Abstract
Introduction:
EEG patterns in chimeric antigen receptor T cell treatment-associated neurotoxicity (immune effector cell-associated neurotoxicity syndrome) have not yet been systematically studied. We tested the hypothesis that EEG background abnormalities in immune effector cell-associated neurotoxicity syndrome correlate with clinical signs of neurotoxicity. In addition, we describe ictal and interictal EEG patterns to better understand the natural history of immune effector cell-associated neurotoxicity syndrome-associated seizures.
Methods:
EEGs were obtained in 19 of 100 subjects in a prospective cohort study of children and young adults undergoing CD19-directed chimeric antigen receptor T cell therapy. We classified the EEG background on a severity scale of 0 to 5 during 30-minute epochs. EEG grades were compared with neurotoxicity scored by Common Terminology Criteria for Adverse Events and Cornell Assessment of Pediatric Delirium scores. Descriptive analysis was conducted for ictal and interictal EEG abnormalities.
Results:
EEG background abnormality scores correlated well with Common Terminology Criteria for Adverse Events neurotoxicity scores (P = 0.0022) and Cornell Assessment of Pediatric Delirium scores (P = 0.0085). EEG was better able to differentiate the severity of coma patterns compared with the clinical scores. The EEG captured electroclinical seizures in 4 of 19 subjects, 3 of whom had additional electrographic-only seizures. Seizures most often arose from posterior head regions. Interictal epileptiform discharges were focal, multifocal, or lateralized periodic discharges. No seizures or interictal epileptiform abnormalities were seen in subjects without previous clinical seizures.
Conclusions:
Continuous EEG monitoring is high yield for seizure detection in high-risk chimeric antigen receptor T cell patients, and electrographic-only seizures are common. Increasing severity of EEG background abnormalities correlates with increasing neurotoxicity grade.
Keywords: EEG, CAR T, Neurotoxicity, ICANS, Seizure
Chimeric antigen receptor (CAR) T cell therapy has shown excellent efficacy in relapsed or refractory hematologic malignancies,1 but the challenges remain in the management of associated toxicities.2 Chimeric antigen receptor T cells are created by genetically modifying T cells with a chimeric receptor that recognizes surface antigens on cancer cells. After the CAR T cells are infused into the patient, they undergo rapid proliferation, which can be associated with a systemic cytokine surge with fever and capillary leak (cytokine release syndrome [CRS]).3 Neurotoxicity affects approximately 30% to 40% of patients who receive CD19-directed CAR T cells for leukemia or lymphoma.4,5 The term “immune effector cell-associated neurotoxicity syndrome” (ICANS) was introduced in 2018 by consensus statement of the American Society for Transplantation and Cellular Therapy (ASTCT) to reflect the fact that a neurologic syndrome can occur with a variety of cell-based immunotherapy modalities.2
Immune effector cell-associated neurotoxicity syndrome most often presents with delirium and/or language impairment. Seizures have been reported in 0% to 30% of patients, with the highest incidence in patients with acute lymphocytic leukemia.5 Cerebral edema is a rare but often fatal complication.4 Neurotoxicity follows a characteristic course, with symptoms developing during the first week after CAR T cell infusion, typically in the setting of ongoing or resolving CRS. Although neurotoxicity is considered a syndrome separate from CRS, the risk of neurotoxicity strongly correlates with the degree of CRS.5 Neurotoxicity symptoms peak around day 7 and, in most cases, resolve by day 21.5 Standard treatment consists of steroids, interleukin-6 blockade, and aggressive management of seizures.5
The ASTCT working group proposed a new ICANS assessment and grading scheme for children and adults, with grades ranging from 0 (no ICANS) to 5 (death) (Table 1). This replaces a variety of grading schemes that were largely based on the NCI Common Terminology Criteria for Adverse Events (CTCAE).6 Pediatric ICANS grading relies to a large degree on Cornell Assessment of Pediatric Delirium (CAPD) scores as a readout of cerebral dysfunction.7 Cornell Assessment of Pediatric Delirium scores ≥ 9 are consistent with delirium, and per the ASTCT working group recommendation, all patients with a CAPD score ≥ 9 are assigned an ICANS score of 3. Grade 3 is also assigned for any seizures, and grade 4 for seizures that are multiple or longer than 5 minutes. By contrast, in the CTCAE, brief focal seizures are grade 1, generalized seizures grade 2, new or multiple seizures despite intervention are grade 3, and life-threatening or prolonged repetitive seizures are grade 4.
TABLE 1.
ASTCT ICANS Consensus Grading
| Neurotoxicity Domain | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| ICE score (for adults and children >12 | 7–9 | 3–6 | 0–2 | 0 (patient is unarousable and unable to perform ICE) |
| OR | ||||
| CAPD (for children ≤12) | <9 | <9 | ≥9 | Unable to perform CAPD |
| Depressed level of consciousness | Awakens spontaneously | Awakens to voice | Awakens only to tactile stimuli | Patient unarousable or requires vigorous or repetitive tactile stimuli to arouse. Stupor or coma. |
| Seizure | Any clinical seizure (focal or generalized) that resolves rapidly or nonconvulsive seizures on EEG that resolve with intervention | Life-threatening prolonged seizure (>5 minutes) or repetitive clinical or electrographic seizures without return to baseline in between | ||
| Motor findings | Deep focal motor weakness such as hemiparesis or paraparesis | |||
| Increased ICP/Cerebral edema | Focal/local edema on neuroimaging | Diffuse cerebral edema on neuroimaging, decerebrate or decorticate posturing, or cranial nerve VI palsy, or papilledema, or Cushing triad |
ICANS grade is determined by the most severe event (ICE score, level of consciousness, seizure, motor findings, increased ICP/cerebral edema) not attributable to any other cause. For example, a patient with an ICE score of 3 who has a generalized seizure is classified as having grade 3 ICANS. Table modified with permission from Lee et al., 2019.2 ICE score, immune effector cell associated encephalopathy score. Adaptations are themselves works protected by copyright. So to publish this adaptation, authorization must be obtained both from the owner of the copyright in the original work and from the owner of copyright in the translation or adaptation.
CAPD, Cornell Assessment of Pediatric Delirium; ICP, intracranial pressure; ICANS, immune effector cell–associated neurotoxicity syndrome.
Although no prospective studies of EEG monitoring in CAR T cell patients have been reported to date, there is evidence that EEGs are frequently abnormal in this population. In adult patients with ICANS after CD19-CAR T cell treatment, diffuse slowing was seen in the majority of EEGs.4,8,9 One group found a high incidence of periodic discharges on the ictal-interictal spectrum, present in 78% of patients who had EEGs.8 Interictal focal epileptiform abnormalities have only been described in a patient with preexisting epilepsy.4 Seizures on EEG have been primarily reported in patients who also had clinical seizures. Nonconvulsive status epilepticus can occur after convulsive seizures are treated with medication, but in some cases, it was seen without preceding clinical seizures.9,10
Based on these data, we identified two key knowledge gaps to address in this study. First, we sought to determine how the EEG background changes as a reflection of brain dysfunction during immunotherapy. We hypothesized that higher CTCAE and CAPD scores correlate with an increasing degree of abnormality in the EEG background. If this is confirmed, EEG may be then validated as a real-time measure of brain dysfunction that can supplement clinical assessments of treatment response.
Second, it is not known which CAR T cell patients should undergo prolonged EEG monitoring to detect electrographic-only seizures that are a treatable cause of depressed mental status and may contribute to adverse outcomes if untreated.11 We hypothesized that patients with clinical seizures are at risk of having electrographic-only seizures, a finding that has been noted in EEG monitoring for other systemic inflammatory illnesses such as sepsis.12
METHODS
Subjects
We conducted a prospective cohort study of all patients who received CAR T cell infusions on the PLAT-02 or PLAT-03 phase 1/2 trials of CD19-directed CAR T cells for pediatric B cell malignancies at Seattle Children’s ( NCT02028455, NCT03186118).13 This cohort includes all 43 PLAT-02 phase 1 patients who have been previously reported.13,14 The study was approved by the Seattle Children’s IRB.
EEG Grading
EEGs were obtained on recommendation of the consulting neurology service for the workup of seizures, abnormal movements concerning for seizures, and/or encephalopathy. Continuous EEG monitoring was initiated per institutional criteria for high risk patients who failed to show improvement in mental status after a seizure or who had prolonged depressed level of consciousness. cEEG monitoring was continued until at least 24 hours of seizure freedom. All EEGs were reviewed by board-certified epileptologists. The EEG background was classified per the work of Synek15 by a board-certified epileptologist who was masked to the subjects’ CTCAE and CAPD scores. Briefly, the Synek scheme designated grade 1 as + posterior dominant rhythm (PDR), alpha and theta slowing, grade 2 as +PDR, predominantly theta, grade 3 as no PDR, predominantly delta, grade 4 as discontinuity or burst suppression, and grade 5 as voltage suppression <5 μV. EEG background was scored for 30-minute epochs every 12 hours, preferentially coinciding with the twice-daily CAPD scoring. For routine EEG, we evaluated the first 30 minutes of the recording.
Neurotoxicity Grading
Because our clinical trial protocols were developed before the institution of ICANS neurotoxicity criteria, neurotoxicity was graded according to the NCI CTCAE, v4.0, with grades ranging from 0 = no impairment to 5 = death. Neurotoxicity grades were assigned for each EEG epoch based on clinical signs and symptoms present at the time of recording. We then assigned an overall neurotoxicity grade for each patient, which reflects the highest grade neurologic sign or symptom occurring over the entire course of neurotoxicity. Criteria deviated from the CTCAE in 2 instances: patients with any seizures were given a grade 3 and patients whose only symptom above grade 2 was headache were given grade 2. Patients with headache but no other neurologic abnormalities were not considered to have neurotoxicity.
Antiseizure Medications
Seizure prophylaxis with levetiracetam was started in all patients who developed severe CRS or any degree of neurotoxicity and continued until resolution of these symptoms. Acute seizures were treated per institutional protocol.
CAPD Scoring
Cornell Assessment of Pediatric Delirium scores were obtained by the bedside nurse once per shift in all patients who were admitted to the ICU, with the score reflecting the patient’s status throughout the shift. For the CAPD, 8 different behavioral domains are scored from 0 = normal to 4 = highly abnormal, and the subdomain scores are added for the overall score. Cornell Assessment of Pediatric Delirium scores ≥ 9 were considered indicative of delirium, in accordance with the original validation studies.7 Cornell Assessment of Pediatric Delirium scores without corresponding EEG within 4 hours were not included in this study.
CRS Grading
Cytokine release syndrome was graded according to the criteria proposed by Lee et al.13,16 and reported on a simplified scale: 0 = none, 1 = mild, and 2 = severe (requiring pressors and/or positive pressure ventilation).
Statistics
Correlations of continuous and/or categorical variables were evaluated by using the Kruskal–Wallis test and uncorrected Dunn post hoc test. Binary variables were evaluated using the chi-squared test.
RESULTS
Duration of EEG Monitoring
One hundred consecutive patients who received CD19-directed CAR T cells were included in the study. Of this group, 19 subjects (8 women, 11 men) underwent EEG ≤28 days after CAR T cell infusion (Fig. 1 and Tables 2 and 3). The decision to obtain EEG was made by the consulting neurology team as part of the management of acute neurotoxicity. EEG was recorded in all neurotoxicity patients who had seizures or concern for subclinical seizures, and cEEG was obtained per ICU protocol in patients with prolonged severe encephalopathy or ongoing seizures (see Methods for details). Seven of 19 subjects had cEEG monitoring lasting 1 to 3 days, and the remainder had 30 to 60 minute EEGs. EEGs were initiated on day 5 to 13 (median day 8) after CAR T cell infusion.
FIG. 1.
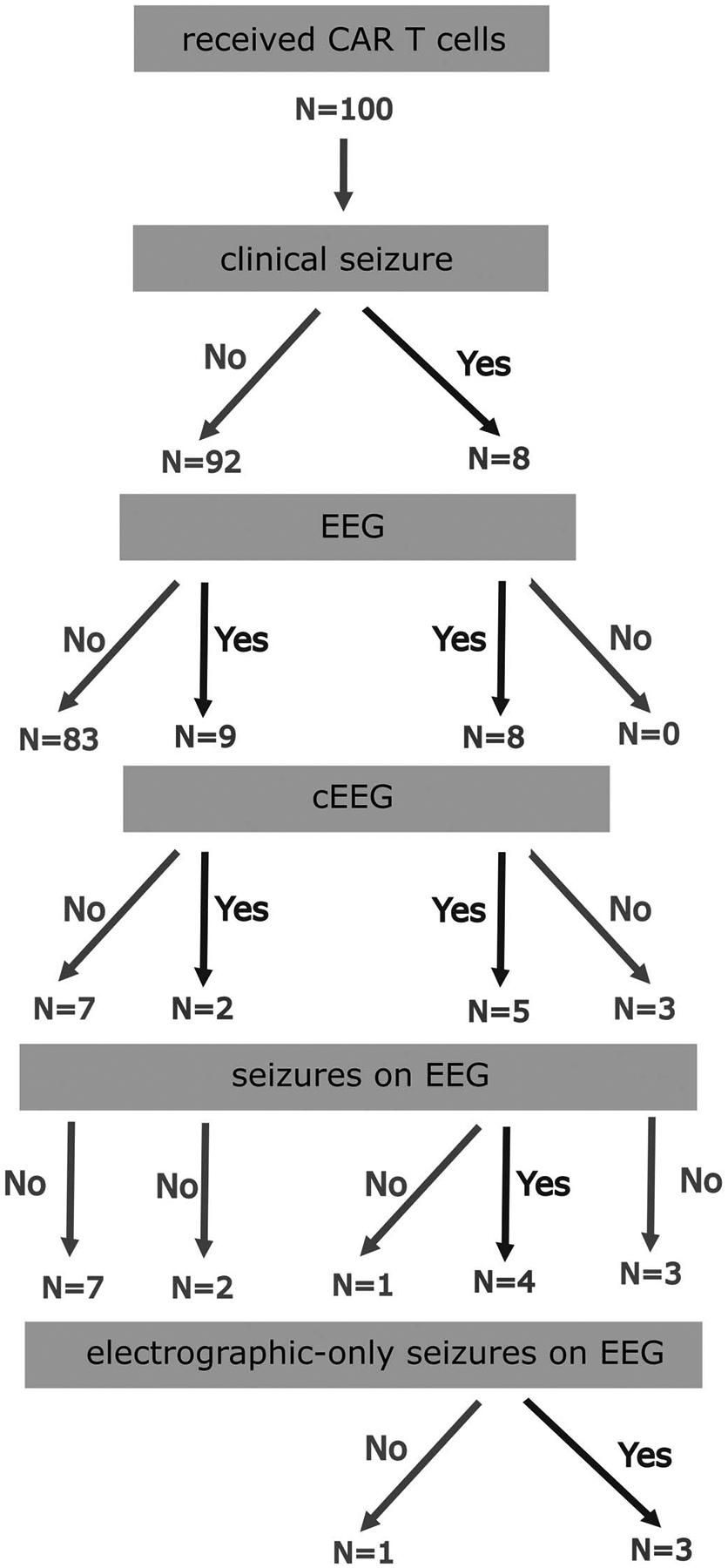
Summary of EEG findings in a 100 subject cohort of pediatric patients treated with CD19-directed CAR T cells. CAR, chimeric antigen receptor.
TABLE 2.
Patient and EEG Characteristics
| cEEG | Routine EEG | P | |
|---|---|---|---|
| N | 7 | 12 | |
| Age at CAR T infusion (mean in years, range) | 14.9 (6–22) | 8.9 (2–23) | 0.025 |
| Female sex | 4 (57%) | 4 (33%) | 0.311 |
| History of epilepsy | 0 (0%) | 1 (8%) | 0.433 |
| Neurotoxicity CTCAE score (mean, range 0–5) | 3.6 | 2.8 | 0.108 |
| CRS grade (mean, range 0–2) | 1.1 | 1.3 | 0.410 |
| Acute MRI abnormality | 5/7 (71%) | 6/8 (75%) | 0.876 |
| Received steroids | 7 | 8 | 0.086 |
| Received tocilizumab | 6 | 7 | 0.216 |
| EEG duration (mean, range) | 48 hours (14–75 hours) | 45 minutes (30–75 minutes) | <0.001 |
| Clinical seizure ≤28 days after CAR T cell infusion | 5 (71%) | 3 (25%) | 0.048 |
| Interictal epileptiform discharges | 4 (57%) | 1 (8%) | 0.020 |
| Seizures on EEG | 4 (57%) | 0 (0%) | 0.003 |
| Electrographic-only seizures | 3 (43%) | 0 (0%) | 0.013 |
| Number of acute antiseizure medications (range) | 2.0 (1–4) | 1.2 (1–2) | 0.050 |
| Number of antiseizure medications after day 28 (range) | 0.85 (0–2) | 0.25 (0–1) | 0.042 |
| Seizures after day 28 | 1 (14%) | 0 (0%) | 0.179 |
CTCAE, common terminology criteria of adverse events, designating most severe score of any symptom throughout the patient’s course. CRS, cytokine release syndrome (none is coded as 0, mild = 1, severe = 2).
CAR, Chimeric Antigen Receptor; MRI, magnetic resonance imaging.
Bolded items denote variables which are statistically significantly different between groups, P < 0.05.
TABLE 3.
Continuous EEG Characteristics and Neurotoxicity Outcomes
| Patient ID | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Age (years) | 22 | 16 | 16 | 6 | 15 | 13 | 16 |
| Neurotoxicity grade | 4 | 4 | 3 | 4 | 4 | 3 | 2 |
| CRS grade | Severe | Severe | Mild | Mild | Mild | Mild | Mild |
| EEG duration | 75 hours | 60 hours | 48 hours | 58 hours | 52 hours | 32 hours | 14 hours |
| Clinical symptoms | Focal seizures, decreased level of consciousness | Coma | Generalized seizure, decreased level of consciousness | Focal seizures, decreased level of consciousness | Focal seizures, decreased level of consciousness | Decreased level of consciousness, abnormal movements | Drowsiness and confusion |
| Background | 2–3 Hz slowing, decreased R sided voltages, slight improvement toward end of recording | 0.5–3 Hz slowing, initially discontinuous, FIRDA, improving over time of recording | 1–3 Hz slowing, no change throughout recording | 0.5–6 Hz slowing, becoming discontinuous after 12 hours, then continuous again after 36 hours | 1–7 Hz slowing, initial voltage attenuation, improving throughout recording | 0.5–6 Hz slowing, improving markedly throughout recording | 2–5 Hz slowing, stable |
| PDR | Present at beginning and end | No | No | No | No | No | No |
| Focal slowing | Yes | No | No | Yes | Yes | Yes | No |
| Sleep architecture | No | No | Intermittent | No | No | No | Yes |
| Interictal epileptiform | R occipital periodic discharges | No | Multifocal sharps and spikes | Lateralized periodic discharges | Focal rhythmic discharges | No | No |
| Ictal-interictal continuum | R occipital periodic discharges at 1–2 Hz, without evolution or response to treatment, persist throughout recording | No | No | Lateralized periodic discharges at 0.5–1 Hz, resolved on day 2 | No | No | No |
| Seizure | Multiple R > L occipital seizures, with and without clinical manifestations | No | Generalized electroclinical seizure at EEG initiation | Multiple focal seizures, with and without clinical manifestations | Many focal seizures, with or without clinical manifestations | No | No |
| Sedating infusions | None | None | None | Midazolam first 12 hours (for seizures) | Midazolam (for seizures), dexmedetomidine, fentanyl (for sedation) | Propofol at the beginning (for sedation) | None |
| Bolus medications during recording | LEV, PHT | LEV | Lorazepam, LEV | Lorazepam, LEV, PHT, PHB | LEV, PHT | LEV | LEV |
| Acute MRI findings | R > L occipital cortical diffusion restriction, bilateral thalamic T2 prolongation | New diffuse symmetric white matter T2 prolongation | No acute changes, chronic white matter increased T2 signal | Bilateral thalamic and symmetric white matter T2 prolongation | Symmetric diffusion restriction and T2 prolongation in thalami, pons, and white matter | Mild symmetric white matter T2 hyperintensities | Normal |
| Neurologic outcome > 28 days | Focal epilepsy | Mild cognitive complaints | Normal | Normal | Normal | Normal | Return to baseline (patient with trisomy 21) |
Details are shown for all 7 patients who had cEEG. Neurotoxicity grade denotes overall neurotoxicity as determined by the most severe sign or symptom on the CTCAE scale that was present during the entire course: 0, no neurotoxicity; 1, mild; 2, moderate; 3, severe; 4, life threatening.
CRS, cytokine release syndrome; CTCAE, Common Terminology Criteria for Adverse Events; FIRDA, frontal intermittent rhythmic delta activity; LEV, levetiracetam; MRI, magnetic resonance imaging; PDR, posterior dominant rhythm; PHB, phenobarbital; PHT, fosphenytoin.
EEG Background Abnormalities
During Neurotoxicity
Neurotoxicity is a dynamic process that is typically monophasic, with rapid onset and gradual improvement over the course of several days.4,9,14 To determine whether EEG background accurately reflects the severity of neurotoxicity compared with standard clinical measures, we graded the background of all study EEGs on the Synek scale from 0 (normal) to 5 (severe amplitude suppression) (see Methods). For cEEGs, we selected 30-minute epochs every 12 hours for evaluation, which allows for correlation with the twice-daily CAPD scoring.
The EEG background was abnormal in all epochs for all 19 subjects. In some of the subjects, pharmacologic sedation might have partially contributed to the abnormal background, but in others, no sedation was administered (Table 3). The severity of background abnormalities on the Synek scale was correlated with the CTCAE neurotoxicity score of the patients during the EEG recording (P = 0.0022, Kruskal-Wallis test) (Fig. 2A). Median EEG background grade was 2 for patients with CTCAE grade 0 to 2 neurotoxicity, 3 for grade 3 neurotoxicity, and 4 for grade 4 neurotoxicity. We also found a positive correlation between EEG score and CAPD (P = 0.0085, Kruskal–Wallis test) (Fig. 2B). The median CAPD score was 12 for grade 1 EEGs, 11 for grade 2, 20 for grade 3, 22 for grade 4, and 24 for grade 5. For patients with CAPD ≥ 20, EEG backgrounds ranged from reactive patterns with delta predominance to nonreactive, discontinuous, or severely suppressed patterns. This indicates a ceiling effect of the CAPD, which is primarily designed to detect delirium in awake patients.
FIG. 2.
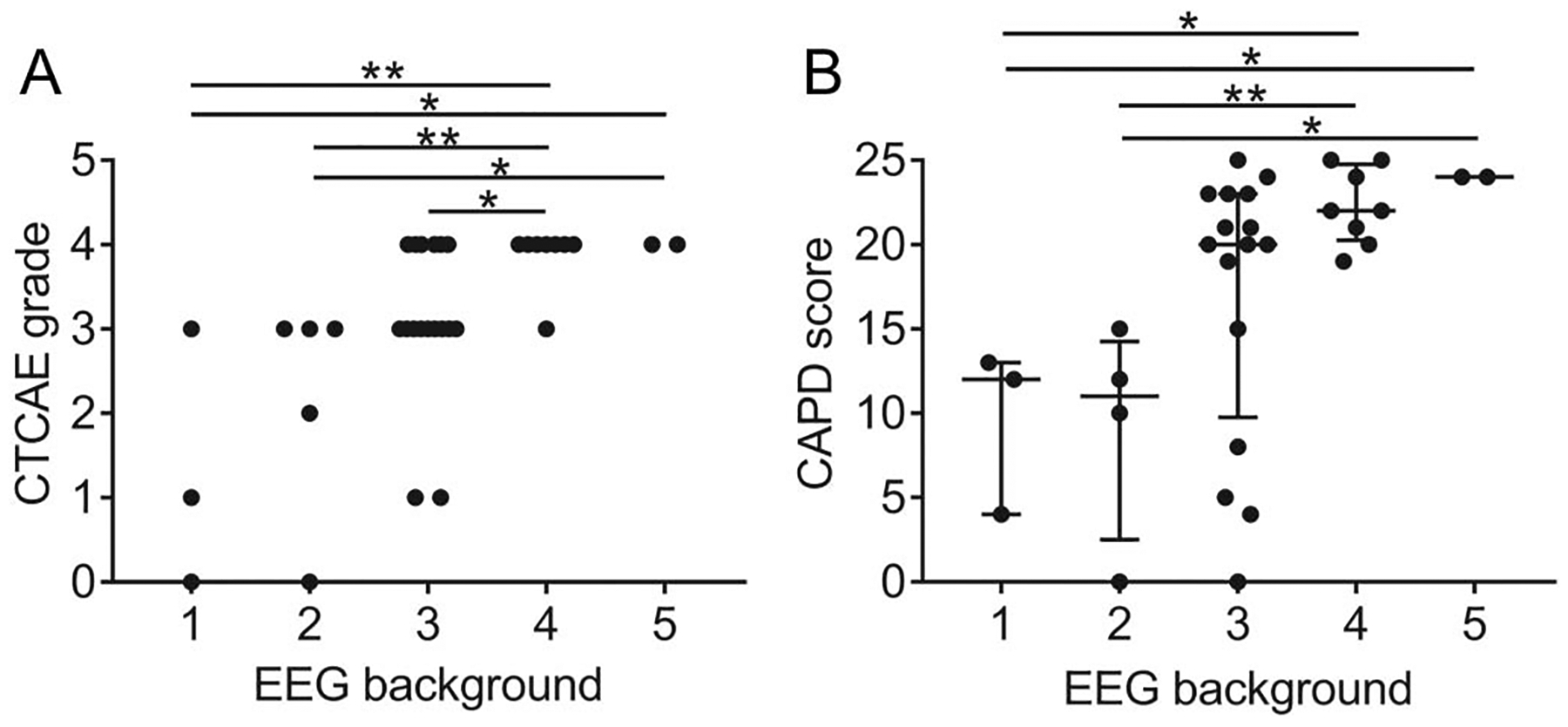
EEG background correlates with clinical neurotoxicity and delirium grading. A, x-axis shows the EEG background score, and y-axis shows CTCAE neurotoxicity grade that was present during the EEG recording. Each symbol indicates an individual 30-minute epoch. B, x-axis shows EEG background score, and y-axis shows CAPD score obtained within 4 hours of the EEG score. Each data point represents an individual 30-minute epoch. Bars show the median and interquartile range. *P < 0.05; **P < 0.01; ***P < 0.001. CAPD, Cornell Assessment of Pediatric Delirium; CTCAE, Common Terminology Criteria for Adverse Events.
A posterior dominant rhythm, indicative of a well-preserved cerebral function, was only identified in patients with low concurrent CAPD scores (N = 9, CAPD range 0–15) but was absent in some with similar scores (N = 3, CAPD range 0–15). This suggests that in some cases the EEG may be more sensitive than the CAPD in detecting subtle neurologic dysfunction.
The EEG background showed a range of patterns of evolution over time (Table 3 and Fig. 3), and in most cases, cEEGs did not capture the entire episode of neurotoxicity. Patients with more severe clinical manifestations (treatment-refractory seizures, coma) had more prolonged background pattern abnormalities lasting several days without significant pattern change. When cEEG was obtained during the lead-in or recovery phase of neurotoxicity, the background showed an overall monophasic pattern of worsening, plateau, and improvement. However, the recovery period was typically not captured in its entirety on cEEG, and we therefore do not know whether the EEG background lags behind the clinical normalization. Subjects with milder neurotoxicity showed a much more dynamic EEG background, with rapid improvement of the background over a range of hours. Treatment with immunomodulation (steroids, IL-6 blockade with tocilizumab and/or IL-1 blockade with anakinra) did not have obvious short-term effects on the EEG background, and evaluation of treatment effects was complicated by the fact that most patients had already been receiving immunomodulation for several hours to days before EEG initiation.
FIG. 3.
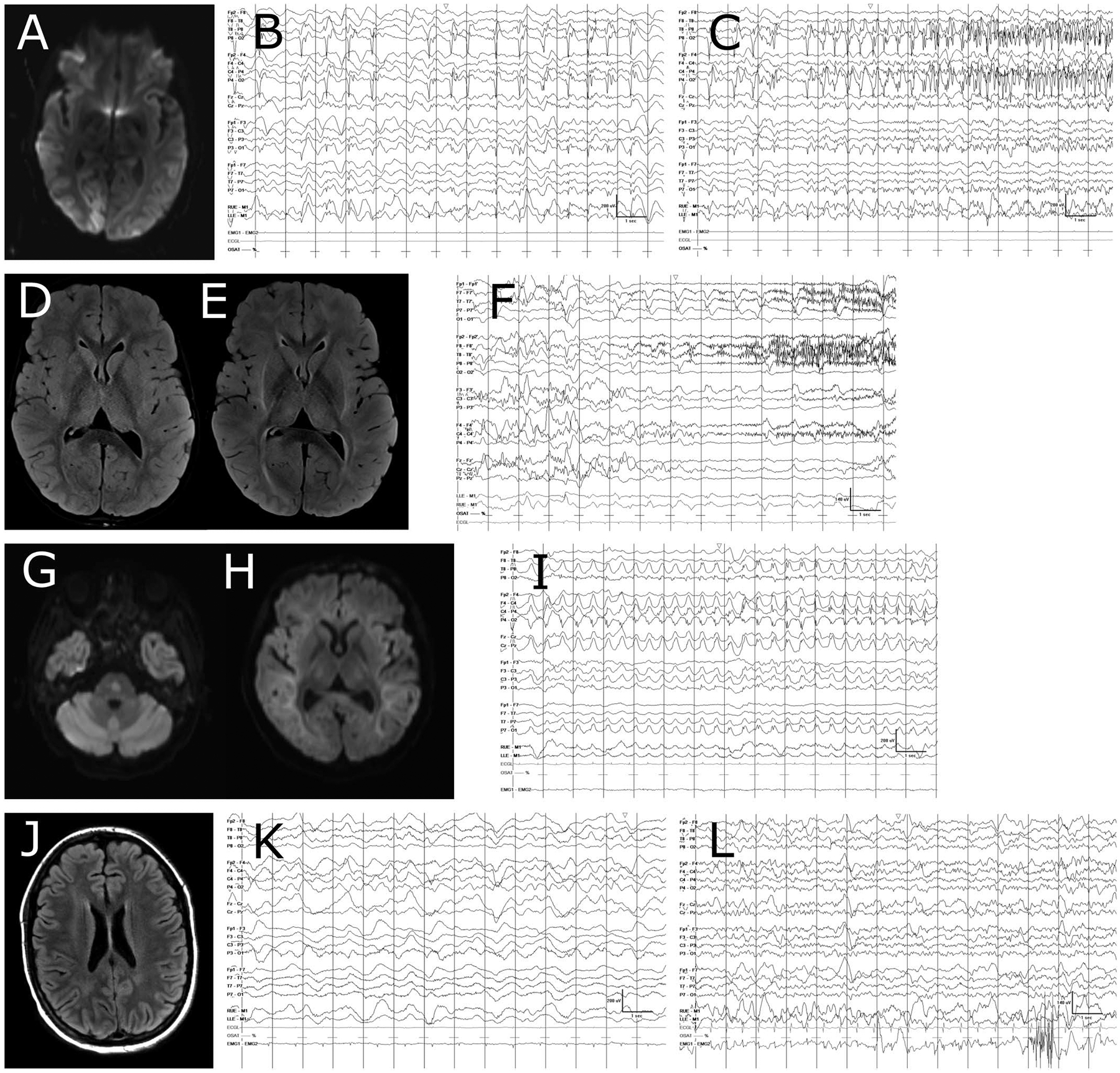
MRI and EEG findings during neurotoxicity. Selected cases are shown, with subject ID numbers corresponding to Table 3. A–C, Subject 1. A, diffusion-weighted imaging (DWI) showing R > L occipital cortical diffusion restriction. B, Shows lateralized periodic discharges without evolution in frequency or field, which were present for several days on cEEG. In (C), onset of a seizure from the background of periodic discharges is shown. D–F, Subject 4. D, Shows fluid-attenuated inversion recovery (FLAIR) sequence obtained on day 6 after CAR T cell infusion. There is mild edema in the bilateral thalami and subtle white matter signal abnormalities. E, Shows the same sequence on day 9, with more prominent white matter FLAIR signal and more pronounced bithalamic edema. This change in imaging was accompanied by a worsening of EEG background toward a more discontinuous pattern. F, Shows left-sided periodic discharges, and a seizure with fast activity arising from the right hemisphere at the same time. The seizure had clinical manifestations of left upper extremity shivering. G-I, Subject 5. G, DWI sequence showing diffusion restriction in the pons. H, DWI sequence showing extensive diffusion restriction throughout the supratentorial white matter, with relative sparing of the cortex. I, Shows the evolution of a typical focal seizure for this subject. J-L, Subject 2. J, FLAIR sequence showing mild diffuse supratentorial white matter T2 hyperintensities, which were not present on MRI before CAR T cell treatment. K, Mildly asymmetric slow wave pattern, the patient was comatose without sedation at the time of this recording. L, EEG obtained 9 days later, at which time the patient was awake and alert, but having difficulty answering orientation questions. EEG grid lines represent 1 second intervals. CAR, Chimeric Antigen Receptor.
cEEG Has High Yield of Seizure Detection in Subjects With Clinical Seizures
Of the 100 subjects who received CAR T cells, clinical seizures occurred in 8 (8%) within 28 days after infusion. Acute abnormalities on brain magnetic resonance imaging were seen in 6 of the 8 patients with seizures, with subcortical-only lesions in all but one (Fig. 3 and Table 3).
EEGs were obtained in all 8 patients who had seizures: 3 had 30- to 60-minute routine EEGs and 5 had cEEG monitoring. Two additional patients with severe encephalopathy but no clinical seizures also had cEEGs. Electroclinical seizures were captured in 4 of the 7 patients who had cEEG monitoring and in none of the patients who had routine EEGs. All 4 of the patients with electroclinical seizures had preceding clinical seizures, and 3 had additional electrographic-only seizures (Table 3). In 2 of these subjects, seizures were still occurring >48 hours after initiation of cEEG, despite the treatment with antiseizure medications.
Ictal and Interictal Patterns
Seizures arose from the parietal and/or occipital head regions in 3 of the 4 patients whose seizures we were able to record, and seizure onset was not captured in 1 patient. The longest recorded seizure lasted 10 minutes. No status epilepticus was recorded on EEG.
Interictal epileptiform discharges were seen in 5 of the 19 subjects (4 with cEEG and 1 with routine EEG). All 5 had previous clinical seizures. The interictal discharges included focal and multifocal spikes and sharp waves, as well as lateralized periodic discharges. Two subjects had a high burden of lateralized or focal periodic discharges on the ictal-interictal continuum, which intermittently evolved into definite seizures that had a well-defined onset and end and were often accompanied by clinical manifestations (Table 3 and Fig. 3). However, both also had epochs of periodic discharges where the distinction between ictal and interictal was ill-defined.
Other focal abnormalities (focal slowing in all cases) occurred in 6 subjects, 3 of whom had preceding seizures. No seizures or interictal epileptiform discharges were found in the subjects who did not have previous clinical seizure activity.
Clinical Seizure Characteristics and Long-Term Seizure Outcomes
The majority of clinical seizures not captured on EEG had focal onset with unilateral limb shaking, often followed by secondary generalization. Seizures resolved within 28 days after CAR T cell infusion in all but one subject who had posterior cortical cytotoxic edema and acute symptomatic seizures arising from the same region (subject 1, Fig. 3). The area of injury evolved into cortical atrophy, and the patient developed focal epilepsy attributable to the injured area and required long-term antiseizure medications. Histopathologic findings from this patient have been previously described.14 This picture represents a very rare form of CAR T cell neurotoxicity that has only been described in one other patient.4
In all others, antiseizure medications were discontinued successfully ≤6 months after CAR T cell treatment. None of the patients who developed seizures had a history of epilepsy, but one patient with a history of epilepsy and nonepileptic spells had a recurrence of nonepileptic spells in the setting of CRS and mild delirium. These were characterized by side-to-side head movements and pelvic thrusting, were distractible by caregiver intervention, and were not accompanied by any EEG changes.
DISCUSSION
We show that EEG background patterns accurately reflect the degree of clinical neurotoxicity during the EEG recording after CD19-directed CAR T cell therapy, confirming our initial hypothesis. In addition, we find that a simple classification scheme for EEG background allows for additional differentiation of the patient’s neurologic status when combined with CAPD and/or CTCAE criteria, especially in patients with severe ICANS who are poorly responsive or comatose. These findings support the need for further investigation of EEG background as a tool for monitoring of ICANS.
We found that the Synek15 EEG background scale provided an appropriate dynamic range for describing the background abnormalities seen in our pediatric CAR T cell patient population. The Synek scale was originally developed for patients with coma after hypoxic brain injury or trauma but has also been used in more clinically comparable settings such as septic encephalopathy.15,17–19 A similar scale by Young et al.17 does not provide a category for mild abnormalities and includes a category of triphasic waves, which did not occur in our pediatric patient population. Based on our data, the Synek scale appears to be a useful tool for clinical and/or research applications because it captures the entire range of abnormalities that would be expected in patients with ICANS, from mild slowing to electrocerebral silence.
The applicability of our findings to other immunotherapy patient populations is uncertain, given the broad age range, diversity of underlying diagnoses, and differences in cell-based immunotherapy products.5 Abnormal EEG backgrounds with diffuse slowing have been described in several other cohorts of CAR T cell patients,4,8,9 but EEG background correlation with concurrent clinical signs or symptoms has not been previously provided. Generalized periodic discharges were not seen in our cohort, in contrast to a series of 4 adults with CAR T cell neurotoxicity who all had generalized periodic discharges.20
There are currently no universally accepted criteria for EEG monitoring of CAR T cell patients. Based on our findings, we propose a 3-tiered risk classification scheme to guide EEG monitoring decisions in the pediatric population. Group 1 would contain high-risk patients with clinical seizures and persistent abnormal mental status (CAPD ≥ 9). In our cohort, all 4 patients who met these criteria had cEEG monitoring, and 100% had additional electrographic seizures, some persisting on days 2 to 3 of monitoring. Thus, continuous EEG monitoring until 24 hours of seizure freedom will likely be high yield in this group of patients.
Group 2 comprises intermediate-risk patients who may have severe encephalopathy or isolated seizures without persistent encephalopathy. In addition, one may also include patients with sedation requirement, acute CNS imaging abnormalities, and/or severe CRS. In our cohort, 3 patients who met these criteria had cEEGs and none showed seizures. Of the 11 who had routine EEG, only one had interictal epileptiform abnormalities. However, our conclusions for this group of patients are limited by the lack of standardized criteria to trigger EEG monitoring and guide duration. It is possible that electrographic-only seizures were missed in patients who received either brief or no EEG monitoring and we likely have underestimated the true incidence of seizures in this patient group.21–23 Others have described electrographic-only seizures in adults with CAR T-related encephalopathy who had no clinically apparent seizures,10 and thus, an increased index of suspicion is likely warranted.
Finally, group 3 captures standard risk patients without the features of group 1 or 2. Only one patient from this group had an EEG in our cohort, which was to characterize nonepileptic spells. Extending EEG monitoring to patients with milder or no clinical neurotoxicity may uncover subtle brain dysfunction and help in early detection of deterioration, which may then trigger additional interventions such as immunomodulatory therapy.
As a next step, we propose the development of EEG monitoring criteria that can be prospectively applied to all patients who receive CAR T cell treatment. Such a study will allow for better understanding of the incidence of seizures in at-risk patients and provide insights into encephalopathy EEG patterns and correlation with outcomes.
Acknowledgments
J. Gust received support by the NIH (CNCDP-K12, 1K12NS098482-02).
Footnotes
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Annesley CE, Summers C, Ceppi F, Gardner RA. The evolution and future of CAR T cells for B-cell acute lymphoblastic leukemia. Clin Pharmacol Ther 2018;103:591–598. [DOI] [PubMed] [Google Scholar]
- 2.Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant 2019;25:625–638. [DOI] [PubMed] [Google Scholar]
- 3.Shimabukuro-Vornhagen A, Gödel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer 2018;6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gust J, Hay KA, Hanafi LA, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov 2017;7:1404–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gust J, Taraseviciute A, Turtle CJ. Neurotoxicity associated with CD19-targeted CAR-T cell therapies. CNS Drugs 2018;32:1091–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Common Terminology criteria for adverse events (CTCAE) Common Terminology criteria for adverse events (CTCAE) protocol development CTEP [online]. Available at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm. Accessed April 27, 2018.
- 7.Traube C, Silver G, Kearney J, et al. Cornell Assessment of Pediatric Delirium: a valid, rapid, observational tool for screening delirium in the PICU*. Crit Care Med 2014;42:656–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karschnia P, Jordan JT, Forst DA, et al. Clinical presentation, management, and biomarkers of neurotoxicity after adoptive immunotherapy with CAR T-cells. Blood 2019;133:2212–2221. [DOI] [PubMed] [Google Scholar]
- 9.Santomasso BD, Park JH, Salloum D, et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov 2018;8:958–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy—assessment and management of toxicities. Nat Rev Clin Oncol 2018;15:47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abend NS, Dlugos DJ, Clancy RR. A review of long-term EEG monitoring in critically ill children with hypoxic-ischemic encephalopathy, congenital heart disease, ECMO, and stroke. J Clin Neurophysiol 2013;30:134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilmore EJ, Gaspard N, Choi HA, et al. Acute brain failure in severe sepsis: a prospective study in the medical intensive care unit utilizing continuous EEG monitoring. Intensive Care Med 2015;41:686–694. [DOI] [PubMed] [Google Scholar]
- 13.Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood 2017;129:3322–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gust J, Finney OC, Li D, et al. Glial injury in neurotoxicity after pediatric CD19-directed chimeric antigen receptor T cell therapy. Ann Neurol 2019;86:42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Synek VM. Prognostically important EEG coma patterns in diffuse anoxic and traumatic encephalopathies in adults. J Clin Neurophysiol 1988;5:161–174. [DOI] [PubMed] [Google Scholar]
- 16.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014;124:188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young GB, McLachlan RS, Kreeft JH, Demelo JD. An electroenceph-alographic classification for coma. Can J Neurol Sci 1997;24:320–325. [DOI] [PubMed] [Google Scholar]
- 18.Roest A, van Bets B, Jorens PG, Baar I, Weyler J, Mercelis R. The prognostic value of the EEG in postanoxic coma. Neurocrit Care 2009;10:318–325. [DOI] [PubMed] [Google Scholar]
- 19.Azabou E, Magalhaes E, Braconnier A, et al. Early standard electroencephalogram abnormalities predict mortality in septic intensive care unit patients. PLoS One 2015;10:e0139969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herlopian A, Dietrich J, Abramson JS, Cole AJ, Westover MB. EEG findings in CAR T-cell therapy-related encephalopathy. Neurology 2018;91:227–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Struck AF, Osman G, Rampal N, et al. Time-dependent risk of seizures in critically ill patients on continuous electroencephalogram. Ann Neurol 2017;82:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Struck AF, Ustun B, Ruiz AR, et al. Association of an electroencephalography-based risk score with seizure probability in hospitalized patients. JAMA Neurol 2017;74:1419–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shafi MM, Westover MB, Cole AJ, Kilbride RD, Hoch DB, Cash SS. Absence of early epileptiform abnormalities predicts lack of seizures on continuous EEG. Neurology 2012;79:1796–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]


