Abstract
Aims
To examine perceptions of extended‐release (XR) buprenorphine injections among people who regularly use opioids in Australia.
Design
Cross‐sectional survey prior to implementation. XR‐buprenorphine was registered in Australia in November 2018.
Setting
Sydney, Melbourne and Hobart.
Participants
A total of 402 people who regularly use opioids interviewed December 2017 to March 2018.
Measurements
Primary outcome concerned the proportion of participants who believed XR‐buprenorphine would be a good treatment option for them, preferred weekly versus monthly injections and perceived advantages/disadvantages of XR‐buprenorphine. Independent variables concerned the demographic characteristics and features of current opioid agonist treatment (OAT; medication‐type, dose, prescriber/dosing setting, unsupervised doses, out‐of‐pocket expenses and travel distance).
Findings
Sixty‐eight per cent [95% confidence interval (CI) = 63–73%] believed XR‐buprenorphine was a good treatment option for them. They were more likely to report being younger [26–35 versus > 55 years; odds ratio (OR) = 3.16, 95% CI = 1.12–8.89; P = 0.029], being female (OR = 1.67, 95% CI = 1.04–2.69; P = 0.034), < 10 years school education (OR = 1.87, 95% CI = 1.12–3.12; P = 0.016) and past‐month heroin (OR = 1.81, 95% CI = 1.15–2.85; P = 0.006) and methamphetamine use (OR = 1.90, 95% CI = 1.20–3.01; P = 0.006). Fifty‐four per cent reported no preference for weekly versus monthly injections, 7% preferred weekly and 39% preferred monthly. Among OAT recipients (n = 255), believing XR‐buprenorphine was a good treatment option was associated with shorter treatment episodes (1–2 versus ≥ 2 years; OR = 3.93, 95% CI = 1.26–12.22; P = 0.018), fewer unsupervised doses (≤ 8 doses past‐month versus no take‐aways; OR = 0.50; 95% CI = 0.27–0.93; P = 0.028) and longer travel distance (≥ 5 versus < 5 km; OR = 2.10, 95% CI = 1.20–3.65; P = 0.009). Sixty‐nine per cent reported ‘no problems or concerns’ with potential differences in availability, flexibility and location of XR‐buprenorphine.
Conclusions
Among regular opioid users in Australia, perceptions of extended‐release buprenorphine as a good treatment option are associated with being female, recent illicit drug use and factors relating to the (in)convenience of current opioid agonist treatment.
Keywords: Buprenorphine depot, buprenorphine injection, depot preparations, medication‐assisted treatment, patient preferences, opioiduse disorder
Introduction
The mainstays of opioid agonist treatment (OAT), methadone and buprenorphine (± naloxone), are well established as effective treatments for opioid use disorder 1, 2. OAT interrupts the cycle of intoxication and withdrawal, greatly reducing illicit opioid use, crime, overdose and mortality risk 3, 4, 5. Despite these clear health benefits, OAT carries some risks, including non‐adherence, injection of medication intended for oral/sublingual administration, diversion and overdose 6, 7, 8.
Newer extended‐release (XR) buprenorphine formulations for the treatment of opioid use disorder have been developed, including subdermal implants and subcutaneous injections 9. In the United States, 6‐monthly subdermal buprenorphine implants have been approved for the treatment of opioid use disorder since 2016 9, 10. More recently, once‐monthly XR‐buprenorphine subcutaneous injections have been approved in the United States 11 and once‐weekly and once‐monthly subcutaneous injections have been approved in Australia and Europe 11, 12.
An emphasis upon supervised daily dosing is a feature of OAT in many countries 13, 14, 15, 16, including Australia, but people in treatment often state that attendance for supervised dosing is burdensome and restrictive on many other aspects of daily life 17, especially if significant travel time is required. In addition, Australian OAT clients frequently pay the costs of pharmacy dispensing fees 18. Although the out‐of‐pocket client costs associated with XR‐buprenorphine are not yet clear, eliminating the need for frequent attendance at the pharmacy or clinic for medication dosing may increase the time available for work and education 9, 17. XR‐buprenorphine delivered by a health‐care professional may also reduce both diversion of the medication and use via unintended routes of administration 9. It is important to understand how changes such as these are perceived among the potential client population 19, 20 to inform the implementation into treatment programmes of this formulation.
To date, two studies have been conducted internationally, examining clients’ perspectives on the impacts of changes to the frequency of dosing for XR‐buprenorphine and potential safety concerns 21, 22. These qualitative studies indicate that perceptions of XR‐buprenorphine injections and implants may be influenced by a range of physical, psychological and social factors 22. Our study aims to develop an understanding of the perceptions of XR‐buprenorphine in a larger sample of potential clients and examine correlates of these perceptions.
The specific aims of this study are to examine:
The proportion of people who use opioids regularly who consider that XR‐buprenorphine would be a good treatment option for them;
The correlates of perceiving XR‐buprenorphine as a good treatment option;
Preferences for a weekly versus monthly injection of XR‐buprenorphine and correlates of such preferences;
Whether current OAT and features of treatment delivery are associated with perceiving XR‐buprenorphine as a good treatment option (among current OAT clients); and
The perceived advantages and disadvantages of XR‐buprenorphine.
Methods
Study design
In this cross‐sectional study to evaluate perception of XR‐buprenorphine, we enrolled participants from three Australian jurisdictions (Sydney, New South Wales; Melbourne, Victoria; and Hobart, Tasmania) between December 2017 and March 2018. These jurisdictions were chosen to ensure adequate representation of the mix of Australian treatment settings (public clinics, general practice/private prescribers and community pharmacy dosing) 23 within the available budget and time constraints. No formal sample size calculations were undertaken, as the study objectives were descriptive. This survey was conducted prior to the registration and marketing of XR‐buprenorphine injection formulations in Australia, although a small number of Australian sites had participated in international Phase 3 trials of a once‐weekly or once‐monthly buprenorphine injection during 2016–17. The STROBE checklist is provided in Supporting information, Appendix S1. The analyses were not pre‐registered; therefore, the results should be considered exploratory.
Ethics
This study was reviewed and approved by South Eastern Sydney Local Health District Human Research Ethics Committee 17/224 (HREC/17/POWH/486), Tasmania Health and Medical HREC (H0017051) and the Alfred Hospital (515/17).
Participants
To be eligible for the study, participants were aged 18 years or older and able to provide voluntary and informed consent; and were either using illicit/extra‐medical opioids regularly or currently receiving treatment for opioid use disorder (or both). In this study, regular opioid use was operationalized as use of any opioid (including heroin use or the extra‐medical use of pharmaceutical opioids) on at least 21 of the past 28 days.
Procedure
Participants were recruited from a range of settings, including needle and syringe programmes, OAT services, snowballing (where eligible participants promote the study via their personal networks 24) and word‐of‐mouth. The study was advertised at services using posters and fliers, but service staff were not directly involved in recruitment. Interested participants contacted the study team directly and were screened for eligibility over the telephone.
Interviewers met participants in person to obtain consent and conduct the interview. Interviews were conducted using a computer‐assisted structured interview schedule administered via computer tablets. Interviews were conducted by trained interviewers who, at a minimum, received training in responding to adverse events such as participant distress or suicidal ideation. The interviews took approximately 1 hour to complete, and participants were reimbursed $AUD50 for their time and out‐of‐pocket expenses.
Measures
Perceptions of XR‐buprenorphine
Interviewers read a brief description of XR‐buprenorphine to all participants (Supporting information, Appendix S2), followed by questions examining perceptions of XR‐buprenorphine. The main outcome in the current study is the item ‘Do you think a long‐acting injection of buprenorphine (‘depot’) would be a good treatment option for you personally?’ (yes/no/not sure).
Participants were also asked a range of additional items to elicit their perceptions of XR‐buprenorphine (see Supporting information, Appendix S2 for the full module of questions and exact item wording), including:
Preferences for the frequency of XR‐buprenorphine injections based on rating the likelihood that they would use weekly versus monthly XR‐buprenorphine injections on a Likert scale from 0–10, where 0 = ‘not at all likely’ and 10 = ‘extremely likely’.
Perceptions of the advantages and disadvantages of treatment with XR‐buprenorphine from a pre‐determined list (multiple responses were allowed), followed by an open‐ended prompt for ‘Other (specify)’.
Willingness to change doctors for treatment with XR‐buprenorphine (yes/no/not sure).
Distance willing to travel for treatment with XR‐buprenorphine measured in travel time (response categories: ‘not interested/not willing to travel at all’, ‘less than 15 minutes’, ‘15–30 minutes’, ‘30–45 minutes’ ‘45–60 minutes’, ‘60–90 minutes’ or ‘more than 90 minutes’).
The extent to which pre‐determined features of treatment with XR‐buprenorphine would raise problems or concerns for them personally (‘no problems or concerns’/‘minor problems or concerns’/‘major problems or concerns’).
Demographic and clinical characteristics
Participants’ age, gender, country of birth (additional data on language and cultural background were not collected), employment status, main source of income, highest level of education and whether or not they were homeless were recorded. ‘Homelessness’ included primary (e.g. sleeping rough), secondary (e.g. couch‐surfing or hostel) and tertiary homelessness (e.g. insecure accommodation such as a caravan) 25. Participants were asked about life‐time substance use and days of use in the past 28 days. ‘Hazardous drinking’ was defined as meeting the cut‐point score of 4 for males and 3 for females on the Alcohol Use Disorder Identification Test, version C (AUDIT‐C) 26.
Treatment history
Participants were asked about their life‐time engagement with a range of possible treatments for opioid use disorder, including OAT. Those currently receiving OAT were asked about the characteristics of their current treatment episode, including medication‐type, length of current treatment episode (years), dosing setting, prescriber setting, main opioid of concern at treatment entry, scheduled doses, missed doses, supervised/unsupervised doses, out‐of‐pocket expenses (dispensing fees, appointment fees, travel costs) and distance travelled for dosing.
Analyses
Data were analyzed using Stata SE version 15.1. Variables were summarized using frequencies and valid percentages, as appropriate. Missing cases for individual variables are reported in the table notes.
Demographic characteristics and substance use of the sample were examined according to (a) whether or not participants believed XR‐buprenorphine might be a good treatment option for them (primary study outcome) and (b) whether or not participants were currently receiving OAT (additional analyses; Supporting information, Appendix S4). Multiple logistic regression controlling for age and gender was used to calculate adjusted odds ratios (aORs) and 95% confidence intervals (95% CI). Characteristics of current OAT episode (among current OAT clients) were examined according to whether or not participants believed XR‐buprenorphine was a good treatment option for them using multiple logistic regression models controlling for age, gender and length of current treatment episode.
Individual differences in Likert scale ratings for weekly versus monthly (i.e. the likelihood rating for weekly injections minus the likelihood rating for monthly injections) were used to determine whether participants held a preference for one over the other. Where the difference equalled zero, the preference was coded as ‘no preference’. Multinomial regression was used to examine whether age, gender, past heroin use, past‐month methamphetamine use and current OAT were associated with displaying no preference, a preference for weekly or a preference for monthly injections. In order to compare groups with a preference for weekly versus monthly injections, the larger of these two groups was selected as the referent group (preference for monthly injections) (Supporting information, Appendix S5).
The pre‐determined list of advantages and disadvantages of XR‐buprenorphine was analyzed using percentages. Where participants endorsed ‘Other (specify)’, free text responses were subsequently re‐categorized where appropriate, or new categories were created and listed by frequency. Additional multiple logistic regression models (controlling for age and gender) were conducted to examine for whether perceptions of XR‐buprenorphine differed according to whether or not participants were currently receiving OAT (Supporting information, Appendix S6).
To check for heterogeneity in key characteristics across the three survey sites (New South Wales, Victoria and South Australia), we (a) examined differences in the primary outcome (‘Perceptions that XR‐buprenorphine was a good treatment option for them’) and (b) included any relevant interaction terms (jurisdiction × characteristic) in each of the logistic regressions shown in Table 1 below. These analyses indicated that there was no substantial evidence of heterogeneity; therefore, the results can be taken as representing similar Australian jurisdictions.
Table 1.
Demographic, clinical and substance use profile of the study sample according to whether participants perceived XR‐buprenorphine was a good treatment option for them (n = 382).
| Perceived XR‐buprenorphine was a good treatment option for them, n = 382a | Adjusted odds ratiosd | |||
|---|---|---|---|---|
| No/not sure n = 122 (32%) | Yes n = 260 (68%) | |||
| n (%) | n (%) | aOR (95% CI) | P‐value | |
| Demographic characteristics | ||||
| Age, years (n = 382) | ||||
| ≤ 25 | 5 (38%) | 8 (62%) | 1.66 (0.39–1.07) | 0.491 |
| 26–35 | 21 (25%) | 63 (75%) | 3.16 (1.12–8.89) | 0.029 |
| 36–45 | 50 (31%) | 112 (69%) | 2.44 (0.93–6.43) | 0.070 |
| 46–55 | 36 (35%) | 68 (65%) | 2.18 (0.81–5.89) | 0.125 |
| > 55 (referent group) | 10 (53%) | 9 (47%) | – | – |
| Gender (n = 382) | ||||
| Male (referent group) | 88 (36%) | 156 (64%) | – | – |
| Female | 34 (25%) | 104 (75%) | 1.67 (1.04–2.69) | 0.034 |
| Education (n = 381) | ||||
| Completed ≥ 10 years school education (referent group) | 94 (36%) | 170 (64%) | – | – |
| Completed < 10 years school education | 27 (23%) | 90 (77%) | 1.87 (1.12–3.12) | 0.016 |
| Main source of incomeb (n = 381) | ||||
| Other (referent group) | 111 (32%) | 236 (68%) | – | – |
| Paid employment | 10 (29%) | 24 (71%) | 1.17 (0.52–2.56) | 0.695 |
| Homelessc (n = 382) | ||||
| No (referent group) | 86 (32%) | 183 (68%) | – | – |
| Yes | 36 (32%) | 77 (68%) | 1.04 (0.64–1.69) | 0.882 |
| Substance use and use disorders | ||||
| Past‐month cocaine use (n = 381) | ||||
| No (referent group) | 112 (33%) | 231 (67%) | – | – |
| Yes | 9 (24%) | 29 (76%) | 1.49 (0.67–3.28) | 0.326 |
| Past‐month (meth)amphetamine use (n = 376) | ||||
| No (referent group) | 69 (39%) | 108 (61%) | – | – |
| Yes | 50 (25%) | 149 (75%) | 1.90 (1.20–3.01) | 0.006 |
| Past‐month heroin use (n = 379) | ||||
| No (referent group) | 52 (40%) | 78 (60%) | – | – |
| Yes | 68 (27%) | 181 (73%) | 1.81 (1.15–2.85) | 0.011 |
| Past‐month morphine use (n = 379) | ||||
| No (referent group) | 98 (32%) | 211 (68%) | – | – |
| Yes | 24 (34%) | 46 (66%) | 1.05 (0.60–1.85) | 0.862 |
| Past‐month oxycodone use (n = 378) | ||||
| No (referent group) | 96 (33%) | 198 (67%) | – | – |
| Yes | 25 (30%) | 59 (70%) | 1.28 (0.75–2.21) | 0.366 |
| Hazardous drinking (AUDIT‐C) (n = 368) | ||||
| No (referent group) | 70 (31%) | 159 (69%) | – | – |
| Yes | 48 (35%) | 91 (65%) | 0.75 (0.48–1.19) | 0.229 |
| Life‐time OAT | ||||
| Ever been in methadone treatment (n = 380) | ||||
| No (referent group) | 24 (32%) | 52 (68%) | – | – |
| Yes | 97 (32%) | 207 (68%) | 0.98 (0.56–1.72) | 0.950 |
| Ever been in buprenorphine ± naloxone treatment (n = 381) | ||||
| No (referent group) | 57 (36%) | 102 (64%) | – | – |
| Yes | 64 (29%) | 158 (71%) | 1.26 (0.80–1.98) | 0.311 |
| Current treatment | ||||
| Current OAT for opioid use disorder (n = 381) | ||||
| No OAT (referent group) | 36 (29%) | 90 (71%) | – | – |
| Methadone | 76 (36%) | 138 (64%) | 0.65 (0.39–1.06) | 0.081 |
| Buprenorphine ± naloxone | 9 (22%) | 32 (78%) | 1.22 (0.52–2.86) | 0.652 |
| Counselling/psychologist/individual CBT sessions (n = 378) | ||||
| No (referent group) | 87 (32%) | 184 (68%) | – | – |
| Yes | 34 (32%) | 73 (68%) | 1.03 (0.63–1.67) | 0.916 |
See Methods for exact item wording; n = 20 participants were missing data on this item.
Main source of income ‘Other’ includes: government benefits or pension, being supported by someone else's income, retirement fund, having no income and ‘other’ source of income.
‘Homeless’ includes primary, secondary or tertiary homelessness 25.
n = 69 cases missing data for this item.
Adjusted odds ratios (AOR) control for age and gender (with the exception of age which controls for gender only, gender which controls for age only).
OAT = opioid agonist treatment (methadone or buprenorphine ± naloxone). AUDIT = Alcohol Use Disorders Identification Test; CBT = cognitive–behavioural therapy.
Results
A total of 402 participants were interviewed in this study [mean age 42 years, standard deviation (SD) = 8.9, 37% female]; demographic and other characteristics are summarized in Supporting information, Appendix S3. The majority of the sample were Australian‐born (85%). Among the 62 participants born elsewhere, 32% were from North‐West Europe, 19% Oceania, 19% South‐East Asia, 11% South and Eastern Europe, 10% North Africa and Middle East and 5% other regions. The vast majority (90%) of participants had life‐time experience of OAT (methadone or buprenorphine ± naloxone) and 67% were currently receiving OAT (56% methadone; 11% buprenorphine ± naloxone). Participants currently receiving OAT were more likely to be female and were less likely to report past‐month use of morphine or oxycodone; however, there were no other significant differences between the in‐ and out‐of‐treatment groups (Supporting information, Appendix S4).
Two‐thirds (68%) of the total sample believed that XR‐buprenorphine was a good treatment option for them after the nature of the treatment was explained to them. Participants who believed XR‐buprenorphine was a good treatment option for them were more likely to be aged 26–35 years (versus > 55 years), female and had completed fewer years of education; they were more likely to report past‐month heroin and methamphetamine use (Table 1). Neither life‐time nor current OAT were associated with participants’ believing that XR‐buprenorphine was a good treatment option for them (Table 1; see Supporting information, Appendix S7 for complete responses, i.e. not dichotomized).
Among the total sample, 15% of participants reported being ‘not at all likely’ (0 on a 0–10 Likert scale) to use a weekly buprenorphine injection and 14% reported being ‘not at all likely’ to use a monthly buprenorphine injection, with no differences according to whether or not they were currently receiving OAT (Fig. 1, Supporting information, Appendix S5). Conversely, 27% of participants reported being ‘extremely likely’ (10 on a 0–10 Likert scale) to use a weekly buprenorphine injection and 47% reported being ‘extremely likely’ to use a monthly buprenorphine injection (Fig. 1). Individual differences in Likert scale scores indicated that 54% of participants reported no difference in their preference for monthly versus weekly injections, 7% showed a preference for weekly injections and 39% showed a preference for monthly injections. There were no significant associations between demographic, drug use or treatment variables and having a preference for weekly or monthly injections (Supporting information, Appendix S5).
Figure 1.
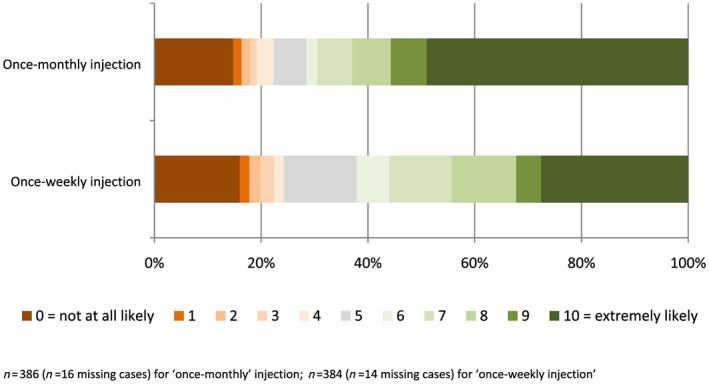
Participant ratings of likelihood of using once‐weekly/once‐monthly XR‐buprenorphine injections (%). [Colour figure can be viewed at wileyonlinelibrary.com]
Among people currently receiving OAT (n = 255), participants who believed XR‐buprenorphine was a good treatment option for them reported a shorter current treatment episode (i.e. higher odds of being in treatment 1–2 years versus ≥ 2 years), travelling greater distances for dosing and fewer unsupervised (or ‘take‐away’) doses (Table 2). Current OAT medication, prescriber setting, dosing setting and out‐of‐pocket costs were not associated with participants’ perceptions that XR‐buprenorphine was a good treatment option for them.
Table 2.
Current OAT clients’ perceptions that XR‐buprenorphine would be a good treatment option for them, according to features of their current OAT.
| Perceived XR‐buprenorphine was a good treatment option for them (n = 255)b | Adjusted odds ratiosc | ||||
|---|---|---|---|---|---|
| Totala N = 266 | No/not suren = 85 (33%) | Yesn = 170 (67%) | |||
| n (%) | n (%) | n (%) | aOR (95% CI) | P‐value | |
| Currently receiving (n = 255): | |||||
| Methadone (referent group) | 224 (84%) | 76 (36%) | 138 (65%) | – | – |
| Buprenorphine ± naloxone | 42 (16%) | 9 (22%) | 32 (78%) | 1.50 (0.62–3.62) | 0.365 |
| Time in current treatment episode, years (n = 229) | |||||
| < 1 | 68 (26%) | 18 (27%) | 49 (73%) | 1.84 (0.95–3.55) | 0.068 |
| 1–2 | 27 (10%) | 4 (15%) | 23 (85%) | 3.93 (1.26–12.22) | 0.018 |
| ≥ 2 (referent group) | 166 (64%) | 59 (38%) | 97 (62%) | – | – |
| Location of last dispensed dose (n = 255) | |||||
| Community pharmacy (referent group) | 115 (43%) | 44 (40%) | 69 (61%) | – | – |
| Other setting (public or private clinic) | 151 (57%) | 41 (29%) | 101 (71%) | 1.64 (0.93–2.91) | 0.089 |
| Prescriber setting (n = 255) | |||||
| Public clinic (referent group) | 173 (65%) | 53 (37%) | 115 (63%) | – | – |
| Other setting | 93 (35%) | 32 (32%) | 55 (69%) | 0.76 (0.43–1.34) | 0.340 |
| Main opioid of concern at treatment entry (n = 252) | |||||
| Heroin (referent group) | 217 (83%) | 68 (33%) | 141 (68%) | – | – |
| Pharmaceutical opioids | 46 (18%) | 16 (37%) | 27 (63%) | 0.83 (0.40–1.73) | 0.618 |
| Dose adherence (n = 254) | |||||
| Took all doses as directed in past 28 days (referent) | 156 (59%) | 51 (34%) | 100 (66%) | – | – |
| Missed a scheduled dose in past 28 days | 108 (41%) | 33 (32%) | 70 (68%) | 0.97 (0.55–1.74) | 0.936 |
| Receiving unsupervised (take‐home) dosesd (n = 254) | |||||
| No (referent group) | 132 (50%) | 36 (28%) | 95 (73%) | – | – |
| 1–7 doses past month | 26 (10%) | 9 (38%) | 15 (63%) | 0.76 (0.27–2.13) | 0.601 |
| ≥ 8 doses past month | 106 (40%) | 40 (40%) | 59 (60%) | 0.50 (0.27–0.93) | 0.028 |
| Out‐of‐pocket expenses for OAT (i.e. pharmacy, travel, and prescriber fees) (n = 242) | |||||
| None (referent group) | 38 (16%) | 13 (34%) | 25 (66%) | – | – |
| $1–35 AUD per week | 96 (40%) | 34 (35%) | 62 (65%) | 0.93 (0.40–2.20) | 0.874 |
| ≥ $35 AUD per week | 110 (45%) | 31 (29%) | 77 (71%) | 1.53 (0.65–3.59) | 0.333 |
| Travel requirements to receive OAT doses (n = 253) | |||||
| 0–5 km per day (referent group) | 130 (49%) | 52 (41%) | 76 (59%) | – | – |
| ≥ 5 km per day | 133 (51%) | 32 (26%) | 93 (74%) | 2.10 (1.20–3.65) | 0.009 |
N = 266 participants reported currently receiving methadone or buprenorphine; of these, n = 255 responded to the item ‘Perceptions that XR‐buprenorphine was a good treatment option for them’.
See Methods for exact item wording
Adjusted odds ratios controlled for age and gender.
‘Unsupervised (take‐home) doses’ = doses dispensed to the patient to take at home (i.e. no direct supervision of consumption by a clinician or pharmacist).
The majority of all participants reported perceived advantages of XR‐buprenorphine from a pre‐specified list (Table 3), most commonly ‘attend treatment services less frequently’, ‘gives me more time to do other things’, ‘allows travel for work or holidays’, ‘prevents cravings for opioids’, ‘feel in control of my treatment’ and ‘suppresses withdrawal symptoms for a long time’ (endorsed by 62–76% of the sample). Additional advantages reported as ‘Other (specify)’ included convenience (n = 4), cost savings (n = 2), enhanced self‐determination (n = 2), enhanced privacy (n = 2), avoiding the stigma of attending for OAT (n = 2), missing fewer doses (n = 2) and removing the logistical challenges of attending for daily dosing with young children (n = 2).
Table 3.
Perceived advantages and disadvantages of XR‐buprenorphine (n = 392).
| % | |
|---|---|
| Advantages | |
| Attend treatment services less frequently | 76 |
| Gives me more time to do other things | 69 |
| Allows travel for work or holidays | 66 |
| Prevents cravings for opioids | 64 |
| Feel in control of my treatment | 63 |
| Suppresses withdrawal symptoms for a long time | 62 |
| Could avoid regular contact with other people in drug treatment | 59 |
| Blocks the effects of other opioids | 54 |
| Reduces the need for willpower to stay in treatment and/or avoid using other opioids | 54 |
| Decreases my risk of overdose (safety) | 52 |
| Disadvantages | |
| Might not hold people for the whole period between doses | 40 |
| Blocks the effects of other opioids | 26 |
| Less flexibility in treatment | 17 |
| Feel less in control of my treatment | 16 |
| Don't like the idea of having the drug/depot inside me for a long time | 16 |
| Reduced opportunity to attend treatment services for dosing | 12 |
| Reduced opportunity to have regular contact with other people in drug treatment | 7 |
10 cases missing data for this section.
Potential disadvantages of XR‐buprenorphine were reported less frequently. The most commonly reported disadvantages from a pre‐specified list (Table 3) were ‘might not hold people for the whole period between doses’ (40%) and ‘blocks the effects of other opioids’ (26%). Additional disadvantages reported as ‘Other (specify)’ included anxiety about the possible side effects (n = 6), not liking buprenorphine (n = 5), pain management concerns (n = 4), concerns about continued drug use (n = 2), concern that the injection may act as a ‘trigger’ for illicit opioid use (n = 2), concern about the risks of overdose (n = 2) and not wishing to be injected as a treatment modality (n = 1).
Compared to those not currently receiving OAT, participants currently receiving OAT were more likely to endorse ‘attend treatment services less frequently’ and less likely to endorse ‘blocks the effects of other opioids’, ‘reduces the need for willpower to stay in treatment and/or avoid using other opioids’ and ‘decreases my risk of overdose’ as advantages. There were no other differences in the reporting of advantages or disadvantages according to current OAT status (Supporting information, Appendix S6).
Among those who responded to the item regarding willingness to change doctors to access XR‐buprenorphine treatment (n = 354; n = 48 missing cases), 68% were willing to change doctors and almost one‐quarter (23%) were not willing to do so (10% ‘don't know’). Among participants in the total sample who responded to the item regarding how far they were willing to travel for treatment with XR‐buprenorphine (n = 388; n = 14 missing cases), 17% were ‘not interested/not willing to travel at all’, 25% were willing to travel up to 30 minutes, 34% 30–60 minutes, 10% 60–90 minutes and 14% more than 90 minutes. To place this in context of current travel time for dosing, among the 51% of current OAT who reported currently travelling ≥ 5 km for dosing (n = 133), 74% believed XR‐buprenorphine was a good treatment option for them (Table 2).
More than two‐thirds of participants (69%) reported no problems or concerns with potential differences in the availability, flexibility and location of services providing this treatment, and 71% reported no problems or concerns with delivery of XR‐buprenorphine via subcutaneous injection (Fig. 2). Almost one in four participants (23%) reported ‘minor’ or ‘major’ concerns about the blockade effects of XR‐buprenorphine (Fig. 2). There were no differences according to whether or not participants were currently receiving OAT (Supporting information, Appendix S6).
Figure 2.
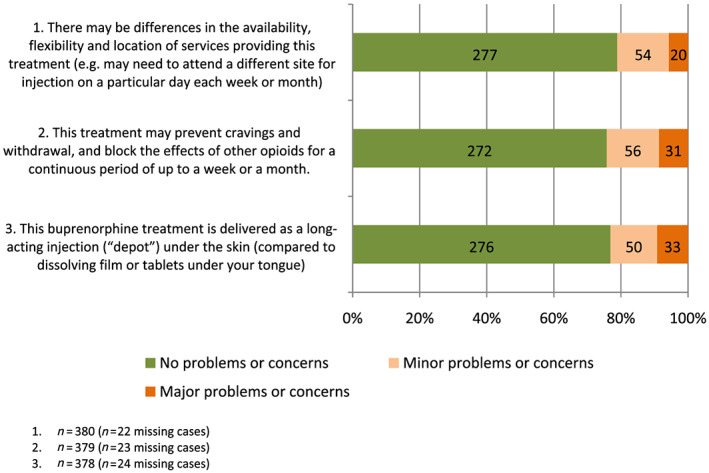
Participants’ reports of the extent to which different features of treatment with XR‐buprenorphine injections raised problems or concerns (%). [Colour figure can be viewed at wileyonlinelibrary.com]
Discussion
Although previous studies have explored OAT client views of XR‐buprenorphine using small samples 21, 22, this study builds on this previous work by undertaking an in‐depth examination of demographic and clinical correlates of these perceptions in a diverse sample of people who regularly use opioids, with varied treatment experiences. Consistent with previous studies 21, 22, perceptions of XR‐buprenorphine were generally positive, with two‐thirds of the current study's sample believing it would be a good treatment option for them if it were available. Overall, less frequent contact with treatment services was viewed positively by participants (endorsed as an advantage by 76%). Although participants endorsed a range of advantages that we identified prior to the study, additional advantages of XR‐buprenorphine were noted, including reducing the burden of attendance for supervised dosing, increasing privacy for OAT clients and reducing stigma.
Participants who believed that XR‐buprenorphine was a good treatment option for them were more likely to report: being younger (26–35 years versus > 55 years); being female; fewer years of school education; and past‐month heroin use and methamphetamine use. Neither life‐time nor current OAT were associated with participants believing XR‐buprenorphine would be a good treatment option. The finding that women were more likely to find this treatment option attractive is consistent with a growing body of research demonstrating that gender impacts on treatment relevance, access and effectiveness, and that women in particular are impacted by stigma, violence and criminalization of substance use 27. It is also consistent with the qualitative reports in the current study indicating that XR‐buprenorphine offers advantages to carers of young children, who are frequently women, by reducing the burden of having to attend for dosing with children present and providing parents with a degree of privacy regarding their drug treatment.
Previous studies have indicated that prior experience with buprenorphine treatment is an important factor influencing perceptions of buprenorphine 20 yet, in the current study, neither life‐time nor current methadone or buprenorphine ± naloxone use were associated with participants believing that XR‐buprenorphine was a good treatment option for them. Participants who reported recent drug use (past‐month heroin and methamphetamine use and methamphetamine dependence), however, were more likely to hold positive views of XR‐buprenorphine. Among current OAT recipients, believing that XR‐buprenorphine was a good treatment option for them was associated with: treatment episodes of 1–2 years (versus ≥ 2 years); fewer take‐away doses and greater travel distance.
Many of these factors appear to be related to less stable patterns of drug use, and are consistent with the perceptions that XR‐buprenorphine may improve treatment adherence and outcomes among those clients who find it difficult to meet current treatment programme requirements and for whom illicit drug use persists. Alternatively, factors such as greater travel distance and fewer unsupervised doses make treatment inconvenient, and policy shifts towards greater restrictions on unsupervised dosing in some jurisdictions (e.g. [28]) may mean that this is an increasing concern for OAT clients. Only 7% of participants reported a preference for weekly XR‐buprenorphine injections (with more than half the sample reporting ‘no preference’ and 39% reporting a preference for monthly injections), possibly reflecting ambivalence about the burden of frequent attendance at drug treatment services. It is interesting that more ‘stable’ OAT client profiles (e.g. receiving more unsupervised doses, treatment episodes longer than 1–2 years) were not associated with viewing XR‐buprenorphine as a good treatment option. It may be that clients who are stable on OAT and have access to unsupervised doses do not perceive additional benefits of XR‐buprenorphine.
In Australia, OAT [methadone and sublingual buprenorphine (± naloxone)] are prescribed from a mix of general practice (or office‐based) and specialist settings, with the majority of clients dispensed their medication in community pharmacies. Although the government fully subsidizes the cost of the medication, there is no government remuneration to community pharmacies for dispensing or supervision of dosing. This cost, approximately AUD$30–70 per week, is passed on to OAT clients. The exception is where clients are accessing State‐government funded public clinics and this cost is covered, although dosing in these settings occurs in only 7% of OAT clients nationally 23. Although the current study did not find an association between OAT clients’ out‐of‐pocket costs and perceptions of whether XR‐buprenorphine might be a good treatment option for them, it is possible that participants were not yet aware of the potential savings.
While overall perceptions of XR‐buprenorphine were positive, views on the benefits and disadvantages varied among participants. Although reported less frequently, participants endorsed important potential disadvantages, such as concerns about the effectiveness of XR‐buprenorphine suppressing withdrawal symptoms for the period between doses, the blockade effect and a sense of loss of control over treatment. In addition to the potential disadvantages that we identified prior to the study, additional disadvantages of XR‐buprenorphine were noted by participants, including anxiety about the possible adverse effects, pain management concerns and concerns that the injection may itself act as a ‘trigger’ for illicit opioid use.
A strength of this study is that it includes participants who were currently engaged and non‐engaged in OAT. A limitation of the survey was that it assessed perceptions and experiences of a range of different treatment approaches for opioid use disorder (e.g. residential rehabilitation, mutual support groups, naltrexone, etc.) as well as XR‐buprenorphine. The initial description of XR‐buprenorphine provided to participants may have primed for more positive responses, and perceptions need to be understood in this light. This study is also subject to the usual limitations associated with cross‐sectional survey designs and convenience sampling methods. Study sites did not routinely collect information about numbers examined for eligibility, confirmed eligible and included in the study, and we are unable to report these details. However, the demographic characteristics of the current study's sample are consistent with those of other large Australian studies of people with OUD (i.e. the majority male, typically aged early 40s, with the majority of OAT clients receiving methadone) 23, 29. The study was conducted in a setting where there is a strong emphasis on the supervised dosing of medication in OAT. The findings on the attractiveness of XR‐buprenorphine may be unique to this context, and may not generalize to other countries or settings where there are fewer restrictions placed on buprenorphine treatment. In addition, although more than half the sample were currently receiving methadone or buprenorphine, the sample of buprenorphine clients (n = 42) was relatively small, limiting both power to detect differences and representativeness. Finally, this study was conducted prior to the implementation of XR‐buprenorphine formulations in Australia, so participants were commenting on hypothetical scenarios and anticipated preferences, benefits and disadvantages. The extent to which these reflect views of XR‐buprenorphine‐experienced clients is yet to be established. Despite these limitations, this study provides important insights into client perspectives on this important and potentially transformative treatment development.
Clients’ perceptions and concerns are essential in informing understanding of who will (and will not) find XR‐buprenorphine an attractive treatment option. They are also important in developing relevant and targeted communication strategies. Clients may be anxious about the introduction of new OAT formulations, and previous research indicates that the way in which change is communicated and managed may impact on their experience. One study has found that in jurisdictions where transfer from buprenorphine–naloxone tablets to the film formulation was mandated, clients reported more adverse effects of medication than in jurisdictions with less stringent policies 19. Australian OAT guidelines recommend that client preferences are taken into account in choosing medications 13. These findings may act as a guide for those discussions that are had on an individual basis. Proactive communication strategies involving peers may help manage anxiety regarding changing buprenorphine formulations among clients.
Declaration of interests
B.L., L.D. and R.A. have received previous untied educational grants from Reckitt Benckiser for studies examining the diversion and injection of buprenorphine–naloxone. R.A. has received previous untied educational grants from Reckitt Benckiser for studies examining the pharmacogenetics of methadone and buprenorphine maintenance treatment and transfer to buprenorphine from high‐dose methadone. B.L., L.D., M.F. and S.N. have received an untied educational grant to examine opioid‐related help‐seeking among people with chronic non‐cancer pain. S.N. has delivered training on opioid use disorder for Indivior for which honoraria were paid to her institution. S.N. has participated in an advisory board meeting Mundipharma relating to intranasal naloxone (sitting fee not taken). B.L., L.D., R.B., R.A. and M.F. have received an untied educational grant from Mundipharma Australia to examine the impacts of Reformulated OxyContin®. B.L., L.D. and M.F. have received an untied educational grant from Seqirus to conduct post‐marketing surveillance of tapentadol. S.N. has received an untied educational grant from Seqirus to study harms related to pharmaceutical opioids. S.L. has received an untied educational grant from Indivior to examine take‐home naloxone for people released from prisons.
Supporting information
Appendix S1 STROBE Statement.
Appendix S2 Module of questionnaire examining perceptions of XR buprenorphine.
Appendix S3 Demographic, clinical and substance use profile of the total study sample, n = 402.
Appendix S4 Demographic and substance use profile according to whether participants are currently receiving OAT (n = 396).
Appendix S5 Multinomial regression model examining predictors of preferences for frequency of XR‐buprenorphine injections (weekly, monthly or no preference) among participants who believed XR‐buprenorphine was a good treatment option for them (n = 286).
Appendix S6 Perception of XR‐buprenorphine according to current OAT status (n = 396).
Appendix S7 Demographic, clinical and substance use profile of participants according to their perceptions of whether XR‐buprenorphine was a good treatment option for them (response categories have not been dichotomised), n = 382.
Acknowledgements
This study was sponsored by UNSW and supported by an Externally Sponsored Collaborative Research grant from Indivior. Indivior contributed to the study design and analysis plan; Indivior had no role in collection, analysis and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication. L.D. (#1135991), S.N. (#1132433 and #1163961) and S.L. (#1140938) are supported by NHMRC research fellowships. LD is also supported by NIH grant NIDA R01DA1104470. S.L. receives additional support from a UNSW Sydney Scientia Fellowship. The National Drug and Alcohol Research Centre at UNSW Sydney is supported by funding from the Australian Government Department of Health under the Drug and Alcohol Program. The authors thank the study participants for generously sharing their views and experiences, Alexander Saeri, who coordinated the project and helped design the questionnaire and Nima Dorkian, Rakin Rahman, Oliver de Angelis, Jessica Forward, Thomas Norman, Jane Akhurst, Luke Ney, Daisy Gibbs, Teleri Moore, Isaac Addo and Kelly Kershaw, who assisted with interviewing participants. Thanks also go to Nick Lintzeris, who made comments on an earlier manuscript draft.
Larance, B. , Degenhardt, L. , Grebely, J. , Nielsen, S. , Bruno, R. , Dietze, P. , Lancaster, K. , Larney, S. , Santo, T. Jr , Shanahan, M. , Memedovic, S. , Ali, R. , and Farrell, M. (2020) Perceptions of extended‐release buprenorphine injections for opioid use disorder among people who regularly use opioids in Australia. Addiction, 115: 1295–1305. 10.1111/add.14941.
References
- 1. Mattick R. P., Breen C., Kimber J., Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev 2009; 2: CD002209. [DOI] [PubMed] [Google Scholar]
- 2. Mattick R. P., Breen C., Kimber J., Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev 2014; 2: CD002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization Guidelines for the Psychosocially Assisted Pharmacological Treatment of Opioid Dependence. Switzerland: WHO Press, World Health Organization; 2009. [PubMed] [Google Scholar]
- 4. Degenhardt L., Bucello C., Mathers B., Briegleb C., Ali H., Hickman M., et al Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta‐analysis of cohort studies. Addiction 2011; 106: 32–51. [DOI] [PubMed] [Google Scholar]
- 5. Degenhardt L., Randall D., Hall W., Law M., Butler T., Burns L. Mortality among clients of a state‐wide opioid pharmacotherapy program over 20 years: risk factors and lives saved. Drug Alcohol Depend 2009; 105: 9–15. [DOI] [PubMed] [Google Scholar]
- 6. Lofwall M. R., Walsh S. L. A review of buprenorphine diversion and misuse: the current evidence base and experiences from around the world. J Addict Med 2014; 8: 315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Larance B., Degenhardt L., Lintzeris N., Bell J., Winstock A., Dietze P., et al Post‐marketing surveillance of buprenorphine‐naloxone in Australia: diversion, injection and adherence with supervised dosing. Drug Alcohol Depend 2011; 118: 265–273. [DOI] [PubMed] [Google Scholar]
- 8. Larance B., Lintzeris N., Ali R., Dietze P., Mattick R., Jenkinson R., et al The diversion and injection of a buprenorphine–naloxone soluble film formulation. Drug Alcohol Depend 2014; 136: 21–27. [DOI] [PubMed] [Google Scholar]
- 9. Rosenthal R. N., Goradia V. V. Advances in the delivery of buprenorphine for opioid dependence. Drug Des Devel Ther 2017; 11: 2493–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rosenthal R. N., Lofwall M. R., Kim S., Chen M., Beebe K. L., Vocci F. J., et al Effect of buprenorphine implants on illicit opioid use among abstinent adults with opioid dependence treated with sublingual buprenorphine: a randomized clinical trial. JAMA 2016; 316: 282–290. [DOI] [PubMed] [Google Scholar]
- 11. Haight B. R., Learned S. M., Laffont C. M., Fudala P. J., Zhao Y., Garofalo A. S., et al Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet 2019; 393: 778–790. [DOI] [PubMed] [Google Scholar]
- 12. Lofwall M. R., Walsh S. L., Nunes E. V., Bailey G. L., Sigmon S. C., Kampman K. M., et al Weekly and monthly subcutaneous buprenorphine depot formulations vs daily sublingual buprenorphine with naloxone for treatment of opioid use disorder: a randomized clinical trial. JAMA Intern Med 2018; 178: 764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gowing L., Ali R., Dunlop A., Farrell M., Lintzeris N. National guidelines for medication‐assisted treatment of opioid dependence. Canberra: Commonwealth of Australia; 2014. [Google Scholar]
- 14. Rhodes T. The becoming of methadone in Kenya: how an intervention's implementation constitutes recovery potential. Soc Sci Med 2018; 201: 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vuong T., Shanahan M., Nguyen N., Le G., Ali R., Pham K., et al Cost‐effectiveness of center‐based compulsory rehabilitation compared to community‐based voluntary methadone maintenance treatment in Hai Phong City, Vietnam. Drug Alcohol Depend 2016; 168: 147–155. [DOI] [PubMed] [Google Scholar]
- 16. Department of Health Clinical Guidelines on Drug Misuse and Dependence Update 2017, Independent Expert Working Group In: Drug Misuse and Dependence: UK Guidelines on Clinical Management. London: Department of Health; 2017. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/673978/clinical_guidelines_2017.pdf [Google Scholar]
- 17. Madden A., Lea T., Bath N., Winstock A. R. Satisfaction guaranteed? What clients on methadone and buprenorphine think about their treatment. Drug Alcohol Rev 2008; 27: 671–678. [DOI] [PubMed] [Google Scholar]
- 18. Rowe J. A raw deal? Impact on the health of consumers relative to the cost of pharmacotherapy. Melbourne: RMIT University, Centre for Applied Social Research; 2007. [Google Scholar]
- 19. Larance B., Dietze P., Ali R., Lintzeris N., White N., Jenkinson R., et al The introduction of buprenorphine–naloxone film in opioid substitution therapy in Australia: uptake and issues arising from changing buprenorphine formulations. Drug Alcohol Rev 2015; 34: 603–610. [DOI] [PubMed] [Google Scholar]
- 20. Uebelacker L. A., Bailey G., Herman D., Anderson B., Stein M. Patients’ beliefs about medications are associated with stated preference for methadone, buprenorphine, naltrexone, or no medication‐assisted therapy following inpatient opioid detoxification. J Subst Abuse Treat 2016; 66: 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gilman M., Li L., Hudson K., Lumley T., Myers G., Corte C., et al Current and future options for opioid use disorder: a survey assessing real‐world opinion of service users on novel therapies including depot formulations of buprenorphine. Patient Prefer Adherence 2018; 12: 2123–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Neale J., Tompkins C. N. E., McDonald R., Strang J. Implants and depot injections for treating opioid dependence: qualitative study of people who use or have used heroin. Drug Alcohol Depend 2018; 189: 1–7. [DOI] [PubMed] [Google Scholar]
- 23. Australian Institute of Health and Welfare (AIHW) National Opioid Pharmacotherapy Statistics Annual Data collection (NOPSAD) 2018, Vol. 2019 Canberra: AIHW, Australian Government. [Google Scholar]
- 24. Penrod J., Preston D. B., Cain R. E., Starks M. T. A discussion of chain referral as a method of sampling hard‐to‐reach populations. J Transcult Nurs 2003; 14: 100–107. [DOI] [PubMed] [Google Scholar]
- 25. Chamberlain C., MacKenzie D. Counting the Homeless 2006. Canberra: Australian Government; 2008. [Google Scholar]
- 26. Reinert D. F., Allen J. P. The alcohol use disorders identification test: an update of research findings. Clin Exp Res 2007; 31: 185–199. [DOI] [PubMed] [Google Scholar]
- 27. Meyer J. P., Isaacs K., El‐Shahawy O., Burlew A. K., Wechsberg W. Research on women with substance use disorders: reviewing progress and developing a research and implementation roadmap. Drug Alcohol Depend 2019; 197: 158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Berends L., Chalmers J., Lancaster K. Trust, agency, and control: perspectives on methadone takeaway dosing in the context of the Victorian policy review. Drug Alcohol Rev 2015; 34: 483–486. [DOI] [PubMed] [Google Scholar]
- 29. Darke S., Marel C., Mills K. L., Ross J., Slade T., Burns L., et al Patterns and correlates of non‐fatal heroin overdose at 11‐year follow‐up: findings from the Australian treatment outcome study. Drug Alcohol Depend 2014; 144: 148–152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 STROBE Statement.
Appendix S2 Module of questionnaire examining perceptions of XR buprenorphine.
Appendix S3 Demographic, clinical and substance use profile of the total study sample, n = 402.
Appendix S4 Demographic and substance use profile according to whether participants are currently receiving OAT (n = 396).
Appendix S5 Multinomial regression model examining predictors of preferences for frequency of XR‐buprenorphine injections (weekly, monthly or no preference) among participants who believed XR‐buprenorphine was a good treatment option for them (n = 286).
Appendix S6 Perception of XR‐buprenorphine according to current OAT status (n = 396).
Appendix S7 Demographic, clinical and substance use profile of participants according to their perceptions of whether XR‐buprenorphine was a good treatment option for them (response categories have not been dichotomised), n = 382.


