Abstract
Lyme disease is the most common vector-borne disease in the northern hemisphere and is caused by spirochetes of the Borrelia burgdorferi sensu lato complex. Lyme borreliae infect diverse vertebrate reservoirs without triggering apparent manifestations in these animals, however Borrelia strains differ in their reservoir hosts. The mechanisms that drive those differences are unknown. To survive in their vertebrate hosts, Lyme borreliae require the ability to escape from host defense mechanisms, in particular complement. To facilitate complement evasion, Lyme borreliae produce diverse proteins at different stages of infection, allowing them to persistently survive without being recognized by hosts and potentially resulting in host-specific infection. This review discusses the current knowledge regarding the ecology and evolutionary mechanisms of Lyme borreliae-host associations driven by complement evasion.
Keywords: Borrelia, Ixodes, Host association, Immune evasion, Lyme disease
Lyme borreliae and complement
Lyme disease (LD) or borreliosis is caused by spirochetes belonging to the Borrelia burgdorferi sensu lato (s.l.) complex [1]. (Note the genus of Borrelia also causes another disease, e.g. relapsing fever, we use the terminology Lyme borreliae here to represent the causative agent of LD). Lyme borreliae are transmitted from vertebrate reservoir hosts (see Glossary) to humans via hard ticks of the genus Ixodes [2]. More than 20 different genospecies of the complex have been identified so far of which six species are confirmed to cause human LD: B. burgdorferi sensu stricto (s.s.), B. afzelii, B. garinii, B. spielmanii, B. bavariensis (formerly referred to as B. garinii OspA serotype 4), and B. mayonii [2]. Within a genospecies, the isolates of Lyme borreliae may differ in their genetic contents and have been genotyped using different methodologies [3-6]. These isolates often vary in their associations with particular host species [7, 8].
Common to B. burgdorferi s.l. species is their ability to counteract the innate immune defense mechanisms of diverse hosts. Some mammalian and avian reservoir hosts can be persistently infected by certain species for prolonged periods without suffering from disease manifestations. In contrast, the immune system of humans and other animals that are non-reservoir hosts can develop disease manifestations, including arthritis, carditis, neurological symptoms (known as neuroborreliosis), and acrodermatitis chronica atrophicans [2, 9]. The ability of B. burgdorferi s.l. to be maintained in these hosts, or cause disease manifestations differ, but the mechanisms that drive such differences remain unclear.
Complement, as an important pillar of innate immunity, forms a powerful surveillance system that comprises a well-organized network of fluid-phase and membrane-bound regulatory proteins circulating in the blood. Upon recognition of invading microorganisms, complement is immediately activated in a cascade-like manner. Despite the effectiveness of complement, Lyme borreliae develop strategies to circumvent this crucial, non-specific barrier of their hosts [10]. However, the heterogeneity in the ability of Lyme borreliae genospecies to survive in sera from different hosts lead to the hypothesis that such Lyme borreliae complement-inhibitory strategies do not necessarily protect spirochetes from killing by serum of every host species [11]. Additionally, the ability to evade complement appears to determines host infectivity of these pathogens [10, 12, 13]. This review thus focuses on the current knowledge of the molecular mechanisms utilized by Lyme borreliae to counteract complement and the potential role of complement evasion in the evolution of host specialization for those bacteria.
Diversity in complement evasion of Lyme borreliae
Complement is a powerful component of vertebrates’ immune defense against invading microorganisms. A Lyme borreliae strain’s ability to evade complement has been determined by testing whether that a particular strain is able to survive in host sera (also described as serum resistance). B. burgdorferi s.l. varies in their ability to inhibit complement from humans and various animals (Table 1) [14-16]. A strain’s ability to avoid complement-mediated killing by a particular host’s serum is strongly correlated with the capability of that strain to survive in that host. For example, the ave-associated species B. garinii and B. valaisiana are generally able to survive in avian but not mammalian sera, while the mammal-associated species B. afzelii, B. bavariensis, B. spielmanii, B. bissettiae, and B. japonica can generally survive in mammalian but not avian sera (reviewed in [17] (Table 1). Additionally, B. burgdorferi, B. afzelii, B. spielmanii, B. bavariensis, and B. mayonii, which have been isolated from humans, are capable of surviving in human sera. Note that the pathogenicity of B. valaisiana and B. lusitaniae for humans remains unclear, but these strains are killed by human serum (reviewed in [18]) (Table 1). A notable exception is B. garinii, which has been isolated from humans with neurological manifestations, yet some B. garinii strains are highly vulnerable to the killing by human sera. Despite several proteins derived from tick saliva were shown to contribute to the resistance of B. burgdorferi s.l. to complement attack [19], the correlation of host-specific serum resistance and the infectivity pattern among B. burgdorferi s.l. supporting the notion of bacterial factor(s) that determines host association.
Table 1.
Serum susceptibility pattern of B. burgdorferi s.l. to human and diverse animal seraa
| Speciesb |
B. burgdorferi |
B. afzelii |
B. bavariensis |
B. japonica |
B. bissettiae |
B. andersonii |
B. garinic |
B. valaisiana |
B. lusitaniae |
|---|---|---|---|---|---|---|---|---|---|
| Human | R | R | R | R | I | S | S | S | S |
| Mouse | R | R | R | R | R | ND | S | S | ND |
| Rat | S | R | R | ND | ND | ND | S | ND | ND |
| Hamster | R | R | R | R | ND | ND | S | S | S |
| Squirrel | R | R | R | R | ND | ND | S | S | ND |
| Rabbit | I | S | ND | ND | I | ND | S | ND | ND |
| Cat | I | R | R | R | ND | ND | I | R | ND |
| Lynx | I | I | R | S | R | I | I | R | S |
| Dog | I | R | R | I | R | R | S/I | I | S |
| Wolve | I | S | R | S | R | I | S/I | S | S |
| Mouflon | I | R | R | R | R | I | R/I | R | R |
| Pheasant | I | S | S | S | ND | ND | R | R | S |
| Blackbird | I | S | S | S | ND | ND | R | R | S |
| Sheep | I | S | S | R | S/R | I | S | S | R |
| Horse | I | S | S | S | ND | ND | S | S | S |
| Pig | I | S | S | S | ND | ND | S | S | S |
| Goat | S | S | ND | ND | ND | ND | S | ND | ND |
| Bovine | S | S | S | S | S | S | S | S | S |
| Deer | S | S | S | S | S | S | S | S | S |
| Eur. Bisond | S | S | S | S | S | S | S | S | S |
| Lizard | S | S | S | S | S | ND | R | R | R |
| Quail | R | ND | ND | ND | S | ND | ND | ND | ND |
Data shown were derived from [13]; R, serum-resistant; I, intermediate serum-resistant; S, serum-sensitive, ND, no data available
B. burgdorferi, B. afzelii, B. bavariensis, B. japonica, B. bissettiae, and B. andersonii are (mainly) rodent-associate species, B. garinii and B. valaisiana are bird-associate species, and B. lusitaniae is a reptile-associate species.
Variations in the serum susceptibility pattern have been reported for the heterogenous genospecies B. garinii [14]. Of note, B. garinii OspA serotype 4 was thereafter referred to as B. bavariensis known to display a serum-resistant phenotype. B. mayonii and B. spielmanii has not been included due to the lack of available data but both species resist complement-mediated killing by human serum [99, 100].
Eur., European.
The factors of Lyme borreliae involved in complement evasion.
Complement can be activated through three canonical routes: the classical (CP), the lectin (LP), and the alternative pathway (AP) (Fig. 1)[20]. The binding of antibody to antigen and C1 protein complex activates CP, whereas the association of mannan-binding lectin, ficolins, or collectins with carbohydrates on a pathogen’s surface induces activation of the LP. Formation of the C3 convertase in the fluid phase, C3bBb and subsequent cleavage of C3 to C3a and C3b triggers the activation of the AP and leads to the deposition of C3b to the microbal or other target surface (Fig. 1). Activation of each of these pathways results in formation of two different types of C3 convertases: C3bBb formed by the AP, and C4b2a generated by the- CP and LP (Fig. 1). Both C3 convertases then promote formation of the central complement component, C3b, which leads to formation of the C5 convertase(s) to cleave C5 into C5a and C5b. C5b deposition on bacterial surfaces initiates the terminal sequence (TS), which recruits the late complement proteins C6, C7, and C8. The association of C5b, C6, C7, and C8 leads to the deposition of C9, which is multimerized to form the bacteriolytic terminal complement complex (TCC, also known as the membrane attack complex, MAC). To protect self surfaces from excessive activation, complement is tightly controlled by a number of soluble and membrane-anchored regulators. These regulators include, but not limited to, C1 esterase inhibitor (C1-INH) and C4b-binding protein (C4BP) that inhibit CP and LP, Factor H (FH) and Factor H-like protein 1 (FHL-1) that inhibit AP, and vitronectin that negatively modulates the formation of the MAC (Fig. 1)[20].
Figure 1. Schematic diagram of vertebrate complement cascades and the particular steps Lyme borreliae anti-complement protein interact.
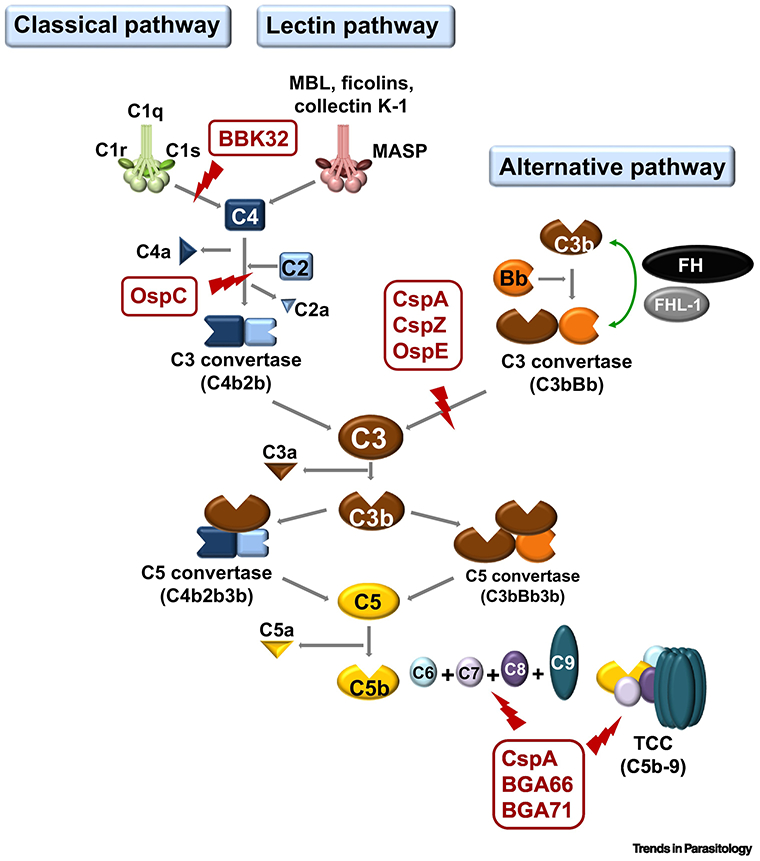
The CspA, CspZ, and OspE of B. burgdorferi s.l. target the host complement regulator, FH, by inhibiting the formation C3bBb to inactivate AP. LD spirochetes also produce BBK32 and OspC that bind to C1r and C4b, respectively. These proteins inhibit CP (for BBK32 and OspC) and LP (for OspC). Additional proteins of B. burgdorferi s.l. (e.g. CspA, BGA66, and BGA71) inactivate TCC by preventing the formation of C5b-9 on the surface of spirochetes (Part of the figure is adapted from [18]). FH, Factor H; AP, alternative pathway; CP, classical pathway; LP, Lectin pathway; TS, terminal sequence; LD, Lyme disease; TCC, terminal complement complex
Lyme borreliae possess a number of structurally diverse outer surface proteins to inactivate complement at different stages of the infection cycle. These proteins target complement proteins/regulators that can modulate different arms of complement (reviewed in [13]). The proteins that inhibit AP include the collectively termed FH/FHL-1-binding Complement-Acquiring Surface Proteins (CRASP): CspA, CspZ, and OspE-related protein (members of a family of proteins collectively known as “Erp”, which include ErpA, ErpC, and ErpP) [21-27](Table 2). The recruitment of FH and/or FHL-1 by these proteins onto the bacterial surface leads to inactivation of the AP, permitting Lyme borreliae to survival in host sera. Additionally, B. burgdorferi s.l. produce at least two additional outer surface proteins to inhibit complement: BBK32 and OspC (Table 2) [28, 29]. BBK32 binds to C1r and thereby inhibits the activation of the C1 complex, resulting in the termination of all downstream activation steps of the CP. OspC of B. burgdorferi s.l. binds to C4b to prevent the formation of C4b2a, the C3 convertase of CP and LP, and thus inhibits activation of those pathways [28, 29]. Of note, formation of the MAC can be down-regulated by several Lyme borreliae proteins [30, 31](Table 2) but the role of TS inhibition to contribute to Lyme borreliae infectivity is as yet unclear.
Table 2.
Characteristics of complement interacting proteins of LD spirochetes
| BBK32 | OspC | CspA | CspZ | OspE paralogs |
BGA66 | BGA71 | p43 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| ErpPa | ErpCa | ErpAa | ||||||||
| synonyms and other designations | none | none | CRASP-1 BBA68 |
CRASP-2 BBH06 |
CRASP-3 BBN38 |
CRASP-4 | CRASP-5 ErpI ErpN BBP38 BBL39 OspE |
none | none | none |
| gene name | bbk32 | ospC | cspA | cspZ | erpP | erpC | erpA | bga66 | bga71 | ND |
| origin | Bb | Bb | Bb, Ba, Bs, Bm | Bb | Bb | Bb | Bb | Bba | Bba | Bb |
| confers serum resistance | yes | yes | yes | yes | unclearb | unclearb | unclearb | yes | yes | ND |
| interaction with complement | C1r | C2 | FH FHL-1 |
FH FHL-1 |
FHR-1 FHR-2 |
FHR-1 FHR-2 |
FHR-1 FHR-2 |
C7, C8, C9, | C7, C8, C9, | C4BP |
| regulators / components | C7, C8, C9, TCC | FHR-5 | FHR-5 | TCC | TCC | |||||
| affected complement pathways | CP | CP | AP, TS | AP | ND | ND | ND | TS | TS | CP/LP(?) |
| Fed larvae | − | − | + | + (LE) | + (HE) | + (HE) | + (HE) | ND | ND | ND |
| Unfed nymphs | − | − | + (HE) | − | − | − | − | ND | ND | ND |
| Fed nymphs | + | + | + (LE) | + (LE) | + | + | + | ND | ND | ND |
| Tick biting sites | + | + | + | + (HE) | + (HE) | + (HE) | + (HE) | ND | ND | ND |
| Distal sites | + | − | − | + (HE) | + (HE) | + (HE) | + (HE) | ND | ND | ND |
| + | + (LE) | + (HE) | + (HE) | + (HE) | ND | ND | ND | |||
Binding of FH has only been confirmed for recombinant proteins.
Confers serum resistance only when ErpP and ErpA are expressed under flaB promoter in a cspA-deficient B. burgdorferi in the infectious background.
CRASP, complement-regulator acquiring surface protein; Erp, OspE/F-like protein; FH, Factor H; FHL, Factor H-like protein, FHR, FH-related protein; TCC, terminal complement complex; Bb, B. burgdorferi; Bba, B. bavariensis; Ba, B. afzelii; Bs, B. spielmanii; Bm, B. mayonii; AP, alternative pathway; CP, classical pathway; LP, lectin pathway; TS, terminal sequence, ND; no data available, HE; high expression, LE; low expression
Multiple regulatory mechanisms control expression of complement inhibitory proteins.
Lyme borreliae proteins that mediate resistance to host complement exhibit different patterns of expression during infection, indicative of several distinct regulatory pathways for the production of these proteins. Lyme borreliae within unfed ticks do not produce OspC, OspE-related proteins, CspA, or CspZ [32-35](Table 2). When an infected tick begins to feed on the blood of a vertebrate host, production of OspC is induced, so that transmitted bacteria possess this protein on their outer surface [32]. However, OspC production is repressed within a few days after establishment of infection [36] (Table 2). In contrast, OspE-related proteins are also induced during tick feeding, but these outer surface proteins continue to be produced throughout vertebrate infection, and bacteria acquired by ticks from infected mammals produce all of their OspE-related proteins [33, 37] (Table 2). Production of CspA is also induced during tick feeding, repressed subsequently after the transmission begins, and the infection establishes at the tick biting site of the skin. The cspA expression is then induced when Lyme borreliae are transmitted from infected vertebrates to feeding ticks [34, 35] (Table 2). CspZ exhibits yet another pattern of expression: its production begins after transmission of bacteria from the tick into the vertebrate, persists throughout vertebrate infection, then is repressed during acquisition by feeding ticks [34, 35](Table 2).
Of the Lyme borreliae complement-resistance mediators, the regulatory networks of OspC and the OspE-related proteins are the most well studied. High-level expression of OspC is dependent upon an alternative sigma factor (RpoS), which has led to a hypothesis that RpoS directly controls ospC transcription [38]. However, ospC is transcribed at low levels in rpoS-deficient mutants, leading to an alternative hypothesis that the effect of RpoS is indirect [39, 40]. Consistent with that second model, a region of DNA 5’ of the ospC promoter is required for RpoS-dependent induction of ospC, and is likely to be a binding site for a regulatory protein that is under control of RpoS [41, 42]. Additionally, bbk32 is also regulated by such a RpoS-dependent mechanism in the similar fashion as ospC [43, 44]. While the operon of ospC is controlled in the RpoS-independent manner [39], this operon contains a highly-conserved operator region, and are under transcriptional regulation of three proteins that bind to erp operator DNA: the BpaB repressor, the BpuR co-repressor, and the EbfC anti-repressor [45-49]. Studies of BpuR and EbfC indicated that each protein regulates its own production, and that production of both proteins is also controlled by the DnaA protein (the master regulator of bacterial replication) [50-52]. In addition, our preliminary studies of CspZ found that a novel Lyme borreliae protein binds near the cspZ transcriptional promoter, which warrants further investigation.
Polymorphisms of complement-interacting proteins influencing Lyme borreliae-host association
CspA, a complement evasion factor operating in the ticks
The transcript encoding CspA is expressed by B. burgdorferi s.s. at the onset of tick feeding and during transmission to vertebrate hosts, and then repressed in the later stages of infection [35] (Table 2). The tick-specific expression profile of cspA is consistent with the previous finding that Lyme borreliae require CspA to survive in ticks’ midgut upon blood feeding [53]. A recent observation indicates that CspA-mediated FH-binding activity is essential for these pathogens to evade complement in the ingested blood, permitting efficient tick-to-host transmission [53] (Fig. 2, Key Figure). The CspA polymorphisms are associated with variable FH-binding activity [53, 54], resulting in the strains that are either highly vulnerable (in the absence of FH) or highly resistant (upon binding of FH) to complement of vertebrate hosts [53, 54]. These findings indicate that CspA is one of the determinants that define host-specific infection. However, whether particular CspA variants that promote inefficient tick-borne transmission to mice have a role in facilitating transmission to other animals remains unknown. The evolutionary mechanisms and amino acid determinants of this protein to drive such host associations need further investigations.
Figure 2, Key Figure. Complement inhibitory proteins and their potential roles in the infection route.
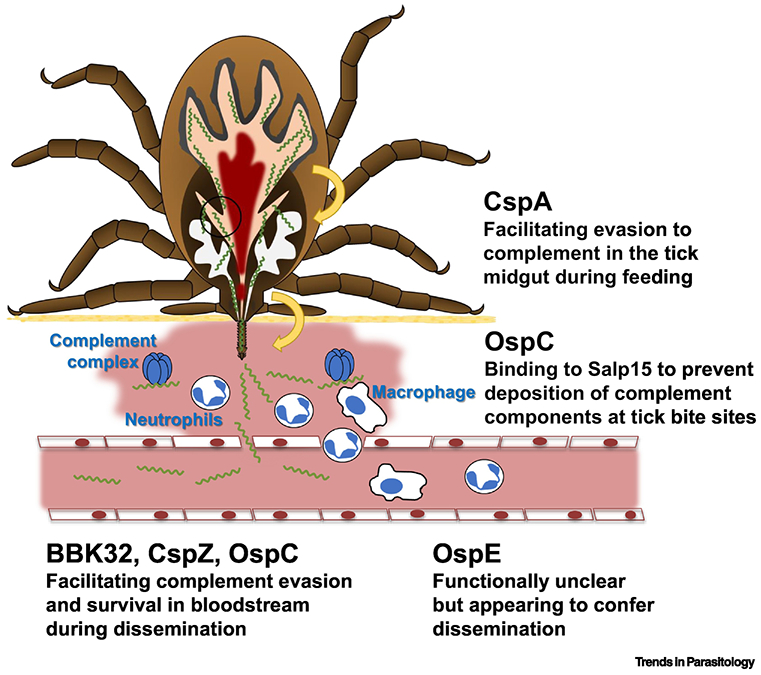
When ticks feed on hosts, B. burgdorferi s.s. produce CspA to facilitate spirochete escape from complement-mediated killing in the blood meal. After transmission to a host, the tick salivary protein, Salp15, binds to OspC on the spirochete surface to prevent opsonophagocytosis at tick bite sites. Additionally, B. burgdorferi s.s. produces OspC, BBK32, and CspZ to promote complement evasion and bloodstream survival of spirochetes. The cell types and complement complex have been indicated on the figure. Though the function of OspE during infection remains unclear, the current evidence supports that this protein may confer spirochete dissemination in vertebrate animals (Part of the figure is adapted from [27]).
CspZ, a complement evasion factor operating in the vertebrate host
In contrast to cspA, expression of cspZ occurs only in the vertebrate host [35] (Table 2). Lyme borreliae that lack cspZ or produce a mutant CspZ without FH-binding activity exhibit reduced colonization of distal tissues during mouse infection. Those results indicate that CspZ-mediated FH-binding activity contributes to spirochete dissemination [55, 56] (Fig. 2). Unlike CspA, the amino acid sequences of CspZ are largely conserved among different B. burgdorferi s.s. strains (> 95% identity) and species of the B. burgdorferi s.l. complex (>70% identity) [57, 58]. However, allelically different human FH-binding activity was observed in CspZ from different B. burgdorferi s.s. strains [57, 58]. Comparisons of the solved structure of CspZ of B. burgdorferi B31 with different B. burgdorferi s.s. strain showed variations in the regions that are involved with FH-binding activity [59]. These results raise an intriguing question: would this host-specific FH-binding activity of CspZ enable this protein as one of the determinants that drive host association? Additionally, CspZ is not carried by every B. burgdorferi s.s. strain, suggesting that additional genes encoding complement-inhibitory proteins are co-expressed with cspZ [57, 60] (Table 2).
OspE paralogs, additional complement evasion factors operating in the vertebrate host?
B. burgdorferi s.l. produces multiple paralogs of OspE [61-63]. Consistent with expression of ospE triggered by host-specific environmental cues (e.g. blood meal), a previous study reported that passive transfer of anti-OspE IgG reduces the levels of spirochetes transmission to mice [64]. A B. burgdorferi s.s. strain with transposon inserted into erpA (one ospE paralog in B. burgdorferi s.s. strain B31-A3) displays a two-week delay in the distal tissue colonization when co-infected with a population of mutant Lyme borreliae strains with transposon inserting in different genes [65]. These findings suggest that OspE promotes spirochetes’ tick-to-host transmission and hematogenous dissemination (Fig. 2). The ospE genes largely differ in the number of copies and sequences among different species or strains of B. burgdorferi s.l. , raising a possibility that OspE determines host-specificity of infection [66, 67].
OspC and BBK32, complement evasion factors operating in the initial phase of infection
OspC is one of the most studied outer surface lipoproteins in B. burgdorferi s.l. This protein is not expressed when Lyme borreliae are in ticks prior to blood feeding but produced upon the blood feeding of ticks and during transmission. After the entry into hosts, the production of OspC remains until Lyme borreliae begin disseminating to distal tissues (Table 2). OspC binds to a tick salivary protein, Salp15, and the decoration of this tick protein on the surface of Lyme borreliae prevents opsonophagocytosis at the tick biting site [68]. OspC also binds to human complement C4b to inactivate CP and LP. Consistent with these activities, OspC is required for Lyme borreliae to survive at infection initiation sites during the first 24 hours of pathogen inoculation and confers spirochetes’ the ability to remain in the mammalian bloodstream [28, 69] (Fig. 2). Nonetheless, the molecular mechanisms leading to such phenotypes need further investigations. Furthermore, OspC is one of the most polymorphic proteins among different strains or species of B. burgdorferi s.l. [1]. However, whether this protein is a determinant of host-specific survival and if so, which mechanisms drive such survival is still unclear.
BBK32 was initially identified as an adhesin that binds to extracellular matrix molecules fibronectin and glycosaminoglycans on the host cell surface and later demonstrated as a C1r-binding protein to inactivate CP [29]. In agreement with a blood meal-induced expression profile of bbk32 (Table 2), BBK32 contributes to the ability to survive in mouse bloodstream at short-term and disseminate to joints at early stages of infection [69, 70] (Fig. 2). Though BBK32 is conserved (close to 90% similarity among strains or species of B. burgdorferi s.l.), the orthologs from B. afzelii and B. garinii differ in their capability to bind to human C1r [71]. Assuming that C1r-binding activity plays a role in conferring spirochete survival in vertebrate bloodstream and promoting dissemination at infection onset, such a strain-to-strain variation of BBK32-mediated C1r-binding activity may support the notion that this protein drives host-specific infectivity.
Host specialism of LD spirochetes at a glance
The spirochetes of the B. burgdorferi s.l. complex are maintained in an enzootic cycle between ticks of the Ixodes ricinus species complex and reservoir hosts, including small and medium-sized mammals, birds and reptiles [9]. In most Lyme disease endemic regions, there is a diverse community of co-circulating Lyme borreliae and an association between different classes of vertebrate hosts and some B. burgdorferi s.l. genospecies has been observed [9, 72, 73]. Some of these observed associations may be due to extrinsic factors such as geographic co-occurrence of hosts with specific B. burgdorferi s.l. genospecies. However, there is strong evidence that at least some of these genospecies differ intrinsically in transmissibility across hosts, i.e. they are “host specialized” [11, 12, 72]. The strongest evidence is provided by experiments demonstrating increased fitness for B. afzelii in mice and B. garinii in birds [11, 12, 73] and, to some extent, field studies demonstrated greater genospecies infection prevalence in certain hosts compared to the background infection prevalence in local populations of Ixodes spp [74].
In contrast to the other genospecies in the B. burgdorferi s.l. complex, B. burgdorferi s.s. is considered a host generalist, as it has been isolated from multiple classes of vertebrate animals (e.g. mammalian and avian hosts) [[72] and summarized in [9]]. However, multiple studies indicate that some genotypes of B. burgdorferi s.s. have higher fitness in some hosts in laboratory studies [75] and are more prevalent in certain mammalian or avian host species [9, 76-82]. Evidence of within-genospecies association of specific genotypes of B. burgdorferi s.l. and certain hosts has also been described for B. garinii and B. afzelii in laboratory experiments [9, 73, 83] and some field studies [84, 85], but not in others [86]. A limitation of field studies is that they represent only snapshots of population structures that are spatially and temporally variable due to stochastic effects or other forces, making inferences of host association difficult [72].
Eco-evolutionary mechanisms driving B. burgdorferi-host specialism
Despite evidence for some level of association between B. burgdorferi s.s. strains and hosts from laboratory infections and field studies [5, 87], the extent to which host adaptation drives the genome-wide diversification in B. burgdorferi s.l. is currently under debate. Particular attention has focused on factors driving polymorphism in OspC, one the most diverse Lyme borreliae antigens that is heavily targeted by the vertebrate immune system [88-90]. Balancing selection has been proposed to maintain ospC alleles at intermediate frequencies, with high sequence diversity within a population [91]. Genome-wide linkage to this single locus may then be responsible for maintaining genetic variation at linked loci [92-94]. It is currently debated which specific mode of balancing selection drives the OspC polymorphism in B. burgdorferi s.s.. Some authors have proposed that, similarly to the process operating across B. burgdorferi s.l. species, host specialization via multiple-niche polymorphism (with hosts acting as different ‘niches’ for B. burgdorferi) could lead to diversification within B. burgdorferi s.s. [72, 80, 81, 95].
Alternatively, the OspC polymorphism could be maintained by negative frequency dependent selection mediated by adaptive immunity, such that bacterial populations carrying rare genotypes have a selective advantage over common genotypes and are thus maintained in the population [91, 95, 96]. Theoretical studies predict that frequency dependent fitness leads to fluctuations in the abundance of spirochete genotypes, which would result in temporal shifts in the population structures; however evidence for these fluctuations is limited [97, 98].
An intriguing question is whether the partial and regionally constrained host associations observed in B. burgdorferi s.s. represent an incipient evolutionary process of host specialization (Fig. 3). That is, is B. burgdorferi s.s. on an evolutionary path to diversify into species-associated ecotypes similar to the B. burgdorferi s.l. genospecies in Europe? B. burgdorferi s.s. generalism, i.e. the ability to infect multiple hosts, has in fact been proposed as a key property allowing it to spread across the northeastern United States following large-scale habitat destruction in the course of the post-Columbian settlement and during the industrial revolution [81]. The more recent geographic expansion of B. burgdorferi s.s may provide additional opportunities for adaptation to different host niches, resulting in the development of species-associated ecotypes similar to the B. burgdorferi s.l. genospecies in Europe [85]. The recent redefinition of B. bavariensis from a genotype of B. garinii to a novel genospecies, after it was shown to infect mice in contrast to B. garinii (a bird-adapted genospecies), provides a glimpse of potential future processes of host specialization and Lyme borreliae speciation by B. burgdorferi s.l. linked to vector or host association [85].
Figure 3. The host-pathogen association for B. burgdorferi s.l. genospecies.
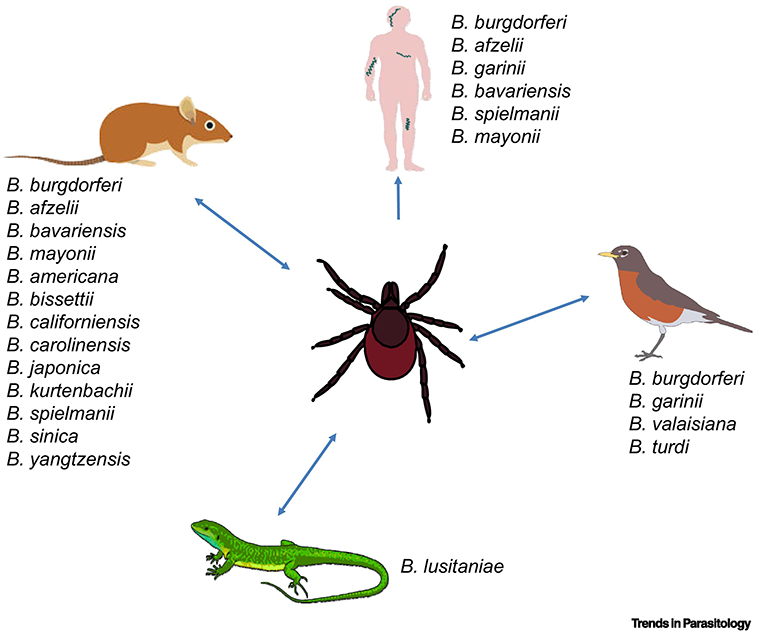
The indicated B. burgdorferi s.l. genospecies are acquired and transmitted between ticks and different vertebrate hosts including humans, small mammals, reptiles, and aves. Shown is the vertebrate hosts that have been demonstrated or suspected to carry respective species of the B. burgdorferi s.l. complex (Part of the figure is adapted from [9]).
Concluding Remarks
Here we summarize the evidence that supports the proteins that contribute to complement evasion-mediated infectious phenotypes in a host-specific manner, leading a question if complement evasion activity of B. burgdorferi s.l. confers the host association (see Outstanding questions). The fact that some of these proteins are functionally redundant and produced simultaneously in the infection cycle, raising the hypothesis that these proteins play in concert in promoting host association of B. burgdorferi s.l. (see Outstanding Questions). Furthermore, the ability of complement to eliminate Lyme borreliae differs among diverse animal species falling in the same taxonomic class (e.g. aves or mammalia) appears to differ. This leads to an intriguing question if complement plays a role in defining the different levels of competence for the hosts within the same taxonomic classes (see Outstanding questions). In addition, though a spirochete-host association has been clearly defined for different Lyme borreliae genospecies, whether this association also applies to different genotypes of spirochetes within the same genospecies (e.g. B. burgdorferi s.s.) is unclear. Teasing apart this question could examine an incipient evolutionary process of B. burgdorferi s.l. toward a more complete host association (see Outstanding questions). Future investigations of the above-mentioned questions will undoubtedly contribute to an insight about the factors contributing in the pathobiology of spirochetes and their diversity in host association.
Outstanding questions.
Does the species-specific polymorphism of complement- or complement regulators-binding activity play a key role in driving host association of B. burgdorferi s.l.?
Why would B. burgdorferi s.l. produce multiple proteins displaying redundant complement-inhibitory activity (e.g. CspZ, OspE, BBK32, and OspC) when they are in the vertebrate hosts? Would they all contribute Lyme borreliae-host association?
Within the same taxonomic classes (e.g. aves or mammalia), would there be a difference in complement evasion for different species/strains of the B. burgdorferi s.l. complex leading to different levels of Lyme borreliae-host association?
Would the partial and regionally constrained host-specific infectivity be observed in B. burgdorferi s.s., representing an incipient evolutionary process toward more complete Lyme borreliae-host association?
Highlights.
Emerging and re-emerging zoonotic diseases have a major impact on global public health including tick-transmitted illnesses such as Lyme disease. The Lyme disease causing pathogens developed a range of sophisticated strategies to overcome the innate immune system of various vertebrate hosts to accomplish their natural host-tick enzootic cycle.
Inactivation of host’s complement in ticks ‘blood meal and host bloodstream are crucial steps to secure spirochetes from killing during transmission and dissemination.
Strain to strain variation of the levels to evade complement may contribute to distinct Lyme borreliae-host association.
Acknowledgement
This work was supported by NSF-IOS1755286 (YL, MDW), DoD-TB170111 (YL), NIH-R21AI144891 (YL), NIH-R01AI121401 (PK), and the LOEWE Center DRUID Novel Drug Targets against Poverty-Related and Neglected Tropical Infectious Diseases, project C3 (PK).
Glossary
- Host infectivity
Efficiency with which infection is transmitted from a tick host population to to feeding ticks.
- Host specialism/specialization (with host specialization as the process and host specialized as the adjective)
Ecological and evolutionary process by which a pathogen becomes differentially adapted and thus restricts its host range to a subset of potential hosts. Intrinsic fitness variation of B. burgdorferi s.l. strains in vertebrate host species is generally cited as evidence of host specialization.
- Lyme borreliae speciation
The evolution of a new Lyme borreliae species.
- Lyme borreliae-host association
Hosts from which the specified Lyme borreliae species/strains has been isolated, i.e. the species/strain is capable of infecting (surviving and disseminating) in the host. These associations represent a pattern (compare with host specialization) that may be due to multiple processes, including differential susceptibility or resistance to serum complement (the topic in this paper) as well as other mechanisms.
- Non-reservoir hosts
Hosts that may have contact with infected ticks and may or may not develop a long-lasting infection but are incapable of transmitting the infection to ticks.
- Opsonophagocytosis
Identification of an invading microorganism by opsonins following phagocytosis.
- Reservoir hosts
Nature hosts that the vector (e.g. ticks) become infected by feeding on such hosts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Radolf JD et al. (2012) Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol 10 (2), 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steere AC et al. (2016) Lyme borreliosis. Nat Rev Dis Primers 2, 16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang IN et al. (1999) Genetic diversity of ospC in a local population of Borrelia burgdorferi sensu stricto. Genetics 151 (1), 15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bunikis J et al. (2004) Sequence typing reveals extensive strain diversity of the Lyme borreliosis agents Borrelia burgdorferi in North America and Borrelia afzelii in Europe. Microbiology 150 (Pt 6), 1741–1755. [DOI] [PubMed] [Google Scholar]
- 5.Hoen AG et al. (2009) Phylogeography of Borrelia burgdorferi in the eastern United States reflects multiple independent Lyme disease emergence events. Proc Natl Acad Sci U S A 106 (35), 15013–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Margos G et al. (2008) MLST of housekeeping genes captures geographic population structure and suggests a European origin of Borrelia burgdorferi. Proc Natl Acad Sci U S A 105 (25), 8730–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanincova K et al. (2008) Fitness variation of Borrelia burgdorferi sensu stricto strains in mice. Appl Environ Microbiol 74 (1), 153–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alghaferi MY et al. (2005) Borrelia burgdorferi ospC heterogeneity among human and murine isolates from a defined region of northern Maryland and southern Pennsylvania: lack of correlation with invasive and noninvasive genotypes. J Clin Microbiol 43 (4), 1879–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tufts DM et al. (2019) Outer surface protein polymorphisms linked to host-spirochete association in Lyme borreliae. Mol Microbiol. 111 (4), 868–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurtenbach K et al. (2002) Host association of Borrelia burgdorferi sensu lato--the key role of host complement. Trends Microbiol 10 (2), 74–9. [DOI] [PubMed] [Google Scholar]
- 11.Kurtenbach K et al. (1998) Differential transmission of the genospecies of Borrelia burgdorferi sensu lato by game birds and small rodents in England. Appl Environ Microbiol 64 (4), 1169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurtenbach K et al. (2002) Differential survival of Lyme borreliosis spirochetes in ticks that feed on birds. Infect Immun 70 (10), 5893–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kraiczy P (2016) Travelling between Two Worlds: Complement as a Gatekeeper for an Expanded Host Range of Lyme Disease Spirochetes. Vet. Sci 3 (2), 12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhide MR et al. (2005) Sensitivity of Borrelia genospecies to serum complement from different animals and human: a host-pathogen relationship. FEMS Immunol Med Microbiol 43 (2), 165–72. [DOI] [PubMed] [Google Scholar]
- 15.Kurtenbach K et al. (1998) Serum complement sensitivity as a key factor in Lyme disease ecology. Infect Immun 66 (3), 1248–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Dam AP et al. (1997) Complement-mediated serum sensitivity among spirochetes that cause Lyme disease. Infect Immun 65 (4), 1228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kraiczy P (2016) Travelling between Two Worlds: Complement as a Gatekeeper for an Expanded Host Range of Lyme Disease Spirochetes. Vet Sci 3 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kraiczy P (2016) Hide and Seek: How Lyme Disease Spirochetes Overcome Complement Attack. Front Immunol 7, 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuijt TJ et al. (2011) Lyme borreliosis vaccination: the facts, the challenge, the future. Trends Parasitol 27 (1), 40–7. [DOI] [PubMed] [Google Scholar]
- 20.Zipfel PF and Skerka C (2009) Complement regulators and inhibitory proteins. Nat Rev Immunol 9 (10), 729–40. [DOI] [PubMed] [Google Scholar]
- 21.Kraiczy P et al. (2004) Complement resistance of Borrelia burgdorferi correlates with the expression of BbCRASP-1, a novel linear plasmid-encoded surface protein that interacts with human factor H and FHL-1 and is unrelated to Erp proteins. J Biol Chem 279 (4), 2421–9. [DOI] [PubMed] [Google Scholar]
- 22.Hartmann K et al. (2006) Functional characterization of BbCRASP-2, a distinct outer membrane protein of Borrelia burgdorferi that binds host complement regulators factor H and FHL-1. Mol Microbiol 61 (5), 1220–36. [DOI] [PubMed] [Google Scholar]
- 23.Kraiczy P and Stevenson B (2013) Complement regulator-acquiring surface proteins of Borrelia burgdorferi: Structure, function and regulation of gene expression. Ticks Tick Borne Dis 4 (1-2), 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hellwage J et al. (2001) The complement regulator factor H binds to the surface protein OspE of Borrelia burgdorferi. J Biol Chem 276 (11), 8427–35. [DOI] [PubMed] [Google Scholar]
- 25.Kraiczy P et al. (2003) Immune evasion of Borrelia burgdorferi: mapping of a complement-inhibitor factor H-binding site of BbCRASP-3, a novel member of the Erp protein family. Eur J Immunol 33 (3), 697–707. [DOI] [PubMed] [Google Scholar]
- 26.Brisson D et al. (2013) Distribution of cp32 prophages among Lyme disease-causing spirochetes and natural diversity of their lipoprotein-encoding erp loci. Appl Environ Microbiol 79 (13), 4115–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin YP et al. (2020) New Insights Into CRASP-Mediated Complement Evasion in the Lyme Disease Enzootic Cycle. Front Cell Infect Microbiol 10, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caine JA et al. (2017) Borrelia burgdorferi outer surface protein C (OspC) binds complement component C4b and confers bloodstream survival. Cell Microbiol. 19 (12). doi: 10.1111/cmi.12786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia BL et al. (2016) Borrelia burgdorferi BBK32 Inhibits the Classical Pathway by Blocking Activation of the C1 Complement Complex. PLoS Pathog 12 (1), e1005404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hammerschmidt C et al. (2016) BGA66 and BGA71 facilitate complement resistance of Borrelia bavariensis by inhibiting assembly of the membrane attack complex. Mol Microbiol 99 (2), 407–24. [DOI] [PubMed] [Google Scholar]
- 31.Hallstrom T et al. (2013) CspA from Borrelia burgdorferi inhibits the terminal complement pathway. MBio 4 (4), e00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwan TG et al. (1995) Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci U S A 92 (7), 2909–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller JC et al. (2003) Temporal analysis of Borrelia burgdorferi Erp protein expression throughout the mammal-tick infectious cycle. Infect Immun 71 (12), 6943–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Lackum K et al. (2005) Borrelia burgdorferi regulates expression of complement regulator-acquiring surface protein 1 during the mammal-tick infection cycle. Infect Immun 73 (11), 7398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bykowski T et al. (2007) Coordinated expression of Borrelia burgdorferi complement regulator-acquiring surface proteins during the Lyme disease spirochete's mammal-tick infection cycle. Infect Immun 75 (9), 4227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang FT et al. (2002) An immune evasion mechanism for spirochetal persistence in Lyme borreliosis. J Exp Med 195 (4), 415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller JC and Stevenson B (2004) Increased expression of Borrelia burgdorferi factor H-binding surface proteins during transmission from ticks to mice. Int J Med Microbiol 293 Suppl 37, 120–5. [DOI] [PubMed] [Google Scholar]
- 38.Hubner A et al. (2001) Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc Natl Acad Sci U S A 98 (22), 12724–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caimano MJ et al. (2004) RpoS is not central to the general stress response in Borrelia burgdorferi but does control expression of one or more essential virulence determinants. Infect Immun 72 (11), 6433–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arnold WK et al. (2018) Transcriptomic insights on the virulence-controlling CsrA, BadR, RpoN, and RpoS regulatory networks in the Lyme disease spirochete. PLoS One 13 (8), e0203286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang XF et al. (2005) Analysis of the ospC regulatory element controlled by the RpoN-RpoS regulatory pathway in Borrelia burgdorferi. J Bacteriol 187 (14), 4822–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drecktrah D et al. (2013) An inverted repeat in the ospC operator is required for induction in Borrelia burgdorferi. PLoS One 8 (7), e68799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He M et al. (2007) Regulation of expression of the fibronectin-binding protein BBK32 in Borrelia burgdorferi. J Bacteriol 189 (22), 8377–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hyde JA et al. (2007) Borrelia burgdorferi alters its gene expression and antigenic profile in response to CO2 levels. J Bacteriol 189 (2), 437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jutras BL et al. (2013) Bpur, the Lyme disease spirochete's PUR domain protein: identification as a transcriptional modulator and characterization of nucleic acid interactions. J Biol Chem 288 (36), 26220–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jutras BL et al. (2012) BpaB and EbfC DNA-binding proteins regulate production of the Lyme disease spirochete's infection-associated Erp surface proteins. J Bacteriol 194 (4), 778–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burns LH et al. (2010) BpaB, a novel protein encoded by the Lyme disease spirochete's cp32 prophages, binds to erp Operator 2 DNA. Nucleic Acids Res 38 (16), 5443–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Babb K et al. (2006) Borrelia burgdorferi EbfC, a novel, chromosomally encoded protein, binds specific DNA sequences adjacent to erp loci on the spirochete's resident cp32 prophages. J Bacteriol 188 (12), 4331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Babb K et al. (2004) Molecular characterization of Borrelia burgdorferi erp promoter/operator elements. J Bacteriol 186 (9), 2745–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riley SP et al. (2009) Borrelia burgdorferi EbfC defines a newly-identified, widespread family of bacterial DNA-binding proteins. Nucleic Acids Res 37 (6), 1973–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jutras BL et al. (2013) Posttranscriptional self-regulation by the Lyme disease bacterium's BpuR DNA/RNA-binding protein. J Bacteriol 195 (21), 4915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jutras BL et al. (2019) The Lyme disease spirochete's BpuR DNA/RNA-binding protein is differentially expressed during the mammal-tick infectious cycle, which affects translation of the SodA superoxide dismutase. Mol Microbiol 112 (3), 973–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hart T et al. (2018) Polymorphic factor H-binding activity of CspA protects Lyme borreliae from the host complement in feeding ticks to facilitate tick-to-host transmission. PLoS Pathog 14 (5), e1007106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hammerschmidt C et al. (2014) Versatile roles of CspA orthologs in complement inactivation of serum-resistant Lyme disease spirochetes. Infect Immun 82 (1), 380–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coleman AS et al. (2008) Borrelia burgdorferi complement regulator-acquiring surface protein 2 does not contribute to complement resistance or host infectivity. PLoS One 3 (8), 3010e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marcinkiewicz AL et al. (2019) Blood treatment of Lyme borreliae demonstrates the mechanism of CspZ-mediated complement evasion to promote systemic infection in vertebrate hosts. Cell Microbiol 21 (2), e12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rogers EA et al. (2009) Comparative analysis of the properties and ligand binding characteristics of CspZ, a factor H binding protein, derived from Borrelia burgdorferi isolates of human origin. Infect Immun 77 (10), 4396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rogers EA and Marconi RT (2007) Delineation of species-specific binding properties of the CspZ protein (BBH06) of Lyme disease spirochetes: evidence for new contributions to the pathogenesis of Borrelia spp. Infect Immun 75 (11), 5272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brangulis K et al. (2014) Structural characterization of CspZ, a complement regulator factor H and FHL-1 binding protein from Borrelia burgdorferi. FEBS J 281 (11), 2613–22. [DOI] [PubMed] [Google Scholar]
- 60.Kraiczy P et al. (2008) Borrelia burgdorferi complement regulator-acquiring surface protein 2 (CspZ) as a serological marker of human Lyme disease. Clin Vaccine Immunol 15 (3), 484–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marconi RT et al. (1996) Molecular and evolutionary analyses of a variable series of genes in Borrelia burgdorferi that are related to ospE and ospF, constitute a gene family, and share a common upstream homology box. J Bacteriol 178 (19), 5615–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akins DR et al. (1999) Molecular and evolutionary analysis of Borrelia burgdorferi 297 circular plasmid-encoded lipoproteins with OspE- and OspF-like leader peptides. Infect Immun 67 (3), 1526–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.El-Hage N et al. (2001) Surface exposure and protease insensitivity of Borrelia burgdorferi Erp (OspEF-related) lipoproteins. Microbiology 147 (Pt 4), 821–30. [DOI] [PubMed] [Google Scholar]
- 64.Nguyen TP et al. (1994) Partial destruction of Borrelia burgdorferi within ticks that engorged on OspE- or OspF-immunized mice. Infect Immun 62 (5), 2079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lin T et al. (2012) Analysis of an ordered, comprehensive STM mutant library in infectious Borrelia burgdorferi: insights into the genes required for mouse infectivity. PLoS One 7 (10), e47532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stevenson B et al. (2002) Differential binding of host complement inhibitor factor H by Borrelia burgdorferi Erp surface proteins: a possible mechanism underlying the expansive host range of Lyme disease spirochetes. Infect Immun 70 (2), 491–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hovis KM et al. (2006) Selective binding of Borrelia burgdorferi OspE paralogs to factor H and serum proteins from diverse animals: possible expansion of the role of OspE in Lyme disease pathogenesis. Infect Immun 74 (3), 1967–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramamoorthi N et al. (2005) The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature 436 (7050), 573–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Caine JA and Coburn J (2015) A short-term Borrelia burgdorferi infection model identifies tissue tropisms and bloodstream survival conferred by adhesion proteins. Infect Immun 83 (8), 3184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin YP et al. (2015) Glycosaminoglycan binding by Borrelia burgdorferi adhesin BBK32 specifically and uniquely promotes joint colonization. Cell Microbiol 17 (6), 860–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhi H et al. (2018) The Classical Complement Pathway Is Required to Control Borrelia burgdorferi Levels During Experimental Infection. Front Immunol 9, 959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kurtenbach K et al. (2006) Fundamental processes in the evolutionary ecology of Lyme borreliosis. Nat Rev Microbiol 4 (9), 660–9. [DOI] [PubMed] [Google Scholar]
- 73.Heylen D et al. (2014) Songbirds as general transmitters but selective amplifiers of Borrelia burgdorferi sensu lato genotypes in Ixodes rinicus ticks. Environ Microbiol 16 (9), 2859–68. [DOI] [PubMed] [Google Scholar]
- 74.Jacquot M et al. (2016) Multiple independent transmission cycles of a tick-borne pathogen within a local host community. Sci Rep 6, 31273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baum E et al. (2012) Experimental infections of the reservoir species Peromyscus leucopus with diverse strains of Borrelia burgdorferi, a Lyme disease agent. mBio 3 (6), e00434–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brinkerhoff RJ et al. (2010) Genotypic diversity of Borrelia burgdorferi strains detected in Ixodes scapularis larvae collected from North American songbirds. Appl Environ Microbiol 76 (24), 8265–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vuong HB et al. (2014) Occurrence and transmission efficiencies of Borrelia burgdorferi ospC types in avian and mammalian wildlife. Infect Genet Evol 27, 594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vuong HB et al. (2017) Influences of Host Community Characteristics on Borrelia burgdorferi Infection Prevalence in Blacklegged Ticks. PLoS One 12 (1), e0167810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mechai S et al. (2016) Evidence for Host-Genotype Associations of Borrelia burgdorferi Sensu Stricto. PLoS One 11 (2), e0149345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brisson D and Dykhuizen DE (2004) ospC diversity in Borrelia burgdorferi: different hosts are different niches. Genetics 168 (2), 713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hanincova K et al. (2006) Epidemic spread of Lyme borreliosis, northeastern United States. Emerg Infect Dis 12 (4), 604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brisson D et al. (2008) Conspicuous impacts of inconspicuous hosts on the Lyme disease epidemic. Proc Biol Sci 275 (1631), 227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Durand J et al. (2017) Fitness estimates from experimental infections predict the long-term strain structure of a vector-borne pathogen in the field. Sci Rep 7 (1), 1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jacquet M et al. (2017) The abundance of the Lyme disease pathogen Borrelia afzelii declines over time in the tick vector Ixodes ricinus. Parasit Vectors 10 (1), 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Norte AC et al. (2019) Host dispersal shapes the population structure of a tick-borne bacterial pathogen. Mol Ecol. 29 (3), 485–501 [DOI] [PubMed] [Google Scholar]
- 86.Raberg L et al. (2017) Evolution of antigenic diversity in the tick-transmitted bacterium Borrelia afzelii: a role for host specialization? J Evol Biol 30 (5), 1034–1041. [DOI] [PubMed] [Google Scholar]
- 87.Spielman A (1994) The emergence of Lyme disease and human babesiosis in a changing environment. Ann N Y Acad Sci 740, 146–56. [DOI] [PubMed] [Google Scholar]
- 88.Xu Q et al. (2006) Constitutive expression of outer surface protein C diminishes the ability of Borrelia burgdorferi to evade specific humoral immunity. Infect Immun 74 (9), 5177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tilly K et al. (2006) Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect Immun 74 (6), 3554–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liang FT et al. (2004) Borrelia burgdorferi changes its surface antigenic expression in response to host immune responses. Infect Immun 72 (10), 5759–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brisson D and Dykhuizen DE (2006) A modest model explains the distribution and abundance of Borrelia burgdorferi strains. Am J Trop Med Hyg 74 (4), 615–22. [PMC free article] [PubMed] [Google Scholar]
- 92.Brisson D et al. (2012) Genetics of Borrelia burgdorferi. Annu Rev Genet 46, 515–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Haven J et al. (2012) Ecological and inhost factors promoting distinct parasite life-history strategies in Lyme borreliosis. Epidemics 4 (3), 152–7. [DOI] [PubMed] [Google Scholar]
- 94.Walter KS et al. (2017) Genomic insights into the ancient spread of Lyme disease across North America. Nat Ecol Evol 1 (10), 1569–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brisson D et al. (2011) Biodiversity of Borrelia burgdorferi strains in tissues of Lyme disease patients. PLoS One 6 (8), e22926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Haven J et al. (2011) Pervasive recombination and sympatric genome diversification driven by frequency-dependent selection in Borrelia burgdorferi, the Lyme disease bacterium. Genetics 189 (3), 951–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qiu WG et al. (2002) Geographic uniformity of the Lyme disease spirochete (Borrelia burgdorferi) and its shared history with tick vector (Ixodes scapularis) in the Northeastern United States. Genetics 160 (3), 833–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.States SL et al. (2014) Lyme disease risk not amplified in a species-poor vertebrate community: similar Borrelia burgdorferi tick infection prevalence and OspC genotype frequencies. Infect Genet Evol 27, 566–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Herzberger P et al. (2007) Human pathogenic Borrelia spielmanii sp. nov. resists complement-mediated killing by direct binding of immune regulators factor H and factor H-like protein 1. Infect Immun 75 (10), 4817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Walter L et al. (2019) Elucidating the Immune Evasion Mechanisms of Borrelia mayonii, the Causative Agent of Lyme Disease. Front Immunol 10, 2722. [DOI] [PMC free article] [PubMed] [Google Scholar]


