Abstract
Introduction
Cosmetovigilance is public health surveillance on cosmetic products with a public health objective. Since the radical development in beautifying products in Saudi Arabia, the Saudi Food and Drug Authority takes the responsibility of regulating cosmetic products and issuing guidelines to ensure its safety. Despite this, there exists a lacuna of Re published reports on cosmetics-related adverse reactions in the Saudi population. We aimed to assess self-reported adverse reactions in the general public of the Eastern Province, Saudi Arabia.
Materials and Method
A cross-sectional study was conducted for three months. The questionnaire for data collection was adopted and modified from previous studies for the cosmetic utilization behaviors and adverse reactions.
Results
Among the 425 participants, 50.6% reported that they had at least one adverse reaction in the past two years. Redness of the skin (19%), pimples (15%), and itching (13%) were the commonly reported adverse reactions. The majority of the adverse reactions were reported with hair care (29%) and skincare products (25%). The majority [n = 181 (84.2%)] of the participants with managed the ARs by the cessation of the product use. The univariate analysis found that gender, age, allergic to medications and food, family history of allergy, mixing cosmetics, and frequent switching of cosmetic brands were associated with adverse events. However, the adjusted analysis found that allergic to medication (adjusted OR: 3.9), family history of allergy (adjusted OR: 1.91), and mixing cosmetics (adjusted OR: 1.70) were significantly associated with cosmetics-related adverse reactions.
Conclusion
Cosmetovigilance is a model of safety monitoring of cosmetics. It can be considered as a one of the element in public health activities. Pharmacists should be more vigil on this issue in the near future. To strengthen the findings further, a national wide prevalence study can be conducted prospectively and analyses causality and report to the pharmacovigilanvce system of the country.
1. Introduction
Cosmetic is the primary aspect of the human daily lifestyle in all generations and is spread among the people for numerous uses and purposes (Saudi Food and Drug Authority, 2008). According to the US Food and Drug Administration (FDA), cosmetics are defined as “articles for beautification, cleansing or altering physical appearance” (U.S. Food and Drug Administration, 2018). Whereas Saudi FDA define the cosmetics as ‘any product contains one or more substance intended to use on the outer parts of the human body (skin, hair, nails, lips and the outer parts of the genital), teeth, and the mucous lining of the oral cavity for cleaning purposes, perfuming, to protect or keep the good condition, to change or improve appearance, or to change or improve the smell of the body.” (Saudi Food and Drug Authority, 2008). Similarly, the requirements of cosmetics are different in US and Saudi Arabia: some personal care products are considered as OTC drug in US where as it is under cosmetics in Kingdome and other courtiers (Saudi Food and Drug Authority, 2008). (U.S. Food and Drug Administration, 2018).
The majority of cosmetic consumers are focused on short-term outcomes of the cosmetics on appearance rather than the long-term consequences on the whole body. It is believed that such products have a reasonable degree of safety and tolerability (Kwa et al., 2017). In recent years, more attention has been given for testing and monitoring of the possible harmful effects of cosmetics. The studies revealed that exposure to various chemical substances present in cosmetics poses a health risk (Alani et al., 2013, Draelos, 2015). It can vary from mild hypersensitivity response to severe anaphylactic reaction or even a lethal intoxication. It may occur immediately or after the prolonged use of cosmetics (Alani et al., 2013, Draelos, 2015). Headache, dizziness, tiredness, and nausea were the frequently reported adverse reactions associated with prolonged exposure to heavy makeup (Al-Fawaz, 2016, Husain, 2019, Orton and Wilkinson, 2004). Cosmetovigilance is a public health surveillance on cosmetic products with public health objectives (Vigan and Castelain, 2014). In US and Canada , manufactures, health care providers and consumers are encouraged to report cosmetics-related ADRs to the FDA or Heath Canada, respectively (FDA, 2020a, MedEffec Canada, 2020). Whereas in the European Union, Post Launch Monitoring and Colipa guidelines targeted to harmonizes the causality assessment of adverse effects of cosmetic products (European Commission, 2013, Zweers et al., 2012).
In the Middle East, beauty and personal care trade is growing twice as faster than any other part of the world (Eye of Riyadh, 2018). Economic and cultural changes in the Kingdom of Saudi Arabia has an effect on cosmetic use habit. Fragrances, haircare, cosmetics, skincare, and men’s grooming are the key categories of cosmetics that growths the beauty and personal care market in the Kingdom (Chęś, 2016, Husain, 2019). The beauty industry in Saudi Arabia has been valued at a staggering US$5.7 billion in 2019 and estimated to grow up to $6.9 billion in 2021 (Chęś, 2016, Maisey, 2018, Statista Research Department, 2018). Since the radical development in beautifying products in the Kingdom, the Saudi FDA (SFDA) takes the responsibility of regulating cosmetic products and issuing guidelines to ensure its safety. In order to regulate manufacturing, importation, and marketing of cosmetic products, SFDA implemented an electronic system called eCosma (https://ecosma.sfda.gov.sa). Furthermore, SFDA assigned a unified call center number to enquire about the safety of food, drugs, and cosmetics (Saudi Food and Drug Authority, 2008). Despite all these efforts, there exists a lacuna of research on cosmetics utilization patterns and cosmetics-related adverse reactions in the Saudi population. Hence this pilot study was aimed to assess cosmetics utilization patterns and self-reported adverse reactions in the general public of the Dammam metropolitan region.
2. Materials and methods
2.1. Study design and settings
A cross-sectional study was conducted, from January to March 2019, among the general population living in the metropolitan area in the Eastern Province of Saudi Arabia. The metropolitan area is formed by three main neighboring cities: Dammam, Dhahran, and Khobar. Study questionnaires were distributed in public as well at working places such as schools, colleges, hospitals, companies, and shopping malls. Participants were requested to read the information about the study and to agree with the informed consent before proceeding to items in the questionnaire.
2.2. Study population and sampling
Sample size calculation was done by using OpenEpi (Version 3). A population size of 700,000 was considered (Bilal et al., 2017). Previous literature shows the prevalence of adverse effects of cosmetics was varied from 8 to 38%. With 95% confidence limit and 5% margin of error, the sample size required for estimating the prevalence of 38% was 362, which we approximated to 400.
Residents of Dammam metropolitan region were included in the study irrespective of their nationality and gender or age. Persons who have a habit of using any categories of cosmetic products and who read/ write either English or Arabic were included in the study. Pediatric populations where considered if their legal guardians can fill the data collection tool. Persons with hearing and sight problems were excluded from the study. Similarly, persons on permanent cosmetic methods like plastic surgery, tattoos, fillers, and Botox were also excluded from the study.
2.3. Data collection
The questionnaire for data collection was adopted and modified from previous studies for the cosmetic utilization behaviors and adverse reactions (Bilal et al., 2017, Di Giovanni et al., 2006, Meharie et al., 2014, Sautebin, 2008). US FDA Med watch ADR reporting forms for the consumers were used as the primary reference for the development of AR section. The questionnaire was translated into Arabic; a back-translation method was used to confirm the phase validity of the original questionnaire. Moreover, expert opinion was considered for further editing after translation.
The questionnaire had three main parts: first part was about general socio-demographic information, second part addressed the cosmetics utilization pattern. The last part dealt with the participant’s experience of cosmetics-related adverse reactions for the last two years.
The types of cosmetics have been divided into skin care, hair care, make-up, personal care, nail care, perfumes and traditional care products. In the AR section participants were asked to provide the nature and type of reaction, onset of the reaction, cosmetics suspected to cause the reaction and how they managed to the reaction. The information on the brand or the details of the cosmetics that caused them the reactions were not obtained.
Three trained pharmacy students who speak both Arabic and English distributed the questionnaire. Participants were informed about the purpose of the study and data confidentiality, and informed consent on their willingness to participate in the study was obtained. Considering the sample size of 400, we targeted for distributing 1000 questionnaires.
2.4. Statistical analysis
Data management and analysis were carried out using SPSS Statistics (Version 24.0. Armonk, NY: IBM Corp.). Descriptive statistics (frequency and percentage) were used to summarize demographic characteristics, the pattern of cosmetic use, and adverse events. Logistic regression model was used to assess the determinants of the occurrence of cosmetics-related adverse events among the respondents. Variables that found significant in the univariate analysis were entered into multivariable logistic regression. Adjusted odds ratio (AOR) and its 95% confidence interval (CI) were used to show the strength of association and statistical significance of predictors.
2.5. Operational definitions
Adverse Reactions are defined as harmful/ noxious outcomes that probably related to the cosmetic use in view of the participants.
Cosmetics are defined as any articles used for beautification, cleansing, and personal care, including skincare, hair care, nail care, personal care, makeups, and perfumes.
3. Results
3.1. Characteristics of the study participants
A total of 1000 data collection forms were distributed to the general public, and 473 were returned. Four hundred twenty-five participants were included in the study after refining the completeness of the data. The demographic details of the study participants were given in Table 1. The male–female ratio was 1:3. The age ranged from 10 to 67 years, with more than two-third below the age of 30 years. More than one-half of our study participants had university-level education (245; 57.65%). The average monthly income of the majority (252; 59.29%) was less than 5000 Saudi riyals. Nearly 20% (n = 83) and 10% (n = 43) of participants had a history of food and drug allergy, respectively.
Table 1.
Demographic details of the study participants.
| Variables | Male [n = 109] | Female [n = 316] | Total [n = 425] | |
|---|---|---|---|---|
| Age (in years) | ||||
| Under or equal to 18 | 15 (13.8%) | 111 (35.1%) | 126 (29.6%) | |
| 19–29 | 38 (34.9%) | 128 (40.5%) | 166 (39.1%) | |
| 30–40 | 33 (30.3%) | 51 (16.1%) | 84 (19.8%) | |
| Above or equal 41 | 23 (21.1%) | 26 (8.2%) | 49 (11.5%) | |
| Education level | ||||
| Intermediate or lower | 6 (5.5%) | 14 (4.4%) | 20 (4.7%) | |
| Secondary | 25 (22.9%) | 135 (42.7%) | 160 (37.6%) | |
| University | 78 (71.6%) | 167 (52.8%) | 245 (57.6%) | |
| Monthly Income | ||||
| Less than 5000 SR | 36 (33%) | 216 (68.4%) | 252 (59.3%) | |
| 5000 – 10,000 SR | 30 (27.5%) | 58 (18.4%) | 88 (20.7%) | |
| >10,000 SR | 43 (39.4%) | 42 (13.3%) | 85 (20%) | |
| Allergic to any medication | 12 (11%) | 31 (9.8%) | 43 (10.1%) | |
| Allergic to food | 22 (20.2%) | 61 (19.3%) | 83 (19.5%) | |
| Family history of allergy | 34 (31.2%) | 150 (47.5%) | 184 (43.3%) | |
3.2. Types of cosmetics: Usage by gender
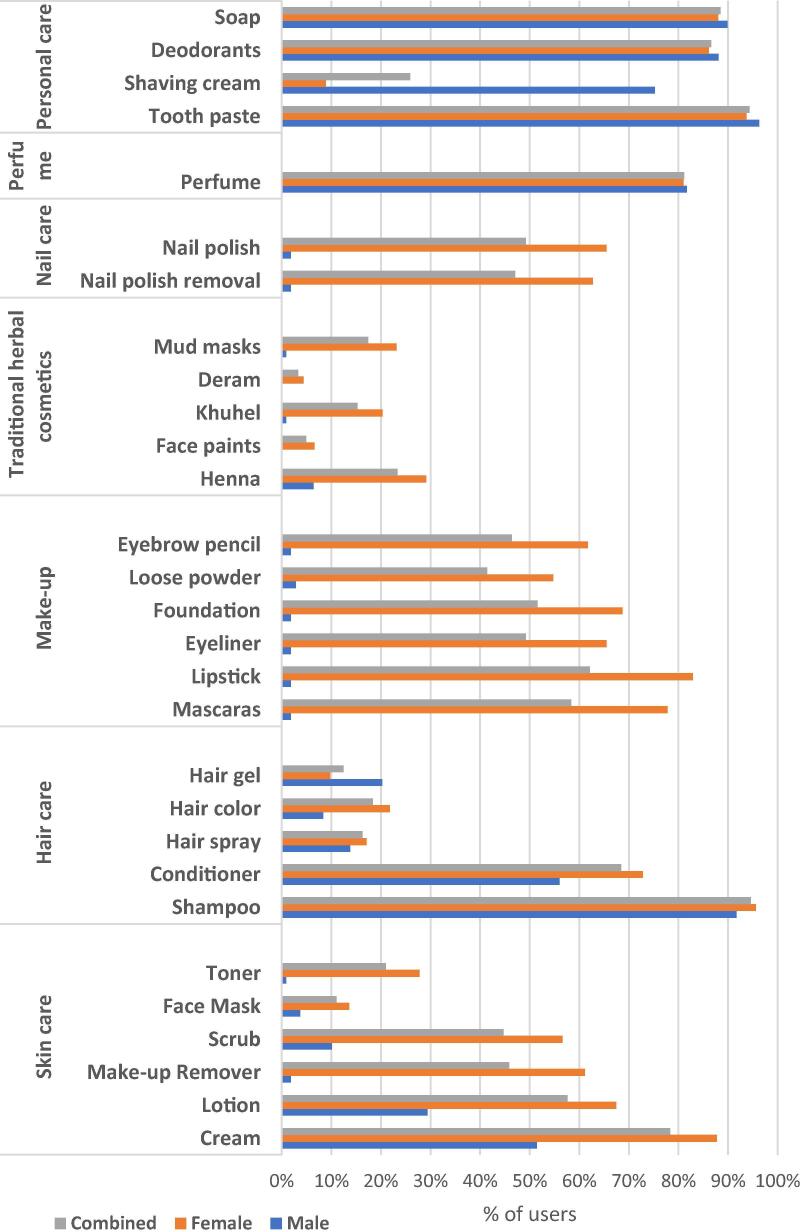
Makeup products [n = 1313 (24.56%)] and personal care products [n = 1255 (22.43%)] were the frequently used cosmetics by the study population. Makeup [n = 1300 (28.61%)] and Skincare [n = 993 (22.5%)], were the favorite choice for females whereas personal care products [n = 318 (47.16%)] and haircare products [207 (25.5)] were preferred by men. Details of preference for cosmetics between the gender were given in Fig. 1.
Fig. 1.
Types of cosmetics: usage by gender.
3.3. Cosmetic utilization behaviors
Cosmetic usage behavior is given in Table 2. Nearly one-half of participants [n = 199 (47%)] used 3–5 cosmetics per day. A large proportion of participants [n = 224 (53%) utilized cosmetic products more than once a day. Nearly 31% (n = 131) and 23% (n = 93) were mix cosmetics either each other or with water, respectively. In addition, 48% (n = 206) of the consumers share make-ups with family members or friends. Local shop [n = 227(26.40%)] and pharmacy [n = 262(26.28%)] were the preferable spot of purchase. Quality [287 (31.43%)] and brand [203 (22.23%)] were the top preferable motive for deciding the cosmetics. A large number of respondents store their products in room cabinet [n = 363(54.50%)].
Table 2.
Cosmetic use behavior.
| Male [n = 109] | Female [n = 316] | Total [n = 425] | |
|---|---|---|---|
| Number of cosmetics used per day.: | |||
| ≤ 2 | 46 (42.2%) | 107 (33.9%) | 153 (36%) |
| 3–5 | 55 (50.5%) | 144 (45.6%) | 199 (46.8%) |
| 6–10 | 8 (7.3%) | 38 (12%) | 46 (10.8%) |
| >10. | 0 (0%) | 27 (8.5%) | 27 (6.4%) |
| Cosmetics utilization per day | |||
| 1 | 46 (42.2%) | 178 (56.3%) | 224 (52.7%) |
| 2 | 46 (42.2%) | 79 (25%) | 125 (29.4%) |
| 3 | 12 (11%) | 43 (13.6%) | 55 (12.9%) |
| ≥4 | 5 (4.6%) | 16 (5.1%) | 21 (4.9%) |
| Mixing cosmetics each other’s | 14 (12.8%) | 117 (37%) | 131 (30.8%) |
| Sharing the Cosmetics. | 33 (30.3%) | 173 (54.7%) | 206 (48.5%) |
| Store cosmetics1: | |||
| Room cabinet. | 81 (74.3%) | 282 (89.2%) | 363 (54.50%) |
| Bathroom. | 67 (61.5%) | 62 (19.6%) | 129 (19.37%) |
| Car. | 21 (19.3%) | 2 (0.6%) | 23 (3.45%) |
| Handbags. | 7 (6.4%) | 108 (34.2%) | 115 (17.27%) |
| Other. | 7 (6.4%) | 29 (9.2%) | 36. (5.41%) |
| Criteria for selecting cosmetics1: | |||
| Brand. | 36 (33.0%) | 167 (52.8%) | 203 (22.23%) |
| Advertisements. | 15 (13.8%) | 54 (17.1%) | 69 (7.56%) |
| Quality. | 70 (64.2%) | 217 (68.7%) | 287 (31.43%) |
| Cost. | 50 (45.9%) | 120 (38.0%) | 170 (18.62%) |
| Recommendation | 32 (29.4%) | 152 (48.1%) | 184 (20.15%) |
| Mode of purchasing1: Shop | 47 (43.1%) | 196 (62.0%) | 243 (28.26%) |
| Online shopping. | 17 (15.6%) | 99 (31.3%) | 116 (13.49%) |
| Pharmacy | 71 (65.1%) | 155 (49.1%) | 226 (26.28%) |
Number exceed the total due to multiple factors allowed
3.4. Safety measures on cosmetic habits
Of the total 425 participants, 38% (n = 163) of them read instructions label before the use of cosmetics, and a quarter of participants [n = 103 (24%)] perform allergy testing prior to cosmetics use (Table 3). A large proportion of respondents, 69% (n = 293), circumspect to check the expiry date of products; while in contrast, 43% (n = 182) used the cosmetic products until it finishes. 41.4% (n = 176) of participants stated that they change the cosmetics brand of sporadically.
Table 3.
Safety measures on cosmetic habits.
| Male [n = 109] | Female [n = 316] | Total [n = 425] | |
|---|---|---|---|
| Duration of cosmetics use: | |||
| ≤6 months. | 22 (20.2%) | 27 (8.5%) | 49 (11.53%) |
| 6–12 months. | 10 (9.2%) | 53 (16.8%) | 63 (14.82%) |
| ≥2years. | 6 (5.5%) | 22 (7.0%) | 28 (6.59%) |
| Until its finishes | 56 (51.4%) | 126 (39.9%) | 182 (42.82%) |
| Till its expiry date. | 15 (13.8%) | 88 (27.8%) | 103 (24.24%) |
| Change the cosmetic brand: | |||
| Yes. | 26 (23.9%) | 103 (32.6%) | 129 (30.35%) |
| Sometimes. | 39 (35.8%) | 137 (43.4%) | 176 (41.41%) |
| No. | 44 (40.4%) | 76 (24.1%) | 120 (28.24%) |
| Check the expiry of cosmetics: | |||
| Yes. | 74 (67.9%) | 219 (69.3%) | 293 (68.94%) |
| No. | 35 (32.1%) | 97 (30.7%) | 132 (31.06%) |
| Read the instructionYes. | 36 (33%) | 127 (40.2%) | 163 (38.4%) |
| Sometimes. | 39 (35.8%) | 111 (35.1%) | 150 (35.3%) |
| No. | 34 (31.2%) | 78 (24.7%) | 112 (26.4%) |
| Testing for allergy | 17 (15.6%) | 86 (27.2%) | 103 (24.2%) |
3.5. Adverse reactions of cosmetics
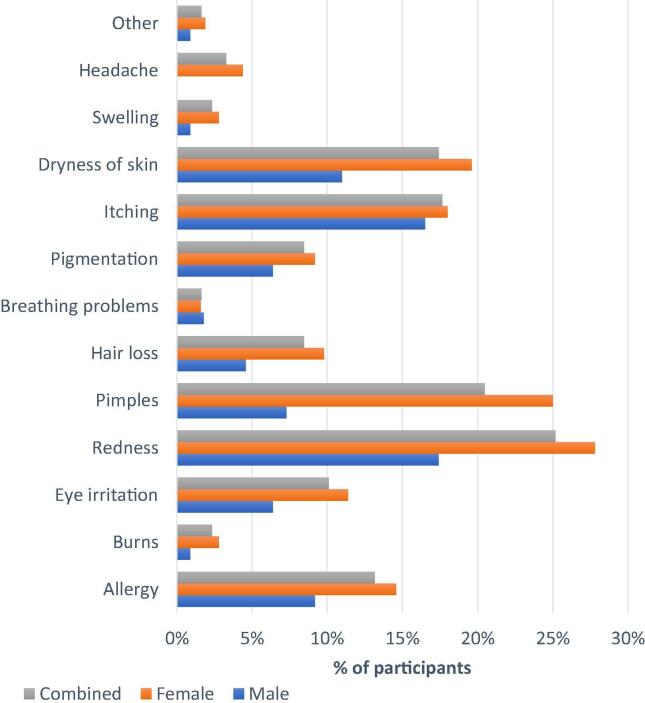
Of the total 425 participants involved in the study, 50.6% (n = 215) developed one or more adverse reactions related to cosmetics usage at least once in the last two years; therefore, the two-year prevalence of AR in our study was 50.5%. A total of 562 adverse reactions were reported among 215 participants giving an average of 2.6 (range1-6) per person. Redness of the skin (n = 107), pimples (n = 87) and itching (n = 75) were the topmost reported ARs by the participants. Fig. 2 enlists the pattern of AR in the study participants.
Fig. 2.
Adverse reactions of cosmetics.
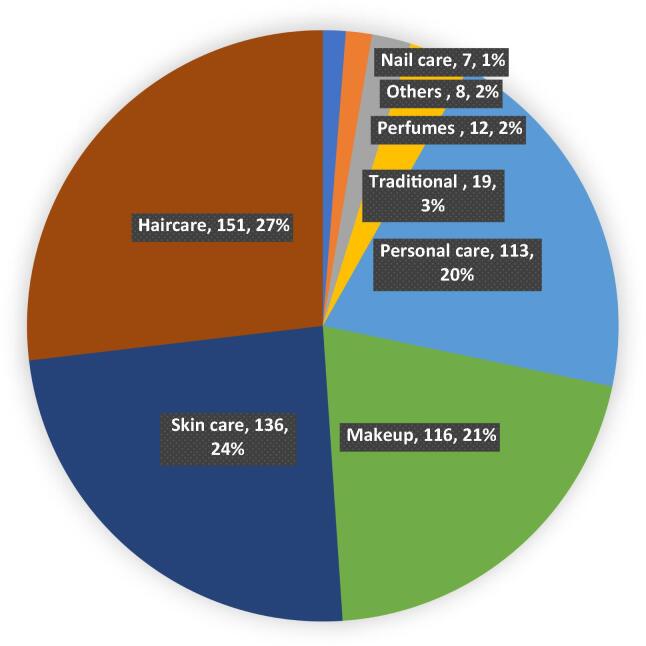
A major proportion of the ARs were related with hair care [n = 151 (27%)] and skincare product [n = 136 (24%)], respectively. The details are given in Fig. 3.
Fig. 3.
Pattern of product-related ARs.
3.6. Management of adverse effects of the cosmetics
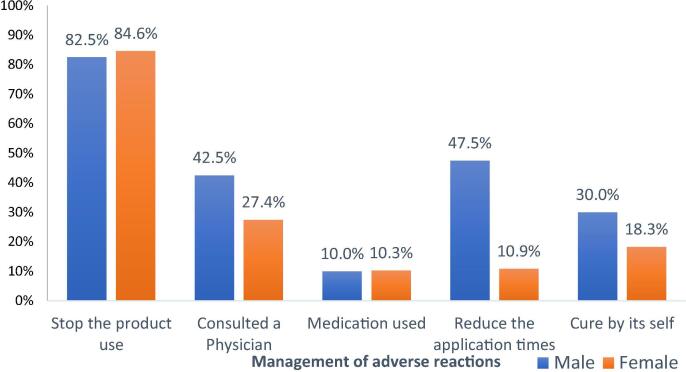
The majority [n = 181 (84.2%)] of the participants with ARs managed the ARs by the cessation of the product use. Several participants had consulted physician [n = 65 (30.9%)] or taken medication [n = 22 (10.2%)] to managing adverse effects of cosmetics. Gender-wise comparison is given in Fig. 4.
Fig. 4.
Management of ARs.
3.7. Predictors of cosmetic adverse events
The univariate analysis found that gender, age, allergic to medications and food, family history of allergy, mixing cosmetics, and frequent switching of cosmetic brands were associated with adverse events (Table 4). However, the adjusted analysis found that allergic to medication (adjusted OR: 3.9), family history of allergy (adjusted OR: 1.91), and mixing cosmetics (adjusted OR: 1.70) were significantly associated with cosmetics-related adverse reactions.
Table 4.
Predictors of ARs.
| Variables | n (%) | p-value1 | Adjusted OR2 (95% CI) | |
|---|---|---|---|---|
| Gender | ||||
| Male. | 40 (36.7%) | 0.001* | Ref | |
| Female. | 175 (55.4%) | 1.61 (0.97, 2.67) | ||
| Age | ||||
| less than19 | 81 (64.3%) | 0.003* | 1.95 (0.92, 4.13) | |
| 19–29 | 73 (44%) | 0.95 (0.47, 1.92) | ||
| 30–40 | 40 (47.6%) | 1.26 (0.58, 2.71) | ||
| >40 | 21 (42.9%) | Ref | ||
| Do you allergic to any medication? | ||||
| No | 180 (47.1%) | 0.000* | Ref | |
| Yes | 35 (81.4%) | 3.9 (1.66, 9.17)* | ||
| Do you allergic to any type of food? | ||||
| No | 161 (47.1%) | 0.003* | Ref | |
| Yes | 54 (65.1%) | 1.29 (0.73, 2.26) | ||
| Family history of allergy | ||||
| No. | 99 (41.1%) | 0.000* | Ref | |
| Yes. | 116 (63%) | 1.91 (1.24, 2.95)* | ||
| Number of cosmetics / days | ||||
| Less than or equal 2. | 69 (45.1%) | 0.275 | – | |
| 3–5. | 108 (54.3%) | |||
| 6–10. | 22 (47.8%) | |||
| >10 | 16 (59.3%) | |||
| Frequency of cosmetics/ day | ||||
| 1 | 108 (48.2%) | 0.134 | – | |
| 2 | 64 (51.2%) | |||
| 3 | 35 (63.6%) | |||
| >3 | 8 (38.1%) | |||
| Mixing Cosmetics | ||||
| No. | 133 (45.2%) | 0.001* | Ref | |
| Yes. | 82 (62.6%) | 1.70 (1.07, 2.68)* | ||
| Sharing cosmetics | ||||
| No | 108 (49.3%) | 0.588 | – | |
| Yes. | 107 (51.9%) | |||
| Read the label of instruction | ||||
| Yes. | 81 (49.7%) | 0.113 | – | |
| Sometimes. | 85 (56.7%) | |||
| No. | 49 (43.8%) | |||
| Changing cosmetic brand frequently | ||||
| Yes. | 77 (59.7%) | 0.023* | 1.59 (0.92, 2.75) | |
| Sometimes. | 87 (49.4%) | 1.19 (0.71, 2.0) | ||
| No. | 51 (42.5%) | Ref | ||
Chi-square test was carried out; 2Adjusted odds ratio was estimated using multiple logistic regression; *statistically significant at 5% level.
4. Discussions
Cosmetovigilance is a growing area under pharmacovigilance an one of the in Saudi Arabia. This study analyses the cosmetic utilization pattern and related ARs by using self reported survey. Longitudinal monitoring of the safety of drugs is practiced in countries like Netherlands (Härmark et al., 2011).
One-half of our study participants reported the occurrence of AR to cosmetics in the past two years. The proportion was much higher than that reported with the previous studies (Getachew and Tewelde, 2018, Huf et al., 2013). The difference might be due to the difference in the pattern and type of cosmetic usage, long duration of the study, low priority on safety of non-medicated cosmetic as well as methodological and cultural difference in the study and studied population. In contrast, study from Ethiopia had reported a much higher incidence of 64% (Bilal et al., 2017). In the present study, similar to other studies (Bilal et al., 2017, Di Giovanni et al., 2006), a higher proportion of ARs was reported among females. One of the reasons could be that the rate and number of cosmetics usages in female group is much higher than the male. In addition, the gender-specific difference in the psychological factors that affect cosmetics use and ARs (Bilal et al., 2017, Korichi et al., 2008). Younger age participants were reported more ARs, and this finding could be highly attributed to a high rate of consumption and more awareness in the younger age group. Moreover, this age group is sprite and have a robust desire for self-care and beautification. A similar trend was observed in previous studies (Bilal et al., 2017, Norudin et al., 2010).
In line with other studies (Bilal et al., 2017, Dibaba et al., 2013, Getachew and Tewelde, 2018, Meharie et al., 2014), hair care and skincare products are found to be more associated with ARs. In contrast, a Brazilian study reported that soap, shampoo, and deodorants as the common culprit for ARs (Huf et al., 2013). It is well documented that these products contain many chemical additives in order to improve performance, effectiveness, and viability of the cosmetics (Alani et al., 2013, Juhász and Marmur, 2014). Exposure to various chemical substances present in cosmetics poses a health risk that varies from a mild hypersensitivity response to a lethal intoxication (Dhavalshankh and Dhavalshankh, 2012, Zainy, 2017). Correspondingly, toxicological studies on cosmetics in Saudi Arabia has been reported the presence of heavy metals and other components more than an approved limit (Al-Saleh et al., 2012, Al-Saleh et al., 2009, AlQuadeib et al., 2018, Zainy, 2017). Also, misbranded and spurious cosmetics are not uncommon in the beautifying market (Dhavalshankh and Dhavalshankh, 2012).
Cosmetics are reported to cause a wide array of adverse reactions, including pigment disorders, irritant, contact urticaria, photosensitization, damage of hair and nails, and acneiform eruptions (Pereira and Pereira, 2018). Similarly, in line with previous studies (Bilal et al., 2017, Di Giovanni et al., 2006), our study sample had experienced various ARs: redness (20%) and pimples (15%) were the topmost ARs. Sites of cosmetic application were the utmost affected by cosmetics-related AR. Again, this finding is supported by literature (Dibaba et al., 2013, Getachew and Tewelde, 2018). One percent of participants reported systemic effects like breathing problems as an AR caused by perfume, deodorant, soap, hair spray, and hair coloring products. Likewise, headache was also reported with certain products such as cream, toner, foundation, deodorant, and soap.However the causal relationship between the reported AR and cosmetics use is not assesed in the study.
The assessment on the management of adverse effects revealed that approximately 85% of the participant managed their ARs by the cessation of the products. Surprisingly 30% of the patients with cosmetic-related adverse events utilize the health care services. A similar finding was reported in previous literature (Bilal et al., 2017, Di Giovanni et al., 2006, Dibaba et al., 2013, Norudin et al., 2010), which highlight the serious nature of the reactions. Allergic to medication and family history of allergy were significantly associated with cosmetic related ARs. In line with previous reports, mixing cosmetics, and changing the brands of cosmetic products were recognized as important predictors for experiencing an adverse event. This could be partly explained by the interaction between cosmetic products or the synergistic effect of the products to each other (Bilal et al., 2017, Dibaba et al., 2013, Norudin et al., 2010).
This study might have some limitations. Firstly, the present study used a self-report questionnaire to collect the data on cosmetic use and the related ARs. Therefore, there will be chance of bias created by the fact that people who had a reaction were more likely to respond to the questionnaire. Similarly, the study requested the participants to report the AR over period of two years, therefore could not exclude the possibility that findings might be affected by recall bias, and it may lead to under-estimation as well. Secondly, participants’ medical illnesses and medication history were also not within the scope of this study. Likewise, some of the adverse reactions stated by the study participants might not have been initiated by the cosmetic product. It could have been assessed by proper further causality assessment, which was beyond the scope of this study
Dermatologist and primary care physicians are the foremost reference for public with any skin complaints. A recent study reported that skin related issues are the most common reason for visits to primary care physicians (St. Sauver et al., 2013). Studies also reported that products labeled as hypoallergenic also contained recognized allergens or irritants (FDA, 2020b, Hamann et al., 2015, Lazzarini et al., 2018). Therefore, there exists a responsibility by the consumer as well as their physician, and a special consideration has to be advised patients who are at risk of contact dermatitis and adverse skin reactions (Ashique and Chandrasekhar, 2017). The role of pharmacist in Public engagements are well documented (Allison et al., 2017). Considering as primary and easy contact by the public, pharmacist can crucially play a role to strengthen the Cosmetovigilance system of a country. Distributing educational leaflets on Cosmetovigilance , conducting awareness classes, mass media activities and direct information providing services to the health care providers and the consumers can be considered as some of the measured to improve the Cosmetovigilance. Similarly an early detection and management of AR of cosmetics may benefits the improve the economic aspects of therapeutics (Ashique and Chandrasekhar, 2017).
5. Conclusion
Cosmetovigilance is a new model of safety monitoring of cosmetics. A substantial percentage of study participants reported having had at least one adverse reaction. Therefore, better methodologies to address this issue might be considered in the future. Awareness creation programs and supporting the Cosmetovigilance model among cosmetic users, sellers, and other stakeholders may help to expand this. Correspondingly Cosmetovigilance can be considered as a one element in public health activities. Pharmacists should be more vigil on this issue in the near future. To strengthen the existing data, a national wide prevalence study can be considered can be prospectively and analyses causality and report to the Saudi Pharmacovigilance system.
CRediT authorship contribution statement
Jisha M. Lucca: Conceptualization, Data curation, Investigation, Methodology, Resources, Supervision, Visualization, Writing - original draft, Writing - review & editing. Royes Joseph: Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Writing - review & editing. Zainab Hussain Al Kubaish: Data curation, Formal analysis, Investigation, Project administration, Resources, Writing - original draft, Writing - review & editing. Sarah Mohammad Al-Maskeen: Data curation, Formal analysis, Investigation, Project administration, Resources, Writing - original draft, Writing - review & editing. Zainab Ali Alokaili: Data curation, Formal analysis, Investigation, Project administration, Resources, Writing - original draft, Writing - review & editing.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Fawaz N. News. Arab News; 2016. Women spend massive sums on cosmetics in Saudi Arabia | Arab. [Google Scholar]
- Al-Saleh I., Al-Enazi S., Shinwari N. Assessment of lead in cosmetic products. Regul. Toxicol. Pharm. 2009;54:105–113. doi: 10.1016/j.yrtph.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Al-Saleh I., Elkhatib R., Al-Rouqi R., Al-Enazi S., Shinwari N. The dangers of skin-lightening creams. Toxicol. Environ. Chem. 2012;94:195–219. doi: 10.1080/02772248.2011.631925. [DOI] [Google Scholar]
- Alani J.I., Davis M.D.P., Yiannias J.A. Allergy to cosmetics: A literature review. Dermatitis. 2013 doi: 10.1097/DER.0b013e3182a5d8bc. [DOI] [PubMed] [Google Scholar]
- Allison D.G., Higginson P., Martin S. Antibiotic resistance awareness: a public engagement approach for all pharmacists. Int J Pharm Pract. 2017;25:93–96. doi: 10.1111/ijpp.12287. [DOI] [PubMed] [Google Scholar]
- AlQuadeib B.T., Eltahir E.K.D., Banafa R.A., Al-Hadhairi L.A. Pharmaceutical evaluation of different shampoo brands in local Saudi market. Saudi Pharm J. 2018;26:98–106. doi: 10.1016/j.jsps.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashique K.T., Chandrasekhar D. Role of clinical pharmacist in cosmeto-vigilance of misuse and abuse of topical corticosteroids. Indian J Dermatol. 2017 doi: 10.4103/ijd.IJD_686_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilal A.I., Tilahun Z., Osman E.D., Mulugeta A., Shekabdulahi M., Berhe D.F. Cosmetics Use-Related Adverse Events and Determinants Among Jigjiga Town Residents, Eastern Ethiopia. Dermatol Ther (Heidelb) 2017;7:143–153. doi: 10.1007/s13555-016-0157-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chęś A. The middle eastern market of cosmetics and toiletries: characteristics underlying demand and potential for growth. Stud Ekon. 2016;303:114–133. [Google Scholar]
- Dhavalshankh A.G., Dhavalshankh G.P. Cosmetovigilance: the study of prevalence & vigilance of adverse cutaneous reactions in hairdye users. Int J Biol Med Res. 2012;2:1704–1707. [Google Scholar]
- Di Giovanni C., Arcoraci V., Gambardella L., Sautebin L. Cosmetovigilance survey: Are cosmetics considered safe by consumers? Pharmacol. Res. 2006;53:16–21. doi: 10.1016/j.phrs.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Dibaba, H., Yadesa, D., Wubishet, B.L., Sabe, Z., Gerima, B., 2013. Cosmetics Utilization Pattern and Related Adverse Reactions Among Female University Students. nternational J Pharm Sci Res 4, 997–1004.
- Draelos, Z.D., 2015. Cosmetics: The Medicine of Beauty. J Cosmet Dermatol 14, 91–91. https://doi.org/10.1111/jocd.12146 [DOI] [PubMed]
- European Commission, 2013. Annex 1: Causality Assessment of Undesirable Effects Caused By Cosmetic Products in ‘SUE Reporting Guidelines’ [WWW Document]. URL https://ec.europa.eu/docsroom/documents/13251/attachments/1/translations/en/renditions/native (accessed 4.5.20).
- Eye of Riyadh, 2018. MENA region’s beauty, personal care industry to record 8.5% annual growth in next 3 years. Eye Riyadh.
- FDA, 2020a. MedWatch: The FDA Safety Information and Adverse Event Reporting Program | FDA [WWW Document]. URL https://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program (accessed 4.4.20).
- FDA, 2020b. “Hypoallergenic” Cosmetics | FDA [WWW Document]. URL https://www.fda.gov/cosmetics/cosmetics-labeling-claims/hypoallergenic-cosmetics#Hypoallergenic_Cosmetics (accessed 4.4.20).
- Getachew M., Tewelde T. Cosmetic Use and Its Adverse Events among Female Employees of Jimma University, Southwest Ethiopia. Ethiop J Health Sci. 2018;28:717–724. doi: 10.4314/ejhs.v28i6.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann C.R., Bernard S., Hamann D., Hansen R., Thyssen J.P. Is there a risk using hypoallergenic cosmetic pediatric products in the United States? J. Allergy Clin. Immunol. 2015;135:1070–1071. doi: 10.1016/j.jaci.2014.07.066. [DOI] [PubMed] [Google Scholar]
- Härmark L., van Puijenbroek E., van Grootheest K. Longitudinal monitoring of the safety of drugs by using a web-based system: The case of pregabalin. Pharmacoepidemiol. Drug Saf. 2011;20:591–597. doi: 10.1002/pds.2135. [DOI] [PubMed] [Google Scholar]
- Huf, G., Rito, P. da N., Presgrave, R. de F., Boas, M.H.S.V., 2013. Adverse reactions to cosmetic products and the Notification System in Health Surveillance: a survey. Brazilian J Epidemiol 16, 1017–20. https://doi.org/10.1590/s1415-790x2013000400021 [DOI] [PubMed]
- Husain K. A survey on usage of personal care products especially cosmetics among university students in Saudi Arabia. J Cosmet Dermatol. 2019;18:271–277. doi: 10.1111/jocd.12773. [DOI] [PubMed] [Google Scholar]
- Juhász M.L.W., Marmur E.S. A review of selected chemical additives in cosmetic products. Dermatol. Ther. 2014 doi: 10.1111/dth.12146. [DOI] [PubMed] [Google Scholar]
- Korichi R., Pelle-De-Queral D., Gazano G., Aubert A. Why women use makeup: Implication of psychological traits in makeup functions. J. Cosmet. Sci. 2008;59:127–137. [PubMed] [Google Scholar]
- Kwa M., Welty L.J., Xu S. Adverse Events Reported to the US Food and Drug Administration for Cosmetics and Personal Care Products. JAMA Intern Med. 2017;177:1202–1204. doi: 10.1001/jamainternmed.2017.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarini, R., Hafner, M. de F.S., Rangel, M.G., 2018. Evaluation of the presence of allergens in children’s products available for sale in a big city. An Bras Dermatol 93, 457–459. https://doi.org/10.1590/abd1806-4841.20187111 [DOI] [PMC free article] [PubMed]
- Maisey S. Natl; 2018. Saudi Arabia beauty market now worth Dh20 billion. [Google Scholar]
- MedEffect Canada, 2020. MedEffect Canada - Canada.ca [WWW Document]. URL https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada.html (accessed 4.5.20).
- Meharie, B.G., Ambaye, A.S., Mengesha, Y., Haimanot, T./, Atnafie, S.A., 2014. A cross sectional study on assessment of cosmetics utilization and self reported adverse reactions among Wollo university Dessie campus female students, Dessie, North East Ethiopia. Eur J Pharm Med Res 2, 49–63.
- Norudin M., Desnika M.A., Rafi M. Cosmetic usage in Malaysia, understanding the major determinants affecting the users. Int J Bus Sci. 2010;1:273. [Google Scholar]
- Orton D.I., Wilkinson J.D. Cosmetic Allergy. Am. J. Clin. Dermatol. 2004;5:327–337. doi: 10.2165/00128071-200405050-00006. [DOI] [PubMed] [Google Scholar]
- Pereira J.X., Pereira T.C. Cosmetics and its Health Risks. Glob J Med Res. 2018;18:63–70. doi: 10.34257/gjmrbvol18is2pg63. [DOI] [Google Scholar]
- Saudi Food and Drug Authority, 2008. Cosmetics [WWW Document]. URL https://www.sfda.gov.sa/en/cosmetic/Pages/cosmetic_product.aspx (accessed 1.6.20).
- Sautebin L. Understanding the Adverse Effects of Cosmetics. Drug Saf. 2008;31:433–436. doi: 10.2165/00002018-200831050-00010. [DOI] [PubMed] [Google Scholar]
- St. Sauver, J.L., Warner, D.O., Yawn, B.P., Jacobson, D.J., McGree, M.E., Pankratz, J.J., Melton, L.J., Roger, V.L., Ebbert, J.O., Rocca, W.A., 2013. Why patients visit their doctors: Assessing the most prevalent conditions in a defined American population. Mayo Clin Proc 88, 56–67. https://doi.org/10.1016/j.mayocp.2012.08.020 [DOI] [PMC free article] [PubMed]
- Statista Research Department, 2018. Value of sales in the beauty industry in Saudi Arabia in 2015 and 2020 [WWW Document]. Statista. URL https://www.statista.com/statistics/664313/saudi-arabia-beauty-industry-sales-value/ (accessed 1.6.20).
- U.S. Food and Drug Administration, 2018. Cosmetics | FDA [WWW Document]. URL https://www.fda.gov/cosmetics (accessed 1.6.20).
- Vigan M., Castelain F. Cosmetovigilance: definition, regulation and use “in practice”. Eur J Dermatology. 2014 doi: 10.1684/ejd.2014.2493. [DOI] [PubMed] [Google Scholar]
- Zainy F.M.A. Heavy Metals in Lipstick Products Marketed in Saudi Arabia. J Cosmet Dermatological Sci Appl. 2017;07:336–348. doi: 10.4236/jcdsa.2017.74030. [DOI] [Google Scholar]
- Zweers P.G.M.A., Gilmour N.J., Hepburn P.A., Gerritsen R.F., van Puijenbroek E.P. Causality methods in Cosmetovigilance: Comparison of Colipa and PLM versus global introspection. Regul. Toxicol. Pharm. 2012;63:409–417. doi: 10.1016/j.yrtph.2012.05.005. [DOI] [PubMed] [Google Scholar]