Abstract
Background and Aims:
Within the context of busy clinical settings, healthcare providers need practical, evidence-based options to engage smokers in quitting. Sampling of nicotine replacement therapy (i.e., provision of NRT starter kits) is a brief, pragmatic strategy to address this need. We aimed to compare the effects of NRT sampling plus standard care (SC), relative to SC alone, provided by primary care providers during routine clinic visits.
Design:
Cluster-randomized clinical trial.
Setting:
22 primary care clinics in South Carolina, USA.
Participants:
Adult smokers (N=1245; 61% female, mean age 50.7, (SD=13.5) both motivated and unmotivated to quit, seen during routine clinical visit.
Interventions:
Provider-delivered SC (n=652, 12 clinics) cessation advice, or SC + a two-week supply of both nicotine patch and lozenge, with minimal instructions on use (n=593; 10 clinics).
Measurements:
The primary outcome was 7-day point prevalence smoking abstinence at 6-month follow-up, using intent-to-treat. Additional outcomes included NRT use and quit attempts, assessed at 1, 3, and 6 months following baseline.
Findings:
Seven-day point prevalence abstinence rates were significantly higher in the NRT sampling group throughout follow-up, including at 6 months (12% vs. 8%; Odds Ratio [OR] = 1.5; 95% Confidence Interval [CI]: 1.0 – 2.4). NRT sampling increased prevalence of any use of NRT (65% vs. 25%; OR = 5.8; 95% CI: 4.3 – 7.7), with higher prevalence of use at 6 months (25% vs. 14%; OR = 2.0; 95% CI: 1.5 – 2.7). NRT sampling increased the rate of quit attempts in the initial month (24% vs. 18%; OR = 1.5; 95% CI: 1.0 – 2.3) but had no significant effect on overall rate of quit attempts (48% vs. 45%; OR = 1.2; 95% CI: 0.8 – 1.7).
Conclusion:
Providing smokers with a free 2-week starter kit of nicotine replacement therapy increased quit attempts, use of stop smoking medications, and smoking abstinence compared with standard care in a primary care setting.
Keywords: Smoking Cessation, Cessation Induction, Primary Care, Pragmatic Clinical Trial
Introduction
Smoking cessation interventions within the context of primary care remain an important public health priority. Brief physician advice has been a mainstay of several clinical practice guidelines (1), with substantial evidence for effectiveness (2). Several studies suggest that healthcare providers consistently ask about smoking status and offer advice to quit but are less well equipped to provide patients with concrete tools to achieve this goal (3). Despite availability of several well-established pharmacotherapies (4, 5), a number of studies consistently show their underuse at the population level (6, 7). For physicians within busy clinical practice settings, getting more smokers to make evidence-based quit attempts remains a significant challenge.
Guided by recommendations for pragmatic approaches (8), i.e. brief, face-valid interventions that are easily deliverable, our group has tested various permutations of medication sampling to induce smokers toward quitting (9). Provision of medication starter-kits (few weeks only), with minimal accompanying instruction, allows the smoker to decide if and how to use them. Conceptually, sampling of medication could increase motivation and/or confidence to quit, allowing smokers to become more familiar with evidence-based treatment options, without pressure to quit. Many smokers continue to hold misperceptions about cessation pharmacotherapy, and particularly NRT. Misperceptions are broad, but typically pertain to concerns about 1) safety (e.g., addictive potential, adverse events), and 2) efficacy (10–14). Provision of medication samples within clinical practice offers a concrete, immediately actionable tool for both smokers and their providers. Sampling is not a substitute for full cessation treatment but rather a catalyst to it. To date, sampling of cessation medications has been restricted to nicotine replacement therapy (NRT), based on the rationale that NRT is well established (4, 5), available over-the-counter (OTC), does not require complex tapering instruction, available in both acute (lozenge, gum) and sustained (patch) delivery, and results in minimal side effects (15).
We have conducted two clinical trials of NRT sampling (2–4 week starter kits), one large (N=849) and focused exclusively on smokers unmotivated to quit (16, 17), which demonstrated improvements along hypothesized mechanisms above (18), and another small (N=157) with separate comparisons among smokers who were and were not motivated to quit (19). Both yielded generally positive effects, with a 30–60% relative increase in various cessation-related outcomes. Others have also demonstrated the feasibility and efficacy of remotely delivering longer-duration medication to a broad spectrum of smokers (20, 21), with consistent results. Each of these trials, however, has been conducted outside the context of real-world healthcare settings, where the potential for widescale dissemination is most opportune. Several trials within primary care have tested proactive interventions but have included additional mailings, telephone calls, and occasionally intensive counseling support (22, 23), which may not be easily scalable. Other prominent trials within healthcare settings have been restricted to smokers wanting to quit (24) or who were attending cessation clinics (25), which represents a limited range of smokers. We herein present a large-scale cluster-randomized clinical trial that examines outcomes from a minimally intensive medication sampling intervention, tested among a broad spectrum of smokers seen within primary care clinics across South Carolina. We hypothesized that NRT sampling would promote a) treatment uptake (use of medications), b) quit attempts, and c) cessation as compared to standard care.
Materials and Methods
Overall Design
Details of major study design considerations have been presented elsewhere (26). Briefly, adult smokers were recruited across 22 primary care clinics, wherein all study procedures were administered by clinic staff during routine healthcare visits. Randomization, administered on a rolling basis as clinics joined, was at the clinic level, and was stratified by rural (vs. urban) and small (vs. large) sites, using stratified randomization lists created at the outset of the study by and accessible only to the study statistician. We considered randomization at both patient and provider level but opted against this to avoid possible within-clinic contamination of treatment effects. As the sampling intervention is ostensibly a behavioral intervention, there was no blinding. Standard care (SC; no medication sampling) served as the control group. Following baseline consent and treatment delivery, all participants were followed for six months via phone (centrally, by research staff) to collect study outcomes. Participants were compensated up to $80 in gift cards across the baseline and three follow-up contacts (+1, +3, +6 months). All procedures were approved by appropriate regulatory oversight, including distally for any clinics (n=2) that were not subsumed by centralized Institutional Review Board (IRB; n=20). Data collection began in July 2014 and terminated in June 2018. The trial is registered with ClinicalTrials.gov: NCT02096029.
Clinic Selection and Training
Clinics were recruited through partnership with the Care Coordination Institute, which offers a network of primary care clinics across South Carolina. The a priori plan was to recruit 20 clinics in total, with requirements within each to recruit between 58–68 participants within a 3-month window. Twenty-four clinics were approached. Two declined to participate, and two more (control condition) were replaced after their recruitment pace fell below expectation. Enrolled participants within these clinics were retained in the study. The 20 remaining clinics all met recruitment goals. Descriptions of individual clinics are presented within the published methods manuscript (26).
To ensure sample representativeness, each clinic was tasked with recruiting participants in proportion to their clinic profile (gender, race), ascertained and provided in advance through Care Coordination Institute. As background, South Carolina is 27% African American (US Census 2017). All clinics met their profile targets.
All consenting personnel within each clinic were IRB approved, inclusive of any necessary ethics and human subjects research training. Prior to initiation of recruitment, cessation-certified research staff gave a one-time, 60–90 minute in-person training of all study procedures, during which an overview of clinical practice guidelines for physician advice (5As)(1), and a brief review of all cessation pharmacotherapies, both NRT and other, was provided. This was undertaken to standardize all information that providers received at study outset, but it was explicitly and repeatedly emphasized that provider/patient communications were meant to be self-determined and occur naturally; i.e., unscripted by research protocol. Thus, all interventions were delivered in the context of naturally occurring smoking cessation advice as done by each provider; i.e., standard care.
Participants
Study eligibility was kept broad to be consistent with the study’s pragmatic intent. Participants were required to be: a) age 18+, b) a smoker of at least five cigarettes per day on ≥25 days out of the last 30 days, c) English speaking, and d) recruited through a primary care site actively enrolled in the study. Exclusion criteria included FDA contraindications for NRT use (pregnancy/breastfeeding; recent cardiovascular trauma). Motivation to quit smoking was not required, nor was willingness to sample a cessation medication.
Interventions
Following tablet-assisted screening, consent, and baseline assessment (managed directly between clinic staff and patient), participants in all clinics received a take-home bag that included basic information on smoking cessation (e.g., “How to Quit Smoking” and other National Cancer Institute brochures) as well as a brochure with referral to the state quitline. Explicit prompts to quit, including setting a quit date, were not required, but were suggested as per pre-study training on clinical practice guidelines. For participants recruited from clinics assigned to the standard care group, there was no other intervention beyond this cessation advice they received from their provider. For clinics assigned to the medication sampling group, these take-home bags additionally included a two-week supply of both nicotine lozenges and patches, provided with the original packaging. Providers could discuss these medications to the extent they wished, again with the intent to keep this as naturalistic as possible. Detailed information of both products was provided in the take-home bag given to patients along with answers to frequently asked questions and information about how to get more medications if needed (general information on medications was also provided to the control group). There was no further provision of medications beyond the initial two-week sample. As per above, explicit prompts to quit, including setting a quit date, were not required, but suggested.
Prior literature on short-term delivery of NRT suggests cost-efficacy with merely one (25) or two (27) weeks duration. We opted for a two week sampling experience in concordance with conventional packaging (i.e., patches predominantly come in 14-day supply), but also because we believed this could provide ample opportunity for smokers to experience any effect. Dosing was kept uniform for all participants: 14mg patch + 4mg lozenge. Tailored dosing was considered but deemed inconsistent with translational intent (all bags were pre-filled for each clinic). Additionally, we did not believe that the 21mg patch would be clinically warranted in the case of adjunctive lozenge, and we further opted against the 2mg lozenge to prevent under-dosing for those who use only that product. We did not include a placebo control to NRT sampling since our intervention is a behavioral experience, specifically inclusive of medication expectancies, vs. the pharmacologic response to it (which is already well established). Consistent with our sampling rationale, use of medications was not required, but merely suggested via written brochure, as this itself was a study outcome.
Assessments
Baseline assessments included standard questions on demographics and smoking history. The latter included the Heaviness of Smoking Index (range 0–6)(28), and motivation and self-efficacy to quit, both measured via 0–10 scales (16, 18). Phone-based follow-up was attempted +1, +3, and +6 months following baseline (within a 7-day window for each), with 5–8 attempts for all, followed by a mailed letter if the participant was still unreachable. These interviews ascertained the following outcomes: a) incidence of quit attempts, both self-defined of any duration and those lasting ≥24hrs, b) cigarettes per day (CPD), averaged over the 7 preceding days of each contact, c) 7-day point prevalence at each follow-up, and d) “floating abstinence;” i.e., any 7-day period of non-smoking at any point in the study. Continuous abstinence is not reported because, unlike traditional cessation trials, there was no quit date on which to anchor it. Additional outcomes pertained to treatment engagement: e) use of any cessation medication, f) ever purchase of patch/lozenge, asked only within the NRT group as a measure of product adoption after sampling (any medication/NRT use within control group was assumed as purchased since it was not study-provided), and g) use of the quitline. Study procedures did not include any biological verification of smoking status, since 1) this was a minimally intensive study (few minutes of intervention with no direct contact between participant and study staff) , 2) several prior commentaries have suggested that verification is unnecessary in such studies(29, 30), and 3) options for remote capture of non-nicotine biomarkers are limited (see Discussion).
Statistical Analysis
Full background on sample size calculations and assumptions for intra-class correlations (ICC) are presented in the background methods text (26). The primary outcome on which our study was powered was 7-day point prevalence at 6 month follow-up, which, unlike continuous abstinence, allows for delayed quitting (30). Drawing on our prior evidence, we a priori estimated a quit rate of 20% in the NRT group and 13% in the SC group. We used data from previous studies to estimate an ICC = 0.005. See results below for actual ICCs calculated following completion of the trial. With power = 0.8 and alpha = 0.05, final planned enrollment was targeted for 20 total clinics, each recruiting a minimum of 58 participants (maximum 68), for a total planned enrollment of 1160 participants.
Potential demographic covariates were examined to determine their individual relationship with the primary outcome of point prevalence abstinence at six months. Variables with a statistically significant (p<0.05) relationship with this outcome (or previously identified as having a relationship to this outcome; see below) were included in each model. All analyses were based on intent-to-treat principles, in which anyone missing an interview was presumed to be smoking, without any quit attempt. This conservative approach, recommended by SRNT guidelines (31, 32), biases all results toward the null hypothesis. No imputation was applied for analyses involving continuous outcomes (e.g., cigarettes per day). All analyses included a random effect for clinic site and were adjusted for individual level characteristics. Baseline demographics were compared between SC and NRT+SC groups via t-tests or chi-square tests, as appropriate. For primary and secondary outcomes, general linear mixed models and generalized linear mixed models were used for both our continuous and binary outcomes, respectively, to account for clustering within site. Each outcome was examined independent of the other outcomes (e.g., point prevalence abstinence at six months was analyzed independently of abstinence at one month and three months). The primary covariate of interest in regression models was treatment group (NRT sampling + SC vs. SC) with adjustments made for potential confounders measured at baseline (see below). Results are presented as unadjusted and adjusted odds ratios (AOR) with 95% confidence intervals (CI); an alpha level of 0.05 was used for all hypothesis tests. All analyses were generated using SAS Software, Version 9.4.
Results
Study sample
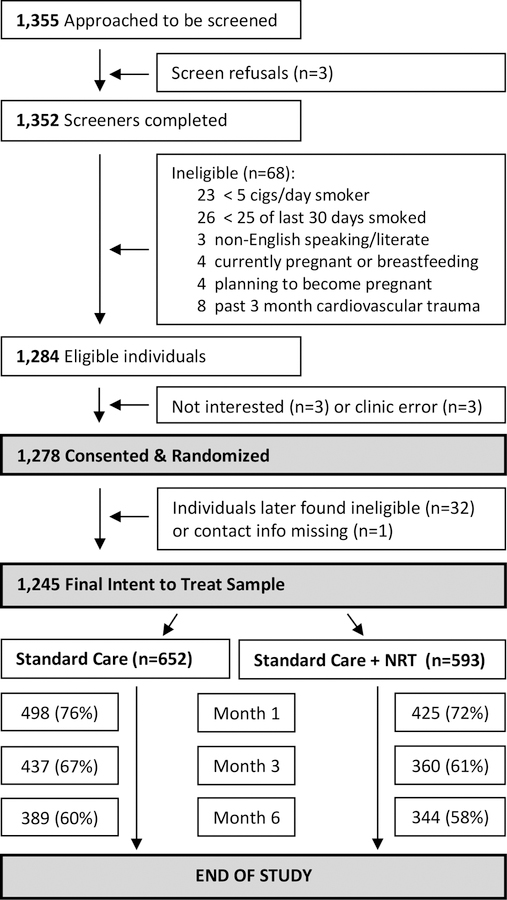
Recruitment flow and retention are depicted in Figure 1. We used clinic personnel to approach individuals who were known to them as established smokers. Only 3 people approached for screening refused (<1%), likely suggesting that personnel selectively approached known smokers who might seem willing to participate. Among those screened, 68 (5%) were deemed ineligible. Of those eligible, only 6 were not enrolled (3 for lack of interest and 3 due to a clinic error). In total, 652 SC and 593 NRT Sampling participants were enrolled, representing the intent-to-treat sample (Figure 1). Retention rates at six months were no different between groups (60% vs. 58%, Chi-square=0.35, df=1, p=0.6).
Figure 1:
Recruitment Flow and Retention
Baseline characteristics
Baseline characteristics are presented in Table 1. Participants tended to be female (61%) with a mean age of roughly 50 years, smoking an average of 15 CPD, moderately dependent, with only 35% having made a quit attempt in the past year, and over one-third (36%) were African American. Measures of motivation and confidence to quit smoking (each on a 0–10 scale), both established predictors of quit attempts and cessation, were similar between groups. Several baseline variables differed significantly between groups, which likely reflects the fact that clinics, and not individuals, were randomized. Thus, we caution against over-interpretation of individual differences between groups given the large sample size and clinic-level randomization with nested demographics within each clinic. Of all baseline variables that differed between groups, only nicotine dependence (Heaviness of Smoking Index; HSI) predicted outcome, and thus HSI is included as a covariate in all following analyses, in addition to the random effect for clinic site as previously noted. However, both gender and race were added as covariates given large baseline differences and their association with quitting documented elsewhere in the literature (33–35).
Table 1:
Baseline Characteristics
| Standard Care (SC) n=652 | SC + NRT n=593 | p† | ||
|---|---|---|---|---|
| Demographics | ||||
| Age: mean (SD) | 51.0 (13.6) | 50.4 (13.4) | 0.5 | |
| Female | 56% | 66% | 0.0009 | |
| Race | <0.0001 | |||
| White | 53% | 73% | ||
| African American | 45% | 26% | ||
| Married or member of unmarried couple | 38% | 46% | 0.007 | |
| Education | 0.5 | |||
| HS, GED, or less | 64% | 63% | ||
| Some College | 27% | 30% | ||
| College Graduate | 8% | 7% | ||
| Insured, of any kind | 85% | 87% | 0.3 | |
| Income | 0.002 | |||
| < $25k | 57% | 50% | ||
| $25 - $50k | 27% | 25% | ||
| > $50k | 16% | 25% | ||
| Smoking History | ||||
| HSI: Heaviness of Smoking Index (0–6): mean (SD) | 2.6 (1.6) | 2.8 (1.4) | 0.001 | |
| Average Cigarettes per Day: mean (SD) | 15.0 (9.3) | 15.3 (8.6) | 0.5 | |
| Quit Attempt (QA) in Past Year | 35% | 36% | 0.7 | |
| Used NRT on last QA | 21% | 22% | 0.6 | |
| Used other cessation medication last QA | 11% | 11% | 0.8 | |
| Other smokers in household | 46% | 49% | 0.3 | |
| Age started smoking: mean (SD) | 18.2 (6.5) | 17.4 (5.7) | 0.03 | |
| Motivation to quit in next month (0–10) | 6.2 (3.7) | 6.4 (3.6) | 0.5 | |
| Confidence to quit in next month (0–10) | 5.8 (3.5) | 6.0 (3.2) | 0.3 | |
T-tests were used for continuous measures, and chi-square tests were used for categorical measures
Cessation Related Outcomes & Changes in Smoking
As depicted in Table 2, smokers who received an NRT sample were more likely to make a quit attempt sooner, within a month of their clinic visit (24% vs. 18%) but were no more likely to make either any quit attempt (48% vs. 45%) or any 24-hr quit attempt (42% vs. 40%) throughout the entire six-month follow-up. However, rates of point-prevalence abstinence were significantly higher for NRT vs. SC participants, at 1- (5% vs. 2%), 3- (10% vs. 5%), and 6-month follow-ups (12% vs. 8%). Rates of floating abstinence (any 7-day period of non-smoking ever in the study) were also higher among NRT participants (26% vs. 22%; see Table 2). Based on the entire sample, the longest quit attempt was about 9 days longer (mean difference=8.98 days; 95% CI: 3.7 to 14.3 days) on average among participants in the NRT vs. the SC condition (21.8 vs. 12.8 days; p<.001). Restricting this same comparison to those who made a quit attempt, the longest duration of abstinence was almost two weeks longer (11.9 days; 95% CI: 4.1 to 19.8) among NRT vs. SC participants (32.8 vs. 20.9 days; p=0.003).
Table 2:
Cessation, Quit Attempts, and Medication Use
| Standard Care | NRT Sampling | Unadjusted* | Adjusted** | |||
|---|---|---|---|---|---|---|
| N (%) | N (%) | OR | 95% CI | AOR | 95% CI | |
| Abstinence, 6 months | 52 (8%) | 70 (12%) | 1.5 | 1.0 – 2.4 | 1.7 | 1.1 – 2.6 |
| Abstinence, 3 months | 30 (5%) | 57 (10%) | 2.2 | 1.4 – 3.5 | 2.6 | 1.6 – 4.2 |
| Abstinence, 1 months | 10 (2%) | 32 (5%) | 3.6 | 1.4 – 8.7 | 4.4 | 1.7 – 11.1 |
| Floating Abstinence | 142 (22%) | 152 (26%) | 1.3 | 0.9 – 2.0 | 1.5 | 1.1 – 2.1 |
| QA within 1 month | 117 (18%) | 142 (24%) | 1.5 | 1.0 – 2.3 | 1.7 | 1.1 – 2.6 |
| Any QA | 296 (45%) | 287 (48%) | 1.2 | 0.8 – 1.7 | 1.3 | 0.9 – 1.8 |
| Any 24hr QA | 259 (40%) | 249 (42%) | 1.1 | 0.8 – 1.7 | 1.3 | 0.9 – 1.8 |
| Medication use, ever within study | 160 (25%) | 386 (65%) | 5.8 | 4.3 – 7.7 | 5.9 | 4.3 – 7.9 |
| Medication use at six months | 93 (14%) | 149 (25%) | 2.0 | 1.5 – 2.7 | 2.0 | 1.5 – 2.8 |
QA: Quit Attempt; All Abstinence measures defined as 7-day point prevalence (self-report); Floating Abstinence defined as any 7-day period of non-smoking throughout follow-up
Odds Ratio including random effect for site
Adjusted Odds Ratio including random effect for site, and controlling for a) nicotine dependence [Heaviness of Smoking Index], b) gender, and c) race.
Analyses evaluating changes over time, again including a random effect for site, and controlling for HSI, gender and race, revealed a significant time x group interaction (p<0.03) for cigarettes smoked per day (average of 7 days prior to each assessment), such that smokers receiving NRT reduced their smoking (relative to baseline) to a greater extent than those in the SC group within a month of their initial visit (average reduction from baseline of 40.3% vs. 27.4%), with greater reductions by the end of the study (52.3% vs. 45.3%).
Subgroup Analyses of Cessation Outcomes
We were specifically interested in whether any of our main cessation outcomes differed as a function of baseline motivation to quit, i.e., to test if the NRT helped only those who endorsed a strong desire to quit (≥7 on 0–10 ladder; n=671) vs. not (n=573). Rates of all cessation-related outcomes were higher among those who endorsed stronger motivation to quit at baseline, and none of these outcomes in either sub-group reached statistical significance, most likely due to diminished power. Importantly however, in all instances the treatment effect, comparing NRT Sampling vs. SC (both OR and AOR) was the same or higher among those who were less vs. more motivated to quit (Table 3), suggesting that medication sampling worked similarly across all smokers (the treatment x baseline motivation interaction was also non-significant). In subgroup analyses of medication uptake (Table 3), NRT sampling significantly promoted medication use for both low and high motivation smokers.
Table 3:
Subgroup Comparisons of Cessation-Related Outcomes by Baseline Motivation to Quit
| Low Motivation to Quit (n=573) | High Motivation to Quit (n=671) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SC (n=315) | NRT (n=258) | OR | 95% CI | AOR* | 95% CI | SC (n=336) | NRT (n=335) | OR | 95% CI | AOR* | 95% CI | ||
| Abstinence, 6 months | 15 (5%) | 20 (8%) | 1.7 | 0.8 – 3.6 | 1.7** | 0.8 – 3.7 | 37 (11%) | 50 (15%) | 1.4 | 0.9 – 2.2 | 1.5 | 0.9 – 2.4 | |
| Floating Abstinence | 44 (14%) | 47 (18%) | 1.4 | 0.8 – 2.5 | 1.6 | 1.0 – 2.7 | 97 (29%) | 105 (31%) | 1.1 | 0.8 – 1.6 | 1.3 | 0.9 – 1.9 | |
| Any Quit Attempt | 109 (35%) | 94 (36%) | 1.1 | 0.7 – 1.8 | 1.2 | 0.8 – 1.9 | 186 (55%) | 193 (58%) | 1.1 | 0.8 – 1.6 | 1.2 | 0.8 –1.8 | |
| Any 24hr Quit Attempt | 92 (29%) | 78 (30%) | 1.1 | 0.6 – 1.8 | 1.2 | 0.8 – 2.0 | 166 (49%) | 171 (51%) | 1.1 | 0.8 – 1.5 | 1.2 | 0.8 – 1.6 | |
| Medication use, ever within study | 65 (21%) | 114 (44%) | 4.9 | 3.4 – 7.0 | 5.0 | 3.4 – 7.4 | 95 (28%) | 242 (72%) | 6.6 | 4.7 – 9.3 | 6.6 | 4.6 – 9.4 | |
| Medication use at six months | 40 (13%) | 60 (23%) | 2.1 | 1.3 – 3.3 | 2.1** | 1.3 – 3.3 | 53 (16%) | 89 (27%) | 2.0 | 1.3 – 3.0 | 2.0 | 1.3 – 3.1 | |
SC: Standard Care
NRT: NRT Sampling
OR: Odds Ratio including random effect for site
AOR: Adjusted Odds Ratio including random effect for site, and controlling for a) nicotine dependence [Heaviness of Smoking Index], b) gender, and c) race.
AOR including random effect for site and controlling for HSI only: Model with all covariates did not converge due to the low numbers of participants in certain groups after stratifying.
Motivation to Quit split Low (0–6 on 10pt scale) vs. High (7–10)
Secondary Outcomes: Medication Usage
As compared to SC, participants who received starter NRT kits were more likely to use cessation medication (of any kind) in the month following their clinic visit (55% vs. 10%; AOR = 12.2 (95% CI: 8.3 – 18.0). Use of medication (in the week preceding each contact) was sustained at both 3- (34% vs. 12%; AOR = 3.8; 95% CI: 2.8 – 5.4) and 6-month follow-ups (25% vs. 14%; see Table 2). Overall, 65% of NRT and 25% of SC participants used cessation medication (AOR = 5.9; 95% CI: 4.3 – 7.9). Restricting to patch and lozenge alone, 63% (NRT + SC) vs. 14% (SC) used these products (AOR = 10.2; 95% CI: 7.4 – 14.2) at any point across follow-up. All of these patch/lozenge users within the SC group (14%) likely obtained it on their own (as it was not provided through study), whereas 19% of participants in the NRT group overall (30% of ever patch/lozenge users within this group) went on to obtain patch/lozenge independently (AOR = 1.4; 95% CI: 0.96 – 2.0), which can be viewed as an index of treatment adoption.
Other Related Outcomes
Longitudinal analyses revealed significant treatment effects on both motivation to quit (NRT: 7.4 SE = 0.2 vs. SC: 6.9; SE = 0.2; p = 0.02) and self-efficacy (NRT: 6.7; SE = 0.2 vs. SC: 6.2; SD = 0.2; p = .03), but no time by group interaction on either (motivation to quit: p=0.2; self-efficacy: p=0.4). Unexpectedly, participants who received NRT were less likely to connect with the state quitline for additional quitting assistance, though there were very low rates of contacting the quitline in either group (4% vs. 8%; AOR: 0.48; 95% CI: 0.3 – 0.9).
Post-hoc analyses completed after trial completion revealed actual ICCs as follows: a) any quit attempt: 0.027; b) any 24hr quit attempt: 0.021; c) floating (i.e., any) abstinence during study duration: 0.019; and d) point prevalence abstinence at six month follow-up: = 0.013.
Discussion
This study found that provision of a free two-week starter kit of NRT (both patch and lozenge) delivered as part of physician advice to quit smoking increased quit attempts, use of stop smoking medications, and smoking abstinence compared to standard care, and that these effects held regardless of a smoker’s motivation to quit. Study strengths include a cluster-randomized design with a large and demographically diverse study sample, recruited through a network of primary clinics in South Carolina.
Two other prominent clinical trials have evaluated strategies to deliver pharmacotherapy-based cessation treatment in primary care (36, 37), both with promising results. However, each of these trials exclusively targeted smokers wanting to quit and delivered interventions in the context of research-intensive infrastructure that was largely out of the hands of providers themselves. We herein took a more pragmatic approach, focusing on smokers across the motivational spectrum, under the belief that simply offering free medication often changes motivation itself (38). We also took a minimalist approach to intervention delivery, with a heightened focus on translational and scalable potential (39) for eventual implementation into clinical practice. The sampling intervention tested here took only a few minutes to deliver, required no complicated instructions to either the patient or the provider, and was easily embedded within the context of busy primary care practices.
The cessation outcomes observed in this study are, as expected, lower than reported in most RCTs of NRT (4) that offer longer duration of medication with added counseling support. The sampling experience herein was a minimally intensive intervention, applied liberally to a broad group of smokers. Expectations of outcomes must be tempered. Nonetheless, our findings have real-world applications suggesting that medication sampling in primary care practice settings can serve as a behavioral catalyst for smoking cessation (40). Recent meta-analyses (41) suggest that pharmacological interventions as delivered to smokers not ready to quit yield a wide range of effect sizes (Number Needed to Treat ranging from 6–70, as compared to 25 herein, all comers). The real value of medication sampling, if any, will derive from thorough cost effectiveness analyses, including analyses of Quality-Adjusted Life Years (QALY). NRT samples provided herein were inexpensive (<$60 per participant), and if implemented in real-world practice, would not require much additional costs. Real intervention costs, inclusive of NRT itself and the logistics of clinic delivery, would be nominal and potentially covered by either insurance providers or clinics themselves. Rudimentary estimates here suggest the total cost per quit attempt is $150, and cost per quit is $475. This is similar to or lower than that estimated for quitline-based medication give-away programs (27, 42–44) presumed to attract smokers wanting to quit. More recent evidence suggests potential cost-effectiveness of medication distribution specifically for smokers not wanting to quit (41).
Limitations
The absence of biological verification of smoking status stands out as a study weakness. Methods exist for remote collection of saliva samples for testing of cotinine, but these options do not lend themselves for NRT interventions, especially when >20% of the sample is using NRT at follow-up. Methods for remote capture of carbon monoxide verification are in development (45) but not yet ready for widespread implementation. Modest retention throughout follow-up is another study weakness, though consistent with other RCTs of smoking cessation within primary care (22, 23, 36, 37). Additionally, while behavioral interventions are difficult to blind, the lack of blinded assessment during follow-up may have introduced bias, which could be particularly compounded in the absence of biochemical verification of outcomes. Finally, models involving binary outcomes included the conservative assumption of missing data as “no event,” and no such assumptions were made for continuous outcomes. As such, the estimates presented here assume data were “missing completely at random,” which we cannot verify as participants may be smoking more (or less) at follow-up, which may have impacted their ability to be available for assessments.
Conclusions
The challenges of delivering effective yet scalable smoking cessation interventions within real-world medical settings are significant, but so are the opportunities. Provision of medication samples to promote smoking abstinence within the context of primary care is wholly consistent with seminal recommendations to do so (46) and may be a practical, disseminable strategy to get more smokers engaged in evidence-based care.
Acknowledgements:
The authors express profound appreciation to the many personnel across all clinics who joined in this effort, as well as the numerous research personnel who assisted with study procedures and specifically to Ms. Amy Boatright (MUSC) and Mr. Douglas Fleming (CCI) who coordinated the entire effort.
Funding: This trial was funded primary through the National Institute on Drug Abuse (R01 DA 021619). Additional research support through NIH UL1 TR001450 and K23 DA 045766 (JD). The funding source had no involvement in the collection, analysis, interpretation, or write-up of study data. The corresponding author (MJC) and statistician (AEW) had full access to all the data in the study, and all authors were responsible for the decision to submit for publication.
Declaration of Interests: Drs. Carpenter, Cummings, and Gray have received consulting honoraria from Pfizer. Dr. Cummings also has served as a paid expert witness in litigation filed against the tobacco industry. Nicotine patches were purchased through GSK Bulk Pricing. All other NRT was purchased through conventional outlets.
Footnotes
References
- 1.Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ. et al. Treating tobacco use and dependence: 2008 Update Clinical Practice Guideline Rockville, MD: US Public Health Service; 2008. [Google Scholar]
- 2.Stead LF, Bergson G, Lancaster T. Physician advice for smoking cessation (Cochrane Review) The Cochrane Library, Issue 3, Oxford: Wiley Publishers; 2008. [DOI] [PubMed] [Google Scholar]
- 3.Kruger J, O’Halloran A, Rosenthal A. Assessment of compliance with US public health service clinical practice guideline for tobacco by primary care physicians, Harm Reduction Journal 2015: 12: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: An overview and network meta-analysis Cochrane Database of Systematice Reviews, Oxford: John Wiley & Sons; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah SD, Wilken LA, Winkler SR, Lin SJ. Systematic review and meta-analysis of combination therapy for smoking cessation, Journal of the American Pharmacists Association 2008: 48: 659–665. [DOI] [PubMed] [Google Scholar]
- 6.Dahne J, Wahlquist AE, Garrett-Mayer E, Heckman BW, Cummings KM, Carpenter MJ. State tobacco policies as predictors of evidence-based cessation method usage: Results from a large, nationally representative dataset, Nicotine & Tobacco Research 2018: 20: 1336–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fix BV, Hyland A, Rivard C, McNeill A, Fong GT, Borland R. et al. Usage patterns of stop smoking medications in Australia, Canada, the United Kingdom, and the United States: Findings from the 2006–2008 International Tobacco Control (ITC) Four Country Survey, International Journal of Environmental Research and Public Health 2011: 8: 222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glasgow RE. What does it mean to be pragmatic? Pragmatic methods, measures, and models to facilitate research translation, Health Education and Behavior 2013: 40: 257–265. [DOI] [PubMed] [Google Scholar]
- 9.Carpenter MJ, Jardin BF, Burris JL, Mathew AR, Schnoll RA, Rigotti NA. et al. Clinical strategies to enhance the efficacy of nicotine replacement therapy for smoking cessation: A review of the literature, Drugs 2013: 73: 407–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balmford J, Borland R, Hammond D, Cummings KM. Adherence to and reasons for premature discontinuation from stop-smoking medications: Data from the ITC Four-Country Survey, Nicotine & Tobacco Research 2011: 13: 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bansal MA, Cummings KM, Hyland A, Giovino GA. Stop-smoking medications: Who uses them, who misuses them, and who is misinformed about them?, Nicotine & Tobacco Research 2004: 6: S303–S310. [DOI] [PubMed] [Google Scholar]
- 12.Cummings KM, Hyland A, Giovino GA, Hastrup JL, Bauer JE, Bansal MA. Are smokers adequately informed about the health risks of smoking and medicinal nicotine?, Nicotine & Tobacco Research 2004: 6: S333–S340. [DOI] [PubMed] [Google Scholar]
- 13.Etter JF, Perneger TV. Pharmacoepidemiology and drug utilization: Attitudes toward nicotine replacement therapy in smokers and ex-smokers in the general public, Clinical Pharmacology Therapy 2001: 69: 175–183. [DOI] [PubMed] [Google Scholar]
- 14.Hammond D, McDonald PW, Fong GT, Borland R. Do smokers know how to quit? Knowledge and perceived effectiveness of cessation assistance as predictors of cessation behaviour, Addiction 2004: 99: 1042–1048. [DOI] [PubMed] [Google Scholar]
- 15.Zapawa LM, Hughes JR, Benowitz NL, Rigotti NA, Shiffman S. Cautions and warnings on the US OTC label for nicotine replacement: What’s a doctor to do?, Addictive Behaviors 2011: 36: 327–332. [DOI] [PubMed] [Google Scholar]
- 16.Carpenter MJ, Hughes JR, Gray KM, Wahlquist AE, Saladin ME, Alberg AJ. Nicotine therapy sampling to induce quit attempts among smokers unmotivated to quit: A randomized clinical trial, Archives of Internal Medicine 2011: 171: 1901–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carpenter MJ, Alberg AJ, Gray KM, Saladin ME. Motivating the unmotivated for health behavior change: A randomized trial of cessation induction for smokers, Clinical Trials 2010: 7: 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burris JL, Heckman BW, Mathew AR, Carpenter MJ. A mechanistic test of nicotine replacement therapy sampling for smoking cessation induction, Psychology of Addictive Behaviors 2015: 29: 392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jardin BF, Cropsey KL, Wahlquist AE, Gray KM, Silvestri GA, Cummings KM. et al. Evaluating the effect of access to free medication to quit smoking: A clinical trial testing the role of motivation, Nicotine & Tobacco Research 2014: 16: 992–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cunningham JA, Kushnir V, Selby P, Tyndale RF, Zawertailo L, Leatherdale ST. Effect of mailing nicotine patches on tobacco cessation among adult smokers: A randomized clinical trial, JAMA Internal Medicine 2016: 176: 184–190. [DOI] [PubMed] [Google Scholar]
- 21.Cunningham JA, Kushnir V, Selby P, Tyndale RF, Zawertailo L, Leatherdale ST. Beyond quitting: Any additional impact of mailing free nicotine patches to current smokers, Nicotine & Tobacco Research 2018: 20: 654–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu SS, van Ryn M, Nelson D, Burgess DJ, Thomas JL, Saul J. et al. Proactive tobacco treatment offering free nicotine replacement therapy and telephone counselling for socioeconomically disadvantaged smokers: a randomised clinical trial, Thorax 2016: 71: 446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu SS, van Ryn M, Sherman SE, Burgess DJ, Noorbaloochi S, Clothier B. et al. Proactive tobacco treatment and population-level cessation: A pragmatic randomized controlled trial, JAMA Internal Medicine 2014: 174: 671–677. [DOI] [PubMed] [Google Scholar]
- 24.An LC, Zhu S-H, Nelson DB, Arikian NJ, Nugent S, Partin MR. et al. Benefits of telephone care over primary care for smoking cessation: A randomized trial, Archives of Internal Medicine 2006: 166: 536–542. [DOI] [PubMed] [Google Scholar]
- 25.Abdullah AS, Hedley AJ, Chan SSC, Lam T-H. A randomized controlled trial of two different lengths of nicotine replacement therapy for smoking cessation, BioMed Research International 2013: 2013. [DOI] [PMC free article] [PubMed]
- 26.Dahne J, Wahlquist AE, Boatright AS, Garrett-Mayer E, Fleming DO, Davis R. et al. Nicotine replacement therapy sampling via primary care: Methods from a pragmatic cluster randomized clinical trial, Contemporary Clinical Trials 2018: 72: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cummings KM, Hyland A, Carlin-Menter S, Mahoney MC, Willett J, Juster HR. Costs of giving out free nicotine patches through a telephone quit line, Journal of Public Health Management and Practice 2011: 17: E16–23. [DOI] [PubMed] [Google Scholar]
- 28.Borland R, Yong HH, O’Connor RJ, Hyland A, Thompson ME. The reliability and predictive validity of the Heaviness of Smoking Index and its two components: findings from the International Tobacco Control Four Country study, Nicotine & Tobacco Research 2010: S45–S50. [DOI] [PMC free article] [PubMed]
- 29.Benowitz N, Jacob P, Ahijevych K, Jarvis M, Hall S, LeHouezec J. et al. Biochemical verification of tobacco use and cessation, Nicotine & Tobacco Research 2002: 4: 149–159. [DOI] [PubMed] [Google Scholar]
- 30.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: Issues and recommendations, Nicotine & Tobacco Research 2003: 5: 13–25. [PubMed] [Google Scholar]
- 31.Hughes J, Keely J, Niaura R, Ossip-Klein D, Richmond R, Swan G. Measures of abstinence in clinical trials: issues and recommendations, Nicotine & Tobacco Research 2003: 5: 13–26. [PubMed] [Google Scholar]
- 32.Meltzer LR, Simmons VN, Sutton SK, Drobes DJ, Quinn GP, Meade CD. et al. A randomized controlled trial of a smoking cessation self-help intervention for dual users of tobacco cigarettes and e-cigarettes: Intervention development and research design, Contemporary Clinical Trials 2017: 60: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cox LS, Okuyemi K, Choi WS, Ahluwalia JS. A review of tobacco use treatments in U.S. ethnic minority populations, American Journal of Health Promotion 2011: 25: S11–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piper ME, Cook JW, Schlam TR, Jorenby DE, Smith SS, Bolt DM. et al. Gender, race, and education differences in abstinence rates among participants in two randomized smoking cessation trials, Nicotine & Tobacco Research 2010: 12: 647–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith PH, Kasza KA, Hyland A, Fong GT, Borland R, Brady K. et al. Gender differences in medication use and cigarette smoking cessation: Results from the ITC Four County Survey, Nicotine & Tobacco Research 2015: 17: 463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith SS, McCarthy DE, Japuntich SJ, Christiansen B, Piper ME, Jorenby DE. et al. Comparative effectiveness of 5 smoking cessation pharmacotherapies in primary care clinics, Archives of Internal Medicine 2009: 14: 2148–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piper ME, Cook JW, Schlam TR, Jorenby DE, Smith SS, Collins LM. et al. A randomized controlled trial of an optimized smoking treatment delivered in primary care, Annals of Behavioral Medicine 2018: 52: 854–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cunningham JA, Kushnir V, McCambridge J. The impact of asking about interest in free nicotine patches on smoker’s stated intent to change: real effect or artefact of question ordering?, Nicotine & Tobacco Research 2016: 18: 1215–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milat AJ, King L, Bauman AE, Redman S. The concept of scalability: Increasing the scale and potential adoption of health promotion interventions into policy and practice, Health Promotion International 2013: 28: 285–298. [DOI] [PubMed] [Google Scholar]
- 40.Kasza KA, Hyland AJ, Borland R, McNeill AD, Bansal-Travers M, Fix BV. et al. Effectiveness of stop-smoking medications: Findings from the International Tobacco Control (ITC) four country survey, Addiction 2013: 108: 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ali A, Kaplan CM, Derefinko KJ, Klesges RC. Smoking cessation for smokers not ready to quit: Meta-analysis and cost-effectiveness analysis, American Journal of Preventive Medicine 2018: 55: 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fellows JL, Bush T, McAfee T, Dickerson J. Cost effectiveness of the Oregon quitline “free patch initiative”, Tobacco Control 2007: 16(Suppl 1): i47–i52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krupski L, Cummings KM, Hyland A, Mahoney MC, Toll BA, Carpenter MJ. et al. Cost and effectiveness of combination nicotine replacement therapy among heavy smokers contacting a quitline, Journal of Smoking Cessation 2014: 1–10.
- 44.Cummings KM, Fix B, Celestino P, Carlin-Menter S, O’Connor R, Hyland A. Reach, efficacy, and cost-effectiveness of free nicotine medication giveaway programs, Journal of Public Health Management Practice 2006: 12: 37–43. [DOI] [PubMed] [Google Scholar]
- 45.McClure EA, Tomko RL, Carpenter MJ, Treiber FA, Gray KM. Acceptability and compliance with a remote monitoring system to track smoking and abstinence among young smokers, American Journal of Drug and Alcohol Abuse 2018: 44: 561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fiore MC, Baker TB. Treating smokers in the health care setting, New England Journal of Medicine 2011: 365: 1222–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]