Abstract
Hyperammonemia syndrome, with high levels of ammonia and neurologic dysfunction, is a syndrome with historically high mortality that may occur after solid organ transplantation. Recently this has been associated with infection due to Ureaplasma, mostly following lung transplantation. We describe the first case of hyperammonemia syndrome due to Ureaplasma infection after liver-kidney transplantation. Our patient rapidly recovered after specific antibiotic treatment. It is important to consider these infections in the differential diagnosis for encephalopathy post-transplant, as these organisms often do not grow using routine culture methods and polymerase chain reaction testing is typically required for their detection. This is particularly critical after liver transplantation, where a number of other etiologies may be considered as a cause of hyperammonemia syndrome.
Keywords: Ureaplasma, hyperammonemia, liver transplantation
1. INTRODUCTION
Hyperammonemia syndrome (HS), characterized by markedly elevated serum ammonia levels and acute, progressive neurologic dysfunction, was discovered as a potential complication of hematopoietic cell transplant as early as 1991.1 Since that time several reports have described a similar phenomenon after solid organ transplantation, primarily following lung transplant,2-4 but also after renal transplant5-6 and rarely heart transplant.2,7 In the past, this syndrome has been difficult to treat, with high mortality rates.3 It is now understood that bacteria from the genera Ureaplasma are implicated as organisms associated with this syndrome.4, 8-9 However, making this diagnosis after liver transplant is more challenging, as hyperammonemia following liver transplantation has many other causes, including portal vascular abnormalities (especially thrombosis),10 inborn errors of metabolism,11 or dysfunction of the liver allograft. We present the first reported case of HS due to Ureaplasma infection in a liver-kidney transplant patient.
2. CASE REPORT
A 53-year-old woman with cirrhosis due to chronic hepatitis B virus (HBV) infection and end stage renal disease due to type 2 diabetes mellitus requiring hemodialysis (HD) underwent a simultaneous liver-kidney transplant from a single deceased standard risk donor. The donor received a single dose of levofloxacin prior to organ procurement. Cultures of donor blood and urine were negative, as were cultures of transport fluid. Peri-operative antibiotics included ceftriaxone and fluconazole. Induction immunosuppression was comprised of basiliximab and methylprednisolone; maintenance immunosuppression consisted of mycophenolate, prednisone and tacrolimus. Subsequent infection prophylaxis included trimethoprim-sulfamethoxazole, ganciclovir and fluconazole. She received HBV immunoglobulin for 7 days postoperatively and was continued on tenofovir and entecavir for active, pre-operative viremia. Her liver function began to normalize within the first post-operative day (POD). However, her kidney transplant suffered significant delayed graft function, eventually requiring HD beginning on POD 9. Biopsy of the renal allograft on POD 12 demonstrated acute tubular necrosis without features of rejection. A computerized tomography (CT) scan without contrast of the abdomen and pelvis on POD 12 revealed large, complex fluid collections around the liver and kidney grafts. Bacterial and fungal cultures of fluid aspirated from the collections on POD 13 were negative.
Repeat CT on POD 22 showed enlarging hematomas around both allografts with mass effect on the kidney, a prominent density along the left segmental portal vein consistent with a hematoma, and a pseudoaneurysm of the recipient hepatic artery (Figure 1). No hepatic vein thrombosis was detected and apart from her prior large splenorenal collaterals, there were no features present to suggest significant portocaval or portosystemic shunting. An angiogram of the hepatic vessels was performed to rule out other sources of bleeding or vascular stenoses and the pseudoaneurysm was stented. On POD 23 her mental status rapidly deteriorated. Neurologic exam revealed non-reactive pupils, a left gaze preference, no withdrawal or localization to pain, unintelligible groaning and spontaneous antigravity movement in the upper extremities only. Electrolytes, blood gas analysis, international normalized ratio, lactate, and hepatic function testing were all normal. Liver duplex ultrasound showed no portal vein thrombosis. Tacrolimus was held (serum level, 8.3 ng/mL) out of concern for neurotoxicity. Magnetic resonance imaging of the brain was normal. Serum ammonia then returned markedly elevated at 278 mcg/dL (normal <65 mcg/dL) and remained persistently elevated with ongoing neurologic dysfunction despite appropriate HD and administration of lactulose and rifaximin.
Figure 1.
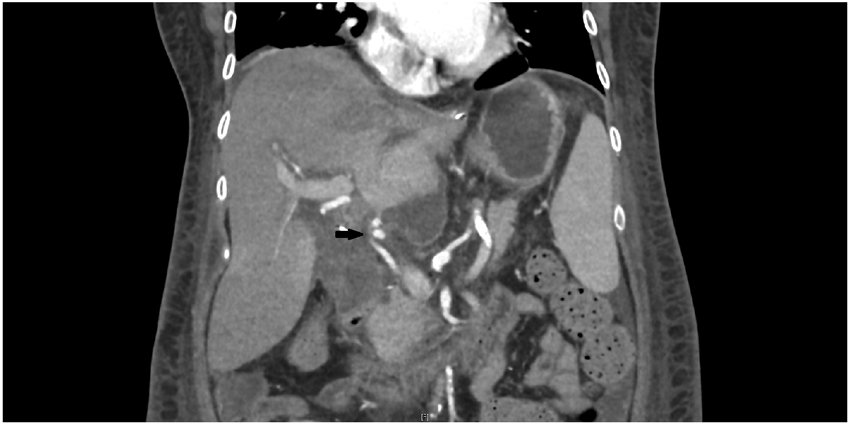
CT on POD 22 demonstrating persistent large fluid collections around the liver and a pseudoaneurysm of the hepatic artery (arrow).
The persistent perihepatic fluid collection was again aspirated on POD 23 and she was started on empiric vancomycin, piperacillin-tazobactam and micafungin. After infectious disease consultation empiric antimicrobials were changed to doxycycline and levofloxacin on POD 25 due to concern for infectious causes of hyperammonemia. Within 24 hours, her neurologic exam began to normalize, and by 2 days later (POD 27) she was completely oriented with resolution of her neurologic dysfunction. Concomitantly her serum ammonia level fell precipitously to 44 mcg/dL by POD 27 and remained normal thereafter. Polymerase chain reaction (PCR) testing on the aspirated perihepatic fluid was positive for Ureaplasma parvum or U. urealyticum DNA. The University of Washington Molecular Microbiology laboratory employs a multiplexed lab-developed PCR assay that detects and identifies Mycoplasma spp. and Ureaplasma spp., with a lower limit of detection of 4 genomes per PCR reaction, but does not distinguish between U. parvum and U. urealyticum. Urine organic acid and plasma amino acid analyses were inconsistent with a urea cycle disorder.
On POD 27, she developed hypotension with decreasing hematocrit, prompting an urgent abdominal exploration. The hepatic artery appeared grossly normal with an intact, nonbleeding anastomosis. Due to the infected perihepatic fluid collection and presumed stent involvement, the stent was removed and the pseudoaneurysm was resected with primary reconstruction of the artery. Samples from the pseudoaneurysm sac, perihepatic and perirenal collections, and the stent itself ultimately tested positive for Ureaplasma by PCR. Both levofloxacin and doxycycline were continued for a total of 6 weeks to treat endovascular infection and peritonitis. She completed antibiotic therapy without issue, had a normal neurologic exam and was doing well when seen in outpatient follow-up one year later. Recipients of the lungs, heart, or other kidney from the same donor did not develop any evidence of hyperammonemia or Ureaplasma infection.
3. DISCUSSION
The development of hyperammonemia syndrome after liver transplantation can be a challenging clinical problem. There are several potential etiologies, including primary dysfunction of the new liver, portal vein thrombosis, portosystemic shunting, or a urea cycle disorder in the donor liver. In our patient, the liver was otherwise functioning well, there was no evidence for shunt or portal vein thrombosis, and testing for urea cycle disorders was negative. Instead, she was found to have abdominal infection with Ureaplasma, involving collections around the liver, kidney and hepatic artery, with PCR tests positive for the same organism from multiple samples over time. Antimicrobial therapy for Ureaplasma infection resulted in rapid normalization of both her ammonia level and mental status, supporting the link between hyperammonemia syndrome and Ureaplasma infection.
Post-transplant hyperammonemia syndrome is a rare condition most commonly observed in the lung transplant population.2-4,9,12-13 It is characterized by elevated ammonia levels in the absence of synthetic liver dysfunction, with altered mental status and often cerebral edema.3 Historically, the condition has been highly fatal, with mortality rates of 67-75%, and refractory to typical therapies for hyperammonemia such as dialysis, bowel decontamination, and treatment with nitrogen scavengers.3 Until recently the underlying etiology of hyperammonemia syndrome in the post-transplant population remained unknown. However, the link between HE and Mycoplasma infection was first suggested in 2013,14 when a lung transplant recipient died from HE and was found to have disseminated infection with M. hominis. Subsequently, hyperammonemia syndrome was linked to disseminated Ureaplasma infection in post-lung transplant patients,4 and it was demonstrated that by hydrolyzing urea to generate adenosine triphosphate (ATP) U. urealyticum and U. parvum caused elevated plasma ammonia levels in infected, immunocompromised mice.8 Interestingly, subsequent analysis of the first patient identified with M. hominis infection and hyperammonemia14 showed that Ureaplasma was also present, raising the question of whether M. hominis truly played a role in the development of hyperammonemia or not. 4
Identifying Ureaplasma in clinical specimens does not always indicate infection, as these organisms may be present as part of the normal flora of the genitourinary tract or oral cavity. Furthermore, their presence does not always lead to hyperammonemia, as post-operative infections with these organisms have been described in thoracic9 or kidney transplantation15 without associated hyperammonemia or encephalopathy. Why hyperammonemia develops in some situations and not others is unknown. In addition, whether these post-transplant infections are donor- or recipient-derived is not always clear. In the lung transplant population, studies have suggested a donor origin for most, if not all, Ureaplasma infections causing hyperammonemia.12 The pathogenesis of infection in our patient is not obvious. Factors favoring potential recipient origin include the use of pre-procurement levofloxacin and the lack of transmission with other organs, as all of the other organ recipients from the same donor, including the lung and other kidney, did not manifest any evidence of hyperammonemia or Ureaplasma infection. However, a single pre-procurement dose of levofloxacin may not have been enough to eradicate Ureaplasma, and the finding of coexistent infection around both the transplanted liver and kidney suggest potential donor origin.
The diagnosis of Ureaplasma infections can be difficult. They lack a cell wall and thus cannot be identified by Gram stain, and they are challenging to grow with standard bacterial culture methods. Diagnosis often requires the use of molecular methods, and clinicians must consider specifically ordering PCR testing for these organisms. In addition, antimicrobial susceptibility testing is not widely available and optimal treatment is often not well defined. Antimicrobial agents that act on the cell wall, such as beta-lactams and vancomycin, have no activity against these infections. Tetracyclines, fluoroquinolones and macrolides all may have activity against certain Ureaplasma species. However, resistance patterns are variable and have changed over time.16-17 Studies suggest that most Ureaplasma isolates remain susceptible to tetracyclines, while 5.2-6.4% are resistant to levofloxacin and up to 68.8% of isolates are resistant to ciprofloxacin.16 Given the variability in resistance patterns and challenges associated with obtaining susceptibilities, our preference is for combination therapy with doxycycline and levofloxacin for most severe infections due to Ureaplasma species. Surgical exploration and drainage should also be considered for management of infected collections and foreign bodies.
In conclusion, we describe the first reported case of hyperammonemia syndrome due to Ureaplasma infection after liver-kidney transplant and its successful treatment. In one prior report, hyperammonemia syndrome occurred after liver transplant, and U. urealyticum and M. hominis infection was also present, but the connection between those two entities was not appreciated.18 Our case highlights the importance of considering Ureaplasma infections in all post-transplant patients with elevated ammonia levels and altered mental status, regardless of the transplanted organ, and specifically testing for them by PCR. This is particularly critical after liver transplantation, where multiple other potential etiologies for hyperammonemia should be considered. These are readily treatable infections with resultant rapid improvement in ammonia levels and mental status, as was seen in our patient. As such, empiric specific antimicrobial treatment should be strongly considered for post-transplant patients with hyperammonemia syndrome. Historically high mortality rates due to HS should be expected to decrease dramatically with better recognition and treatment of this entity. While some institutions have implemented protocols to universally screen lung transplant donors and recipients for Ureaplasma infection,12 further studies are needed to determine the best practices for diagnosis, treatment and prevention of hyperammonemia syndrome in all transplant recipients.
ACKNOWLEDGMENTS/FUNDING
C.A.C. was supported in part by an institutional training grant (T32 AI007140-41) from the National Institute of Allergy and Infectious Diseases. M.A.C. was supported in part by an institutional training grant (T32 DK007742-22) from the National Institute of Diabetes and Digestive and Kidney Diseases. S.G. was supported in part by an institutional training grant (T32 HL007287) from the National Heart, Lung, and Blood Institute.
Abbreviations
- ATP
adenosine triphosphate
- CT
computerized tomography
- HBV
hepatitis B virus
- HD
hemodialysis
- HS
hyperammonemia syndrome
- PCR
polymerase chain reaction
- POD
, post-operative day
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose.
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1.Tse N, Cederbaum S, Glaspy JA. Hyperammonemia following allogeneic bone marrow transplantation. Am J Hematol. 1991;38(2):140–1. [DOI] [PubMed] [Google Scholar]
- 2.Krutsinger D, Pezzulo A, Blevins AE, et al. Idiopathic hyperammonemia after solid organ transplantation: Primarily a lung problem? A single-center experience and systematic review. Clin Transplant. 2017;31(5):e12957. [DOI] [PubMed] [Google Scholar]
- 3.Chen C, Bain KB, Iuppa JA, et al. Hyperammonemia syndrome after lung transplantation: a single center experience. Transplantation. 2016;100(3):678–84. [DOI] [PubMed] [Google Scholar]
- 4.Bharat A, Cunningham SA, Budinger GRS, et al. Disseminated Ureaplasma infection as a cause of fatal hyperammonemia in humans. Sci Transl Med. 2015;7(284):284re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiberenge RK, Lam H. Fatal hyperammonemia after repeat renal transplantation. J Clin Anesth. 2015;27(2):164–7. [DOI] [PubMed] [Google Scholar]
- 6.Li GZ, Tio MC, Pak LM, et al. Noncirrhotic hyperammonemia after deceased donor kidney transplantation: a case report. Am J Transplant. 2019;19:3197–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madathil RJ, Gilstrap LG, Pelletier MC, et al. Isolated hyperammonemic encephalopathy in heart transplantation. J Heart Lung Transplant. 2018;37(3):427–29. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Greenwood-Quaintance KE, Karau MJ, et al. Ureaplasma parvum causes hyperammonemia in a pharmacologically immunocompromised murine model. Eur J Clin Microbiol Infect Dis. 2017;36(3):517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker AW, Messina JA, Maziarz EK, et al. Epidemiology of invasive Mycoplasma and Ureaplasma infections early after lung transplantation. Open Forum Infect Dis. 2019;6 (Suppl 2): S646. [Google Scholar]
- 10.Belenky A, Igov I, Konstantino Y, et al. Endovascular diagnosis and intervention in patients with isolated hyperammonemia, with or without ascites, after liver transplantation. J Vasc Interv Radiol. 2009;20:259–263. [DOI] [PubMed] [Google Scholar]
- 11.Ramanathan M, Uppalapu S, Patel NM. Hiding in plain sight: a case of ornithine carbamylase deficiency unmasked post-liver transplantation. Am J Transplant. 2017;17:1405–1408. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez R, Chi M, Ison MG, et al. Sequelae of donor-derived mollicutes transmission in lung recipients. Am J Respir Crit Care Med. 2017;195(5):687–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matson KM, Sonetti DA. Successful treatment of Ureaplasma-induced hyperammonemia syndrome post-lung transplant. Transpl Infect Dis. 2018;e13022. [DOI] [PubMed] [Google Scholar]
- 14.Wylam ME, Kennedy CC, Hernandez NM, et al. Fatal hyperammonaemia caused by Mycoplasma hominis. Lancet. 2013;382(9908):1956. [DOI] [PubMed] [Google Scholar]
- 15.Ekiel AM, Pietrzak B, Kamiński P, et al. Prevalence of urogenital Mycoplasmas and Ureaplasmas in women after kidney transplantation. Transplantation. 2009;87(6):848–51. [DOI] [PubMed] [Google Scholar]
- 16.Fernández J, Karau MJ, Cunningham SA, et al. Antimicrobial susceptibility and clonality of clinical Ureaplasma isolates in the United States. Antimicrob Agents Chemother. 2016;60(8):4793–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krausse R, Schubert S. In-vitro activities of tetracyclines, macrolides, fluoroquinolones and clindamycin against Mycoplasma hominis and Ureaplasma ssp. isolated in Germany over 20 years. Clin Microbiol Infect. 2010;16(11):1649–55. [DOI] [PubMed] [Google Scholar]
- 18.Haller M, Forst H, Ruckdeschel G, et al. Peritonitis due to Mycoplasma hominis and Ureaplasma urealyticum in a liver transplant recipient. Eur J Clin Microbiol Infect Dis. 1991;10:172. [DOI] [PubMed] [Google Scholar]


