Abstract
Heightened risk-taking tendencies during adolescence have been hypothesized to be attributable to physiological differences of maturation in key brain regions. The socioemotional system (e.g., (nucleus accumbens), which is instrumental in reward response, shows a relatively earlier development trajectory than the cognitive control system (e.g., medial prefrontal cortex), which regulates impulse response. This developmental imbalance between heightened reward seeking and immature cognitive control potentially makes adolescents more susceptible to engaging in risky activities. Here, we assess brain structure in the socioemotional and cognitive control systems through viscoelastic stiffness measured with magnetic resonance elastography (MRE) and volumetry, as well as risk-taking tendencies measured using two experimental tasks in 40 adolescents (mean age = 13.4 years old). MRE measures of regional brain stiffness reflect brain health and development via myelin content and glial matrix makeup, and have been shown to be highly sensitive to cognitive processes as compared to measures of regional brain volume and diffusion weighted imaging metrics. We find here that the viscoelastic and volumetric differences between the nucleus accumbens and the prefrontal cortex are correlated with increased risk-taking behavior in adolescents. These differences in development between the two brain systems can be used as an indicator of those adolescents who are more prone to real world risky activities and a useful measure for characterizing response to intervention.
Keywords: magnetic resonance elastography, risk taking, reward seeking, adolescent, viscoelasticity, brain stiffness
1. Introduction
It has been well documented that individuals in adolescence engage in heightened levels of health compromising risk-taking behaviors; however, research shows that these actions are not the result of adolescents being irrational, acting unconcerned about risk, or using different reasoning than adults (Reyna and Farley, 2010; Steinberg, 2007), and, in fact, adolescents even engage in some thoughtful and positive risks (Duell & Steinberg, 2018). However, despite expensive educational programs designed to address excessive negative risk taking, statistics continue to show heightened risky activity in this population. Therefore, a clearer understanding of the mechanisms driving risk taking is needed. Owing to recent advances in neuroimaging, physiological indicators have been identified as potential key mediators of risk-taking tendencies (Luciana, 2013). Risk-taking behaviors are suggested to be controlled by two counterbalancing brain systems, the reward-system, often referred to as the socioemotional system, and the cognitive control system, which is responsible for regulation of reward-seeking impulses. However, developmental trajectories vary between regions, including with respect to volume, functional activation, and myelin content (Bartzokis, 2008; Blakemore et al., 2010), and advanced structural neuroimaging of these two key reward and control regions, and how they differ in individuals at their peak risk-taking age, may provide a sensitive assessment of the neural regions supporting risk taking in adolescence.
Developmental differences in these key brain regions, including the reward and control systems, have been hypothesized to drive risk-taking tendencies in adolescents. The reward system in the brain encompasses several different structures, but most notably the nucleus accumbens (NAcc) (Knutson et al., 2018). The NAcc has a large number of dopaminergic neurons, and shows strong functional activation during reward tasks (Rademacher et al., 2010; Willuhn et al., 2013), particularly among adolescents relative to children and adults (Galvan et al., 2006). and to mature earlier than cognitive control brain regions (Ikemoto, 2007; Mills et al., 2014). The cognitive control system is responsible for self-regulation and impulse control, and includes both the ventromedial prefrontal cortex (vmPFC) and the orbitofrontal cortex (OFC), both structures display similar patterns of activation during self-regulatory behavior and decision making (Bechara et al., 2000; Hare et al., 2009), including when the decision is based on the value of the reward (Bouret and Richmond, 2010; Elliott et al., 2000; Wallis and Kennerley, 2011). The cognitive control system is among the last of the brain systems to reach maturity (Johnson et al., 2010), leaving a period of maturational imbalance where an individual is particularly prone to reward seeking without the benefit of a fully developed regulatory system (Casey et al., 2008; Steinberg, 2010; Steinberg et al., 2008; Strang et al., 2013).
While the majority of prior research examining links between adolescent brain development and risk taking have been done using fMRI (Shulman et al., 2016; van Duijvenvoorde et al., 2016), a more convincing index of maturation of these neural systems may be the structural, rather than functional, aspect of brain health and development (Strang et al., 2013; DeWitt et al., 2014; Jacobus et al., 2013). Neuroimaging measures of structural integrity that probe intrinsic properties of neural tissue may overcome challenges in interpreting activation data from fMRI that depends on response to stimulus, and thus potentially provide more direct and sensitive metrics of brain maturation (Somerville, 2016). In this work, we propose brain viscoelastic mechanical properties as sensitive measures of neural tissue microstructural integrity to investigate regions of the reward and control systems and how they relate to indices of risk-taking behavior. These mechanical properties, such as stiffness, are measured in vivo with magnetic resonance elastography (MRE) and are highly sensitive to brain health in aging and neurological disorders (Hiscox et al., 2016; Murphy et al., 2019). These mechanical properties have recently been discovered to correlate with cognitive performance including memory, fluid intelligence, and rule-learning (Hiscox et al., 2018; Johnson et al., 2018; Schwarb et al., 2019, 2017, 2016), and these correlations appear to be stronger than correlations between the same cognitive task and brain volume (Johnson and Telzer, 2018). In a mouse model, accompanied by ex vivo histology, it is concluded that viscoelastic properties are sensitive to changes to myelination and extracellular matrix composition (Schregel et al., 2012), and thus may provide unique signatures of brain tissue integrity.
Pediatric brain mechanics have only recently been measured through MRE (McIlvain et al., 2018; Yeung et al., 2019), however this early work shows sizeable potential for MRE to be used as a beneficial neuroimaging tool for characterizing neurodevelopment and associated disabilities (Chaze et al., 2019; Johnson and Telzer, 2018; McIlvain et al., 2020). It is expected that as the brain matures it also stiffens, and stiffness will reflect myelin content, neuron density, and glial matrix integrity (Guo et al., 2019). Average global brain stiffness appears to be the same in adolescents as in adults (McIlvain et al., 2018; Yeung et al., 2019), though regional stiffness may follow slightly different trajectories through development, with subcortical regions of the deep gray matter and deep white matter being more stiff during development than in adulthood (McIlvain et al., 2018). For example, Guo et al. (2019) reported an increase in mouse brain stiffness during adolescence in the hippocampus, the globus pallidus, and the primary motor cortex. Although the subcortical structures appear to increase in stiffness during adolescence, regional brain volumes generally decrease during adolescence. Volume of the NAcc and cortical thickness are both observed to decrease during adolescence (Goddings et al., 2014; Raznahan et al., 2014); therefore, it is expected that trends seen in volumetric measures are opposite those seen in brain stiffness. Volume with respect to reward and control systems in adolescent risk taking has been previously explored but only qualitatively (Mills et al., 2014). In conjunction with brain viscoelasticity, these two approaches to assessing brain integrity can provide a thorough examination of the role of neural structure in adolescent risk taking. This study examines relative differences in the development of reward and control regions through their viscoelasticity in order to shed light on how mechanical properties contribute to risk taking in adolescents individually and together.
2. Methods
2.1. Participants
Forty-four (22/22 male/female; 12–14 (13.4 ± 0.6) years old) cognitively normal, healthy, right-handed adolescents participated in both the imaging and risk-taking assessments as part of this study. Of these 44 participants, two were excluded due to low MRE signal-to-noise ratio (SNR) (McGarry et al., 2011) or artifacts in the reconstructed property maps (see section 2.3 Image Processing), and two were determined to be statistical outliers in one of the measured values (see section 2.6 Statistical Analysis). The final sample included data from 40 adolescents (21/19 male/female). This study was approved by the University of Illinois at Urbana-Champaign Institutional Review Board and all participants and their guardians gave informed written assent/consent prior to being tested. These data were collected as a subset of participants from a larger study where only a small portion completed the MRE scan. This same subset was used in our previous MRE study comparing brain mechanical properties in adolescents and adults (McIlvain et al., 2018).
2.2. Imaging
Each participant completed an imaging session on a Siemens 3T Trio MRI scanner (Siemens Medical Solutions; Erlangen, Germany) that included an MRE acquisition using a 3D multislab, multishot spiral sequence (Johnson et al., 2014): FOV = 240 × 240 mm2; 60 total slices; TR/TE = 1800/73 ms; 2.0×2.0×2.0 mm3 resolution; 10 slabs; 8 slices per slab; 25% slab overlap; 1 in-plane spiral shot (R=3), bilateral, flow-compensated, matched-period motion-encoding gradients. G = 26 mT/m; 4 evenly-spaced phase offsets). Displacements from 50 Hz vibrations were delivered to the head via a pneumatic actuator system with passive pillow driver (Resoundant, Inc.; Rochester, MN). The total MRE acquisition time was 6 minutes. In addition, a high-resolution T1-weighted MPRAGE (magnetization-prepared rapidly-acquired gradient echo) scan was collected on all participants for volumetric analysis and anatomical segmentation (0.9 × 0.9 × 0.9 mm3; TR/TI/TE = 1900/900/2.32 ms).
MRE image reconstruction included SENSE parallel imaging, iterative field inhomogeneity correction, and navigator correction for motion-induced phase errors between shots (Johnson et al., 2014). Poor quality data, including low displacement amplitude or excessive head motion, was excluded through calculation of the octahedral shear strain-based signal to noise ratio (OSS-SNR); an OSS-SNR > 3.0 was deemed sufficient for stable inversion (McGarry et al., 2011).
2.3. Image Processing
Region-of-interest (ROI) masks were obtained via automatic segmentation of the T1-weighted MPRAGE images using the recon-all pipeline in Freesurfer image analysis suite (v 6.0.0) (Fischl, 2012). The MPRAGE images were registered to their corresponding MRE magnitude images through a rigid-body affine transformation (6-degrees of freedom, tri-linear interpolation and a correlation ratio cost function) using the FLIRT tool within FSL (FMRIB Software Library v.6.0) (Jenkinson et al., 2012). Masks of the OFC, comprising the medial and lateral orbital frontal cortex labels in Freesurfer, the vmPFC, comprising the rostral middle frontal cortex and rostral anterior cingulate cortex labels, and the NAcc were created by registering their segmented masks to MRE space.
The nonlinear inversion algorithm (NLI) was used to convert MRE displacement data into estimates of brain tissue viscoelastic properties (McGarry et al., 2012; Van Houten et al., 2001). NLI returns the complex shear modulus (G = G’ + iG”) comprising the storage (G’) and loss (G”) modulus of the tissue, from which we calculated viscoelastic shear stiffness as and damping ratio as (Manduca et al., 2001). Here we additionally incorporated a priori spatial information during inversion by using soft prior regularization (SPR) (McGarry et al., 2013), so that property variation is penalized during NLI optimization. Masks of the NAcc, OFC, and vmPFC were separately provided as priors during NLI. This method has been seen to reduce uncertainty in measures and is the same pipeline which was previously used for subcortical structure property estimation (Johnson et al., 2016; McIlvain et al., 2018; Schwarb et al., 2016).
Measures of subcortical structure volume (Buckner et al., 2004) and cortical thickness (Fischl and Dale, 2000) were calculated using Freesurfer (Fischl, 2012). NAcc volumes were normalized by total intracranial volume, but cortical thickness was not, in accordance with the Freesurfer recommendations (Buckner et al., 2004). Prior to statistical analysis, each structural measure was standardized (z-scores) for use in comparing structures while accounting for differences in measurement scales.
2.4. Risk Susceptibility Tasks
Each participant completed modified versions of two computerized behavioral tasks while in the MRI scanner, the Balloon Analogue Risk Task (BART) and the Yellow Light Game (YLG). These tasks both assessed risk-taking tendencies and were chosen as they provide a model for the unpredictable rewards that exemplify real-world risky behaviors (Goldenberg et al., 2017; McCormick and Telzer, 2015; Op de Macks et al., 2018).
On each trial of the BART, participants were presented with a computer-generated balloon. When a balloon appeared, participants chose to either pump the balloon or to cash-in save their points for that trial. When choosing to pump the balloon, there was the possibility that the balloon would either inflate or explode. A successful inflation gained the subject an additional point, an explosion would result in no points from the entire trial. The balloons were one of three colors (red, blue, green) which each represent one of the following: 1) low-risk balloons (explode after 10–12 pumps); 2) high risk balloons (explode after 4–6 pumps); or 3) variable risk balloons (explode after 5–11 pumps). The color representing each condition was varied between subjects but stayed consistent between trials for a single participant so that subjects had the opportunity to use the history of pop-rate to inform their decisions. The task was self-paced, and the balloon stayed on the screen until the participant made a decision. Participants were told that they could win a $10 gift card at the end of the session if they earned enough points during the task. The number of points needed to win the prize was not disclosed so that participants were motivated to continue earning points throughout the task. However regardless of performance, all participants were given a $10 gift card after completing the scan session. We examined both the average number of pumps a subject took before ending the trial on variable risk balloons (BART-Pumps) and the number of total points received for the game (BART-Points), two indices that have been associated with real-life risk-taking tendencies (McCormick and Telzer, 2015; Qu et al., 2016). Whereas average pumps on variable balloons represents only a subjects willingness to take risks, total points encompasses the adaptive response in relation to previous reward success or failure and may acknowledge a higher level of risk-taking and reward-seeking in the context of both reward and control (Schonberg et al., 2012a).
The second task, the yellow light task (YLG) (Op de Macks et al., 2018; Rogers et al., 2018), is an adaption of the Stoplight Task (Chein et al., 2011; Gardner and Steinberg, 2005). Each participant completed two-runs of a simulated driving course with 20 intersections each, with the goal to get through all of the intersections in the shortest amount of time. At each intersection, the participant saw a yellow light and had the decision to ‘stop’ or ‘go’ by pressing one of two buttons. If the participant chose to ‘stop’ at an intersection, they do not risk crashing, but delay their course time by 2.5 seconds and see either an approaching car which honks, or a blue tilde if no car is in the intersection. If the participant made the decision to ‘go’ through the intersection, they do not have this time delay, but they risk the possibility of crashing, which causes a 5-second delay. If the participant did not make a decision, this resulted in a 1 second delay and red cross on the screen. Participants were explicitly informed about the associated time delays, or lack thereof, of the decisions and outcomes. The probability of a car being in any given intersection was kept constant at 50%, and the perceived distance of the yellow light varied between 200 and 250 feet although these parameters were unknown to participants prior to the task. At the end of each run, participants were told their overall driving course time and the number of crashes. Past research indicates that, due to learning effects, participants tend to show higher behavioral risk performance during the initial rounds of the task and a lower and more stable pattern of risk taking thereafter (Kahn et al., 2014). Therefore, participants in the present study played two practice rounds of the task, prior to scanning. The percentage of ‘go’ decisions at the yellow light was used as the metric to assess willingness to take risks.
2.5. Pubertal Development Measures
To examine the role of puberty in adolescent risk taking, participants and guardians of the participants completed the Pubertal Development Scale (Petersen et al., 1988). The questionnaire measured development by asking participants to report levels of pubic hair, axial growth, skin changes, facial hair (male only), voice changes (male only), breast development (female only), and age of menarche (female only) on a 4-point scale ranging from ‘not begun development’ to ‘completely developed’. Pubertal status scores could range from 1 to 4, with higher scores indicating greater pubertal development. The mean of all items was calculated and final scores between adolescents and their guardians were averaged. Three participants did not have reports of pubertal status.
2.6. Statistical Analysis
All analyses were performed using JMP Pro 14.0.0 (SAS Institute, Inc. Cary, NC). Multivariate robust outlier analysis (10% tail quantile; data excluded at three times the interquartile range) included all stiffness, volume, and risk-taking scores. Any participant who was identified as a statistical outlier in any of these categories was removed from all analyses resulting in two participants being removed and a final sample of n=40. Measures of volume, stiffness and damping ratio were z-scored prior to subtraction for comparison between structures with different value ranges. Pearson partial correlation analyses were conducted between brain structural measures and risk assessment tasks for each of the ROIs individually, as well as the differences in structural measures between the socioemotional and cognitive control regions (i.e. NAcc value minus OFC value, and NAcc value minus vmPFC value). Correlation coefficients, r, and associated p-values were reported for each of the hypothesized regions and their differences, and significance was determined at p < 0.05. To strengthen our analysis, we incorporated analysis of regions not expected to contribute to risk-taking behavior, including an alternate subcortical structure (thalamus) and two alternate frontal regions (superior frontal and caudal middle frontal cortices). These structures were analyzed in relation to risk taking measures, as well as their differences and the differences between these regions and the originally hypothesized regions were assessed.
3. Results
Structural measures of stiffness, damping ratio, and volume were analyzed for their correlations with risk-taking measures from the BART and YLG. Table 1 presents statistical distributions of age, puberty, risk-taking scores and brain structural measurements. Age and pubertal status were correlated with each other, even within our narrow age range (r = 0.346, p < 0.031); however, neither age nor puberty were significantly correlated with our tasks nor our structural measures, so they were not included as covariates in any additional analyses. These results can be found in Supplemental Information.
Table 1:
Adolescent population demographics, demonstrating uniform distribution among age, puberty, and risk-taking scores. NAcc, nucleus accumbens; OFC, orbitofrontal cortex; vmPFC, ventromedial prefrontal cortex.
| Mean | St. Dev. | Min/Max | Skew | |
|---|---|---|---|---|
| Age (Male N = 21 / Female N = 19) | 13.43 | 0.640 | 12.33/14.75 | 0.033 |
| Puberty Questionnaire – Adolescent | 2.59 | 0.768 | 1.0/3.8 | −0.453 |
| Puberty Questionnaire – Guardian | 2.73 | 0.777 | 1.0/4.0 | −0.741 |
| Risk Taking – BART Total Points | 155.87 | 17.17 | 123/193 | 0.234 |
| Risk Taking – BART Pumps on Save | 4.91 | 0.77 | 3.28/6.42 | −0.042 |
| Risk Taking – YLG Percent Go Decisions | 50.17 | 16.422 | 10.8/88.8 | 0.189 |
| MRE Stiffness (kPa) – NAcc | 2.94 | 0.36 | 2.35/3.66 | 0.234 |
| MRE Stiffness (kPa) – OFC | 2.75 | 0.21 | 2.40/3.44 | 0.997 |
| MRE Stiffness (kPa) – vmPFC | 2.97 | 0.22 | 2.61/3.55 | 0.905 |
| MRE Damping Ratio – NAcc | 0.254 | 0.053 | 0.15/0.40 | 0.370 |
| MRE Damping Ratio – OFC | 0.256 | 0.043 | 0.16/0.33 | −0.453 |
| MRE Damping Ratio – vmPFC | 0.229 | 0.032 | 0.15/0.29 | −0.284 |
| Volume (mm3) – NAcc | 507.49 | 70.69 | 351.1/649.3 | 0.008 |
| Thickness (mm) – OFC | 2.87 | 0.135 | 2.59/3.19 | 0.121 |
| Thickness (mm) – vmPFC | 2.97 | 0.121 | 2.76/3.32 | 0.571 |
As seen in Figure 4, zero-order correlations between risk-taking scores on the BART-Points and YLG and stiffness of the NAcc, OFC, and vmPFC independently were not significant, although do trend in the expected directions. For example, a stiffer (i.e. more developed) NAcc was generally associated with more willingness to engage in risky behavior in these tasks (BART-Points: r = 0.28, p = 0.09; YLG: r = 0.28, p = 0.08). Conversely, stiffness of the cognitive control system was associated with more cautiousness in the risk assessing tasks: OFC (BART-Points: r = −0.27, p = 0.09; YLG: r = −0.11, p = 0.48), vmPFC (BART-Points: r = −0.06, p = 0.69; YLG r = 0.01, p = 0.94). Interestingly, we found that the difference in stiffness between the NAcc and the OFC or vmPFC was the strongest predictor of risk taking in these tasks. Specifically, the difference in stiffness between NAcc and OFC was significantly correlated with risk assessments (BART-Points: r = 0.44, p < 0.01; YLG r = 0.36, p = 0.02). Difference in stiffness between NAcc and vmPFC was only statistically significant for the BART-Points (r = 0.33, p = 0.03), but approached significance for the YLG (r = 0.29, p = 0.07). These results were such that a greater difference in stiffness between structures (i.e. NAcc being stiffer than OFC or vmPFC) was correlated with more risky behavior. The BART-Pumps measure was not significantly correlated with stiffness of any of the structures independently (NAcc: r = 0.08, p = 0.61; OFC: r =−0.31, p = 0.05, vmPFC: r = −0.11, p = 0.51) nor with the difference between structures (NAcc-OFC: r = 0.26, p = 0.10, NAcc-vmPFC: r = 0.16, p = 0.33).
Figure 4:
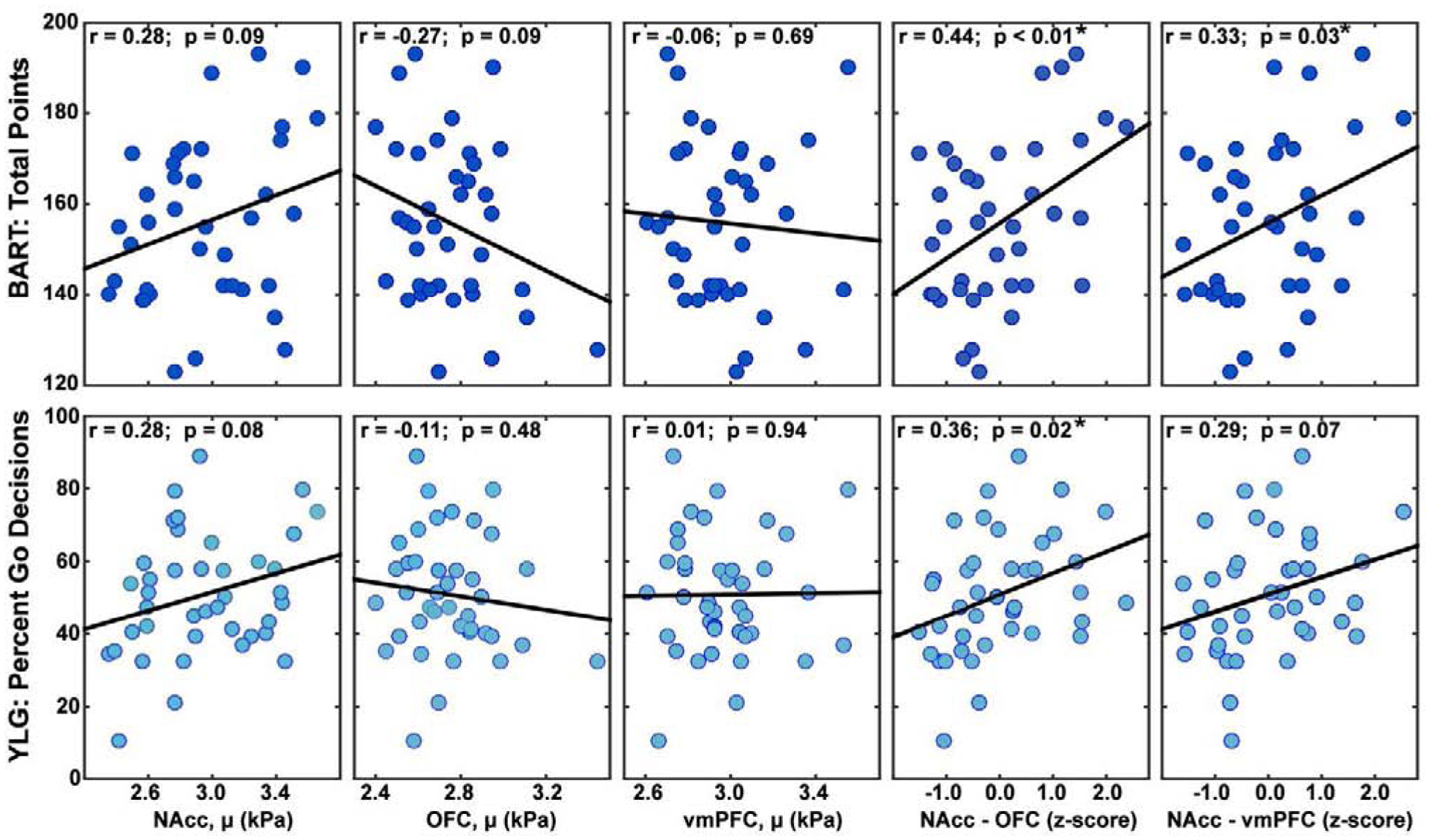
Correlations of brain stiffness with score from the BART (total points) and YLG (percent go decisions) for nucleus accumbens (NAcc), orbitofrontal cortex (OFC), ventromedial prefrontal cortex (vmPFC), and differences between z-scores of the NAcc and the other two structures.
As seen in Figure 5, we again found that risk task scores did not correlate with the volumes of the NAcc (BART-Points: r = −0.17, p = 0.28; BART-Pumps: r = 0.02, p = 0.89, YLG: r = −0.10, p = 0.54) or the thickness of the vmPFC (BART-Points: r = −0.10, p = 0.56; BART-Pumps: r < −0.01, p = 0.95, YLG: r = 0.27, p = 0.09). The only significantly correlation was between the YLG and the OFC thickness (BART-Points: r = 0.07, p = 0.68; BART-Pumps: r = 0.01, p = 0.95; YLG: r = 0.40, p = 0.01). However, the volumetric differences between structures was again correlated with risk taking in the YLG. Specifically, YLG risk taking was negatively correlated with the difference between NAcc volume and OFC thickness (r = −0.35, p = 0.02) and the difference between NAcc volume and vmPFC thickness (r = −0.32, p = 0.04). These results were such that a greater difference in volume between structures (i.e., NAcc being smaller than OFC or vmPFC) was correlated with more risky behavior. Interestingly, volumetric differences did not significantly correlate with BART performance for either BART-Points (NAcc-OFC: r = −0.17, p = 0.30; NAcc-vmPFC: r = −0.14, p = 0.41) or BART-Pumps (NAcc-OFC: r = −0.08, p = 0.65; NAcc-vmPFC: r = −0.09, p = 0.58).
Figure 5:
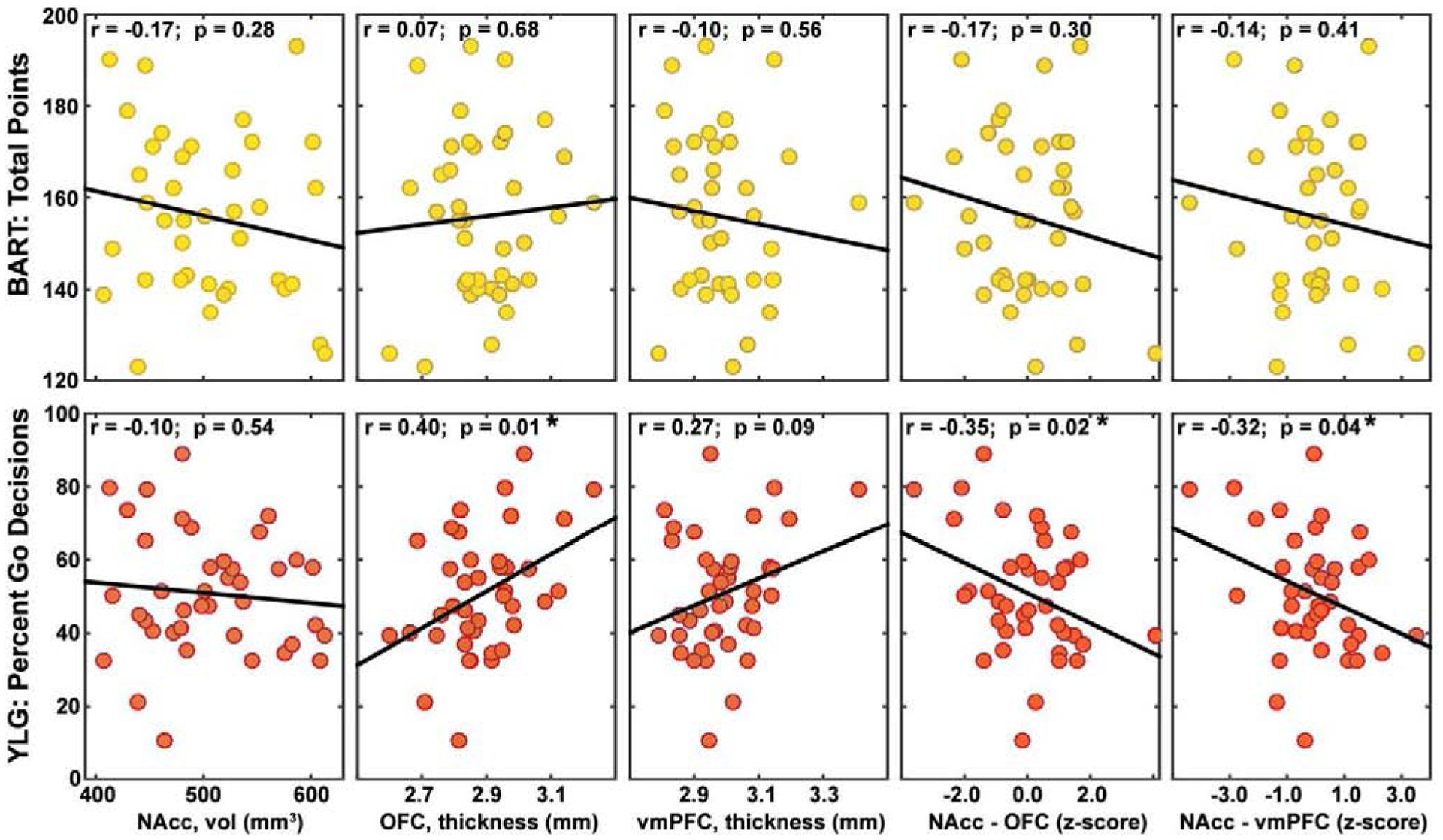
Correlations of brain volume and cortical thickness with score from the BART (total points) and YLG (percent go decisions) for nucleus accumbens (NAcc), orbitofrontal cortex (OFC), ventromedial prefrontal cortex (vmPFC), and differences between z-scores of the NAcc and the other two structures.
We did not find any significant correlations between risk tasks and damping ratio as measured through MRE in the structures independently, for NAcc (BART-Points: r = −0.11, p = 0.56; BART-Pumps: r < 0.01, p = 0.93; YLG: r =−0.01, p = 0.97), OFC (BART-Points: r = −0.06, p = 0.81; BART-Pumps: r < −0.01, p =0.98; YLG: r = 0.01, p =0.95), or vmPFC (BART-Points: r = −0.30, p = 0.62; BART-Pumps: r =0.01, p =0.94; YLG r = −0.04, p = 0.79). Difference in damping ratio values between the two systems were also not significantly correlated for either the difference between the NAcc and the OFC (BART-Points: r = 0.09, p = 0.17; BART-Pumps: r = 0.07, p = 0.89 ;YLG: r = 0.02, p = 0.90) or for the difference between the NAcc and the vmPFC (BART-Points: r = −0.02, p = 0.30; BART-Pumps: r = 0.01, p = 0.96; YLG: r = −0.02, p = 0.89).
We additionally examined the stiffness of alternative regions, which were not expected to be involved in risk-taking behavior, including the thalamus and superior frontal and caudal middle frontal cortices, and their relation to task scores. None of these regions exhibited significant relationships between their stiffness and YLG, BART-Points, or BART-Pumps. Additionally, none of the differences between these regions, nor their differences with NAcc, OFC, or vmPFC, were significantly related to task scores. The complete results of these analyses can be found in the Supplemental Information.
4. Discussion
Here we present, for the first time, an analysis of viscoelastic brain properties in the context of risk-taking behavior in adolescents. Despite numerous functional MRI studies which analyze the relationship between regional brain activity and risk-taking tendencies in adolescents, a surprisingly limited number of risk-taking studies focus on brain structure. It has been acknowledged that, in addition to functional measures, a measure of brain structure is necessary to achieve a more complete understanding of how physiology affects risk taking in adolescents (Strang et al., 2013). Confirming how reward and control neural substrates contribute to risk-taking tendencies can provide a new method for assessing brain maturation, which will allow for identification of subsets of the population who may be at greater likelihood to take risks and can be useful for characterizing response to intervention.
We found here that risk-taking tendencies, evaluated by both the yellow light game and the balloon analogue risk task, significantly correlate with difference in stiffness between the brain regions known to support the socioemotional and cognitive control systems. No significant correlations with risk taking were seen for either stiffness or volume of the NAcc, OFC, or vmPFC individually, though slopes trended in the expected direction. This suggests that a more-developed socioemotional center or a more-developed cognitive control center independently are not the mediators of risk, but rather the interaction in development between the two systems is correlated with risk-taking tendencies. In this sense, absolute values of development are not as important as relative values of development of brain regions. This is consistent with theoretical perspectives of a maturational imbalance between reward and cognitive control being important in adolescent risk taking, as proposed by dual systems models of adolescent risk taking (Casey et al., 2008; Steinberg, 2010, 2007).
Brain stiffness has been posited as a measure of microstructural brain health, such that individuals with a denser glial matrix or healthier myelin sheaths will display higher brain stiffness (Johnson and Telzer, 2018). In particular, during maturation there is development of proteins which promote myelin, extracellular matrix, and the cellular cytoskeleton, each of these contributes to brain stiffening in adolescence (Guo et al., 2019). These changes in stiffness during development can be measured noninvasively using MRE, which may provide sensitive indicators of brain development, and which are potentially expected to change prior to other structural metrics, such as loss of volume. Additionally, measures of brain viscoelasticity have been reported to be more strongly correlated with changes in cognitive performance than measures of volume, potentially due to heightened sensitivity to aspects of extracellular matrix (Johnson and Telzer, 2018). Therefore, analysis of brain mechanical properties in the context of risk taking may potentially provide a more sensitive metric than other imaging modalities for understanding individual differences in adolescent behavior and brain maturation.
We have previously observed that the stiffness of the brain differs between adolescence and adulthood, and regions such as the subcortical gray matter are significantly different between age groups (McIlvain et al., 2018). Interestingly, the caudate and putamen are both stiffer in adolescents than in adults, but have been cited to decrease in volume during maturation and puberty, along with other subcortical structures like the NAcc (Goddings et al., 2014; Johnson and Telzer, 2018). Consistent with these observations, we find here that stiffness and volume measures correlated with our tasks in opposing directions – i.e. greater stiffness and smaller volumes indicated heightened maturity.
The connecting pathway between the cognitive control center and the socioemotional center is a commonly known dopamine pathway, which is thought to invigorate reward-seeking behavior (Clark and Dagher, 2014), and the NAcc has been seen to play a key role in impulsive behavior and is activated for performance dependent rewards (Zink et al 2004). In studies done in rats who have lesions on the NAcc, the rats almost always choose options with less risk associated (Cardinal and Howes, 2005). An fMRI study performed using a modified version of the BART assessment shows that voluntary increases in the level of risk of the game are correlated with increases in neural activation in the ventral striatum region, including the NAcc. However, involuntary risk did not show this same activation pattern and it was concluded that passive risk alone does not activate the NAcc as strongly, likely because the cognitive control system is also at play (Rao et al., 2008). We see here that as a person trends toward more risky behavior, we see an upward trend in NAcc stiffness, and a downward trend in NAcc volume. There is only one previous MRE study that has focused on any brain region associate with reward seeking, which concluded that there is an inverse correlation between striatum stiffness and body mass index (Hetzer et al., 2019). However, this study was on adults and did not specifically assess any measures of reward-seeking behavior, thus limiting the ability to compare with the results presented here.
Active myelination is occurring in the brain throughout adolescence and the prefrontal cortex is one of the last regions to attain full myelin content, therefore we expect processes controlled by the prefrontal cortex to develop later in life (Kelley et al., 2004). Both the vmPFC and the OFC have been well-cited as key structures in decision making and in the dual systems model of adolescent risk taking, therefore both were considered in the analysis presented here (Casey et al., 2008; Hartley and Somerville, 2016). Control functions of both structures are dependent upon the reward valuation of the task (Bouret and Richmond, 2010) and both structures are relied upon in decision making when the outcome of the decision is ambiguous or poses a risk (Rao et al., 2008; Smith and Huettel, 2012). However, it is thought that the vmPFC may be activated first and then information is filtered through the OFC (Bechara et al., 2000). There also appears to be evidence that the vmPFC is more predominant in the immediate and emotional response to decision making, while the OFC is activated for more conscientious choices (Pushkarskaya et al., 2015; Roy et al., 2013; Wallis, 2007). The vmPFC may be responsible for internally-driven motivational processes which are self-centered, and the vmPFC likely relies on a subject’s intrinsic knowledge, whereas the OFC is critical for externally-driven motivational processes which are environment centered (Bouret and Richmond, 2010). The OFC is notable for the ability to encode a value of a choice based the on recent history of choices; that is, if a decision is being made with no prior information, the OFC is not as engaged as when a person has already seen similar information relevant to this decision, such as in the risk-taking tasks used in our study (Elliott et al., 2000; Wallis and Kennerley, 2011). In this study, we found upward trends in stiffness and downward trends in volume in both the OFC and vmPFC with less risky behavior, indicative that people with more developed OFC and vmPFC are less likely to make risky decisions.
Volumetric data from this population serve to further support the relationship seen in regional brain stiffness and risk-taking tendencies. Volume of NAcc and thickness of the OFC and vmPFC have been studied independently for their roles in risk and reward, but only limited reports of their relative contributions exist. Mills et al. (2014) is one of the first studies to examine volumetric mismatch as a predictor of risk taking, though the study focuses on the developmental mismatch in structures, and only begins to touch on how that relates to individual differences in willingness to take risks. The same study also reports that age of peak risk taking might be later in development (16.6 years) than the population in our study, which may account for the lack of correlations we are seeing between brain volume and the BART task.
Of our two task-based measures, the YLG, with output of number of times a participant took a risk, focuses solely on the willingness of a person to take a risk and less on their reward-seeking behavior, whereas the total points measure from the BART may acknowledge a higher level of reward-seeking behavior which focuses on adaptive response in relation to previous reward success or failure. The choice was made to include two tasks as there is a need in the field of neuroscience of risk taking and reward seeking to identify and use simulated risk-taking tasks that are stimulating, economically advantageous for the subject, and predictive of real world risk taking outside of just monetary gains or losses (Schonberg et al., 2012b). Interestingly, the traditional BART measure of total pumps was not significantly correlated with any of our measures, however this is reasonable as we are using a modified version of BART, with three different colored balloons, each programed to pop at different rates. This format allows participants the opportunity to learn from their history of results in order to inform their future risk taking, as opposed to the traditional BART task that limits the ability to learn from past decisions. This modified BART task specifically allows us to capture the higher-order reward and control systems interaction (measured through total points), which is more indicative of real-world risk scenarios, as opposed to the more single-system measure captured through the traditional pumps measure. Stiffness has been shown to be a more sensitive measure of structural integrity than volume and it is expected that stiffness may change prior to any volumetric changes are observed. It is possible that due to our population being slightly younger than age of peak risk-taking, in conjunction with volume being a less sensitive measure of development accounts, for no significant correlations found between the BART and volumetric measures. Therefore, a study on a population spanning a greater age-range would prove beneficial in the study of volume and stiffness differences with respect to behavior.
This study has several limitations. Most importantly, a more robust examination of risk-taking behaviors and brain mechanical properties would rely on longitudinal measures from pre-pubescence into adulthood. These longitudinal measures would provide a better understanding of how stiffness changes, potentially prior to changes in functional measures, and confirm the assertion that the results presented in this work are related to brain maturation. Longitudinal measures would also provide understanding into individual trajectories, including whether a person is more likely to be on the higher end of risk taking throughout his or her life. Identification of socioeconomic, ethnic, or genetic groups of people and correlations between their neural substrates and risk-taking tendencies can be useful towards providing intervention to those groups with the highest inclination for risky behavior based on differences in structural maturation of their brain. Finally, it is possible that other quantitative imaging metrics, such as from relaxometry or diffusion-weighted imaging, may exhibit similar or complementary relationships, and thus future work may examine the unique information provided by MRE and other modalities in understanding the neural bases of adolescent risk taking.
5. Conclusion
It has been acknowledged that sensitive indices of brain structural maturation during adolescence are needed to better understand the relation to risk taking for the purpose of reward. Brain stiffness as measured through MRE is a robust measure of structural brain integrity, and higher regional stiffness is thought to be indicative of greater integrity and development of that structure. We used MRE sensitivity to probe the maturational imbalance between the reward and the control systems and show a relationship with risk-taking tendencies. We found that neither the stiffness of the NAcc, vmPFC, or OFC independently are significantly correlated with risk-taking measures; however, the difference in stiffness between the NAcc of the socioemotional system and either the OFC and vmPFC of the cognitive control system significantly correlated with both risk-taking assessments. In addition, this same trend is seen for volumetric data, providing strong evidence that structural neuroimaging is necessary in the context of reward and control systems contributions to risk-taking. Assessing the relationship between brain mechanical properties and risk. taking allows for better understanding of individual differences in adolescent behavior and potentially informs the design of effective intervention mechanisms.
Supplementary Material
Figure 1:

A) Magnetic resonance elastography (MRE) experiment with 50 Hz vibration delivered to the head with a pneumatic actuator; B) wave displacement images are inverted to create mechanical property maps of viscoelastic shear stiffness and damping ratio; and C) region-of-interest segmentation including the nucleus accumbens (NAcc), the ventromedial prefrontal cortex (vmPFC), and the orbitofrontal cortex (OFC).
Figure 2:
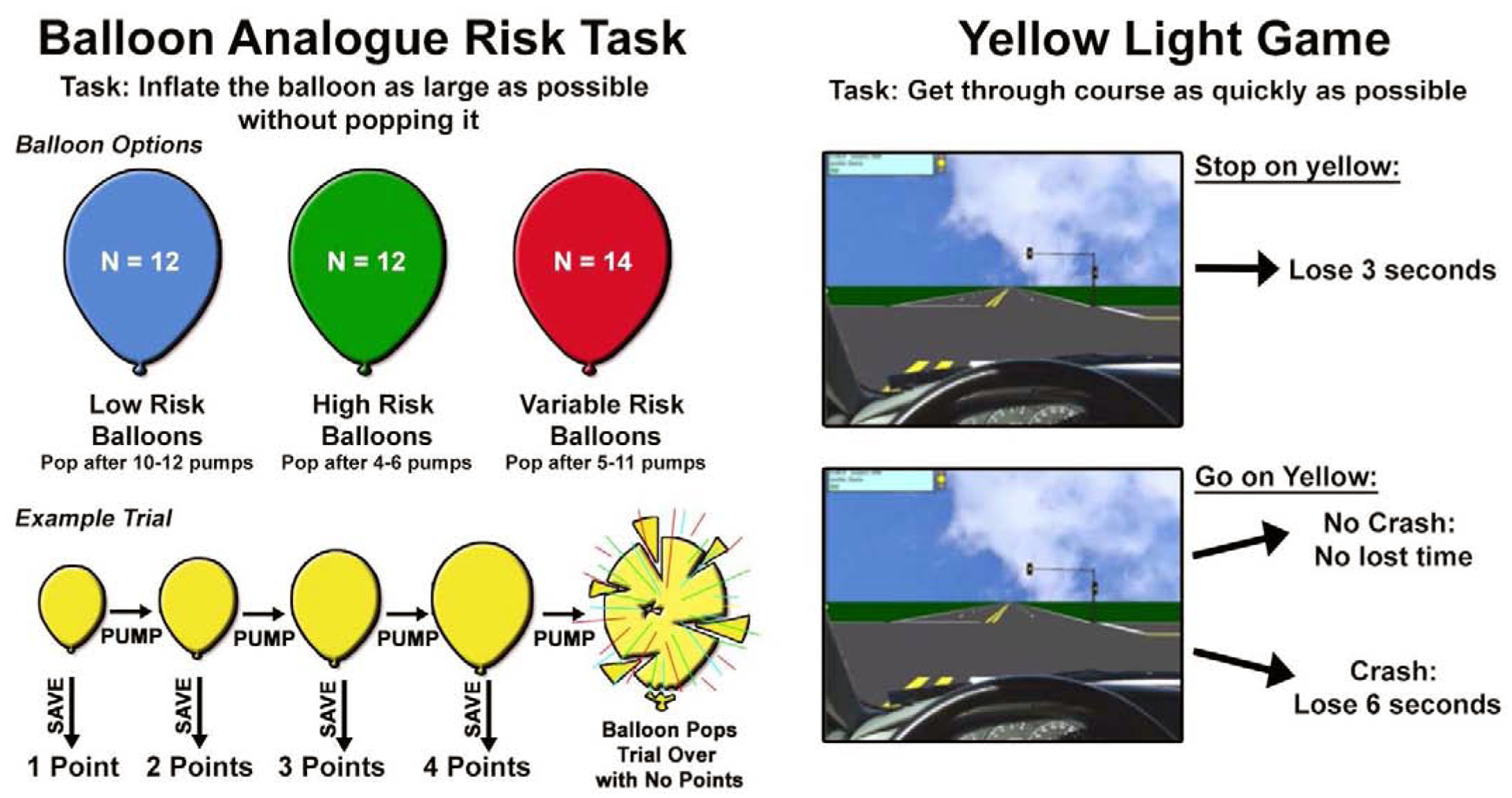
Depictions of risk-taking tasks completed by participants during the scan session including 1) the Balloon Analogue Risk Task (BART) and 2) the Yellow Light Game (YLG). These games are indicative of real-world risk-taking tendencies in adolescents and monetary reward was given dependent on performance to encourage risk taking.
Acknowledgments
Support for this study was partially provided by NSF (SES-1459719), NIH/NIDA (R01-DA039923), the Delaware INBRE Program (NIH/NIGMS P20-GM103446), and the Delaware CTR ACCEL Program (NIH/NIGMS U54- GM104941).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability Statement
Data will be made available upon reasonable request.
Conflict of Interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Ethical statement
This research was conducted in accordance with The Code of Ethics of the World Medical Association. This study was approved by the University of Illinois at Urbana-Champaign Institutional Review Board and all participants, and guardians of the adolescent participants, gave informed written consent prior to being studied.
References
- Bartzokis G, 2008. Brain Myelination in Prevalent Neuropsychiatric Developmental Disorders: Primary and Comorbid Addiction. Adolesc. Psychiatry (Hilversum) 29, 55–96. [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, 2000. Emotion, Decision Making and the Orbitofrontal Cortex. Cereb. Cortex 10, 295–307. 10.1093/cercor/10.3.295 [DOI] [PubMed] [Google Scholar]
- Blakemore S, Burnett S, Dahl RE, 2010. The Role of Puberty in the Developing Adolescent Brain. Hum. Brain Mapp 933, 926–933. 10.1002/hbm.21052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret S, Richmond BJ, 2010. Ventromedial and orbital prefrontal neurons differentially encode internally and externally driven motivational values in monkeys Sebastien. J. Neurosci 30, 8591–8601. 10.123/JNEUROSCI.0049-10.2010.Ventromedial [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ, 2004. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: Reliability and validation against manual measurement of total intracranial volume. Neuroimage 23, 724–738. 10.1016/j.neuroimage.2004.06.018 [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Howes NJ, 2005. Effects of lesions of the nucleus accumbens core on choice between small certain rewards and large uncertain rewards in rats. BMC Neurosci. 6–37, 1–19. 10.1186/1471-2202-6-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A, 2008. The adolescent brain. Dev. Rev 28, 62–77. 10.1016/j.dr.2007.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaze CA, McIlvain G, Smith DR, Villermaux GM, Delgorio PL, Miller F, Crenshaw JR, Johnson CL, 2019. Altered brain tissue stiffness in pediatric cerebral palsy measured by magnetic resonance elastography. NeuroImage Clin. 22 10.1016/j.nicl.2019.101750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein J, Albert D, Brien LO, Uckert K, Steinberg L, 2011. Peers increase adolescent risk taking byenhancing activity in the brain’s reward circuitry. Dev Sci 14, 1–16. 10.1111/j.1467-7687.2010.01035.x.Peers [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CA, Dagher A, 2014. The role of dopamine in risk taking: a specific look at Parkinsonâ€TMs disease and gambling. Front. Behav. Neurosci 8, 1–12. 10.3389/fnbeh.2014.00196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, Frith CD, 2000. Dissociable Functions in the Medial and Lateral Orbitofrontal Cortex: Evidence from Human Nueroimaging Studies. Cereb. Cortex 10, 308–317. [DOI] [PubMed] [Google Scholar]
- Fischl B, 2012. FreeSurfer. Neuroimage 62, 774–781. 10.1016/j.neuroimage.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM, 2000. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Spine (Phila. Pa. 1976) 36, 1–18. 10.1073/pnas.1001504107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M, Steinberg L, 2005. Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: An experimental study. Dev. Psychol 41, 625–635. 10.1037/0012-1649.41.4.625 [DOI] [PubMed] [Google Scholar]
- Goddings A, Mills KL, Clasen LS, Giedd JN, Viner RM, Blakemore S, 2014. NeuroImage The influence of puberty on subcortical brain development. Neuroimage 88, 242–251. 10.1016/j.neuroimage.2013.09.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddings AL, Mills KL, Clasen LS, Giedd JN, Viner RM, Blakemore SJ, 2014. The influence of puberty on subcortical brain development. Neuroimage 88, 242–251. 10.1016/j.neuroimage.2013.09.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg D, Telzer EH, Lieberman MD, Fuligni AJ, Galv A, 2017. Greater response variability in adolescents is associated with increased white matter development. Soc. Cogn. Affect. Neurosci 436–444. 10.1093/scan/nsw132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Bertalan G, Meierhofer D, Klein C, Schreyer S, Steiner B, Wang S, Vieira R, Infante duarte C, Koch S, Boehm-sturm P, Braun J, Sack I, 2019. Brain maturation is associated with increasing tissue stiffness and decreasing tissue fluidity. Acta Biomater 10.1016/j.actbio.2019.08.036 [DOI] [PubMed] [Google Scholar]
- Hare T. a, Camerer CF, Rangel A, 2009. Self-Control in Decision-Making Involves Modulation of the vmPFC Valuation System. Science (80-. ) 324, 646–648. 10.1126/science.1168450 [DOI] [PubMed] [Google Scholar]
- Hartley CA, Somerville LH, 2016. The neuroscience of adolescent decision-making. Curr. Opin. Behav. Sci 5, 108–115. 10.1016/j.cobeha.2015.09.004.The [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetzer S, Hirsch S, Braun J, Sack I, Weygandt M, 2019. Viscoelasticity of striatal brain areas reflects variations in body mass index of lean to overweight male adults. Brain Imaging Behav. [DOI] [PubMed] [Google Scholar]
- Hiscox L, Johnson CL, McGarry MDJ, Schwarb H, Van Beek EJR, Roberts N, Starr JM, 2018. Hippocampal viscoelasticity and episodic memory performance in healthy older adults examined with magnetic resonance elastography. Brain Imaging Behav 10.1007/s11682-018-9988-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscox LV, Johnson CL, Barnhill E, Mcgarry MDJ, Huston J, Beek E.J.R. Van, Starr JM, Roberts N, 2016. Magnetic resonance elastography (MRE) of the human brain : technique , findings and clinical applications. Phys. Med. Biol 61, R401–R437. 10.1088/0031-9155/61/24/R401 [DOI] [PubMed] [Google Scholar]
- Ikemoto S, 2007. Dopamine reward circuitry: Two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res. Rev 56, 27–78. 10.1016/j.brainresrev.2007.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJJ, Woolrich MW, Smith SM, 2012. Fsl. Neuroimage 62, 782–790. 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- Johnson CL, Holtrop JL, McGarry MDJ, Weaver JB, Paulsen KD, Georgiadis JG, Sutton BP, 2014. 3D multislab, multishot acquisition for fast, whole-brain MR elastography with high signal-to-noise efficiency. Magn. Reson. Med 71, 477–485. 10.1002/mrm.25065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CL, Schwarb H, McGarry, M. DJ, Anderson AT, Huesmann GR, Sutton BP, Cohen NJ, 2016. Viscoelasticity of subcortical gray matter structures. Hum. Brain Mapp 37, 4221–4233. 10.1002/hbm.23314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CL, Schwarb H, Horecka KM, McGarry MDJJ, Hillman CH, Kramer AF, Cohen NJ, Barbey AK, 2018. Double dissociation of structure-function relationships in memory and fluid intelligence observed with magnetic resonance elastography. Neuroimage 171, 99–106. 10.1016/j.neuroimage.2018.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CL, Telzer EH, 2018. Magnetic resonance elastography for examining developmental changes in the mechanical properties of the brain. Dev. Cogn. Neurosci 33, 176–181. 10.1016/j.dcn.2017.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S, Blum RW, Giedd JN, 2010. Adolescent Maturity and the Brain: The Promise and Pitfalls of Neuroscience Research in Adolescent Health Policy. J. Adolesc. Heal 45, 216–221. 10.1016/j.jadohealth.2009.05.016.Adolescent [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn LE, Peake SJ, Dishion TJ, Stormshak EA, Pfeifer JH, 2014. Learning to Play It Safe (or Not): Stable and Evolving Neural Responses during Adolescent Risky. J. Cogn. Neurosci 27, 13–25. 10.1162/jocn [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Schochet T, Landry CF, 2004. Risk Taking and Novelty Seeking in Adolescents. Ann. N. Y. Acad. Sci 11, 27–32. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D, 2018. Anticipation of Increasing Monetary Reward Selectively Recruits Nucleus Accumbens. J. Neurosci 21, RC159–RC159. 10.1523/jneurosci.21-16-j0002.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M, 2013. Adolescent brain development in normality and psychopathology. Dev. Psychopathol 25, 1325–1345. 10.1017/S0954579413000643.Adolescent [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manduca A, Oliphant TE, Dresner MA, Mahowald JL, Kruse SA, Amromin E, Felmlee JP, Greenleaf JF, Ehman RL, 2001. Magnetic resonance elastography: Non-invasive mapping of tissue elasticity. Med. Image Anal 5, 237–254. 10.1016/S1361-8415(00)00039-6 [DOI] [PubMed] [Google Scholar]
- McCormick EM, Telzer EH, 2015. Adaptive Adolescent Flexibility: Neurodevelopment of Decision-making and Learning in a Risky Context. J. Cogn. Neurosci 29, 413–423. 10.1162/jocn [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry M, Johnson CL, Sutton BP, Van Houten EE, Georgiadis JG, Weaver JB, Paulsen KD, 2013. Including spatial information in nonlinear inversion MR elastography using soft prior regularization. IEEE Trans. Med. Imaging 32, 1901–1909. 10.1109/TMI.2013.2268978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry MDJ, Houten E.E.W. Van, Johnson CL, Georgiadis JG, Sutton BP, Weaver JB, Paulsen KD, 2012. Multiresolution MR elastography using nonlinear inversion. Med. Phys 39, 6388–6396. 10.1118/1.4754649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry MDJJ, Van Houten EEW, Perrĩez PR, Pattison AJ, Weaver JB, Paulsen KD, 2011. An octahedral shear strain-based measure of SNR for 3D MR elastography. Phys. Med. Biol 56, 153–164. 10.1088/0031-9155/56/13/N02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlvain G, Schwarb H, Cohen NJ, Telzer EH, Johnson CL, 2018. Mechanical properties of the in vivo adolescent human brain. Dev. Cogn. Neurosci 34, 27–33. 10.1016/j.dcn.2018.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlvain G, Tracy JB, Chaze CA, Petersen D, Wright H, Miller F, Crenshaw JR, Johnson CL, 2020. Brain Stiffness Relates to Dynamic Balance Reactions in Children with Cerebral Palsy. J. Child Neurol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Goddings A, Clasen LS, Giedd JN, Blakemore SJ, 2014. The Developmental Mismatch in Structural Brain Maturation during Adolescence. Dev. Neurosci 36, 147–160. 10.1159/000362328 [DOI] [PubMed] [Google Scholar]
- Murphy MC, Iii JH, Ehman RL, 2019. MR elastography of the brain and its application in neurological diseases. Neuroimage 187, 176–183. 10.1016/j.neuroimage.2017.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op de Macks ZA, Flannery JE, Peake SJ, Flournoy JC, Mobasser A, Alberti SL, Fisher PA, Pfeifer JH, 2018. Novel insights from the Yellow Light Game: Safe and risky decisions differentially impact adolescent outcome-related brain function. Neuroimage 181, 568–581. 10.1016/j.neuroimage.2018.06.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen C, Crockett L, Richards M, Boxer A, 1988. A Self-Report Measure of Pubertal Status: Reliability, Validity, and Initial Norms. J. Youth Adolesc 17, 117–133. 10.1103/PhysRevLett.78.1396 [DOI] [PubMed] [Google Scholar]
- Pushkarskaya H, Smithson M, Joseph JE, Corbly C, Levy I, 2015. Neural Correlates of Decision-Making Under Ambiguity and Conflict. Front. Behav. Neurosci 9, 1–15. 10.3389/fnbeh.2015.00325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Fuligni AJ, Galvan A, Lieberman MD, Telzer EH, 2016. Links between parental depression and longitudinal changes in youths’ neural sensitivity to rewards. Soc. Cogn. Affect. Neurosci 1262–1271. 10.1093/scan/nsw035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher L, Krach S, Kohls G, Irmak A, Gründer G, Spreckelmeyer KN, 2010. Dissociation of neural networks for anticipation and consumption of monetary and social rewards. Neuroimage 49, 3276–3285. 10.1016/j.neuroimage.2009.10.089 [DOI] [PubMed] [Google Scholar]
- Rao H, Korczykowski M, Pluta J, Hoang A, Detre JA, 2008. Neural correlates of voluntary and involuntary risk taking in the human brain: An fMRI Study of the Balloon Analog Risk Task (BART). Neuroimage 42, 902–910. 10.1016/j.neuroimage.2008.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Shaw PW, Lerch JP, Clasen LS, Greenstein D, Berman R, Pipitone J, Chakravarty MM, Giedd JN, 2014. Longitudinal four-dimensional mapping of subcortical anatomy in human development. Proc. Natl. Acad. Sci 111, 1592–1597. 10.1073/pnas.1316911111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyna V, Farley F, 2010. Risk and Rationality in Decision Making. Handb. Environ. Risk Decis. Mak 7, 1–44. 10.1201/9781420048735.ch16 [DOI] [Google Scholar]
- Rogers CR, Mccormick EM, Hoorn J. Van, Ivory SL, Telzer EH, 2018. Neural correlates of sibling closeness and association with externalizing behavior in adolescence. Soc. Cogn. Affect. Neurosci 977–988. 10.1093/scan/nsy063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager T, 2013. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn. Sci 16, 147–156. 10.1016/j.tics.2012.01.005.Ventromedial [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonberg T, Fox CR, Mumford JA, Congdon E, Trepel C, Poldrack RA, 2012a. Decreasing ventromedial prefrontal cortex activity during sequential risk-taking: An FMRI investigation of the balloon analog risk task. Front. Neurosci 6, 1–11. 10.3389/fnins.2012.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonberg T, Fox CR, Poldrack RA, 2012b. Mind the Gap: Bridging economic and naturalistic risk-taking with cognitive neuroscience. Trends Cogn. Sci 15, 11–19. 10.1016/j.tics.2010.10.002.Mind [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schregel K, Wuerfel E, Garteiser P, Gemeinhardt I, Prozorovski T, 2012. Demyelination reduces brain parenchymal stiffness quantified in vivo by magnetic resonance elastography. Proc. Natl. Acad. Sci 109, 6650–6655. 10.1073/pnas.1200151109/-/DCSupplemental.www.pnas.org/cgi/doi/10.1073/pnas.1200151109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarb H, Johnson CL, Daugherty AM, Hillman CH, Kramer AF, Cohen NJ, Barbey AK, 2017. Aerobic fitness, hippocampal viscoelasticity, and relational memory performance. Neuroimage 153, 179–188. 10.1016/j.neuroimage.2017.03.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarb H, Johnson CL, Dulas MR, Mcgarry MDJ, Holtrop JL, Watson PD, Wang JX, Voss JL, Sutton B, Cohen NJ, 2019. Structural and Functional MRI Evidence for Distinct Medial Temporal and Prefrontal Roles in Context-dependent Relational Memory. J. Cogn. Neurosci 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarb H, Johnson CL, McGarry MDJ, Cohen NJ, 2016. Medial temporal lobe viscoelasticity and relational memory performance. Neuroimage 132, 534–541. 10.1016/j.neuroimage.2016.02.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman EP, Smith AR, Silva K, Icenogle G, Duell N, Chein J, Steinberg L, 2016. The dual systems model: Review, reappraisal, and reaffirmation. Dev. Cogn. Neurosci 17, 103–117. 10.1016/j.dcn.2015.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DV, Huettel SA, 2012. Decision Neuroscience: Neuroeconomics. Wiley Interdiscip Rev Cogn Sci 10.1002/wcs.73.Decision [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, 2016. Searching for Signatures of Brain Maturity : What Are We Searching For? Neuron 92, 1164–1167. 10.1016/j.neuron.2016.10.059 [DOI] [PubMed] [Google Scholar]
- Steinberg L, 2010. A dual systems model of adolescent risk-taking. Dev. Psychobiol 52, 216–224. 10.1002/dev.20445 [DOI] [PubMed] [Google Scholar]
- Steinberg L, 2007. Risk Taking in Adolescence New Perspectives From Brain and Behavioral Science. Curr. Dir. Psychol. Sci 16, 55–59. 10.1111/j.1467-8721.2007.00475.x [DOI] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J, 2008. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: Evidence for a dual systems model. Dev. Psychol 44, 1764–1778. 10.1037/a0012955 [DOI] [PubMed] [Google Scholar]
- Strang NM, Chein JM, Steinberg L, 2013. The value of the dual systems model of adolescent risk-taking. Front. Hum. Neurosci 7, 1–4. 10.3389/fnhum.2013.00223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijvenvoorde ACK, Achterberg M, Braams BR, Peters S, Crone EA, 2016. Testing a dual-systems model of adolescent brain development using resting-state connectivity analyses. Neuroimage 124, 409–420. 10.1016/j.neuroimage.2015.04.069 [DOI] [PubMed] [Google Scholar]
- Van Houten EEW, Miga MI, Weaver JB, Kennedy FE, Paulsen KD, 2001. Three-dimensional subzoned-based reconstruction algorithm for MR elastography. Magn. Reson. Med 45, 827–837. 10.1002/mrm.1111 [DOI] [PubMed] [Google Scholar]
- Wallis JD, 2007. Orbitofrontal Cortex and Its Contribution to Decision-Making. Annu. Rev. Neurosci 30, 31–56. 10.1146/annurev.neuro.30.051606.094334 [DOI] [PubMed] [Google Scholar]
- Wallis JD, Kennerley SW, 2011. Contrasting reward signals in the orbitofrontal cortex and anterior cingulate cortex. Ann. N. Y. Acad. Sci 1239, 33–42. 10.1111/j.1749-6632.2011.06277.x [DOI] [PubMed] [Google Scholar]
- Willuhn I, Wanat MJ, Clark JJ, Phillips PEM, 2013. Dopamine Signaling in the Nucleus Accumbens of Animals Self- Administering Drugs of Abuse. Curr. Top Behav. Neurosci 3, 29–71. 10.1007/7854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung J, Jugé L, Hatt A, Bilston LE, 2019. Paediatric brain tissue properties measured with magnetic resonance elastography. Biomech. Model. Mechanobiol 10.1007/s10237-019-01157-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


