly involved in care of people with hemophilia (rows shaded in green); n = 45 other respondents 9 (no row shading).
Abbreviations: AAV, adeno‐associated viral; FIX, factor IX; FVIII, factor VIII.
3.3. Self‐reported educational needs
All 131 respondents to a question about educational needs related to gene therapy identified at least 1 need, with an average of 5.6 identified needs per respondent. Specific self‐reported needs are summarized in Figure 1.
Figure 1.
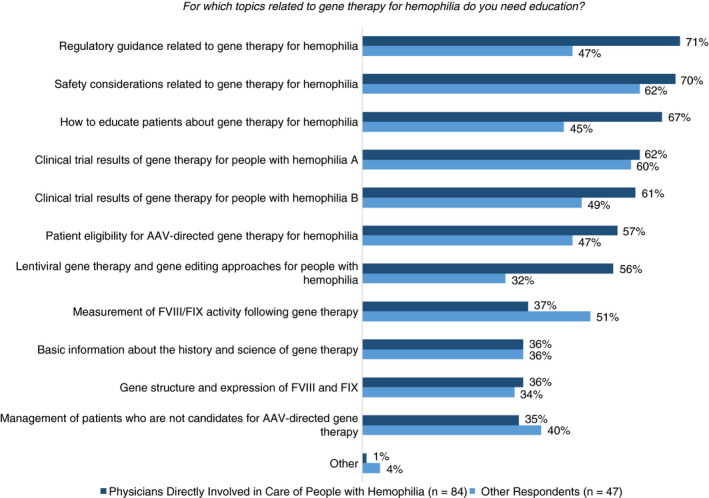
Self‐identified educational needs related to gene therapy for hemophilia. For which topics related to gene therapy for hemophilia do you need education? AAV, adeno‐associated viral; FIX, factor IX; FVIII, factor VIII
4. DISCUSSION
This study was conducted to better understand perceptions and knowledge related to gene therapy as a treatment approach for people with hemophilia among health care providers and scientists in the thrombosis and hemostasis professional community. The survey results indicated that many respondents are lacking in their abilities to explain hemophilia gene therapy or answer patient questions about this emerging treatment approach for people with hemophilia. On knowledge questions, 25% of respondents overall, and 22% of physicians directly involved in the care of people with hemophilia incorrectly answered or were not sure of correct answers. Survey respondents recognized a range of educational needs related to gene therapy. These results expand on what has been previously reported about the knowledge level in the hemophilia care community about gene therapy. A different survey was conducted prior to the first World Federation of Hemophilia (WFH) Gene Therapy Round Table (convened in April 2018) to better understand the needs and knowledge level of the hemophilia community.8 WFH survey respondents included 109 treating physicians from 76 countries. Of the physician treater respondents, 44% had “basic” or “intermediate” understanding of gene therapy, with 12% indicating an “advanced” understanding (specific survey questions were not reported).8 The authors noted that a recurrent theme throughout the roundtable discussions was the need for education at all levels. In a poster presentation, Hurst et al9 reported results of a Continuing Medical Education–certified online clinical practice assessment that included 25 multiple‐choice questions exploring knowledge, attitudes, and perspectives about gene therapy. Based on responses from hematology/oncology respondents, the authors concluded that a majority of providers lack confidence in their understanding of gene therapy for hemophilia A.9 The current survey study adds to these previous reports with additional specific knowledge‐based questions, queries about self‐reported abilities, ranking of perceived barriers/concerns, and respondent‐identified educational needs related to gene therapy for hemophilia.
Gene therapy represents a potentially transformative approach to hemophilia treatment that is distinct from existing therapies including EHL products and nonreplacement strategies. Notably, gene therapy is potentially curative, producing long‐term persistent outcomes, yet with the potential limitation of single treatment only, due to the immune response associated with vector exposure. These unique aspects of gene therapy highlight the importance of education to provide the bridge between evolving basic and clinical trial research and clinical practice. Our findings highlight gaps in knowledge related to gene therapy, including a need for better understanding of the fundamentals of gene therapy in general, and specifically how gene therapy is being developed as a treatment approach for hemophilia A and B. Our results suggest that it would be a mistake to assume that there is universal understanding of the basics about gene therapy; indeed, these basics should be well understood before learning about specific clinical trials and related outcomes. Further, knowledge of current hemophilia gene therapy clinical trials is lacking, including the specific patient populations eligible for enrollment, efficacy and safety outcomes, recent results, and ultimately the implications of results for clinical practice. Along with improved understanding of hemophilia gene therapy clinical trials and results, it will be important for the hemophilia care community to also be knowledgeable about outstanding questions and unknowns that require more data. The top barriers or concerns related to gene therapy for hemophilia identified by physician respondents directly involved in the care of people with hemophilia included long‐term safety and monitoring, durability of expression/response, and challenges associated with use in specific populations such as children or patients with preexisting immunity to AAV. Over 50% of survey respondents in the current study recognized their own need for education on clinical trial results for people with hemophilia, on patient eligibility for AAV‐directed gene therapy for hemophilia, and safety considerations related to gene therapy for hemophilia.
In light of the rapid advances occurring with clinical trials of gene therapy for hemophilia, there is an important need for education so that the hemophilia care team is prepared for the potential integration of gene therapy into the treatment armamentarium for people with hemophilia. Fifty‐nine percent of overall survey respondents indicated a need to learn how to educate patients about gene therapy for hemophilia. Evidence‐based education can help to address misinformation and potential preexisting biases so that people with hemophilia get fair and balanced information about gene therapy from health care providers.
Limitations of the current study include the short time frame for participation in the survey and sample size. Survey participants were not required to answer all questions (thus, the sample size is not consistent across all questions), nor provide an explanation if they chose to skip a particular question. Survey participants may have a particular interest in gene therapy, representing selection bias that should be considered when interpreting the study results. The survey was available only in English, representing an additional limitation and potential for selection bias. For the above reasons, the results may not be generalizable to all professional roles, practice settings, and geographic locations.
Providing education to address hemophilia gene therapy knowledge gaps is not without challenges. There is a “culture of excellence” within the medical profession, in which learners may be embarrassed to admit deficiencies in knowledge or competence and may gravitate toward topics and material with which they are already familiar.10 Limitations of time, distance/travel, and varied learning styles represent additional challenges in meeting individual learning needs. In the current study, respondents with little to no understanding of gene therapy as well as those who self‐identified as “experts” recognized educational needs related to hemophilia gene therapy; therefore, a “one‐size‐fits‐all” approach will not meet the range of educational needs. The results of this study have been used, among other inputs, for the development of a multiphased educational road map by the ISTH Gene Therapy for Hemophilia Steering Committee entitled “Gene Therapy in Hemophilia: An ISTH Education Initiative” (https://genetherapy.isth.org/). Incorporating a variety of educational formats and resources, this dynamic platform was designed for the global hemophilia community to close existing knowledge gaps and to stay abreast of the evolving science and clinical advancements in gene therapy in hemophilia.
RELATIONSHIP DISCLOSURE
DL has received honoraria for academic consulting from Bioverativ, CSL Behring, and Octapharma Plasma. He has received research funding from Bayer, BioMarin, Bioverative, CSL‐Behring, and Octapharma Plasma. JM has held leadership positions with ISTH and WFH. He has served on the speakers’ bureau for Alnylam, Bayer, Biotest, Biogen, ISTH, Novo Nordisk, Pfizer, Sobi, Shire, Roche, and WFH. He has received research funding from Alnylam, Bayer, Biotest, Biogen, ISTH, Novo Nordisk, Pfizer, Sobi, Shire, Roche, and WFH. He has received honoraria from Amgen, Bayer, Biotest, Biogen, Baxalta, CSL‐ Behring, Catalyst Biosciences, Chugai, Freeline, LFB, Novo Nordisk, Roche, and Spark. KJP has received research funding from the BioMarin GeneR8 program, uniQure HOPE‐B program, and Sanofi–ATLAS fitusiran program. He has received honoraria from Alnylam, ApcinteX, BioMarin, Catalyst Bio, Chugai, Novo Nordisk, Octapharma Plasma, Pfizer, Roche, Sanofi, Shire, and Sobi. FP has held leadership positions with EHC, EMA, and EAHAD. She has served on the speakers’ bureau for Bioverativ, CSL‐Behring, Grifols, Novo Nordisk, Roche, Sanofi, Sobi, Spark, Sysmex, and Takeda. She is an advisory board member for Sanofi. GFP has received honoraria for academic consulting from BioMarin, Genentech/Roche, Pfizer, St. Jude, and VarmX. He has held leadership positions with Global Blood Therapeutics, NHF MASAC, and World Federation of Hemophilia. SWP has received honoraria for academic consulting from CSL‐Behring, Novo Nordisk, and Pfizer. He has held leadership positions with MASAC‐National Hemophilia Foundation. Dr Pipe has received research funding from Siemens and Shire. He has received honoraria from ApcinteX, Bayer, Biomarin, Bioverativ, Catalyst, CSL‐Behring, HEMA Biologics, Freeline, Novo Nordisk, Pfizer, Roche/Genentech, Sanofi, Shire, Spark, and uniQure. AS has received honoraria for academic consulting from Bayer, Novo Nordisk, Roche, and Shire/Takeda. He has held leadership positions as Chair of Gene Therapy Task Force of SSC of the ISTH and Chair of the Steering Committee of the Asia Pacific Hemophilia Working Group. He has received research funding from Alnylam, Bayer, and Shire. TVD has held leadership positions with NHF and ISTH. He has received research funding from Pfizer and Takeda. He has received honoraria from Baxalta/Shire/Takeda, Bayer, Biotest, and Pfizer. CM and WS report nothing to disclose.
AUTHOR CONTRIBUTIONS
All authors participated in discussions that resulted in the development of this survey and contributed to the analysis and interpretation of the work. WS developed an initial draft of the manuscript; all authors revised the manuscript and provided approval for the final submission.
Supporting information
Table S1
Peyvandi F, Lillicrap D, Mahlangu J, et al. Hemophilia gene therapy knowledge and perceptions: Results of an international survey. Res Pract Thromb Haemost. 2020;4:644–651. 10.1002/rth2.12326
Handling Editor: Cihan Ay.
Funding information
Supported in part by grants from BioMarin and Shire.
Contributor Information
David Lillicrap, Email: david.lillicrap@queensu.ca, @DavidLillicrap.
Claire McLintock, @doctormclintock.
References
- 1. Mahlangu J, Cerquiera M, Srivastava A. Emerging therapies for haemophilia‐global perspective. Haemophilia. 2018;24(S6):15–21. [DOI] [PubMed] [Google Scholar]
- 2. Pipe SW. Gene therapy for hemophilia. Pediatr Blood Cancer. 2018;65:e26865. [DOI] [PubMed] [Google Scholar]
- 3. Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, et al. Adenovirus‐associated virus vector‐mediated gene transfer in hemophilia B. N Engl J Med. 2011;365:2357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nathwani AC, Reiss UM, Tuddenham EG, Rosales C, Chowdary P, McIntosh J, et al. Long‐term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med. 2014;371:1994–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. George LA, Sullivan SK, Giermasz A, Rasko JEJ, Samelson‐Jones BJ, Ducore J, et al. Hemophilia B gene therapy with a high‐specific‐activity factor IX variant. N Engl J Med. 2017;377:2215–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rangarajan S, Walsh L, Lester W, Perry D, Madan B, Laffan M, et al. AAV5–factor VIII gene transfer in severe hemophilia A. N Engl J Med. 2017;377(26):2519–30. [DOI] [PubMed] [Google Scholar]
- 7. Miesbach W, Meijer K, Coppens M, Kampmann P, Klamroth R, Schutgens R, et al. Gene therapy with adeno‐associated virus vector 5‐human factor IX in adults with hemophilia B. Blood. 2018;131:1022–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pierce GF, Coffin D, Members of the WFH Gene Therapy Round Table Program Committee and Organizing Committee . The 1st WFH gene therapy round table: understanding the landscape and challenges of gene therapy for haemophilia around the world. Haemophilia. 2019;1–6. [DOI] [PubMed] [Google Scholar]
- 9. Hurst S, Warren C, Pasi KJ. Gene therapy in hemophilia: an assessment of hematologists’ knowledge gaps and attitudes. Blood. 2018;132:3485. [Google Scholar]
- 10. Dionyssopoulos A, Karalis T, Panitsides EA. Continuing medical education revisited: theoretical assumptions and practical implications: a qualitative study. BMC Med Ed. 2014;14:1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


