Abstract
Macrophages fuse into multinucleated giant cells (MGC) in many pathological conditions. Despite MGC correlations with granulomas, their functional contribution to inflammation is relatively unknown. An in vitro mouse model of IL-4-induced bone marrow-derived macrophage fusion and microfiltration were used to generate enriched MGC and macrophage populations. Phenotypes were compared in response to well-known inflammatory stimuli, including lipopolysaccharide and crocidolite asbestos. Surface markers were assessed by flow cytometry: CD11b, CD11c, F4/80, and MHC II. Secreted cytokines were assessed by multiplex immunoassay: IFN-γ, IL-1β, IL-6, TNF-α, IL-10, IL-13, and IL-33. Results show that MGC maintained macrophage surface protein expression but lost the ability to produce a cytokine response. This suggests a potentially beneficial role of MGC in isolating the host from a foreign body without contributing to excessive inflammation. This study and future research using other stimulants and environments are important to gaining a fundamental MGC cell biology understanding. This will inform approaches to controlling the foreign body response to particle exposure, medical implants, and many diseases associated with granulomas.
Keywords: Multinucleated giant cell, Macrophage, In vitro, Mouse Phenotype, Surface marker, Cytokine
Graphical abstract
Introduction
Macrophage fusion into multinucleated giant cells (MGC) occurs in pathological events associated with granulomas. MGC most often form in response to persistent microorganisms or materials, but are also found in certain autoimmune or idiopathic conditions (Trout et al., 2016). One example is medical implants, where MGC have been observed to persist on the device for over 15 years post-implantation (McNally and Anderson, 2011a). The well-established correlation with granulomatous conditions may lead to the assumption that MGC actively contribute to inflammation and fibrosis, but their physiological role remains unclear (Franz et al., 2011; Helming and Gordon, 2008). A better understanding of MGC functions in disease is important for future development of therapeutics and approaches to control the foreign body response.
Phagocytosis and extracellular degradation of foreign material are among the few MGC functions that are more commonly described. Studies using mouse cells in vitro have shown MGC can phagocytose larger polystyrene beads than macrophages (Milde et al., 2015; Moreno et al., 2007; Nakanishi-Matsui et al., 2012). Human clinical (Davison et al., 1983; Moriyama et al., 2007; Takemura et al., 1989) and mouse in vivo (Kinaret et al., 2017; Porter et al., 2010) particle inhalation toxicology studies have provided microscopic images of MGC with internalized coal, hard metals, and multiwalled carbon nanotubes. When macrophages encounter foreign bodies too large to be engulfed, it is hypothesized that they fuse into MGC to degrade or sequester them. Podosomes form a sealed compartment that is filled by lysosome exocytosis with degradative enzymes, reactive oxygen species, and an acidic pH (Trout et al., 2016). This process, occasionally termed “frustrated phagocytosis,” occurs in MGC and osteoclasts (Khan et al., 2013; ten Harkel et al., 2015). Similar to MGC, osteoclasts are multinucleated cells formed by macrophage fusion. Osteoclasts are distinguished by their presence in non-pathological conditions where they function to resorb bone and are commonly identified by tartrate-resistant acid phosphatase (TRAP) expression. Some MGC express enzymes associated with degradation, including cathepsin K (Bühling et al., 2001; Park et al., 2013) and matrix metalloproteinase-9 (MacLauchlan et al., 2009; Zhu et al., 2007), but levels in osteoclasts are usually higher, coinciding with increased bone resorption capacity (Khan et al., 2014; ten Harkel et al., 2015). It is possible that MGC degradative activity is similar to macrophages during initial formation, then diminishes shortly afterwards (Jones et al., 2007; McNally and Anderson, 2011a).
There is a lack of research describing phenotypic differences between MGC and the macrophages from which they originate, especially in the context of their inflammatory activity. In vivo and ex vivo studies on this topic are limited because MGC are difficult to isolate in sufficient quantity and purity for successful analysis. Controlled in vitro environments are favorable for investigating effects of specific treatments directly on the cells and analyzing cells using a broader range of techniques (e.g. flow cytometry). The most frequently published in vitro MGC models use interleukin (IL)-4 to stimulate MGC formation from human blood monocytes or mouse bone marrow-derived macrophages (BMdM). We have identified two studies that assess immune profile differences between macrophage and MGC. Khan et al (2014) compared macrophages, osteoclasts, and MGC using mouse BMdM. Although the focus was on osteoclasts and related markers, CC chemokine and CC receptor gene expression was also assessed. MGC expressed higher CCL3, CCL4, CCL5, and CCL9 than macrophages, while others were generally similar depending on timepoint. McNally and Anderson (2011b) compared macrophages and MGC derived from human monocytes. Western blots of whole cell lysates and cell immunostaining were used to detect lymphocyte co-stimulatory, osteoclast, and dendritic cell related markers. Results of interest include MGC displaying increased human leukocyte antigen (HLA-DR), slightly increased CD11c, and loss of CD14. A common limitation of these studies is that they do not control for confounding effects of IL-4 treatment. For example, HLA-DR in MGC may not necessarily be increased as a result of multinucleation, rather this is likely attributed the culture receiving IL-4, a known inducer of HLA-DR (Gerrard et al., 1990).
Questions remain about MGC phenotype and function. Ultimately, it is important to know whether the presence of MGC in granulomatous conditions is beneficial or damaging. The answers would influence therapeutic approaches. The objective of this study was to determine how the phenotype of MGC unique from their macrophage precursors in the context of inflammation. This was investigated using our recently described model of IL-4-induced fusion of mouse BMdM and techniques for MGC enrichment and analysis (Trout and Holian, 2019). Comparing enriched macrophages and MGC from the same original culture controlled for confounding effects. Inflammatory response was assessed by stimulation with conventional lipopolysaccharide (LPS) treatment methods and via the phagocytic pathway using crocidolite asbestos. Key macrophage-related surface markers and cytokines were analyzed: integrin alpha M (CD11b), integrin alpha X (CD11c), adhesion G protein-coupled receptor E1 (F4/80), histocompatibility 2 class II (MHC II), interferon (IFN)-γ, interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, IL-10, IL-13, and IL-33.
Methods
Cell culture materials and methods
General methods for mouse BMdM macrophage and MGC culture, enrichment, and quantification have been previously described by our laboratory (Trout and Holian, 2019). Cells were grown in a humidified, water jacketed incubator (Thermo Fisher Scientific, Waltham, MA) at 37°C and 5% CO2. Sterile 0.2 μm filtered culture media consisted of RPMI-1640 with 10% heat-inactivated fetal bovine serum (FBS), 25 mM HEPES, 2 mM L-glutamine, 1 mM sodium pyruvate, 100 I.U./mL penicillin, and 100 μg/mL streptomycin (FBS: VWR Seradigm, Radnor, PA; all others: Corning subsidiary Mediatech, Manassas, VA). Cells were suspended by using 0.05% trypsin with 0.53 mM EDTA in HBSS (Corning) or Accutase® with 0.5 mM EDTA in Dulbecco’s PBS (BioLegend, San Diego, CA), followed by physical dislodging of cells as necessary using a cell scraper or pipette action. Treatment concentrations of 30 ng/mL were used for recombinant murine proteins macrophage colony-stimulating factor (M-CSF; R&D Systems, Minneapolis, MN) and IL-4 (R&D Systems). Centrifugations of cells in tubes were performed at RCFavg 300 × g for 5 min. Cytocentrifugations of cells onto slides were performed at approximately RCFavg 250 × g for 5 min. Cell counting was completed using a Beckman Coulter (Indianapolis, IN) Z2 cell counter.
Permanox is a trademarked (Nalge Nunc International, Rochester, NY) polymethylpentene tissue culture-treated growth surface with a silicone gasket connecting removable polystyrene chamber walls. Media working volumes per well or vessel were as follows: 8-chamber permanox slides at 0.4 mL, 96-well tissue culture-treated polystyrene (TCPS) plates (Greiner Bio-One, Monroe, NC) at 0.1 mL, and T75 TCPS flasks at 20 mL.
Mice
C57BI/6 mice (Jackson Laboratories, Bar Harbor, ME) aged 10–13 weeks were used for all experiments. Mice were housed in microisolator cages with ad libitum access to food and water in a specific-pathogen-free facility maintained at 22 ± 2°C, 30–40% humidity, and 12-hour light/12-hour dark cycle. Mice were euthanized by intraperitoneal injection of sodium pentobarbital followed by a secondary mechanical means of euthanasia prior to removal of rear legs for bone marrow isolation in a tissue culture hood. Experimental protocols were approved by the University of Montana Institutional Animal Care and Use Committee.
Particle preparation
Asbestos was selected as a model treatment because it is well-established by our laboratory and others to be a stimulator of macrophage activity in rodents and humans. Crocidolite asbestos (diameter 160nm, length 5 μm) was obtained from Research Triangle Institute (Research Triangle Park, NC). Asbestos was prepared into a homogenous dispersion in phosphate-buffered saline (PBS, pH 7.4) immediately prior to in vitro exposure by sonicating for one minute with a 500W, 20 kHz Qsonica Q500 (Newtown, CT) cup-horn system at 30% amplitude pulse.
Macrophage and MGC culture
Bone marrow was flushed from the tibiae and femora in a sterile environment, pooled, centrifuged, resuspended in media, and seeded at 4 × 105 cells/cm2 in a T75 flask. Cells were incubated at 37°C overnight. Adherent stromal cells were discarded, and suspended macrophage progenitor cells were collected. Progenitor cells were added to T75 flasks at 2 × 105 cells/cm2 with M-CSF for four days to mature into BMdM. Mature BMdM were collected using trypsin and were seeded at 9 × 105 cells/cm2 in permanox chamber slides with IL-4 for four days. Media was replaced with fresh media containing IL-4, then cells were cultured for five more days. The culture now consisted of a mixture of BMdM macrophages and MGC.
Cell detachment was completed using Accutase instead of trypsin for the remainder of the experiment, with efforts to handle cells gently to better preserve cell surface protein integrity. The suspended macrophage and MGC mixture was transferred onto a pre-rinsed 20 μm cell strainer (PluriStrainer by PluriSelect; Leipzig, Germany) and washed twice with 4 mL/wash into a tube. The strainer was inverted and washed twice with 4 mL/wash into a new tube. Cells small enough to pass through the strainer into the first tube were designated as the macrophage-enriched population, while cells blocked by the strainer were designated as the MGC-enriched population. A sample of cells was used to confirm enrichment. Remaining cells were seeded at 2.5 × 105 nuclei/mL (≈7.8 × 104 nuclei/cm2) in a 96-well plate. Specified wells were treated with 20 ng/mL LPS from Escherichia coli (MilliporeSigma) and 25 μg/mL asbestos. After 24 hours, cells were prepared for flow cytometry and supernatants were collected for multiplex immunoassay.
Quantification of microfiltration enrichment
Samples of macrophage-enriched and MGC-enriched populations were cytocentrifuged and stained using a method similar to Wright-Giemsa (PROTOCOL™ Hema 3™; Fisher Scientific, Kalamazoo, MI) by submerging slides in a methanol-based fixative for 90 s, “Solution I” for 120 s, “Solution II” for 30 s, and water for 90 s. At least five random, independent (non-overlapping) images were acquired per sample using a Zeiss Axioskop upright microscope with AxioCamMR3 camera (Carl Zeiss, Jena, Germany) at 100× magnification. MGC were defined morphologically as containing three or more nuclei within a common cytoplasm. MGC quantification results are expressed as percent fusion by dividing the number of nuclei within MGC by the total macrophage and MGC nuclei, then multiplying by 100. Alternatively, a purity index was calculated by dividing the number of MGC by total cells.
Flow cytometry
Combined live macrophage and MGC cells were stained with HCS NuclearMask Blue according to manufacturer (Thermo) recommendations. Antibody staining was performed in 100 μl buffer consisting of 1% w/v bovine serum albumin and 0.1% w/v sodium azide in PBS, sterile-filtered. Anti-mouse CD16/CD32 (Tonbo Biosciences, San Diego, CA) was added at 5 μg/mL for 10 minutes to block non-specific antibody binding. The following is a list of fluorochrome conjugated anti-mouse monoclonal antibodies obtained from BioLegend, staining concentrations (μg/mL), and corresponding wavelengths (nm) of lasers and filters: APC CD11b clone M1/70 at 2.5 (637, 670/14), PerCP/Cy5.5 CD11c clone N418 at 10 (488, 695/40), PE F4/80 clone BM8 at 10 (561, 585/16), and FITC MHC II (I-A/I-E) clone M5/114.15.2 at 2.5 (488, 530/30). Wavelengths for NuclearMask were 405, 440/50. Laser power was 100 mW for 637 nm and 50 mW for others. Cell staining time was 20 minutes, followed by two washes. Controls included unstained cells, cells with NuclearMask only, fluorescence minus one, and antibody-capture beads (Thermo) for multi-color compensation. At least 10,000 events per sample were analyzed using an Attune NxT (Thermo) flow cytometer with v2.6 software, with further analysis using FlowJo v10.4.2 software. Note that this acoustic-assisted hydrodynamic focusing cytometer had a configuration that accommodated large MGC cells, while other cytometers with small nozzles may clog.
Forward and side scatter gating was used to remove debris. Macrophages and MGC were separated according to nuclear fluorescence using methods described previously in our laboratory (Trout and Holian, 2019), which is similar to methods used for analysis of osteoclasts (Madel et al., 2018) and megakaryocytes (Drachman et al., 2000). Preliminary experiments indicated that cells could be gated into categories of one nucleus (1N), two nuclei (2N), or three or more nuclei (≥3N) according to nuclear fluorescence, without the need for pre-enrichment by microfiltration. Therefore, MGC and macrophages were combined during staining to improve consistency. Flow cytometry data normalization was completed based on each individual event (e.g. cell) by dividing surface protein fluorescence by nuclear fluorescence. The median normalized ratio of all events within a sample was then used for graphing and statistical analysis.
Multiplex immunoassay
Cytokines secreted by macrophages and MGC were measured using a custom mouse U-PLEX Biomarker Group 1 kit (Meso Scale Discovery, Rockville, MD), which is a multiplex sandwich immunoassay consisting of biotinylated capture antibodies and SULFO-TAG conjugated detection antibodies. The 96-well plate assay was completed according to manufacturer protocol. The plate was washed with 300 μl/well using a Thermo Wellwash 4 MK 2 and shaken at approximately 715 RPM on a Thermo 4625 shaker. Electrochemiluminescence was measured using the MESO QuickPlex SQ 120 with Discovery Workbench 4.0 software. Secreted protein concentrations were calculated using a four-parameter logistic standard curve. Some concentrations were below the limit of detection and could not be estimated from the curves, particularly in unstimulated cells. These nondetect values were considered to be zero for graphing and statistical analysis.
Statistics
Statistical analysis involved comparison of means using a two-way ANOVA followed by multiple comparisons using Tukey’s honest significant difference (HSD) test to compensate for increased type I error. Statistical significance was defined as a probability of type I error occurring at less than 5%. Significant simple contrasts of predetermined scientific interest are displayed on graphs, including treatment differences for each cell type and cell type differences for each treatment; complex and cross-group contrasts are not shown (e.g. Mac with LPS versus MGC control). Significant treatment differences compared to control of corresponding cell type are shown as *p < 0.05, **p < 0.01, and ***p < 0.001. The same p-value scheme is used for other contrasts indicated by daggers (†) with bars. Data is represented as the mean ± standard error of three independent replicate groups of mice for each condition. All analysis was completed in R v3.6.1 statistical software.
Results
Enrichment by microfiltration
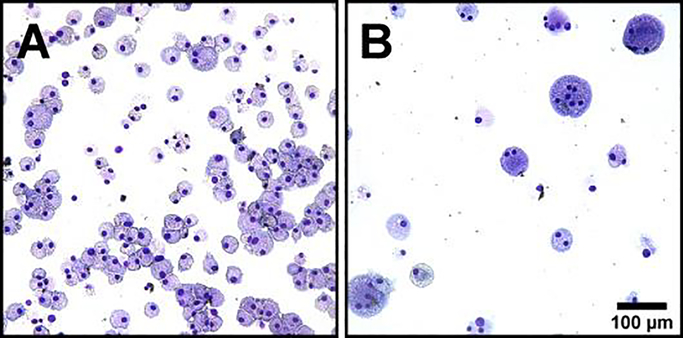
Mature mouse BMdM were treated with IL-4 to induce macrophage fusion into MGC. Then, the mixed culture was separated into macrophage-enriched and MGC-enriched populations by microfiltration, as previously described (Trout and Holian, 2019). A portion of these cells were collected for cytocentrifugation, staining, and morphological assessment of enrichment efficiency (Fig. 1). The percent fusion of each population was 33.1% for MGC-enriched and 0.5% for macrophage-enriched. The purity index was increased 71-fold. Remaining cells from each population were seeded according to number of nuclei/well for subsequent experiments. Two groups of these cells received treatments known to stimulate macrophages: LPS and a combination of LPS and crocidolite asbestos. Supernatants containing secreted cytokines were collected after 24 hours and cells were prepared for flow cytometry.
Fig. 1. Enrichment by microfiltration.
Representative images of purified cell populations resulting from microfiltration separation. (A) Cells that passed through the filter were macrophage-enriched. (B) Cells blocked by the filter were MGC-enriched. After filtration, cell concentrations were adjusted to seed 96-well plates with equal numbers of nuclei/well for subsequent experiments. Scale is same for both images, bar 100 μm.
Surface markers
Surface proteins on macrophages and MGC were analyzed by flow cytometry 24 hours after stimulation with LPS and asbestos. In order to compare marker expression between these two cell types, data normalization was required due to the difference in cell size. To illustrate the importance of normalization, cells were gated into categories of one nucleus (1N), two nuclei (2N), or three or more nuclei (≥3N) for comparisons. The increase in surface marker fluorescence correlates with an increase in the number of nuclei (Fig. 2). MGC are formed by the fusion of macrophages into a larger cell, so it is logical that the nucleus to membrane ratio remains proportional. Evidence of this has been shown by live cell imaging of fusion (Faust et al., 2017) and results suggesting similar buoyant densities (Trout and Holian, 2019). Therefore, surface protein fluorescence was normalized according to nuclear fluorescence of each individual cell.
Fig. 2. Surface markers before normalization.
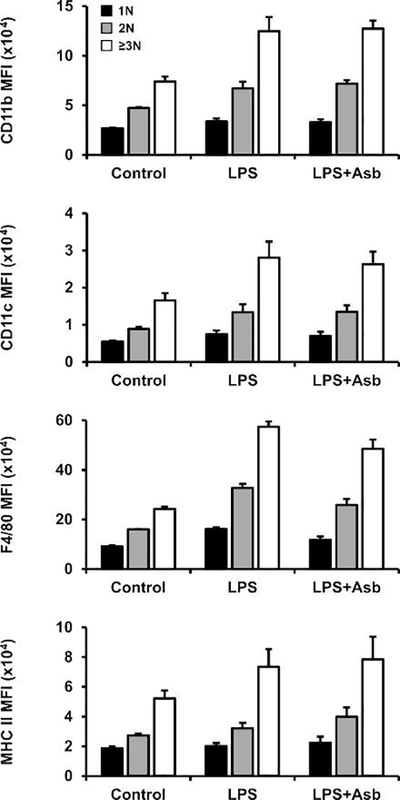
Cells were gated according to nuclear fluorescence into three groups: one nucleus (1N; black bars), two nuclei (2N; gray), and three or more nuclei (≥3N; white). Median fluorescence intensity (MFI) of all surface markers increases in binucleated macrophages (2N) and MGC (≥3N), which may lead to the conclusion that marker expression is increased in these cells. However, the increase in fluorescence may simply be a result of larger cell size, with actual membrane density distribution of markers remaining unchanged. This demonstrates the importance of data normalization.
Normalized surface marker expression in macrophages and MGC was compared for unstimulated cells, stimulated with LPS, and stimulated with LPS and asbestos (Fig. 3). Results of a two-way ANOVA indicate that cell-type effects were not significant. Treatment with LPS resulted in significant increases in F4/80 and a slightly increasing trend in CD11b and CD11c, consistent with previous BMdM studies (Lombardo et al., 2007; Oppong-Nonterah et al., 2019). Macrophage activation by particle exposure has been shown to increase antigen-presentation (Migliaccio et al., 2005), so LPS and asbestos treatment results showing increased MHC II were expected, though the increase was not statistically significant.
Fig. 3. Surface markers after normalization.
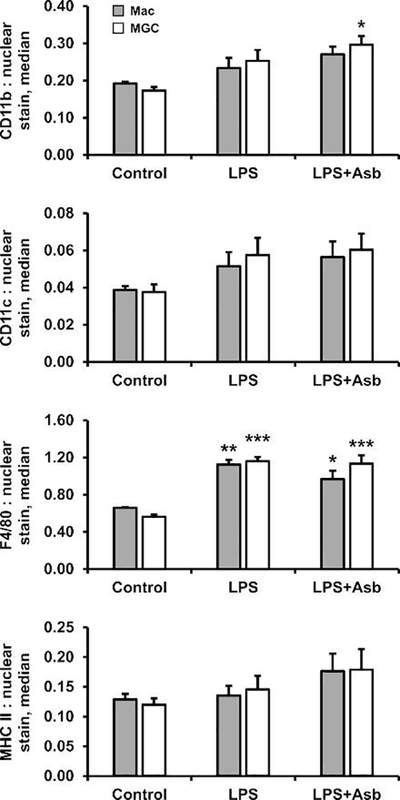
Normalized protein expression by macrophages (gray bars) and MGC (white bars) that were unstimulated (control) or stimulated with LPS or LPS plus asbestos for 24 h. No significant differences in surface markers were observed between macrophages and MGC. Asterisks(*) indicate significant treatment effects versus corresponding cell-type control.
Cytokine secretion
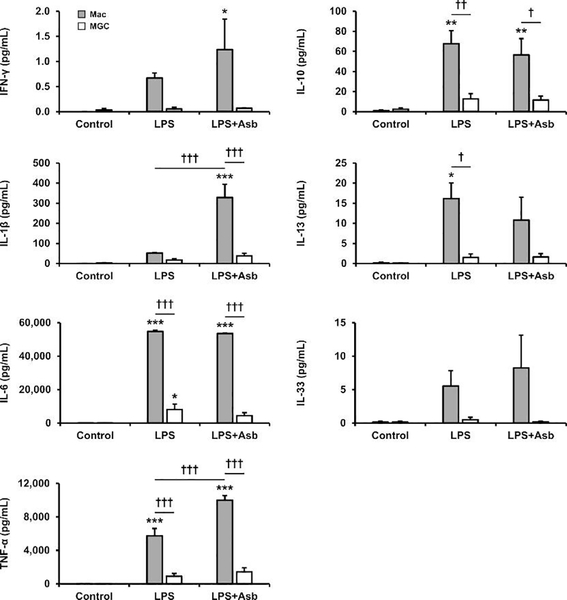
Cytokines secreted by enriched populations of macrophages and MGC were analyzed by multiplex immunoassay 24 hours after stimulation with LPS and asbestos. In contrast to surface marker data, cytokine results did not require normalization because this was already controlled by the experimental design: supernatants were collected from cells seeded at an equal number of nuclei per well with an equal volume of media per well. Comparisons according to nuclei number is consistent with methods for flow cytometry normalization and is appropriate because MGC contain organelles from multiple cells, with the nucleus having a central role in protein production.
Baseline secretion of all cytokines was very low (Fig. 4), as expected. This is why it was important to include treatments known to stimulate macrophages. Results of a two-way ANOVA indicate that cell-type effects were significant (p<0.05) for all proteins analyzed. Therefore, MGC in this study have an impaired ability to either produce or secrete these cytokines compared to macrophages. Although certain cytokines secreted by stimulated MGC were slightly increased from baseline, only IL-6 from the LPS-treated group showed a statistically significant increase.
Fig. 4. Cytokine secretion.
Supernatant protein concentrations from enriched populations of macrophages (gray bars) and MGC (white bars) that were unstimulated (control) or stimulated with LPS or LPS plus asbestos for 24 h. The immunoassay included cytokines generally associated with classical (M1; left column) and alternative (M2; right column) activation. Asterisks(*) indicate significant treatment effects versus corresponding cell-type control. Daggers(†) indicate other significant contrasts as shown by bars.
Macrophages secreted higher concentrations of multiple cytokines when stimulated compared to baseline macrophages (Fig. 4). Many increases resulted from LPS treatment alone, but other cytokines were further increased when asbestos was added. Particularly, IL-1β and TNF-α were significantly higher in the LPS plus asbestos group compared to LPS alone, consistent with our previous studies (Brown et al., 2016; Perkins et al., 1993). It was anticipated that IL-6 would also be increased by asbestos, but levels remained the same as with LPS alone. This was likely because the response to LPS was already very high.
Discussion
Our recent investigation of in vitro MGC formation (Trout and Holian, 2019) helped establish techniques that facilitated the completion of this study. Mouse BMdM were treated with IL-4 in permanox chamber slides to induce macrophage fusion into MGC. In order to effectively compare macrophages and MGC, it was important to purify populations arising from the same original culture. Purity was increased 71-fold by simple microfiltration, which allowed for continuation of the cell culture without stains or other treatments that would interfere with remaining experiments. Additional purification steps may have improved purity, but at the sacrifice of reduced total cell numbers. Enrichment allowed for more appropriate phenotype comparisons between macrophages and MGC. The main objective of this study was to assess common surface markers and cytokines at baseline and in response to stimulation. Stimulation was especially important for meaningful cytokine comparisons because both macrophage and MGC baseline secretions are very low.
Previous MGC phenotype studies (Khan et al., 2014; McNally and Anderson, 2011b) used IL-4 to stimulate macrophage fusion into MGC, then compared these cultures with untreated macrophages. Results from using this experimental design cannot be used to distinguish whether any observed cell-type differences were a consequence of multinucleation or an artifact of IL-4 treatment. In the current study, enrichment methods allowed for macrophages and MGC to be sourced from the same IL-4-stimulated culture. Cell culture could be continued after enrichment by microfiltration without interfering factors present in other separation methods, such as stains required for fluorescence-activated cell sorting. This is the first MGC phenotype study, to our knowledge, that is controlled in a manner that removes confounding effects of treatment differences and other manipulations.
Normalized surface marker expression results indicate that common macrophage markers are present in a similar density on MGC. This was not surprising considering that MGC are formed by macrophage fusion and both cell types were sourced from the same cultures. A particularly interesting result was that MGC-enriched cultures had a similar response to stimulation as macrophage-enriched cultures. For example, MGC had the capacity to respond to LPS by upregulating F4/80. This suggests that MGC have similar levels of toll-like receptor (TLR) 4 and functional LPS-TLR4 signaling pathways.
Baseline cytokine secretion was very low in both macrophages and MGC, as expected. However, the observation that MGC have a diminished ability to produce/secrete cytokines in response to stimulation was somewhat unexpected. Surface marker results suggested that MGC retain the capacity of reacting to LPS stimulation, indicating that those intracellular signaling pathways were not impaired. However, it is possible that those surface proteins were already loaded in intracellular vesicle pools, transferred from macrophages during fusion, that translocated to the plasma membrane during stimulation. Increased cytokine secretion is more dependent on novo synthesis, followed by secretion via exocytosis. Notably, release of cytokines from both conventional and unconventional (e.g. IL-1β after LPS+Asb) secretion pathways were impaired in MGC.
One potential explanation of the impaired cytokine response in MGC is that the presence of multiple nuclei disrupts intracellular signaling and transcription factor localization. The organelle organization and nuclear coordination following fusion is not well understood. An osteoclast study claimed that only certain nuclei within the multinucleated cell are transcriptionally active (Youn et al., 2010), but another study said all nuclei were active (Boissy et al., 2002). Numerous cell functions could become disrupted throughout the complex pathway from cell signaling in response to stimulation to transcriptional upregulation protein processing, and secretion. Further investigation is needed to determine how these mechanisms are altered in MGC. Some example approaches include flow cytometry to assess relevant membrane receptors, microscopic observation of transcription factor localization with nuclei, RT-qPCR, immunoassays using cell lysates, and fluorescence microscopy of organelles and secretory vesicles.
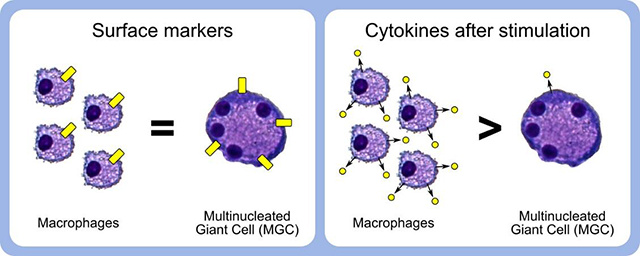
Overall, MGC appear to maintain certain macrophage surface protein characteristics, while losing the ability to promote an inflammatory response. These results fit with the theory that MGC form a “wall” to isolate the host from a foreign body without causing excessive inflammation. In this case, the presence of MGC would be beneficial. However, more research is needed to better understand how the MGC phenotype may differ in response to other stimulants and according to their environment. This fundamental cell biology knowledge will help to develop methods to control the foreign body response, as well as provide insight into several other granulomatous conditions in which the role of MGC is unclear.
Highlights.
Macrophage and multinucleated giant cell (MGC) phenotypes are compared.
MGC maintain expression of macrophage surface proteins.
MGC release fewer cytokines than macrophages during inflammatory stimulation.
Acknowledgements
Special thanks for technical laboratory support from Pam Shaw, Center for Environmental Health Sciences (CEHS) Fluorescence Cytometry core facility. Research funding was provided by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health (NIH) under grant number P30GM103338. Contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NIGMS or NIH.
Abbreviations
- MGC
multinucleated giant cell
- BMdM
BM-derived macrophage
- LPS
lipopolysaccharide
- Asb
crocidolite asbestos
- IL
interleukin
- IFN
interferon
- TNF
tumor necrosis factor
- CD11b
integrin alpha M
- CD11c
integrin alpha X
- F4/80
adhesion G protein-coupled receptor E1
- MHC II
histocompatibility 2 class II
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boissy P, Saltel F, Bouniol C, Jurdic P, Machuca-Gayet I, 2002. Transcriptional activity of nuclei in multinucleated osteoclasts and its modulation by calcitonin. Endocrinology 143, 1913–1921. 10.1210/endo.143.5.8813 [DOI] [PubMed] [Google Scholar]
- Brown TA, Holian A, Pinkerton KE, Lee JW, Cho YH, 2016. Early life exposure to environmental tobacco smoke alters immune response to asbestos via a shift in inflammatory phenotype resulting in increased disease development. Inhal Toxicol 28, 349–356. 10.1080/08958378.2016.1175526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bühling F, Reisenauer A, Gerber A, Krüger S, Weber E, Brömme D, Roessner A, Ansorge S, Welte T, Röcken Ch., 2001. Cathepsin K – a marker of macrophage differentiation? J Pathol 195, 375–382. 10.1002/path.959 [DOI] [PubMed] [Google Scholar]
- Davison AG, Haslam PL, Corrin B, Coutts II, Dewar A, Riding WD, Studdy PR, Newman-Taylor AJ, 1983. Interstitial lung disease and asthma in hard-metal workers: bronchoalveolar lavage, ultrastructural, and analytical findings and results of bronchial provocation tests. Thorax 38, 119–128. 10.1136/thx.38.2.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachman JG, Jarvik GP, Mehaffey MG, 2000. Autosomal dominant thrombocytopenia: incomplete megakaryocyte differentiation and linkage to human chromosome 10. Blood 96, 118–125. [PubMed] [Google Scholar]
- Faust JJ, Christenson W, Doudrick K, Ros R, Ugarova TP, 2017. Development of fusogenic glass surfaces that impart spatiotemporal control over macrophage fusion: direct visualization of multinucleated giant cell formation. Biomaterials 128, 160–171. 10.1016/j.biomaterials.2017.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz S, Rammelt S, Scharnweber D, Simon JC, 2011. Immune responses to implants – A review of the implications for the design of immunomodulatory biomaterials. Biomaterials 32, 6692–6709. 10.1016/j.biomaterials.2011.05.078 [DOI] [PubMed] [Google Scholar]
- Gerrard TL, Dyer DR, Mostowski HS, 1990. IL-4 and granulocyte-macrophage colony-stimulating factor selectively increase HLA-DR and HLA-DP antigens but not HLA-DQ antigens on human monocytes. J Immunol 144, 4670–4674. [PubMed] [Google Scholar]
- Helming L, Gordon S, 2008. The molecular basis of macrophage fusion. Immunobiology 212, 785–793. 10.1016/j.imbio.2007.09.012 [DOI] [PubMed] [Google Scholar]
- Jones JA, Chang DT, Meyerson H, Colton E, Kwon IK, Matsuda T, Anderson JM, 2007. Proteomic analysis and quantification of cytokines and chemokines from biomaterial surface-adherent macrophages and foreign body giant cells. J Biomed Mater Res A 83A, 585–596. 10.1002/jbm.a.31221 [DOI] [PubMed] [Google Scholar]
- Khan UA, Hashimi SM, Bakr MM, Forwood MR, Morrison NA, 2013. Foreign body giant cells and osteoclasts are TRAP positive, have podosome-belts and both require OC-STAMP for cell fusion. J Cell Biochem 114, 1772–1778. 10.1002/jcb.24518 [DOI] [PubMed] [Google Scholar]
- Khan UA, Hashimi SM, Khan S, Quan J, Bakr MM, Forwood MR, Morrison NM, 2014. Differential expression of chemokines, chemokine receptors and proteinases by foreign body giant cells (FBGCs) and osteoclasts. J Cell Biochem 115, 1290–1298. 10.1002/jcb.24781 [DOI] [PubMed] [Google Scholar]
- Kinaret P, Ilves M, Fortino V, Rydman E, Karisola P, Lähde A, Koivisto J, Jokiniemi J, Wolff H, Savolainen K, Greco D, Alenius H, 2017. Inhalation and oropharyngeal aspiration exposure to rod-like carbon nanotubes induce similar airway inflammation and biological responses in mouse lungs. ACS Nano 11, 291–303. 10.1021/acsnano.6b05652 [DOI] [PubMed] [Google Scholar]
- Lombardo E, Alvarez-Barrientos A, Maroto B, Boscá L, Knaus UG, 2007. TLR4-mediated survival of macrophages is MyD88 dependent and requires TNF-α autocrine signalling. J Immunol 178, 3731–3739. 10.4049/jimmunol.178.6.3731 [DOI] [PubMed] [Google Scholar]
- MacLauchlan S, Skokos EA, Meznarich N, Zhu DH, Raoof S, Shipley JM, Senior RM, Bornstein P, Kyriakides TR, 2009. Macrophage fusion, giant cell formation, and the foreign body response require matrix metalloproteinase 9. J Leukoc Biol 85, 617–626. 10.1189/jlb.1008588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madel M-B, Ibáñez L, Rouleau M, Wakkach A, Blin-Wakkach C, 2018. A novel reliable and efficient procedure for purification of mature osteoclasts allowing functional assays in mouse cells. Front Immunol 9, 2567 10.3389/fimmu.2018.02567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally AK, Anderson JM, 2011a. Macrophage fusion and multinucleated giant cells of inflammation, in: Dittmar T, Zänker KS (Eds.), Cell Fusion in Health and Disease, Advances in Experimental Medicine and Biology. Springer Netherlands, Dordrecht, pp. 97–111. 10.1007/978-94-007-0763-4_7 [DOI] [PubMed] [Google Scholar]
- McNally AK, Anderson JM, 2011b. Foreign body-type multinucleated giant cells induced by interleukin-4 express select lymphocyte co-stimulatory molecules and are phenotypically distinct from osteoclasts and dendritic cells. Exp Mol Pathol 91, 673–681. 10.1016/j.yexmp.2011.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio CT, Hamilton RF Jr, Holian A, 2005. Increase in a distinct pulmonary macrophage subset possessing an antigen-presenting cell phenotype and in vitro APC activity following silica exposure. Toxicol Appl Pharmacol 205, 168–176. 10.1016/j.taap.2004.11.005 [DOI] [PubMed] [Google Scholar]
- Milde R, Ritter J, Tennent GA, Loesch A, Martinez FO, Gordon S, Pepys MB, Verschoor A, Helming L, 2015. Multinucleated giant cells are specialized for complement-mediated phagocytosis and large target destruction. Cell Rep 13, 1937–1948. 10.1016/j.celrep.2015.10.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JL, Mikhailenko I, Tondravi MM, Keegan AD, 2007. IL-4 promotes the formation of multinucleated giant cells from macrophage precursors by a STAT6-dependent, homotypic mechanism: contribution of E-cadherin. J Leukoc Biol 82, 1542–1553. 10.1189/jlb.0107058 [DOI] [PubMed] [Google Scholar]
- Moriyama H, Kobayashi M, Takada T, Shimizu T, Terada M, Narita J-I, Maruyama M, Watanabe K, Suzuki E, Gejyo F, 2007. Two-dimensional analysis of elements and mononuclear cells in hard metal lung disease. Am J Respir Crit Care Med 176, 70–77. 10.1164/rccm.200601-134OC [DOI] [PubMed] [Google Scholar]
- Nakanishi-Matsui M, Yano S, Matsumoto N, Futai M, 2012. Lipopolysaccharide induces multinuclear cell from RAW264.7 line with increased phagocytosis activity. Biochem Biophys Res Commun 425, 144–149. https://doi.org/10.10167j.bbrc.2012.07.050 [DOI] [PubMed] [Google Scholar]
- Oppong-Nonterah GO, Lakhdari O, Yamamura A, Hoffman HM, Prince LS, 2019. TLR activation alters bone marrow-derived macrophage differentiation. J Innate Immun 11, 99–108. 10.1159/000494070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JK, Rosen A, Saffitz JE, Asimaki A, Litovsky SH, Mackey-Bojack SM, Halushka MK, 2013. Expression of cathepsin K and tartrate-resistant acid phosphatase is not confined to osteoclasts but is a general feature of multinucleated giant cells: systematic analysis. Rheumatology (Oxford) 52, 1529–1533. 10.1093/rheumatology/ket184 [DOI] [PubMed] [Google Scholar]
- Perkins RC, Scheule RK, Hamilton R, Gomes G, Freidman G, Holian A, 1993. Huma. Alveolar macrophage cytokine release in response to in vitro and in vivo asbestos exposure. Exp Lung Res 19, 55–65. 10.3109/01902149309071080 [DOI] [PubMed] [Google Scholar]
- Porter DW, Hubbs AF, Mercer RR, Wu N, Wolfarth MG, Sriram K, Leonard S, Battelli L, Schwegler-Berry D, Friend S, Andrew M, Chen BT, Tsuruoka S, Endo M, Castranova V, 2010. Mouse pulmonary dose- and time course-responses induced by exposure to multi-walled carbon nanotubes. Toxicology 269, 136–147. 10.1016/j.tox.2009.10.017 [DOI] [PubMed] [Google Scholar]
- Takemura T, Rom WN, Ferrans VJ, Crystal RG, 1989. Morphologic characterization of alveolar macrophages from subjects with occupational exposure to inorganic particles. Am Rev Respir Dis 140, 1674–1685. 10.1164/ajrccm/140.6.1674 [DOI] [PubMed] [Google Scholar]
- ten Harkel B, Schoenmaker T, Picavet DI, Davison NL, de Vries TJ, Everts V, 2015. The foreign body giant cell cannot resorb bone, but dissolves hydroxyapatite like osteoclasts. PLoS One 10, e0139564 10.1371/journal.pone.0139564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trout KL, Holian A, 2019. Factors influencing multinucleated giant cell formation in vitro. Immunobiology 224, 834–42. 10.1016/j.imbio.2019.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trout KL, Jessop F, Migliaccio CT, 2016. Macrophage and multinucleated giant cell classification, in: Otsuki T, Yoshioka Y, Holian A (Eds.), Biological Effects of Fibrous and Particulate Substances, Current Topics in Environmental Health and Preventive Medicine. Springer; Japan, Tokyo, pp. 1–26. 10.1007/978-4-431-55732-6_1 [DOI] [Google Scholar]
- Youn M-Y, Takada I, Imai Y, Yasuda H, Kato S, 2010. Transcriptionally active nuclei are selective in mature multinucleated osteoclasts. Genes Cells 15, 1025–1035. 10.1111/j.1365-2443.2010.01441.x [DOI] [PubMed] [Google Scholar]
- Zhu XW, Price NM, Gilman RH, Recarvarren S, Friedland JS, 2007. Multinucleate giant cells release functionally unopposed matrix metalloproteinase-9 in vitro and in vivo. J Infect Dis 196, 1076–1079. 10.1086/521030 [DOI] [PubMed] [Google Scholar]