Abstract
Polycystic ovary syndrome (PCOS) is one of the most common causes of subfertility, and it is characterized by hormonal dysregulation like insulin resistance. Various measures have been taken in the past to overcome this insulin resistance to improve fertility treatment outcomes. The current paper aims to review and compare the existing studies and literature to assess the impact of myo-inositol (MI) on oocyte and embryo quality in assisted reproductive technology (ARTs). We thoroughly searched the PubMed and Google Scholar databases by using the keywords “PCOS, polycystic ovarian syndrome, inositol, oocyte quality, embryo quality, assisted conception, ART, IVF, and in vitro fertilization.” Nine articles were finalized for review in this paper. Many of the reviewed studies have shown a trend toward the improvement of embryo quality in women with PCOS after MI supplementation; however, there is a lack of statistically significant evidence to support the use of MI in enhancing the quality of oocyte and/or embryo. Clear evidence regarding the role of MI in enhancing the quality of oocyte and embryo in PCOS is limited. A well-controlled, large, randomized controlled trial is required to definitively accept or refute its role.
Keywords: pcos, polycystic ovary syndrom, inositol, oocyte quality, embryo quality, ivf, art, assisted conception, invitrofertilisation
Introduction and background
Polycystic ovary syndrome (PCOS) is a common reproductive condition associated with chronic anovulation; it commonly manifests as oligomenorrhea, irregular menstrual cycle, and androgen excess, with typical ovarian ultrasound features [1]. It is the most prevalent cause of disorder of ovulation and subfertility in females and affects approximately 6-10% of childbearing women population [2]. Although its pathogenesis is poorly understood, the role of insulin in the pathogenesis of hyperandrogenemia in PCOS is central. Insulin resistance in association with luteinizing hormone (LH) increases the production of androgen in theca cells [3]. Therefore, treatment with an insulin-sensitizing agent like inositol, troglitazone, or metformin in women with PCOS may lead to the resumption of spontaneous ovulation [4-8].
Many recent studies have shifted interest toward the two main inositol stereoisomers out of the nine isomers of the inositol family, namely myo-inositol (MI) and D-chiro-inositol (DCI). This inositol complex acts as a second messenger of insulin signaling. Both of these isomers have insulin-like action and have therefore been claimed to improve various menstrual and hormonal parameters in PCOS. Studies on DCI has shown its ability to improve the chances of ovulation and reduction of androgen production in women with PCOS.
PCOS cannot be merely considered as a local ovarian dysfunction, but it is the expression of a complex functional alteration of the whole reproductive system [4,5]. Various randomized and nonrandomized cohort studies have shown that inositol complex (MI and DCI) improves menstrual irregularities and ovarian activity in women with PCOS [8,9]. The benefit of MI supplementation in infertility treatment is due to its ability to increase the intracellular calcium ion oscillation. It has been shown that the follicular fluid of oocytes containing a higher concentration of MI is of better quality in humans [9]. This has also been demonstrated in mouse germinal vesical oocytes by improved meiotic progression [10]. Additionally, it has been stated that the mechanism of inositol could prove beneficial in ways other than its action on the reduction of insulin resistance. For instance, it has been shown that MI is important for follicle-stimulating hormone (FSH) signaling and, therefore, for oocyte maturation and embryo development. The international consensus conference has stated that pretreatment with inositol(s) supplementation could improve oocyte quality and obstetric outcome for in vitro fertilization (IVF) patients [4,11].
In this paper, we aimed to review the role of inositol complex on oocyte and embryo quality in women with PCOS who had undergone various assisted reproductive technology (ART) treatments.
Review
Methods
We systematically searched Pubmed and Google Scholar databases for relevant published articles to find studies assessing the effect of inositol complex supplementation and its effect on oocyte quality in IVF. Keywords we used for the search include “PCOS, polycystic ovarian syndrome, inositol(s), oocyte quality, embryo quality, in vitro fertilization, assisted conception, IVF, and ART.” There was no restriction regarding the time period in our search for the articles. A summary of the outcomes of the search is as follows: a) the keyword “polycystic ovarian syndrome” returned 16,263 peer-reviewed articles; b) “PCOS” yielded 14,659 peer-reviewed articles; c) 185 articles were returned for the combined keywords “PCOS and inositol”; d) 21 articles were found with combined keywords “PCOS, inositol, and in vitro fertilization”; e) when the combined keywords “PCOS, inositol, in vitro, and oocyte quality” were searched, 17 articles were listed. Nine articles were ultimately finalized for review. In this study, systematic review and meta-analysis of randomized controlled trials in which inositol supplementation was used in PCOS cases that underwent ART cycles are included, along with medical hypotheses, observational studies, and prospective trials.
Discussion and results
PCOS and Insulin Resistance
PCOS is a disorder characterized by insulin resistance and hyperinsulinemia. These features occur in both obese and nonobese women. Insulin resistance and hyperinsulinemia are considered to play an important role in the pathogenesis of hyperandrogenic production, ovulatory dysfunction, and various factors of metabolic syndrome in PCOS [2]. These are found in up to 75% of lean PCOS and around 95% of obese PCOS women [12]. The relationship between hyperinsulinemia and hyperandrogenism in polycystic ovarian disease has been identified by Burghen et al. [13]. In PCOS, insulin resistance leads to compensatory hyperinsulinemia. The reduced level of sex hormone-binding globulin and excess ovarian androgen production in PCOS women are the result of compensatory hyperinsulinemia. It plays a prominent role in the pathogenesis of various metabolic syndromes and anovulatory cycle [14,15]. Various actions have been recommended to overcome this insulin resistance as a first-line intervention, such as physical exercise, dietary and lifestyle modification, and insulin sensitizers. However, they are usually unable to overcome insulin resistance, and further interventions are often required [12,16,17].
Inadequate insulin action could be because of the deficiency of DCI. DCI is a component of inositol phosphoglycans (IPGs), which are the second messengers in the insulin pathway. MI, an insulin-sensitizing agent, helps in the restoration of ovulation and on oocyte meiotic maturation. Inositol affects the process of steroidogenesis and reduces the production of androgen from theca cell and decreases the serum concentration of testosterone [4,6,18,19].
Myo-Inositol and Its Role in Insulin Resistance
In 1850, Johann Joseph von Scherer discovered a new compound from a muscle cell and called it inositol, which was coined by combining various Greek words [20,21]. Inositol belongs to the vitamin B complex family. The chemical formula of inositol substance is C6H12O6OR (-CHOH)6. The food items that naturally contain the highest concentration of inositols are fruits, beans, corn, and nuts, indicating that the inositol is widely available in nature [22]. Nine stereoisomers of the inositol family are currently known, and MI is the most common isomer available; DCI is the second most common form. These isomers are formed by the epimerization of six hydroxyl groups of inositol, and MI and DCI are used in the treatment of PCOS as insulin-sensitizing agents [4,11,23-25]. Inositol was formerly known as “myometrial sugar,” although it is not a member of the carbohydrate family. Indeed, the direct involvement of the inositol molecule in insulin signaling has been proven in various studies.
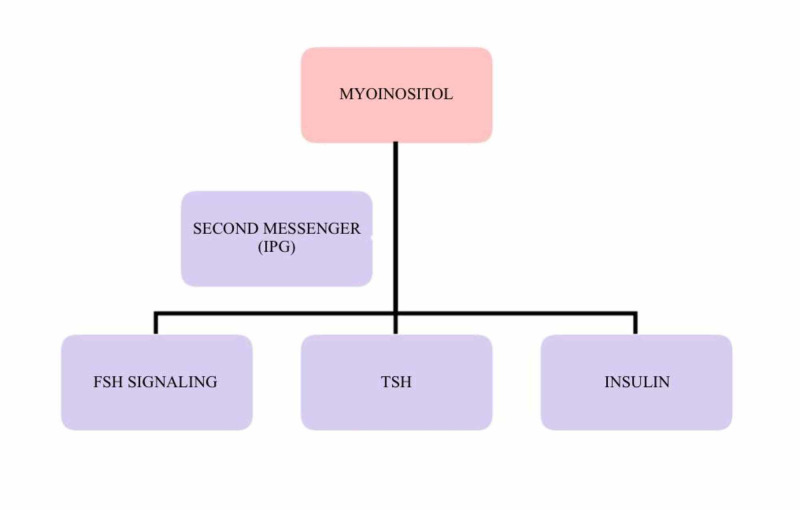
Inositol is involved in regulating a multitude of hormonal signals and metabolic pathways in human beings [25,26]. Over the last few decades, various studies have emphasized the “insulin-sensitizing properties” of inositols. Both MI and DCI appear to be capable of activating the main enzymes involved in the metabolism and uptake of glucose [26-28]. MI is converted into DCI by the activity of the epimerase enzyme [29]. IPGs are the second messengers of insulin. MI and DCI are incorporated intracellularly into IPG, and these IPG mediators mediate some of the actions of the insulin. Phosphatidyl-myo-inositol is the phosphatidyl-insphosphate precursor, and its hydrolysis results in inositol triphosphate. This acts as a second messenger in the regulation of various hormone activities such as those of thyroid-stimulating hormone (TSH), FSH, and insulin. It also helps in improving their signals, as shown in Figure 1 [30,31].
Figure 1. Role of myo-inositol as a second messenger.
IPG: inositol phosphoglycan; FSH: follicle-stimulating hormone; TSH: thyroid-stimulating hormone
It has been evident from various studies that defect in IPGs' second messenger results in insulin pathway impairment [32,33]. This is because IPGs play a role in the activation of enzymes that control the metabolism of glucose [34,35]. Insulin resistance in women with PCOS is due to the defect in either tissue availability or altered metabolism of IPGs' mediator or inositol. Various studies have shown that MI is capable of restoring spontaneous ovarian activity in women with PCOS and, therefore, fertility in many of these cases [25].
PCOS, Oocyte Quality, and Inositol
One of the main challenges in ART is the difficulty in obtaining good-quality oocyte and embryos. Various studies have been conducted to find out the factors that can predict IVF outcomes. The most common reason for unsuccessful IVF-embryo transfer procedures is insufficient oocyte and embryo quality. Other then this, several other reasons like social and environmental factors, aging, and other pathologies can also negatively affect the outcome of the procedure.
In women with PCOS, the oocytes retrieved are often found to be of poor quality [36]. Interestingly, many of these women with PCOS need ARTs to achieve pregnancy. However, over two-thirds of ART cycles result in adverse pregnancy outcomes. This is primarily attributed to the failure in fertilization due to poor oocyte quality [37]. Any treatment capable of improving the quality of the oocyte could, therefore, be considered as a “crowning achievement” for ART procedures. This is the reason why IVF techniques now focus mainly on getting a better quality of oocytes rather than higher numbers of eggs and embryos [38]. PCOS is one of the most common ovulatory disorders, and it is characterized by hormonal dysfunction. It is commonly seen that a higher proportion of women with PCOS have hyperinsulinemia and insulin resistance. Therefore, insulin sensitizers are used to counteract the above-mentioned hormonal signs due to the pathophysiological link between insulin resistance and PCOS aberrations.
MI has been found to be essential for the proper maturation of oocytes, and its higher concentration in human follicular fluid is considered as a marker of high oocyte quality [10,19]. Studies have shown that MI supplementation is positively correlated with the meiotic progression of germinal vesicle oocytes of the mouse by increasing intracellular Ca2+ oscillation [10]. It is also important to note that the ovary maintains normal sensitivity to insulin despite systematic insulin resistance [36]. In women with PCOS, an increase in insulin leads to the stimulation of ovarian epimerase activity. This increased epimerase activity results in a higher level of DCI and a lower level of MI in a follicular fluid, which is called “the ovarian paradox” [39]. As MI is involved in FSH signaling, its depletion in follicular fluid in PCOS could lead to impaired FSH signaling [18,40]. The studies included in this review are summarized in Table 1.
Table 1. Studies included in the review.
MI: myo-inositol; PCOS: polycystic ovary syndrome; IVF: in vitro fertilization; DCI: D-chiro-inositol; ICSI: intracytoplasmic sperm injection; ET: embryo transfer
| No. | Journal | Author | Type of study | year | Aim of the study | Patient population (if applicable) | Conclusions |
| 1 | International Journal of Endocrinology | Lesoine B et al. [41] | Original article | 2016 | To find out if the MI + folic acid combination was able to improve the quality of oocyte, the ratio between follicles and retrieved oocytes, the fertilization rate, and embryo quality | 29 patients with PCOS | PCOS women with MI supplementation showed better fertilization rates and improved embryo quality |
| 2 | European Review for Medical and Pharmacological Sciences | Ciotta et al. [42] | Original article | 2011 | To determine the effects of MI on oocytes quality in women with PCOS | 34 patients with PCOS | A higher number of oocytes were retrieved in the MI group. The number of immature oocytes was also less in the inositol group |
| 3 | Hormone Molecular Biology and Clinical Investigation | Regidor et al. [43] | Original article | 2018 | The second part of the trial aimed to investigate the oocyte quality, the ratio between follicles and retrieved oocytes, fertilization rate, and embryo quality in PCOS women undergoing IVF treatment | 29 patients with PCOS | The placebo group showed a higher number of retrieved oocytes. MI and folic acid group (control) showed a better follicle/retrieved oocyte ratio, more metaphase II oocytes, and more grade 1 quality of the embryo. The control group also showed a nonsignificant increase in fertilization rate |
| 4 | European Review for Medical and Pharmacological Sciences | Unfer et al. [44] | Original article | 2011 | To compare the effect of MI and DCI on oocyte quality in euglycemic PCOS women | The study showed that there was no difference in the number of oocytes collected in two groups. However, the number of immature oocytes was less in the MI group along with an increase in the number of mature (metaphase II) oocyte, compared to DCI. Moreover, the number of top quality embryo and pregnancy rate was also higher in the MI group | |
| 5 | International Journal of Endocrinology | Unfer et al. [45] | Review article | 2016 | To investigate the role of MIl and DCI in physiological involvement in PCOS and potential therapeutic use with assisted reproductive technologies | Inositol may have a role in improving hormonal and reproductive disturbance in PCOS women. It may also have a role in oocyte follicular development and oocyte maturation | |
| 6 | Fertility and Sterility | Papaleo et al. [11] | Original article | 2009 | To determine the effects of oocyte quality in PCOS women undergoing ICSI | 60 patients with PCOS | Total gonadotrophin required for stimulation was less in the MI group. However, the number of oocytes retrieved was not significantly different between the two groups. The mean number of degenerated vesicles and degenerated oocyte was less in the inositol group with the trend toward an increase in metaphase II oocytes |
| 7 | Reproductive BioMedicine Online | Mendoza et al. [46] | Review article | 2017 | To assess the effectiveness of MI and MI in improving oocyte or embryo quality and pregnancy rates for women with PCOS undergoing ICSI | MI was insufficient to improve the oocyte quality and embryo quality | |
| 8 | Archives of Gynecology and Obstetrics | Laganà et al. [47] | Review article | 2018 | To evaluate whether oral MI supplementation is able to reduce the amount of gonadotropins and the length of controlled ovarian hyperstimulation in both PCOS and non-PCOS women undergoing IVF | In MI vs no intervention group, there was no difference in the number of oocytes collected and mature oocytes. In MI vs DCI, the number of mature oocytes was significantly higher in the MI group; however, there was no difference in total oocyte retrieved between the two groups | |
| 9 | Medicine | Zheng et al. [48] | Review article | 2017 | To find out the effectiveness of inositol in IVF-ET, ovulation induction in infertile women | As a secondary outcome, the MI group had more number of grade 1 oocyte and less number of the germinal vesicles and degenerated oocytes. There was no difference in the number of oocytes collected between the two groups |
As substantiated by the above data, the role of MI and DCI supplementation in improving oocyte and embryo quality in women with PCOS undergoing IVF has been investigated in the past in various studies. We aimed to review the association between inositol and its effect on oocyte or/and embryo quality in PCOS women who had undergone IVF and found some interesting results. The studies mentioned in Table 1 have been reviewed extensively, and we aimed to focus on oocyte and embryo quality results. Only IVF and intracytoplasmic injection (ICSI) cycles have been reviewed as our objective was to find out the effect of inositol complex on oocyte or embryo quality in IVF procedures. The number of metaphase II oocytes was taken as the marker for oocyte quality, and embryo quality was assessed based on the number of morphologically grade-one embryos.
The study by Lesione et al. was a prospective randomized study, and it aimed to find out the effect of MI and folic acid versus folic acid-only on oocyte quality, fertilization rate, embryo quality, and the ratio between follicles and retrieved oocytes in PCOS women undergoing IVF treatment [41]. This study revealed that a higher number of metaphase II and I are retrieved in MI and folic acid (MI + FA) group compared to the folic acid-only group. However, the results were not statistically significant. Regarding the number of good quality (grade one) embryo, there was a statistically significant (p<0.05) result in MI + FA group. Similarly, the fertilization rate was also significant in the MI + FA group. It was also noted that the number of oocytes retrieved was higher in the placebo (FA) group, and the ratio of follicle/retrieved oocyte was also lower in the test (MI + FA) group (p<0.05). The study concluded that MI supplementation resulted in a higher fertilization rate and, more importantly, a higher number of top-quality embryos and, therefore, has an overall effect on the quality of oocyte [41].
The study by Ciotta et al. showed that the number of oocytes retrieved was significantly higher in the MI group, and the number of immature oocytes (degenerated oocytes and germinal vesicles) was lower. However, there was no statistical significance in the number of metaphase II oocytes (though the trend was on the higher side) and fertilization rate. This study also revealed that the mean number of grade-one embryos available for transfer was also high in the inositol group (p<0.01). The authors concluded that MI has a role in oocyte maturation due to its insulin-sensitizing property [42].
It was demonstrated by Regidor et al. in their study that the ratio of follicle/retrieved oocyte was lower in the MI group (p<0.05); the fertilization rate was also statistically significant in the MI group [43]. Regarding the number of metaphase II oocyte, the trend favored the MI group, although it was not statistically significant, and the number of grade-one embryos was higher in the inositol group. This result was similar to the findings of Lesione et al. [41]. The authors suggested that MI has a positive role in maintaining the quality of the oocyte pool and increasing the fertilization rate [43].
Furthermore, Unfer et al. compared the effect of DCI and MI on oocyte quality in euglycemic PCOS women. They concluded that the number of mature oocytes and good-quality embryos was higher in the MI group compared to the DCI group [44]. These results support the hypothesis of the ovarian paradox effect of DCI [39]. Unfer et al., in another review, speculated that the inositol complex (MI and DCI) is a safe and effective treatment to increase follicular development and oocyte maturation [45]. However, the findings regarding the number of metaphase II oocyte and grade one embryo were not statistically significant.
In a study involving 60 women with PCOS, Papaleo et al. evaluated the effect of MI on the quality of oocyte in women undergoing ICSI. There was no difference in the number of oocytes retrieved between the two groups; however, there was a significant reduction in the number of degenerated oocytes and germinal vesicles, with a trend towards the increased percentage of metaphase II oocytes [11].
Conversely, two recent systematic reviews and metanalysis showed some different results. The review study by Mendoza et al. on the effect of inositol on women with PCOS undergoing ICSI argued that there is a lack of evidence to support the role of inositol complex in improving oocyte and embryo quality [46]. However, there was significant heterogeneity, and a small number of randomized control trials were included in the review. Another systematic review by Lagana et al. mentioned that MI improves the amount of gonadotrophin usage in the IVF cycle, although it shows no improvement in the total number of oocyte or mature oocytes. The improvement in the usage of gonadotrophins can be explained by the theory that inositol has a role in FSH signaling. However, there was heterogeneity among the studies included, and the quality of embryo was not assessed in all the studies [47]. Interestingly, another review by Zheng et al. stated that MI has a role in improving the number of good-quality embryos and reducing the number of germinal vesicles and degenerated oocytes. However, both PCOS and non-PCOS trials were included in their study [48].
Conclusions
In this article, after reviewing the relevant literature, we can conclude that MI, an insulin-sensitizing agent, has a role in improving the quality of embryos in women with PCOS who undergo various ART procedures. However, the evidence is still not very clear for us to unequivocally recommend it for the improvement of oocyte and embryo quality. The quality of oocyte and, therefore, the embryo is one of the main rate-limiting and stressful factors associated with IVF success in PCOS cases. A large, multicentric, randomized controlled trial is therefore required before we can categorically accept or refute the role of MI in the betterment of oocyte and embryo quality in women with PCOS.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.Polycystic ovary syndrome. Ehrmann DA. N Engl J Med. 2005;352:1223–1236. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 2.Polycystic ovary syndrome. Franks S. N Engl J Med. 1995;333:853–861. doi: 10.1056/NEJM199509283331307. [DOI] [PubMed] [Google Scholar]
- 3.Polycystic ovary syndrome: a syndrome of ovarian hypersensitivity to insulin? Baillargeon JP, Nestler JE. J Clin Endocrinol Metab. 2006;91:22–24. doi: 10.1210/jc.2005-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ovulatory and metabolic effects of D-chiro-inositol in the polycystic ovary syndrome. Nestler JE, Jakubowicz DJ, Reamer P, Gunn RD, Allan G. N Engl J Med. 1999;340:1314–1320. doi: 10.1056/NEJM199904293401703. [DOI] [PubMed] [Google Scholar]
- 5.Randomized double blind placebo-controlled trial: effects of myo-inositol on ovarian function and metabolic factors in women with PCOS. Gerli S, Papaleo E, Ferrari A, Di Renzo GC. http://www.europeanreview.org/wp/wp-content/uploads/458.pdf. Eur Rev Med Pharmacol Sci. 2007;11:347–354. [PubMed] [Google Scholar]
- 6.Effects of inositol on ovarian function and metabolic factors in women with PCOS: a randomized double blind placebo-controlled trial. Gerli S, Mignosa M, Di Renzo GC. https://www.fine-modus.com/wp-content/uploads/2012/02/inositol-and-PCO.pdf. Eur Rev Med Pharmacol Sci. 2003;7:151–159. [PubMed] [Google Scholar]
- 7.Effects of metformin on ovulation rate, hormonal and metabolic profiles in women with clomiphene-resistant polycystic ovaries: a randomized, double-blinded placebo-controlled trial. Ng EH, Wat NM, Ho PC. Hum Reprod. 2001;16:1625–1631. doi: 10.1093/humrep/16.8.1625. [DOI] [PubMed] [Google Scholar]
- 8.Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. Legro RS, Barnhart HX, Schlaff WD, et al. N Engl J Med. 2007;356:551–566. doi: 10.1056/NEJMoa063971. [DOI] [PubMed] [Google Scholar]
- 9.Follicular fluid and serum concentrations of myo-inositol in patients undergoing IVF: relationship with oocyte quality. Chiu TT, Rogers MS, Law EL, Briton-Jones CM, Cheung LP, Haines CJ. Hum Reprod. 2002;17:1591–1596. doi: 10.1093/humrep/17.6.1591. [DOI] [PubMed] [Google Scholar]
- 10.Effect of myo-inositol on the in-vitro maturation and subsequent development of mouse oocytes. Chiu TT, Rogers MS, Briton-Jones C, Haines C. Hum Reprod. 2003;18:408–416. doi: 10.1093/humrep/deg113. [DOI] [PubMed] [Google Scholar]
- 11.Myo-inositol may improve oocyte quality in intracytoplasmic sperm injection cycles. A prospective, controlled, randomized trial. Papaleo E, Unfer V, Baillargeon JP, Fusi F, Occhi F, De Santis L. Fertil Steril. 2009;91:1750–1754. doi: 10.1016/j.fertnstert.2008.01.088. [DOI] [PubMed] [Google Scholar]
- 12.Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Stepto NK, Cassar S, Joham AE, Hutchison SK, Harrison CL, Goldstein RF, Teede HJ. Hum Reprod. 2013;28:777–784. doi: 10.1093/humrep/des463. [DOI] [PubMed] [Google Scholar]
- 13.Correlation of hyperandrogenism with hyperinsulinism in polycystic ovarian disease. Burghen GA, Givens JR, and Kitabchi AE. J Clin Endocrinol Metab. 1980;50:113–116. doi: 10.1210/jcem-50-1-113. [DOI] [PubMed] [Google Scholar]
- 14.Polycystic ovary syndrome: a common hormonal condition with major metabolic sequelae that physicians should know about. Shorakae S, Boyle J, Teede H. Intern Med J. 2014;44:720–726. doi: 10.1111/imj.12495. [DOI] [PubMed] [Google Scholar]
- 15.Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Diamanti-Kandarakis E, Dunaif A. Endocr Rev. 2002;33:981–1030. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. [May;2020 ];Tang T, Lord JM, Norman RJ, Yasmin E, Balen AH. https://www.ncbi.nlm.nih.gov/pubmed/22592687. Cochrane Database Syst Rev. 2012 :0. doi: 10.1002/14651858.CD003053.pub5. [DOI] [PubMed]
- 17.Metformin in women with PCOS, cons. Misso ML, Teede HJ. Endocrine. 2015;48:428–433. doi: 10.1007/s12020-014-0394-8. [DOI] [PubMed] [Google Scholar]
- 18.Effects of myo-inositol in women with PCOS: a systematic review of randomized controlled trials. Unfer V, Carlomagno G, Dante G, Facchinetti F. Gynecol Endocrinol. 2012;28:509–515. doi: 10.3109/09513590.2011.650660. [DOI] [PubMed] [Google Scholar]
- 19.Effects of d-chiro-inositol in lean women with the polycystic ovary syndrome. Iuorno MJ, Jakubowicz DJ, Baillargeon JP, Dillon P, Gunn RD, Allan G, Nestler JE. Endocr Pract. 2002;8:417–423. doi: 10.4158/EP.8.6.417. [DOI] [PubMed] [Google Scholar]
- 20.Johann Joseph von Scherer (1814-69). The early history of clinical chemistry. (Article in German) Büttner J. https://www.ncbi.nlm.nih.gov/pubmed/361923. J Clin Chem Clin Biochem. 1978;16:478–483. [PubMed] [Google Scholar]
- 21.The first demonstration of lactic acid in human blood in shock by Johann Joseph Scherer (1814-1869) in January 1843. Kompanje EJ, Jansen TC, van der Hoven B, Bakker J. Intensive Care Med. 2007;33:1967–1971. doi: 10.1007/s00134-007-0788-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myo-inositol content of common foods: development of a high-myo-inositol diet. Clements RS Jr, Darnell B. Am J Clin Nutr. 1980;33:1954–1967. doi: 10.1093/ajcn/33.9.1954. [DOI] [PubMed] [Google Scholar]
- 23.Myo-inositol in patients with polycystic ovary syndrome: a novel method for ovulation induction. Papaleo E, Unfer V, Baillargeon JP, et al. Gynecol Endocrinol. 2007;23:700–703. doi: 10.1080/09513590701672405. [DOI] [PubMed] [Google Scholar]
- 24.Treatment of hirsutism with myo-inositol: a prospective clinical study. Minozzi M, D’Andrea G, Unfer V. Reprod Biomed Online. 2008;17:579–582. doi: 10.1016/s1472-6483(10)60248-9. [DOI] [PubMed] [Google Scholar]
- 25.Pharmacodynamics and pharmacokinetics of inositol(s) in health and disease. Bizzarri M, Fuso A, Dinicola S, Cucina A, Bevilacqua A. Expert Opin Drug Metab Toxicol. 2016;12:1181–1196. doi: 10.1080/17425255.2016.1206887. [DOI] [PubMed] [Google Scholar]
- 26.SnapShot: inositol phosphates. Hatch AJ, York JD. Cell. 2010;143:1030. doi: 10.1016/j.cell.2010.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inositol’s and other nutraceuticals’ synergistic actions counteract insulin resistance in polycystic ovarian syndrome and metabolic syndrome: state-of-the-art and future perspectives. Paul C, Laganà AS, Maniglio P, Triolo O, Brady DM. Gynecol Endocrinol. 2016;32:431–438. doi: 10.3109/09513590.2016.1144741. [DOI] [PubMed] [Google Scholar]
- 28.Is it time to consider patients suffering from endometriosis-related infertility as “novel candidates” for targeted peri-conceptional d-chiro inositol supplementation? Hypothesis, rationale and some considerations. Vitagliano A, Noventa M, Gizzo S. J Assist Reprod Genet. 2015;32:407–408. doi: 10.1007/s10815-014-0412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Comparison between effects of myo-inositol and D-chiro-inositol on ovarian function and metabolic factors in women with PCOS. Pizzo A, Laganà AS, Barbaro L. Gynecol Endocrinol. 2014;30:205–208. doi: 10.3109/09513590.2013.860120. [DOI] [PubMed] [Google Scholar]
- 30.Phosphoinositides in cell regulation and membrane dynamics. Di Paolo G, De Camilli P. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 31.The adapter protein APPL1 links FSH receptor to inositol 1,4,5-trisphosphate production and is implicated in intracellular Ca(2+) mobilization. Thomas RM, Nechamen CA, Mazurkiewicz JE, Ulloa-Aguirre A, Dias JA. Endocrinology. 2011;152:1691–1701. doi: 10.1210/en.2010-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Low urinary chiro-inositol excretion in non-insulin-dependent diabetes mellitus. Kennington AS, Hill CR, Craig J, et al. N Engl J Med. 1990;323:373–378. doi: 10.1056/NEJM199008093230603. [DOI] [PubMed] [Google Scholar]
- 33.Chiro-inositol-deficiency and insulin resistance: a comparison of the chiro-inositol- and myo-inositol-containing insulin mediators isolated from urine, hemodialysate, and muscle of control and type II diabetic subjects. Asplin I, Galasko G, Larner J. Proc Natl Acad Sci U S A. 1993;90:5924–5928. doi: 10.1073/pnas.90.13.5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The twentieth century struggle to decipher insulin signalling. Cohen P. Nat Rev Mol Cell Biol. 2006;7:867–873. doi: 10.1038/nrm2043. [DOI] [PubMed] [Google Scholar]
- 35.Greek hyperinsulinemic woman, with or without polycystic ovary syndrome, display altered inositols metabolism. Baillargeon JP, Nestler JE, Ostlund RE, Apridonidze T, Diamanti-Kandarakis E. Hum Reprod. 2008;23:1439–1446. doi: 10.1093/humrep/den097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Effect of follicular fluid oxidative stress on meiotic spindle formation in infertile women with polycystic ovarian syndrome. Chattopadhayay R, Ganesh A, Samanta J, Jana SK, Chakravarty BN, Chaudhury K. Gynecol Obstet Invest. 2010;69:197–202. doi: 10.1159/000270900. [DOI] [PubMed] [Google Scholar]
- 37.Oocyte morphology predicts outcome of intracytoplasmatic sperm injection. Serhal PF, Ranieri DM, Kinis A, Marchant S, Davies M, Khadum IM. Hum Reprod. 1997;12:1267–1270. doi: 10.1093/humrep/12.6.1267. [DOI] [PubMed] [Google Scholar]
- 38.Mild stimulation in in vitro fertilization. Macklon NS, Frauser BC. Ann N Y Acad Sci. 2003;997:105–111. doi: 10.1196/annals.1290.012. [DOI] [PubMed] [Google Scholar]
- 39.The D-chiro-inositol paradox in the ovary. Carlomagno G, Unfer V, Roseff S. Fertil Steril. 2011;95:2515–2516. doi: 10.1016/j.fertnstert.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 40.Reflections on inositol(s) for PCOS therapy: steps toward success. Nestler JE, Unfer V. Gynecol Endocrinol. 2015;31:501–505. doi: 10.3109/09513590.2015.1054802. [DOI] [PubMed] [Google Scholar]
- 41.Prospective randomized study on the influence of myoinositol in PCOS women undergoing IVF in the improvement of oocyte quality, fertilization rate, and embryo quality. Lesoine B, Regidor PA. Int J Endocrinol. 2016;2016:4378507. doi: 10.1155/2016/4378507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Effects of myo-inositol supplementation on oocyte’s quality in PCOS patients: a double blind trial. Ciotta L, Stracquadanio M, Pagano I, Carbonaro A, Palumbo M, Gulino F. https://www.ncbi.nlm.nih.gov/pubmed/21744744. Eur Rev Med Pharmacol Sci. 2011;15:509–514. [PubMed] [Google Scholar]
- 43.Management of women with PCOS using myo-inositol and folic acid. New clinical data and review of the literature. Regidor PA, Schindler AE, Lesoine B, Druckman R. Horm Mol Biol Clin Investig. 2018;34:67. doi: 10.1515/hmbci-2017-0067. [DOI] [PubMed] [Google Scholar]
- 44.Myo-inositol rather than D-chiro-inositol is able to improve oocyte quality in intracytoplasmic sperm injection cycles. A prospective, controlled, randomized trial. Unfer V, Carlomagno G, Rizzo P, Raffone E, Roseff S. http://en.vittoriounfer.it/wp-content/uploads/2016/04/Myo-inositol-rather-than-D-chiro-inositol-is-able-to-improve-oocyte-quality.pdf. Eur Rev Med Pharmacol Sci. 2011;15:452–457. [PubMed] [Google Scholar]
- 45.Effects of Inositol(s) in women with PCOS: a systematic review of randomized controlled trials. Unfer V, Nestler JE, Kamenov ZA, Prapas N, Facchinetti F. Int J Endocrinol. 2016;2016:1–12. doi: 10.1155/2016/1849162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Inositol supplementation in women with polycystic ovary syndrome undergoing intracytoplasmic sperm injection: a systematic review and meta-analysis of randomized controlled trials. Mendoza N, Pérez L, Simoncini T, Genazzani A. Reprod Biomed Online. 2017;35:529–535. doi: 10.1016/j.rbmo.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 47.Myo-inositol supplementation reduces the amount of gonadotropins and length of ovarian stimulation in women undergoing IVF: a systematic review and meta-analysis of randomized controlled trials. Laganà AS, Vitagliano A, Noventa M, Ambrosini G, D'Anna R. Arch Gynecol Obstet. 2018;298:675–684. doi: 10.1007/s00404-018-4861-y. [DOI] [PubMed] [Google Scholar]
- 48.Inositol supplement improves clinical pregnancy rate in infertile women undergoing ovulation induction for ICSI or IVF-ET. Zheng X, Lin D, Zhang Y, Lin Y, Song J, Li S, Sun Y. Medicine (Baltimore) 2017;96:0. doi: 10.1097/MD.0000000000008842. [DOI] [PMC free article] [PubMed] [Google Scholar]