Abstract
Objective
Hypothalamic arcuate proopiomelanocortin (Arc-POMC) neurons are involved in different physiological processes such as the regulation of energy balance, glucose homeostasis, and stress-induced analgesia. Since these neurons heterogeneously express different biological markers and project to many hypothalamic and extrahypothalamic areas, it is proposed that Arc-POMC neurons could be classified into different subpopulations having diverse physiological roles. The aim of the present study was to characterize the contribution of the subpopulation of Arc-POMC neurons cosecreting gamma-aminobutyric acid (GABA) neurotransmitter in the control of energy balance.
Methods
Arc-Pomc expression restricted to GABAergic-POMC neurons was achieved by crossing a reversible Pomc-deficient mouse line (arcPomc−) with a tamoxifen-inducible Gad2-CreER transgenic line. Pomc expression was rescued in the compound arcPomc−/−:Gad2-CreER female and male mice by tamoxifen treatment at postnatal days 25 (P25) or 60 (P60), and body weight, daily food intake, fasting glycemia, and fasting-induced hyperphagia were measured. POMC recovery was quantified by immunohistochemistry and semiquantitative RT-PCR. Neuropeptide Y (NPY) and GABAergic neurons were identified by in situ hybridization. Arc-POMC neurons projecting to the dorsomedial hypothalamic nucleus (DMH) were studied by stereotactic intracerebral injection of fluorescent retrobeads into the DMH.
Results
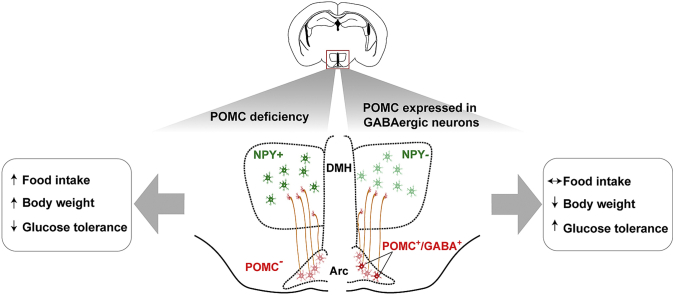
Tamoxifen treatment of arcPomc−/−:Gad2-CreER mice at P60 resulted in Pomc expression in ∼23–25% of Arc-POMC neurons and ∼15–23% of Pomc mRNA levels, compared to Gad2-CreER control mice. Pomc rescue in GABAergic-POMC neurons at P60 normalized food intake, glycemia, and fasting-induced hyperphagia, while significantly reducing body weight. Energy balance was also improved in arcPomc−/−:Gad2-CreER mice treated with tamoxifen at P25. Distribution analysis of rescued POMC immunoreactive fibers revealed that the DMH is a major target site of GABAergic-POMC neurons. Further, the expression of the orexigenic neuropeptide Y (NPY) in the DMH was increased in arcPomc−/− obese mice but was completely restored after Pomc rescue in arcPomc−/−:Gad2-CreER mice. Finally, we found that ∼75% of Arc-POMC neurons projecting to the DMH are GABAergic.
Conclusions
In the present study, we show that the expression of Pomc in the subpopulation of Arc-GABAergic-POMC neurons is sufficient to maintain normal food intake. In addition, we found that DMH-NPY expression is negatively correlated with Pomc expression in GABAergic-POMC neurons, suggesting that food intake may be regulated by an Arc-GABAergic-POMC → DMH-NPY pathway.
Keywords: Proopiomelanocortin, GABA, Energy balance, Dorsomedial hypothalamic nucleus, Arcuate hypothalamic nucleus, Obesity
Abbreviations: POMC, Proopiomelanocortin; GABA, Gamma-aminobutyric acid; GLU, Glutamate; Arc, Arcuate nucleus; DMH, Dorsomedial hypothalamic nucleus; PVH, Paraventricular hypothalamic nucleus; LH, Lateral hypothalamic nucleus; NPY, Neuropeptide Y; TAM, Tamoxifen
Graphical abstract
Highlights
-
•
The subpopulation of arcuate GABAergic-POMC neurons is sufficient to maintain normal food intake.
-
•
Overweight induced by Pomc deficiency is reduced by arcuate Pomc expression restricted to GABAergic-POMC neurons.
-
•
DMH-Npy overexpression in POMC-deficient mice is restored by Pomc rescue restricted to GABAergic-POMC neurons.
-
•
Arcuate POMC neurons projecting to the DMH are mainly GABAergic.
1. Introduction
Overweight is caused by multiple factors leading to the disruption of cerebral circuits that sense energy availability and regulate energy balance [1]. Proopiomelanocortin (POMC) anorexigenic neurons, located in the arcuate nucleus (Arc) of the hypothalamus, are the main regulators of food intake and energy expenditure [2]. Recent studies have shown that Arc-POMC neurons are heterogeneous and can be divided into subpopulations expressing diverse biomarkers and neurotransmitters [[3], [4], [5]]. However, the physiological roles of the different subpopulations, their anatomical distribution and targets, remain unknown.
POMC is a propeptide predominantly expressed in the pituitary gland and the hypothalamus. After posttranslational processing, POMC gives rise to bioactive products with different actions. In particular, hypothalamic melanocortins α- and β-melanocyte-stimulating hormones (α- and β-MSH) suppress food intake, stimulate energy expenditure, and enhance insulin sensitivity through melanocortin receptor 4 (MC4R) [6]. Mutations in Pomc or Mc4r genes lead to severe hyperphagia and early-onset obesity in both humans and mice [[7], [8], [9], [10], [11], [12], [13]]. Moreover, diet-induced obesity triggers hypothalamic inflammation and endoplasmic reticulum stress which, in turn, impairs POMC processing, leading to decreased production of α-MSH and, consequently, to hyperphagia and overweight [[14], [15], [16]]. Notably, the characterization of POMC neurons and their circuits in mouse models has contributed to the design and study of different drugs for the treatment of obesity and type 2 diabetes, some of which are already available for patients [[17], [18], [19], [20], [21], [22]].
Arc-POMC neurons project to different hypothalamic areas involved in energy balance such as the paraventricular (PVH), ventromedial, dorsomedial (DMH), and lateral (LH) hypothalamic nuclei, as well as extrahypothalamic regions involved in other functions like autonomic control, reward, and analgesia [[23], [24], [25]]. The DMH is known to be involved in the regulation of food intake and energy expenditure [26]. It receives afferent projections from the Arc and, in turn, sends efferent projections to the PVH and the LH [26]. Lesions of the DMH result in hypophagia and weight loss, supporting a role for this region in the stimulation of food intake [27]. Moreover, it has been shown that the orexigenic neuropeptide Y (NPY) is involved in DMH induction of food intake in lactating rats and in some models of obese mice [[28], [29], [30], [31]]. Finally, there is some evidence showing that melanocortin receptors mediate an inhibitory tone onto DMH-NPY expression [32]. However, a link between Arc-POMC expression and DMH-NPY expression remains to be established.
It has been shown that POMC neurons are heterogeneous regarding their electrophysiological response to peripheral signals (e.g., glucose) and the receptors they express (e.g., leptin, serotonin, insulin, and estrogen receptors) [[33], [34], [35]]. Moreover, subsets of hypothalamic POMC neurons express and corelease either gamma-aminobutyric acid (GABA) or glutamate (Glu) [[36], [37], [38]], encompassing 45–54% and 7–43% of POMC neurons, respectively [39,40]. Given the antagonistic responses elicited by GABA and Glu in neuronal excitability, it is speculated that these subpopulations carry out different functions. Recent studies applying single-cell RNA sequencing showed that Arc-POMC neurons can be classified into subpopulations with different transcriptomic profiles [[3], [4], [5]]. In particular, Campbell ` showed that Arc-POMC neurons can be classified into three clusters. While two of these clusters express mainly GABAergic markers (Gad1 and Gad2), the third one expresses principally a glutamatergic marker (Vglut2). Interestingly, there is a preponderant response to the fasting of GABAergic-POMC clusters, evidenced by changes in their gene expression profiles [3]. However, the physiological roles of these subpopulations in the regulation of energy homeostasis remain to be elucidated. In the present study, we aimed to dissect the contribution of the subpopulation of Arc-POMC neurons cosecreting GABA in the control of energy balance by expressing Pomc exclusively in GABAergic-POMC neurons of Pomc-deficient mice.
2. Materials and methods
2.1. Animal care
Mice were kept under standard laboratory conditions, with controlled photoperiod (lights on from 7 a.m. to 7 p.m.), tap water, and standard lab chow available ad libitum. Mice were weaned at P21. All procedures were approved by the Institutional Animal Care and Use Committee of the Facultad de Medicina, Universidad de Buenos Aires.
2.2. Mouse lines
Ai14:Gad2-CreER. To characterize the expression pattern of Cre recombinase in the knock-in Gad2-CreERT2 mouse line (Gad2-CreER, for simplicity), we used Ai14:Gad2-CreER mice obtained by crossing heterozygous Gad2-CreER (Gad2tm1(cre/ERT2)Zjh; The Jackson Laboratory, stock: 010,702 [41]) and homozygous Ai14 mice (B6. Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J, The Jackson Laboratories, stock 7914 [42]). Gad2-CreER mice express a tamoxifen-inducible Cre in GABAergic neurons. Cre-expressing neurons were reported by the fluorescent protein tdTomato after tamoxifen treatment at P60 (see Section 2.3).
arcPomc−/−:Gad2-CreER. arcPomc+/− mice [11] were crossed with Gad2-CreER mice to obtain arcPomc+/−:Gad2-CreER mice. Thereafter, arcPomc+/−:Gad2-CreER mice were mated with arcPomc+/− mice to obtain arcPomc−/−:Gad2-CreER reversible POMC-deficient mice and obese arcPomc−/− or normal weight Gad2-CreER control groups.
arcPomc−/−:CreER mice were obtained as described in [11]. Briefly, the arcPomc- line was crossed with CreER transgenic mouse line (B6. Cg-Tg[cre/Esr1]5Amc/J; The Jackson Laboratory, stock: 004682), in which Cre is driven by a ubiquitously active CAAG promoter and induced by tamoxifen.
2.3. CRE induction by tamoxifen
Tamoxifen (Sigma) was prepared in sesame oil (Sigma) as described previously [43]. Gad2-CreER mice were i. p. injected with 150 mg/kg/day tamoxifen at p60 or with 100 mg/kg/day at P25, during five consecutive days, with a solution of 15 or 10 mg tamoxifen/mL, respectively. CreER mice were i. p. injected with one dose of tamoxifen (50 mg/kg).
2.4. General study design
Two cohorts of arcPomc−/−:Gad2-CreER mice and their control littermates were generated by breeding strategies described above and treated with tamoxifen at postnatal days 25 or 60 ± 3 days (P25 and P60 cohorts, respectively). Mice were individually housed within the first week after weaning, and body weight and food intake were registered twice a week. Basal glycemia and glucose tolerant tests (GTTs) were measured after overnight fasting (6 pm–10 am) at the 7th (P60-PRE) and 14th weeks of age (P25 and P60-POST) by collecting blood samples from the tail with a OneTouch® glucometer (LifeScan, Johnson & Johnson). For GTTs, mice received an i. p. injection of glucose (2 g/kg; Sigma) as described previously [44]. Fasting-induced hyperphagia was measured in P60-treated mice at the 15th week of age, as described [11]. Briefly, after a 24-hour fast (10 am–10 am) ad libitum food intake of the next 24 h was measured and expressed relative to the average daily food intake of the previous week. At the 16th week of age, mice were anesthetized with 5% chloral hydrate at 3 pm and perfused with 4% paraformaldehyde (PFA) for subsequent immunohistochemistry or in situ hybridization. Alternatively, mice were killed by cervical dislocation at 11 am, and fresh hypothalami were removed and fast-frozen for RNA extraction, while inguinal (unilateral) and gonadal (bilateral) fat pads as well as livers were weighed.
2.5. Hypothalamic Pomc mRNA expression
Hypothalamic total RNA was prepared following phenol-chloroform extraction using TriPure (Roche) and then treated with RNase-free DNase I (Ambion). First-strand cDNA was synthesized with random primers using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Pomc mRNA was identified by relative quantitative RT-PCR using PowerUp SYBR Green Master Mix (Thermo Fisher Scientific) with specific primers (F- 5′-CTCCTGCTTCAGACCTCCAT-3′ and R- 5′-CAGTCAGGGGCTGTTCATCT-3′; amplicon size: 169 bp), relative to endogenous β-actin (primers F-5′-AGAGGGAAATCGTGCGTGAC-3′ and R- 5′- CAATAGTGATACCTGGCCGT-3’; amplicon size: 138bp). Samples were run on the 7500 Real-Time PCR machine (Applied Biosystem), and the results were analyzed by the 2−ΔΔCT relative quantitation method [45].
2.6. Immunohistochemistry
PFA fixed brains were collected and cut into 35 μm coronal sections with a frozen microtome (Leica). For immunohistochemistry, floating brain sections were treated with 1% H2O2 for 30 min and, after PBS washing, sections were incubated with rabbit polyclonal anti-rat-ACTH antibody (1:1000, A.F. Parlow, National Hormone and Peptide Program, Harbor-UCLA Medical Center, Torrance, CA, USA), overnight at 4 °C. The following day, sections were incubated first with the secondary antibody (biotinylated anti-rabbit IgG made in goat, 1:200, Vector Labs) and then with avidin-radish peroxidase complex (Vectastain ABC Kit, Vector Labs). Finally, slices were developed with diaminobenzidine (DAB) (Vector Labs), mounted on 1% gelatin (Sigma) coated glass slides with Canada balsam, and analyzed with an Olympus BX53 microscope, coupled with a Q-Color5 digital camera and Capture Q software. The quantitative rostrocaudal analysis was performed manually and blinded by two investigators, between −1.22 mm and −2.06 mm from bregma with Fiji software [46]. Results were expressed as the percentage of POMC + neurons in arcPomc−/−:Gad2-CreER- or arcPomc−/−:CreER-rescued mice relative to POMC + neurons in Gad2-CreER or CreER control mice, respectively. For anti-ACTH immunofluorescence, sections were incubated with AlexaFluor488-coupled anti-rabbit IgG (1:1000, Invitrogen) and colocalized with endogenous red fluorescent signal (tdTomato) of Ai14 mice. In this case, images were obtained with an Olympus FV1000 confocal microscope, and slices between −1.34 mm and −1.7 mm from bregma were analyzed with Fiji software.
2.7. Chromogenic (ISH) and fluorescent in situ hybridization (FISH)
For Npy probe cloning, an insert of 493 bp (NM_023456.3, 32–524 bp) was amplified by RT-PCR from RNA of mouse embryos using gene-specific primers: mNpyF: 5′ TCTCACAGAGGCACCCAGAG 3′ and mNpyR: 5′ CAACAACAACAAGGGAAATG 3′ . The PCR product was cloned (pGEM-T Easy Vector, Promega, Cat. A1360) and sequenced (SAI, University of Murcia). Gad1 probe (NM_008077.5, 2198–2949 bp) was kindly provided by Dr. Z. Katarova. For both ISH and FISH, PFA-fixed brains were embedded in 15% gelatin (Sigma) in PBS and then collected and cut into 20 μm coronal sections with a frozen cryostat (Thermo Fisher Scientific). ISH was performed as described [47] with minor modifications. Briefly, Npy probe was synthesized using a digoxigenin- (DIG-) labeling kit (Roche), incubated with ON at 72 °C, and developed with NBT/BCIP (Roche). For FISH, mounted sections were incubated with DIG-labeled Gad1 RNA probe overnight at 55 °C. DIG probe was detected with an anti-Dig-POD antibody (1:150, Roche) and developed with Alexa-488 Tyramide Signal Amplification Kit (1:100, Life Technologies). After FISH protocol, sections were incubated with rabbit polyclonal anti-rat-ACTH antibody (1:100, A.F. Parlow, National Hormone and Peptide Program, Harbor-UCLA Medical Center, Torrance, CA, USA) followed either by an anti-rabbit-Cy3 antisera (1:500, Jackson ImmunoResearch, Figure 1) or by a biotinylated anti-rabbit IgG antisera (1:200, Vector Labs) combined with streptavidin-Alexa Fluor-647 (1:1000, Jackson ImmunoResearch, Figure 6). Sections were mounted with VECTASHIELD (Vector Labs) and analyzed with Axio Imager M2 motorized fluorescent microscope with Apotome2 structured illumination (Zeiss). Photographs were obtained with a monochromatic camera, and fluorophores were artificially colored by Neurolucida Software (MBF Bioscience). For double-labeling analysis (Gad1+/POMC+), one image per hemisphere was taken and manually analyzed with Fiji software. Results were reported as the percentage of POMC + neurons expressing Gad1. For triple-labeling analysis (retrobeads+/Gad1+/ACTH+), z-stack images were taken every 5 μm for a total of 15 μm (one per hemisphere of arcuate nucleus). Stack images were collapsed in a unique plane and analyzed with Fiji software. Quantitative analysis was manually performed by two investigators in sections between −1.4 and −1.9 mm distance to bregma. 32.4 ± 2.5 POMC neurons per hemisection of the hypothalamus were quantified. Results were expressed as the percentage of cells labeled with red (retrobeads) and blue (POMC) signals (presumably POMC neurons projecting to the DMH) that were also green (Gad1+).
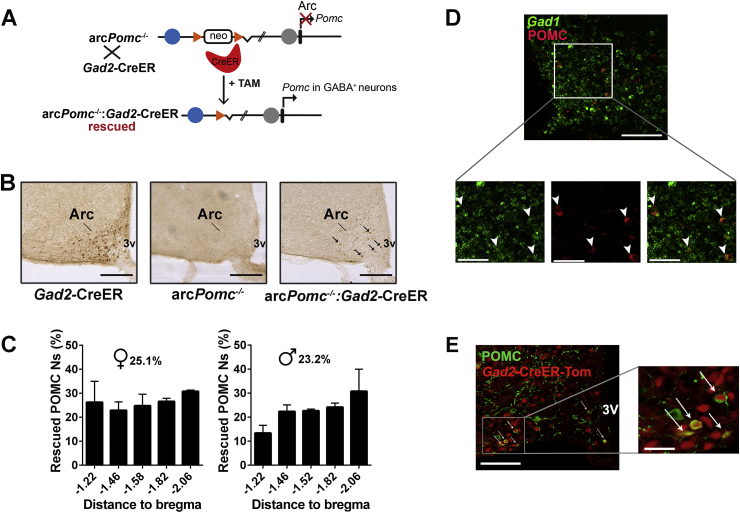
Figure 1.
Generation of a mouse line expressing Pomc restricted to GABAergic-POMC neurons. (A)Pomc− allele contains an insertion of a neomycin resistance cassette (neo), flanked by loxP sites (triangles), interrupting Pomc neuronal enhancer activity (blue circle: nPE1 enhancer; gap after neo: Deleted nPE2 enhancer). Gad2-CreER line drives Pomc reactivation restricted to GABAergic-POMC neurons after i. p. injection of tamoxifen (TAM) in arcPomc−/−:Gad2-CreER mice (rescued). The POMC-cellular specificity of Pomc reactivation is due to the endogenous Pomc regulatory elements in the knock-in arcPomc– allele. Gray circle: Pomc pituitary promoter. Arc: arcuate Pomc transcription. Black rectangle: Pomc exon 1. (B) Representative coronal brain sections of Gad2-CreER, Pomc−/−, and rescued arcPomc−/−:Gad2-CreER mice showing ACTH immunopositive neurons. The arrows indicate rescued POMC expression in GABAergic neurons. 3V, third ventricle; Arc: arcuate nucleus. Magnification bars: 200 μm. (C) Percentage of POMC neurons in arcPomc−/−:Gad2-CreER-rescued mice (GABAergic-POMC neurons), relative to POMC neurons in Gad2-CreER control mice, at different coronal levels of the hypothalamus. The rostrocaudal analysis shows no significant differences (OWA). Error bars: ± SEM; n = 3. (D) Representative coronal brain section of an arcPomc−/−:Gad2-CreER-rescued mice subjected to fluorescent hybridization for Gad1 (GABAergic neurons, green) and immunohistochemistry for ACTH (POMC neurons, red). The white rectangle depicts the area of magnified images shown below. Magnification bars: 200 μm (upper picture) and 50 μm (bottom pictures). The white arrowheads point to GABAergic-POMC neurons. (E) Representative coronal brain section of Ai14:Gad2-CreER mice showing recombination in GABAergic cells (red, “Gad2CreER-Tom”) and ACTH immunopositive neurons (green, “POMC”). The white rectangle depicts the area of the magnified image shown on the right. The white arrows point to GABAergic-POMC neurons. Magnification bars: 200 μm (left picture) and 50 μm (right picture).
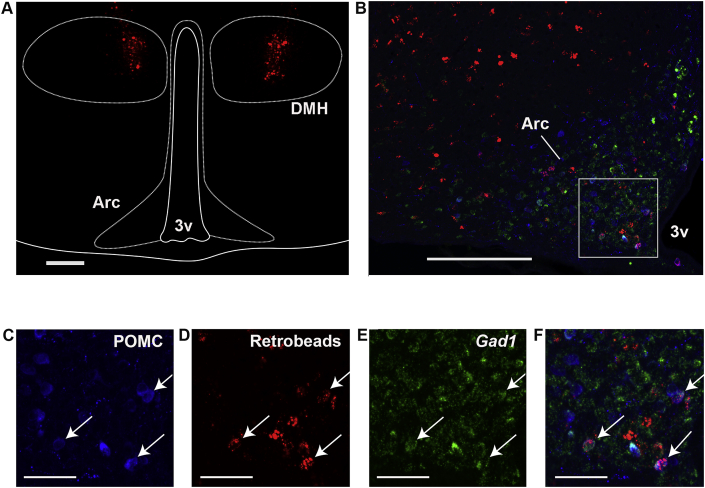
Figure 6.
Arcuate POMC neurons projecting to DMH are mainly GABAergic. (A) Representative image of coronal brain sections showing bilateral injection of retrobeads (red) in the DMH of a wild-type female mouse. Magnification bar: 200 μm. (B) Representative fluorescent microphotograph of a coronal brain section at −1.58 mm from bregma, subjected to POMC immunostaining (blue) and in situ hybridization against Gad1 (green). Red: retrobeads coming from the DMH. Magnification bar: 200 μm. (C–F) Magnified images of the square shown in (B). Arc: arcuate nucleus. 3V: third ventricle. Magnification bar: 50 μm.
2.8. Stereotactic surgery
Red fluorescent latex microspheres (Lumafluor Inc.) were used as a retrograde neuronal tracer to track projections to DMH. Adult wild-type C57Bl/6 (10–12 weeks old) female mice (n = 4) were anesthetized using isoflurane in O2 (2.5% induction, 0.8% maintenance) and placed in stereotactic head apparatus (Kopf Instruments). Ophthalmic ointment was applied to both eyes to prevent drying, and 2% lidocaine hydrochloride was injected subcutaneously for local analgesia. Multiple rounds of betadine and 70% ethanol wipes were applied to the scalp and then a midline 8 mm incision was made to access the skull. Then, a 1 mm diameter craniotomy was drilled. The retrobeads solution (500 nl, 1:200 in saline) was injected bilaterally at 100 nl/min at the following stereotaxic coordinates: 1.7 mm anteroposterior, ±1.3 mm mediolateral relative to bregma, and −4.80 mm dorsoventral from the cortical surface using a 10° angle to avoid ventricles and damage of the superior sagittal sinus. After surgery, the skin was sutured and postoperative care included analgesia (i.p. administration of flunixin 5 mg/kg) for three consecutive days. Two weeks after surgery (14–19 days), mice were perfused and brains were removed and fixed in 4% PFA to prepare them for in situ hybridization and immunohistochemistry.
2.9. Statistical analysis
All data are presented as the mean ± SEM and were analyzed by Student's unpaired two-tailed t-test, one-way or two-way ANOVA (OWA, TWA), or repeated measures ANOVA (RMA), using GraphPad Prism version 6.00 for Windows (GraphPad Software, La Jolla California, USA). Post hoc Bonferroni's test was used when necessary. P < 0.05 was considered significant.
3. Results
3.1. Pomc expression restricted to GABAergic neurons
In order to elucidate the physiological role of POMC expressed in the subpopulation of arcuate (Arc) GABAergic-POMC neurons, we used a previously developed mouse model carrying a mutant conditional Pomc allele (arcPomc-), which harbors a loxP-flanked neomycin cassette within Pomc neuronal enhancer region, which selectively prevents neuronal transcription in the Arc but not in the pituitary gland or the nucleus of the solitary tract (Figure 1A and [11]). Homozygous arcPomc−/− mice are hyperphagic and develop early-onset extreme obesity [11]. However, eutopic Pomc expression can be achieved by crossing arcPomc−/− mouse line with another one expressing Cre recombinase [11]. Interestingly, we previously found that food intake and body weight can be greatly improved after Pomc recovery by a ubiquitously expressed Cre recombinase [11]. In the present study, in order to assure Pomc expression restricted to GABAergic neurons, arcPomc- mouse line was crossed with another knock-in line expressing a tamoxifen-inducible Cre driven by the Gad2 promoter (Gad2-CreER mouse line [41], Figure 1A).
In order to selectively recover Pomc expression in GABAergic neurons, we treated arcPomc−/−:Gad2-CreER mice with tamoxifen at P60, which led to POMC expression in 25.1 ± 3.3% and 23.2 ± 2.3% (mean ± SEM) of POMC neurons in female and male mice, respectively, compared to Gad2-CreER control mice (Figure 1B,C). Moreover, hypothalamic Pomc rescue restricted to GABAergic neurons resulted in 23.3 ± 7.1% and 14.8 ± 1.3% of Pomc mRNA compared to Gad2-CreER control mice (mean ± SEM) in females and males, respectively. POMC immunopositive neurons in rescued mice showed homogeneous distribution across the anterior–posterior axis of the arcuate nucleus (OWA: P > 0.05 for both sexes, Figure 1C). In order to establish the specificity of the Gad2-CreER driver, we performed double labeling by in situ hybridization and immunohistochemistry in brain slices of arcPomc−/−:Gad2-CreER mice which revealed that at least 94.2 ± 0.7% of rescued POMC neurons are GABAergic (Figure 1D).
Since, according to previous quantifications of Gad2+-POMC neurons [39], we expected Pomc recovery in ∼45% of POMC neurons, to determine the extension of Gad2-CreER activity, we generated Ai14:Gad2-CreER reporter mice. After tamoxifen administration, we found tdTomato expression in 26.7 ± 1.9% (mean ± SEM, n = 3) of POMC neurons (Figure 1E), which suggests incomplete recombination induced by the Gad2-CreER driver.
3.2. GABAergic-POMC neurons regulate energy balance
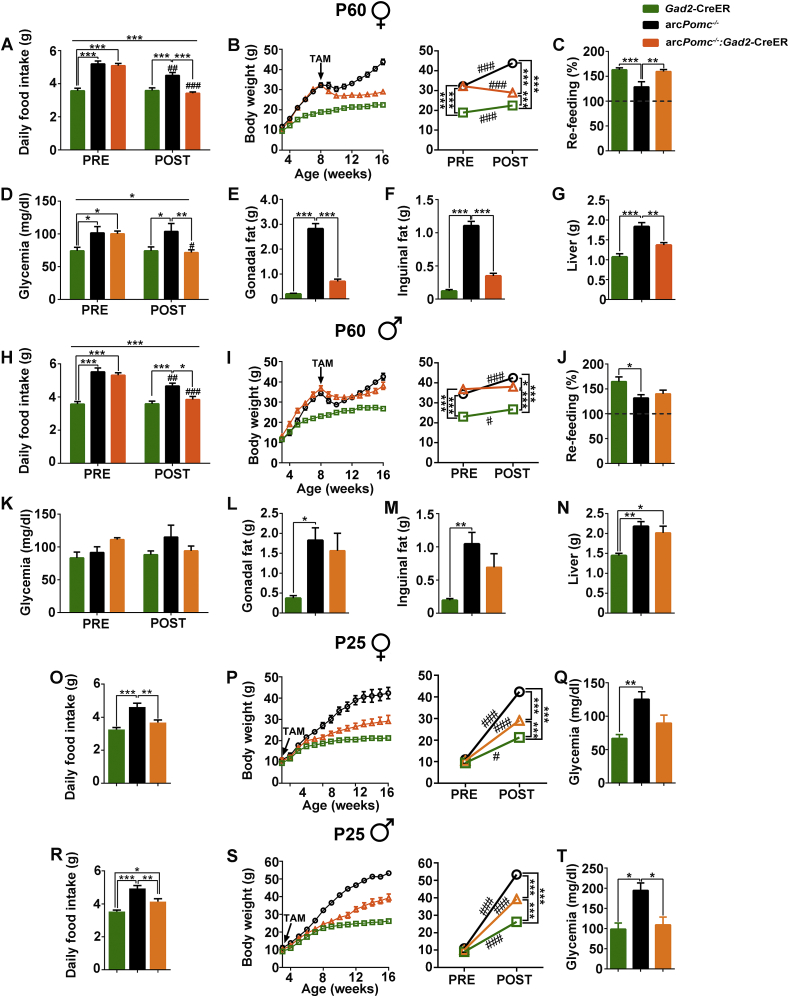
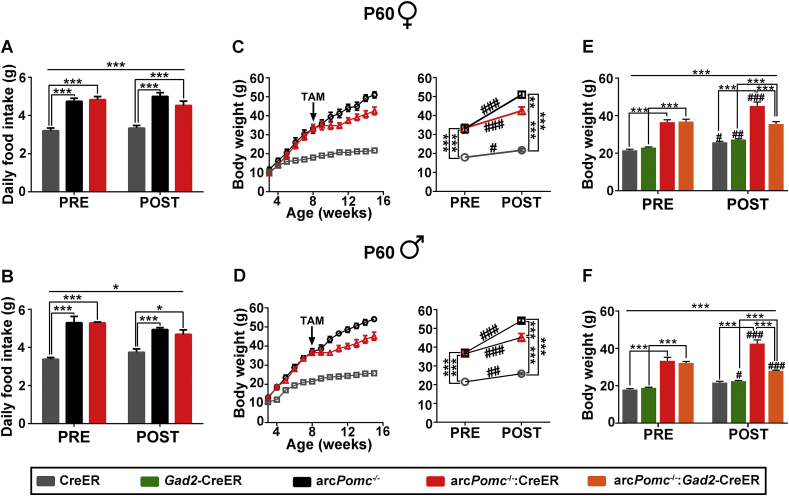
With the aim of studying the role of Arc-POMC expressed in GABAergic neurons in the regulation of energy balance, we rescued Pomc expression in arcPomc−/−:Gad2-CreER mice. After tamoxifen treatment at P60, we found that both female and male arcPomc−/−:Gad2-CreER-rescued mice normalized food intake (Figure 2A,H). Remarkably, female arcPomc−/−:Gad2-CreER mice greatly improved body weight after Pomc rescue: they were ∼70% overweight before treatment and, after losing weight, they remain only ∼29% overweight, compared to Gad2-CreER control littermates (Figure 2B). Furthermore, while arcPomc−/− female mice showed decreased fasting-induced hyperphagia, rescued arcPomc−/−:Gad2CreER mice displayed normal response (Figure 2C). Fasting glycemia and glucose tolerance, which were increased and decreased, respectively, in Pomc-deficient female mice, were completely restored by Pomc recovery in GABAergic-POMC neurons (Figure 2D and Supplementary Figures 1A–1D). In females, body weight improvement was due to fat loss, since inguinal and gonadal fat pads as well as liver weights were significantly lower in arcPomc−/−:Gad2-CreER-rescued mice compared to obese arcPomc−/− control mice (Figure 2E–G).
Figure 2.
Pomc restoration in GABAergic-POMC neurons improves energy balance and glycemia. (A, H) Average of daily food intake from the 6th to 8th and from the 12th to 14th weeks of age (before (PRE) and after (POST) treatment, respectively) of mice treated i. p. with tamoxifen (TAM) at P60. N = 9–14. (B, I) Body weight curves of mice treated with TAM at P60. Graphs on the right show comparisons of body weights before (PRE, 7 weeks old) and after (POST, 16 weeks old) TAM treatment. N = 9–14. (C, J) Fast induced hyperphagia of mice previously treated with TAM at P60. Bars correspond to averages of 24-hour refeeding data expressed as a percentage of prefasting food intake. The degree of hyperphagia can be estimated above the dashed lines. N = 9–14. (D, K) Basal glycemia measured before (PRE, 7 weeks old) and after (POST, 14 weeks old) TAM treatment. N = 5–9. (E-G and L-N) Gonadal and inguinal fat pad weights and liver weights of mice treated with TAM at P60. N = 4–10. (O, R) Average of daily food intake (from the 12th to 14th weeks of age) of mice treated i. p. with TAM at P25. N = 4–11. (P–S) Body weight curves of mice treated with TAM at P25. Graphs on the right show comparisons of body weights before (PRE, 3 weeks old) and after (POST, 16 weeks old) TAM treatment. N = 4–12. (Q, T) Basal glycemia measured at the 14th weeks of age of mice treated with TAM at P25. N = 3–9. (A–G) and (O–Q) correspond to females and (H–N) and (R–T) correspond to males. In all cases, error bars: ±SEM. (A, B, D, H, I, K, P, S) RMA: ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001; #P < 0.05, ##P < 0.01, and ###P < 0.001: pairwise comparisons within the same group, before (PRE) versus after (POST) Pomc restoration (Bonferroni). (C, E–G, J, L–N, O, Q, R, T) OWA: ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 (Bonferroni).
ArcPomc−/−:Gad2-CreER male mice were rescued at P60 completely normalized food intake (Figure 2H). However, they showed mild body weight loss, with body weights that become significantly different from those of arcPomc−/− mice seven weeks after Pomc recovery (RMA, Bonferroni post hoc test: P < 0.01; Figure 2I). Regarding fasting-induced hyperphagia, while arcPomc−/− male mice showed decreased food intake in the refeeding period, rescued arcPomc−/−:Gad2-CreER mice showed no significant differences with either arcPomc−/− or Gad2-CreER control mice (Figure 2J). Fasting glycemia was not increased in Pomc-deficient male mice, at least until the 14th week of age (Figure 2K). However, these mice were intolerant to a glucose overload, a condition that was reverted by Pomc rescue (Supplementary Figures 1E–1H). Finally, weights of inguinal and gonadal fat pads of rescued arcPomc−/−:Gad2-CreER male mice were not significantly different from those of arcPomc−/− or Gad2-CreER mice, while liver weights were higher than those of Gad2-CreER mice (Figure 2L–N).
The above results show that the Pomc expression in GABAergic-POMC neurons is sufficient to maintain normal food intake. However, despite Pomc rescue in obese mice at P60 leads to body weight loss, arcPomc−/−:Gad2-CreER-rescued mice remain heavier than Gad2-CreER littermates. Since we previously found that Pomc recovery by a ubiquitously expressed CreER only leads to normal body weight when tamoxifen is injected to normal weight mice [11], we sought to elucidate if Pomc rescue restricted to GABAergic-POMC neurons in juvenile mice has similar consequences. Therefore, another cohort of mice was injected with tamoxifen at P25, before the onset of obesity. The magnitude of Pomc recovery in arcPomc−/−:Gad2-CreER mice at P25 was similar to that of mice treated at P60: 22.31 ± 1.83% and 26.17 ± 3.94% (mean ± SEM) of POMC + cells compared to control Gad2-CreER mice in females and males, respectively. We found that Pomc rescue in GABAergic-POMC neurons at P25 prevented hyperphagia in females and decreased hyperphagia in male arcPomc−/−:Gad2-CreER mice compared to arcPomc−/− controls (Figure 2O,R). Moreover, Pomc rescue at P25 delayed the onset of obesity in both female and male arcPomc−/−:Gad2-CreER mice: while arcPomc−/− mice became significantly heavier than Gad2-CreER control mice since the 7th and 4th week of age (RMA, Bonferroni post hoc test: P < 0.001 and P < 0.05, female and male, respectively), both female and male arcPomc−/−:Gad2-CreER mice rescued at P25 became overweight only since the 9th week of age (RMA, Bonferroni post hoc test: P < 0.05 for both sexes; Figure 2P,S). Finally, Pomc rescue at P25 prevented hyperglycemia in both female and male arcPomc−/−:Gad2-CreER mice (Figure 2Q,T). Altogether, these results suggest a key role of GABAergic-POMC neurons in the control of energy balance.
3.3. Partial nonspecific Pomc rescue leads to mild body weight improvement
In order to emphasize the significance of GABAergic-POMC neurons in the regulation of energy balance, we compared the improvement of obesity after Pomc rescue, specifically in GABAergic neurons, with that achieved by rescuing POMC in a similar number of neurons but in a nonspecific manner. With the purpose of inducing partial Pomc rescue driven by a ubiquitously expressed Cre recombinase, we generated arcPomc−/−:CreER mice and treated them with a single dose of tamoxifen (50 mg/kg) at P60. Tamoxifen treatment led to 32 ± 3.6% and 35.2 ± 7.2% (mean ± SEM; females and males, respectively) of POMC + neurons in arcPomc−/−:CreER mice compared to CreER controls (Supplementary Figure 2). Contrary to Pomc rescue in GABAergic neurons, nonspecific Pomc rescue failed to improve food intake in both female and male arcPomc−/−:CreER mice (Figure 3A,B). Furthermore, nonspecific POMC rescue led to a mild loss of body weight in both female and male arcPomc−/−:CreER mice compared to arcPomc−/− mice (Figure 3C,D). Remarkably, body weight was significantly lower after Pomc rescue in GABAergic neurons compared to the nonspecific rescue (Figure 3E,F). Moreover, while arcPomc−/−:Gad2-CreER mice maintained or decreased body weight compared to their pretreatment stage (female and male, respectively), arcPomc−/−:CreER mice further increased body weight after Pomc nonspecific rescue (Figure 3E,F). Altogether, the comparison of obesity improvement after GABAergic-specific versus nonspecific Pomc expression suggests a preponderant role of GABAergic-POMC neurons in the regulation of food intake and body weight.
Figure 3.
Partial nonspecific rescue of Pomc fails to normalize food intake and body weight. (A, B) Average of daily food intake from the 6th to 8th and from the 12th to 14th weeks of age (before (PRE) and after (POST) treatment, respectively) in female (A) and male (B) mice treated i. p. with a single low dose of tamoxifen (TAM) at P60). N = 4–7. (C, D) Body weight curves of female (C) and male (D) mice. The graphs on the right show comparisons of body weights before (PRE, 7 weeks old) and after (POST, 16 weeks old) TAM treatment. N = 4–7. (E, F) Comparison of body weights of female (E) and male (F) mice before and after GABA-specific or nonspecific Pomc rescue at P60. The data of arcPomc−/−:Gad2-CreER and Gad2-CreER phenotypes correspond to the same mice shown in Figure 2B (females) and Figure 2I (males). N = 4–14. Error bars: ±SEM. RMA: ∗P < 0.05, and ∗∗P < 0.01, ∗∗∗P < 0.001. Pre versus Post: #P < 0.05, ##P < 0.01, and ###P < 0.001 (Bonferroni).
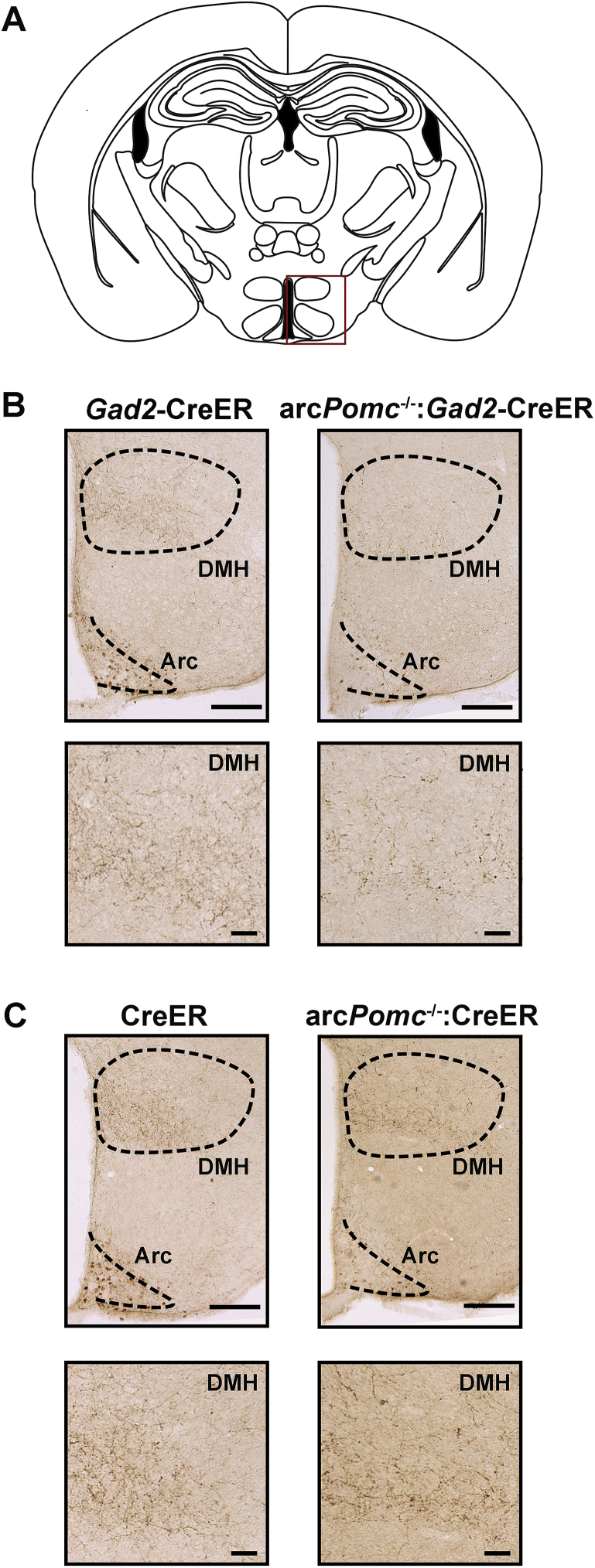
3.4. Distribution of GABAergic-POMC immunoreactive fibers
In order to dissect the anatomical distribution of GABAergic-POMC immunoreactive fibers (POMC-IRFs), coronal and sagittal brain sections of rescued arcPomc−/−:Gad2-CreER mice were subjected to immunohistochemistry against ACTH. As expected, since POMC is rescued in only ∼25% of the total POMC neurons in these mice, in general, few isolated POMC-IRFs were detected. Remarkably, we consistently found POMC-IRFs in the DMH of all analyzed female and male rescued arcPomc−/−:Gad2-CreER mice (Table 1, Figure 4B, and Supplementary Figure 3). To a lesser extent, paraventricular hypothalamic nucleus and the posterior hypothalamic nucleus, which are highly innervated by POMC neurons in wild-type mice, were also the main target sites of GABAergic-POMC neurons (Table 1 and Supplementary Figure 3). Surprisingly, other areas that are highly innervated by POMC neurons in wild-type mice showed POMC-IRFs only in a few proportion of arcPomc−/−:Gad2-CreER-rescued mice (e.g., the bed nucleus of the stria terminalis, retrochiasmatic area, and the periventricular hypothalamic nucleus) (Table 1). On the contrary, all male and female Gad2-CreER control mice showed POMC-IRFs in the brain areas listed in Table 1 (n = 3 and 6, males and females, respectively; data not shown).
Table 1.
Brain areas with GABAergic POMC-immunopositive fibers (POMC-IRF). Numbers indicate the quantity of arcPomc−/−:Gad2-CreER female and male mice rescued at P60 showing POMC-IRF projecting to a specific brain area, from a total of 6 analyzed mice.
| BRAIN AREAS WITH POMC-IRF | FEMALES | MALES |
|---|---|---|
| Dorsomedial hypothalamic nucleus | 6 | 6 |
| Paraventricular hypothalamic nucleus | 4 | 4 |
| Posterior hypothalamic nucleus | 3 | 4 |
| Bed nucleus of the stria terminalis | 2 | 3 |
| Periventricular hypothalamic nucleus | 0 | 2 |
| Paraventricular thalamic nucleus | 2 | 0 |
| Anterior commissure | 2 | 0 |
| Suprachiasmatic nucleus | 0 | 2 |
| Preoptic nucleus | 1 | 1 |
| Tuber cinereum area | 1 | 0 |
| Lateroanterior hypothalamic nucleus | 1 | 0 |
| Retrochiasmatic area | 1 | 0 |
| Premammillary nucleus | 1 | 0 |
Figure 4.
POMC fibers projecting to the dorsomedial hypothalamic nucleus (DMH) of POMC rescued mice. (A) Schematic coronal brain section adapted from [57]. The red rectangle signalizes the brain area shown in the upper pictures of (B) and (C). (B) Upper panels: representative coronal brain sections of Gad2-CreER and rescued arcPomc−/−:Gad2-CreER female mice showing POMC immunopositive neurons in the arcuate nucleus (Arc) and POMC immunoreactive fibers (POMC-IRFs) in the DMH. Magnification bars: 200 μm. (C) Upper panels: representative coronal brain sections of CreER and rescued arcPomc−/−:CreER female mice showing POMC immunopositive neurons in the Arc and POMC-IRFs in the DMH. Magnification bars: 200 μm. (B and C) Bottom panels: magnified areas of DMH taken from the upper pictures showing POMC-IRsF. Magnification bars: 50 μm.
3.5. Pomc rescue in GABAergic-POMC neurons prevents DMH-Npy overexpression
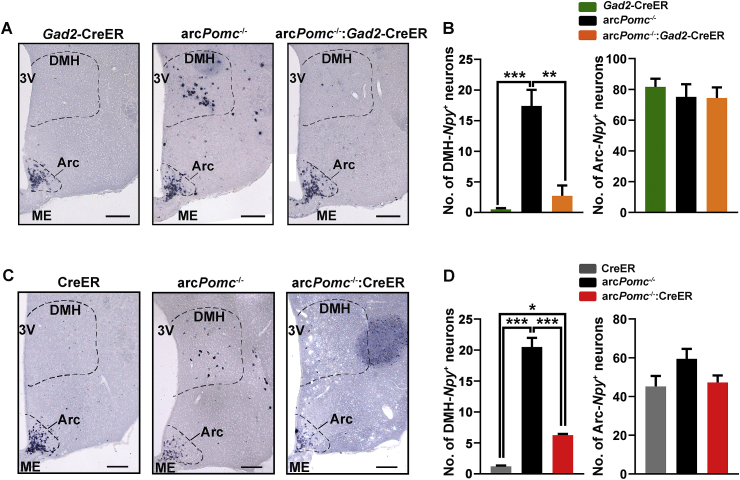
Since we found that the DMH is a major target site of GABAergic-POMC neurons and there is some evidence suggesting that DMH-NPY neurons induce food intake in different models of obese mice, including MC4-RKO [[28], [29], [30], [31]], we hypothesized that POMC expression restricted to GABAergic neurons could normalize food intake by inhibiting DMH-NPY expression. In order to study if there is a correlation between DMH-Npy expression and Arc-POMC expression, we performed in situ hybridization (ISH) for Npy in the brains of female mice previously treated with tamoxifen at P60 (Figure 5). As expected for normal-weight mice, Gad2-CreER mice showed very low expression in the DMH (Figure 5A,B). However, DMH-Npy expression raised more than 30 times in obese arcPomc−/− mice compared to Gad2-CreER siblings. Interestingly, DMH-Npy expression was reestablished by Pomc expression restricted to GABAergic-POMC neurons in arcPomc−/−:Gad2-CreER-rescued mice (Figure 5A,B).
Figure 5.
DMH-NPY expression is normalized after POMC expression restricted to GABAergic neurons. (A, C) Coronal brain sections of mice subjected to in situ hybridization, showing Npy mRNA (purple) in the dorsomedial hypothalamic nucleus (DMH) and arcuate nucleus (Arc) of the hypothalamus. 3V, third ventricle. ME: median eminence. Magnification bars: 200 μm. (B, D) Average of DMH-Npy and Arc-Npy neurons per hemisection for each genotype. Error bars: ±SEM. OWA: ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 (Bonferroni). N = 3–4.
Partial nonspecific Pomc rescue also led to DMH-Npy reduction (Figure 5C,D) and POMC-IRFs in the DMH of arcPomc−/−:CreER mice (Figure 4C). However, contrary to GABAergic-specific Pomc rescue (which leads to DMH-Npy normalization), the expression remains higher in arcPomc−/−:CreER mice compared to CreER controls (Figure 5D).
It is important to note that while the number of DMH-NPY neurons is increased in arcPomc−/− mice, the number of Arc-NPY neurons is not significantly altered (Figure 5B,D). This finding suggests a preponderant role for POMC in the regulation of DMH-NPY expression compared to Arc-NPY expression.
In order to characterize Arc-POMC→DMH projections, we performed a triple labeling experiment in coronal brain sections of wild-type mice. First, neurons projecting to the DMH were labeled by stereotactic guided injections of retrobeads (RBs) to the DMH of anesthetized mice (n=4). Two weeks later, POMC neurons were identified by immunohistochemistry and GABAergic neurons, by in situ hybridization with a Gad1 probe, in fixed coronal brain sections of injected mice (Figure 6A). Our results show that 16.5 ± 2.0% (mean ± SEM) of Arc-POMC neurons are RB+, which presumably project to the DMH. Notably, 74.9 ± 5.4% (mean ± SEM) of POMC+/RB+ neurons are Gad1+, revealing that most of Arc-POMC→DMH projections are GABAergic (Figure 6B–F). Altogether, these results support the hypothesis of an inhibitory tone of arcuate GABAergic-POMC neurons on DMH-NPY expression which may contribute to normal control of food intake.
4. Discussion
In the present study, we show that the subpopulation of arcuate GABAergic-POMC neurons is involved in body weight regulation. Moreover, we found that the DMH is a major target site of GABAergic-POMC neurons followed by the PVN. In addition, we found that DMH-NPY expression is highly increased in POMC-deficient mice and it can be restored by Pomc expression restricted to GABAergic-POMC neurons. Finally, we determined that Arc-POMC neurons projecting to the DMH are mainly GABAergic.
Two previous studies showed the physiological consequences of arcuate Pomc expression restricted to well-defined subpopulations. Burke et al. showed that congenital Pomc expression in the subpopulation of neurons expressing 5-hydroxytryptamine 2c receptor (5-HT2CR), which encompasses about 40% of POMC neurons, prevents hyperphagia in both sexes and obesity in male mice [48]. In another study, it was shown that constitutive Pomc expression restricted to neurons expressing the leptin receptor (50%–80% of POMC neurons) leads to normal food intake and body weight [49]. In these studies, Pomc expression was recovered in 40%–70% of POMC neurons leading to more than 40% of Pomc mRNA expression, which is consistent with previous data showing that there is a threshold of 30% of Pomc mRNA and 30% of POMC + neurons above which mice maintain normal food intake and body weight [50,51]. In this regard, here we show the first example of food intake normalization in adult mice with less than 25% of hypothalamic Pomc mRNA expression. It is important to note that, in the present work, we show improvement of obesity phenotype by Pomc rescue in postnatal life rather than prevention of hyperphagia and overweight by Pomc expression during embryological development. Moreover, by inducing Cre activity in adult mice, we bypassed the developmental stage in which shifts between glutamate and GABA expression can occur, as has been previously shown for POMC neurons [52,53]. Most importantly, we found that Pomc expression in only 23%–25% of POMC neurons restricted to the GABAergic subpopulation has a similar impact as the nonspecific rescue of Pomc in ∼75% of POMC neurons, in mice treated at P60 (compared to [11]).
Although it has been previously shown that Gad2+ POMC cells encompass ∼50% of Arc-POMC neurons [39,40], Gad2-CreER driver induced Pomc expression only in ∼23–25% of POMC neurons. Since we found similar results in Ai14:Gad2-CreER (only ∼26% of POMC neurons were dtTomato+), the incomplete Pomc recovery may be attributed to low Cre expression in the knock-in mouse line, or to incomplete tamoxifen bioavailability, or both. In either case, it is interesting to note that Pomc expression restricted to half of GABAergic-POMC neurons is enough to normalize food intake, while partial nonspecific POMC rescue in arcPomc−/−:CreER mice, with ∼32–35% of POMC neurons expressing Pomc, is not. These results suggest that GABAergic-POMC neurons have a key role in the regulation of food intake.
We previously found that Pomc recovery in arcPomc−/−:CreER at P25 completely prevented obesity [11]. Conversely, arcPomc−/−:Gad2-CreER mice treated at P25, despite showing lower body weight and food intake than arcPomc−/− mice, become overweight by the 9th week of age. However, since only half of GABAergic-POMC neurons recovered Pomc expression in these mice, we cannot exclude that this subpopulation could be sufficient to maintain normal body weight in wild-type mice. Moreover, the participation of glutamatergic-POMC neurons in the regulation of energy balance cannot be ruled out. In this regard, Jones et al. rescued Pomc expression in arcPomc−/− mice with a Vglut2-Cre driver constitutively expressed since the embryological developmental period [53]. However, they found Pomc expression also in GABAergic neurons, probably because of the postnatal shift from glutamate to GABAergic subpopulation.
Regarding glucose homeostasis, female arcPomc−/− and arcPomc−/−:Gad2-CreER mice show fasting hyperglycemia when they are 7 weeks old. Interestingly, only rescued arcPomc−/−:Gad2-CreER mice normalized glycemia after tamoxifen treatment. In addition, both female and male Pomc-deficient mice were intolerant to a glucose overload, a condition that was reverted by Pomc rescue. These results can be attributed not only to body weight loss but also to Pomc recovery in GABAergic-POMC neurons, since we previously showed that normal-weight arcPomc−/−:CreER female mice are intolerant to glucose overload and display decreased insulin sensitivity, both conditions that can be reverted by Pomc rescue [44].
Our results show that a major target site of GABAergic-POMC neurons is the DMH, which is known to be involved in the regulation of food intake and energy expenditure. In mice, DMH-Npy mRNA expression is only found in some models of genetic or high-fat diet-induced obesity and in mice subjected to chronic energy restriction. For example, Ay obese mice, which have an ectopic expression of the orexigenic agouti-signaling protein, show Npy mRNA expression in DMH [29]. It has been suggested that DMH-Npy mRNA expression may be caused by agouti inhibition of MC3/4R in DMH neurons. This hypothesis is supported by results showing that targeted disruption of the MC4-R gene leads to increased expression of Npy mRNA in the DMH [29]. Moreover, injection of MC3/4R-selective agonist melanotan II (MTII) in the DMH suppresses fasting or suckling-induced hyperphagia and suckling-induced Npy mRNA expression in the DMH of lactating rats [54]. Finally, α-MSH analog decreases food intake 1 h after injection in the DMH [55].
Although it is clear that MC3/4R stimulation inhibits DMH-Npy mRNA expression, it remained to be elucidated if Arc-POMC neurons mediate this regulation. Here, we show that obese arcPomc−/− mice exhibit highly increased DMH-Npy mRNA expression. We propose that in wild-type mice α-MSH and/or other neuropeptides derived from POMC are secreted by Arc-POMC neurons and, by interacting with postsynaptic receptors (either directly on DMH-NPY neurons or indirectly on other DMH-intermediate neurons), inhibit DMH-NPY expression assuring normal food intake and body weight (Figure 7, upper panel). Accordingly, Pomc deficiency in arcPomc−/− mice would lead to DMH-Npy mRNA overexpression which, in turn, would contribute to hyperphagia and obesity (Figure 7, middle panel). Finally, given that, as we found, ∼75% of Arc-POMC neurons projecting to the DMH are GABAergic, it is probable that the normalization of DMH-NPY expression after Pomc restoration in GABAergic neurons accounts for food intake normalization leading to body weight loss (Figure 7, lower panel). This model could also explain the reduction in DMH-Npy expression in partially rescued arcPomc−/−:CreER mice, since we expect ∼40% of neurons recovering POMC with the nonspecific driver to be GABAergic. However, we cannot exclude a contribution of non-GABAergic neurons to the inhibition of DMH-Npy expression either in these mice or in wild-type mice. In addition, the Arc-POMC → DMH-NPY inhibition may also be involved in the regulation of fasting-induced hyperphagia, which is also disrupted in arcPomc−/− mice and restored by Pomc rescue in GABAergic-POMC neurons. Although close appositions between α-MSH-immunoreactive fibers and DMH-NPY neurons were previously observed in female rats [54], it remains to be elucidated if Arc-POMC neurons synapse with DMH-NPY neurons in mice. Alternatively, Arc-POMC-derived peptides may exert an indirect inhibitory regulation of DMH-Npy mRNA expression. Although here we propose that food intake may be regulated by an Arc-GABAergic-POMC → DMH-NPY pathway, an Arc-GABAergic-POMC → PVN circuit may also be involved in the normalization of food intake in rescued mice. In this regard, we found POMC-IRFs in the PVN of rescued mice and it has been demonstrated that POMC projections in the PVN increase the activity of MC4R neurons leading to a decrease in food intake [56].
Figure 7.
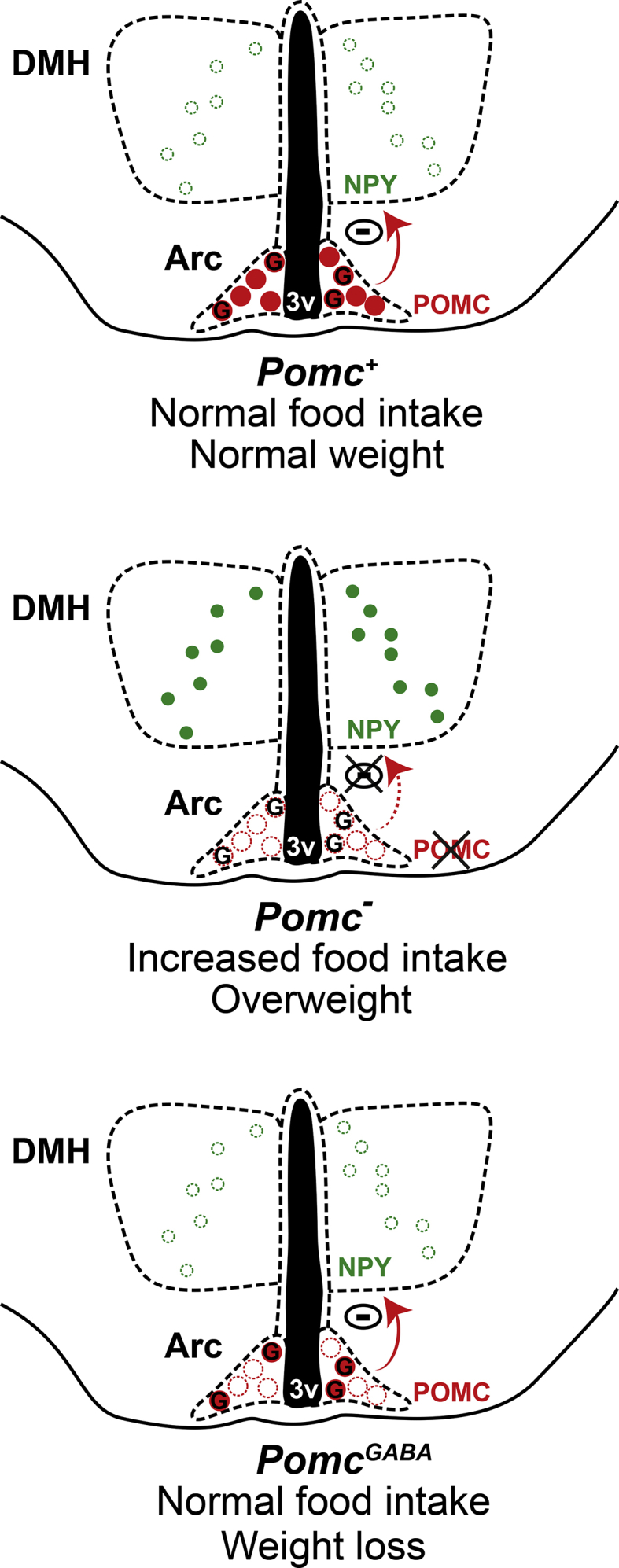
Model of an arcuate GABAergic-POMC → DMH-NPY circuit for the regulation of food intake. Upper panel: in wild-type mice (Pomc+), GABAergic-POMC neurons (red-filled circles with G) of the arcuate nucleus (Arc) suppress food intake by inhibiting NPY expression in dorsomedial hypothalamic nucleus (DMH) neurons (green open circles), maintaining a normal weight. Middle panel: in Pomc knockout mice (Pomc−), lack of Pomc expression in Arc neurons (red-open circles) results in DMH-NPY expression (green-filled circles) and increased food intake and obesity. Lower panel: Pomc expression restricted to GABAergic neurons (PomcGABA) recovers NPY inhibition resulting in normal food intake and weight loss.
In summary, our results show that the subpopulation of Arc-GABAergic-POMC neurons is a major regulator of food intake and body weight. Since Pomc reactivation in these neurons normalizes DMH-NPY expression and food intake, the circuit between Arc-POMC and DMH-NPY neurons could be a potential target for the treatment of obesity.
Authors’ contributions
VFB designed the study. MT, RA, EPB, MBT, and VFB performed the experiments. VFB, MR, EPB, and JLF contributed new reagents and analytic tools. MT, EPB, RA, MBT, and VFB analyzed the data. VB and MT wrote the paper. All authors revised and edited the manuscript and approved the final article.
Acknowledgments
We thank Dr. Juan Belforte, Dr. Lorena Rela, Dr. Gustavo Murer, Dr. Guillermo Lanuza, and Dr. Lucía Franchini for valuable mice and reagents; Jesica Unger, Verónica Risso, Analía López Díaz, and Germán N. La Iacona, for technical assistance; Dr. Mario C. Perelló and Pablo N. De Francesco, for technical advice; and Johnson&Johnson Medical S.A., Argentina, for donating glucometer strips. This work was supported in part by Agencia Nacional de Promoción Científica y Tecnológica (PICT2014-2000), Consejo Nacional de Investigaciones Científicas y Técnicas (PIP 2014–487), Universidad de Buenos Aires, Argentina, The Committee for Aid and Education in Neurochemistry (CAEN-ISN), 2015–2016, and NARSAD Young Investigator Grant Award from Brain and Behavior Research Foundation (#23983, EPB), The Pew Charitable Trusts Repatriation Grant (EPB), Mario Hirsch from Fundación Bunge y Born/Fundación Williams (EPB), and The Spanish Ministry of Science and Innovation and European Regional Fund (MICINN and FEDER-PGC2018-098229-B-100, JLF). This study was awarded by the Fundación Gador (2017) and Fundación Florencio Fiorini (2018).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2020.100985.
Contributor Information
Milagros Trotta, Email: mili.trotta@gmail.com.
Estefanía Pilar Bello, Email: estefania.bello@gmail.com.
Ramiro Alsina, Email: ramiroalsina@gmail.com.
María Belén Tavella, Email: belentavella@gmail.com.
José Luis Ferrán, Email: jlferran@um.es.
Marcelo Rubinstein, Email: mrubins@dna.uba.ar.
Viviana Florencia Bumaschny, Email: vbumaschny@fmed.uba.ar.
Conflict of interest
None.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Berthoud H.R., Münzberg H., Morrison C.D. Blaming the brain for obesity: integration of hedonic and homeostatic mechanisms. Gastroenterology. 2017 doi: 10.1053/j.gastro.2016.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waterson M.J., Horvath T.L. Neuronal regulation of energy homeostasis: beyond the hypothalamus and feeding. Cell Metabolism. 2015;22(6):962–970. doi: 10.1016/j.cmet.2015.09.026. S1550-4131(15)00483-0 [pii] [DOI] [PubMed] [Google Scholar]
- 3.Campbell J.N., Macosko E.Z., Fenselau H., Pers T.H., Lyubetskaya A., Tenen D. A molecular census of arcuate hypothalamus and median eminence cell types. Nature Neuroscience. 2017 doi: 10.1038/nn.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen R., Wu X., Jiang L., Zhang Y. Single-cell RNA-seq reveals hypothalamic cell diversity. Cell Reports. 2017;18(13):3227–3241. doi: 10.1016/j.celrep.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lam B.Y.H., Cimino I., Polex-Wolf J., Nicole Kohnke S., Rimmington D., Iyemere V. Heterogeneity of hypothalamic pro-opiomelanocortin-expressing neurons revealed by single-cell RNA sequencing. Molecular Metabolism. 2017 doi: 10.1016/j.molmet.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cawley N.X., Li Z., Loh Y.P. Biosynthesis, trafficking, and secretion of pro-opiomelanocortin-derived peptides. Journal of Molecular Endocrinology. 2016 doi: 10.1530/JME-15-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biebermann H., Castaneda T.R., van Landeghem F., von Deimling A., Escher F., Brabant G. A role for beta-melanocyte-stimulating hormone in human body-weight regulation. Cell Metabolism. 2006;3(2):141–146. doi: 10.1016/j.cmet.2006.01.007. S1550-4131(06)00031-3 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Krude H., Biebermann H., Luck W., Horn R., Brabant G., Gruters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nature Genetics. 1998;19(2):155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 9.Yaswen L., Diehl N., Brennan M.B., Hochgeschwender U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Natura Med. 1999;5(9):1066–1070. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]
- 10.Yeo G.S., Farooqi I.S., Aminian S., Halsall D.J., Stanhope R.G., O'Rahilly S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nature Genetics. 1998;20(2):111–112. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]
- 11.Bumaschny V.F., Yamashita M., Casas-Cordero R., Otero-Corchón V., De Souza F.S.J., Rubinstein M. Obesity-programmed mice are rescued by early genetic intervention. Journal of Clinical Investigation. 2012;122(11):4203–4212. doi: 10.1172/JCI62543. 62543 [pii] 10.1172/JCI62543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huszar D., Lynch C.A., Fairchild-Huntress V., Dunmore J.H., Fang Q., Berkemeier L.R. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997 doi: 10.1016/S0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 13.Challis B.G., Coll A.P., Yeo G.S., Pinnock S.B., Dickson S.L., Thresher R.R. Mice lacking pro-opiomelanocortin are sensitive to high-fat feeding but respond normally to the acute anorectic effects of peptide-YY(3-36) Proceedings of the National Academy of Sciences of the U S A. 2004;101(13):4695–4700. doi: 10.1073/pnas.0306931101. 0306931101 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cakir I., Nillni E.A. Endoplasmic reticulum stress, the hypothalamus, and energy balance. Trends in Endocrinology and Metabolism. 2019 doi: 10.1016/j.tem.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Araujo E., Moraes J., Cintra D., Velloso L. Mechanisms IN endocrinology: hypothalamic inflammation and nutrition. European Journal of Endocrinology. 2016;175(3):R97–R105. doi: 10.1530/EJE-15-1207. [DOI] [PubMed] [Google Scholar]
- 16.Schneeberger M., Dietrich M.O., Sebastián D., Imbernón M., Castaño C., Garcia A. Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance. Cell. 2013 doi: 10.1016/j.cell.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D'Agostino G., Lyons D., Cristiano C., Lettieri M., Olarte-Sanchez C., Burke L.K. Nucleus of the solitary tract serotonin 5-HT2C receptors modulate food intake. Cell Metabolism. 2018 doi: 10.1016/j.cmet.2018.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burke L.K., Doslikova B., D'Agostino G., Garfield A.S., Farooq G., Burdakov D. 5-HT obesity medication efficacy via POMC activation is maintained during aging. Endocrinology. 2014 doi: 10.1210/en.2014-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Secher A., Jelsing J., Baquero A.F., Hecksher-Sorensen J., Cowley M.A., Dalboge L.S. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. Journal of Clinical Investigation. 2014;124(10):4473–4488. doi: 10.1172/JCI75276. 75276 [pii] 10.1172/JCI75276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kühnen P., Clément K., Wiegand S., Blankenstein O., Gottesdiener K., Martini L.L. Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. New England Journal of Medicine. 2016 doi: 10.1056/NEJMoa1512693. [DOI] [PubMed] [Google Scholar]
- 21.Zhou L., Sutton G.M., Rochford J.J., Semple R.K., Lam D.D., Oksanen L.J. Serotonin 2C receptor agonists improve type 2 diabetes via melanocortin-4 receptor signaling pathways. Cell Metabolism. 2007;6(5):398–405. doi: 10.1016/J.CMET.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doslikova, B., Garfield, A.S., Shaw, J., Evans, M.L., Burdakov, D., Billups, B., et al, n.d. 5-HT2C receptor agonist anorectic efficacy potentiated by 5-HT1B receptor agonist coapplication: an effect mediated via increased proportion of pro-opiomelanocortin neurons activated. Journal of Neuroscience 33(23): 9800–9804, Doi: 33/23/9800 [pii] 10.1523/JNEUROSCI.4326-12.2013. [DOI] [PMC free article] [PubMed]
- 23.O'Donohue T.L., Miller R.L., Jacobowitz D.M. Identification, characterization and stereotaxic mapping of intraneuronal alpha-melanocyte stimulating hormone-like immunoreactive peptides in discrete regions of the rat brain. Brain Research. 1979;176(1):101–123. doi: 10.1016/0006-8993(79)90873-4. 0006-8993(79)90873-4 [pii] [DOI] [PubMed] [Google Scholar]
- 24.Bagnol D., Lu X.Y., Kaelin C.B., Day H.E., Ollmann M., Gantz I. Anatomy of an endogenous antagonist: relationship between Agouti-related protein and proopiomelanocortin in brain. Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1999;19(August 2016):RC26. doi: 10.1523/JNEUROSCI.19-18-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King C.M., Hentges S.T. Relative number and distribution of Murine hypothalamic proopiomelanocortin neurons innervating distinct target sites. PloS One. 2011;6(10) doi: 10.1371/journal.pone.0025864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Branch A., Shen P. CRC Press; 2017. Appetite and food intake. [Google Scholar]
- 27.Bellinger L.L., Williams F.E. Aphagia and adipsia after kainic acid lesioning of the dorsomedial hypothalamic area. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2017 doi: 10.1152/ajpregu.1983.244.3.r389. [DOI] [PubMed] [Google Scholar]
- 28.Li C., Chen P., Smith M.S. The acute suckling stimulus induces expression of neuropeptide Y (NPY) in cells in the dorsomedial hypothalamus and increases NPY expression in the arcuate nucleus. Endocrinology. 1998 doi: 10.1210/endo.139.4.5905. [DOI] [PubMed] [Google Scholar]
- 29.Kesterson R.A., Huszar D., Lynch C.A., Simerly R.B., Cone R.D. Induction of neuropeptide Y gene expression in the dorsal medial hypothalamic nucleus in two models of the agouti obesity syndrome. Molecular Endocrinology. 1997;11(5):630–637. doi: 10.1210/mend.11.5.9921. [DOI] [PubMed] [Google Scholar]
- 30.Guan X.-M., Yu H., Van der Ploeg L.H. Evidence of altered hypothalamic pro-opiomelanocortin/neuropeptide Y mRNA expression in tubby mice. Molecular Brain Research. 1998;59(2):273–279. doi: 10.1016/S0169-328X(98)00150-8. [DOI] [PubMed] [Google Scholar]
- 31.Guan X.M., Yu H., Trumbauer M., Frazier E., Van Der Ploeg L.H.T., Chen H. Induction of neuropeptide Y expression in dorsomedial hypothalamus of diet-induced obese mice. NeuroReport. 1998 doi: 10.1097/00001756-199810260-00015. [DOI] [PubMed] [Google Scholar]
- 32.Grove K.L., Smith M.S. Ontogeny of the hypothalamic neuropeptide Y system. Physiology & Behavior. 2003;79(1):47–63. doi: 10.1016/s0031-9384(03)00104-5. [DOI] [PubMed] [Google Scholar]
- 33.Sohn J.W., Xu Y., Jones J.E., Wickman K., Williams K.W., Elmquist J.K. Serotonin 2C receptor activates a distinct population of arcuate pro-opiomelanocortin neurons via TRPC channels. Neuron. 2011;71(3):488–497. doi: 10.1016/j.neuron.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams K.W., Margatho L.O., Lee C.E., Choi M., Lee S., Scott M.M. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. Journal of Neuroscience. 2010;30(7):2472–2479. doi: 10.1523/JNEUROSCI.3118-09.2010. 30/7/2472 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu J., Jiang L., Low M.J., Rui L. Glucose rapidly induces different forms of excitatory synaptic plasticity in hypothalamic POMC neurons. PloS One. 2014 doi: 10.1371/journal.pone.0105080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hentges S.T., Nishiyama M., Overstreet L.S., Stenzel-Poore M., Williams J.T., Low M.J. GABA release from proopiomelanocortin neurons. Journal of Neuroscience. 2004;24(7):1578–1583. doi: 10.1523/JNEUROSCI.3952-03.2004. 24/7/1578 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hentges S.T., Otero-Corchon V., Pennock R.L., King C.M., Low M.J. Proopiomelanocortin expression in both GABA and glutamate neurons. Journal of Neuroscience. 2009;29(43):13684–13690. doi: 10.1523/JNEUROSCI.3770-09. 29/43/13684 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dicken M.S., Tooker R.E., Hentges S.T. Regulation of GABA and glutamate release from proopiomelanocortin neuron terminals in intact hypothalamic networks. Journal of Neuroscience. 2012;32(12):4042–4048. doi: 10.1523/JNEUROSCI.6032-11.2012. 32/12/4042 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jarvie B.C., Hentges S.T. Expression of GABAergic and glutamatergic phenotypic markers in hypothalamic proopiomelanocortin neurons. The Journal of Comparative Neurology. 2012;520(17):3863–3876. doi: 10.1002/cne.23127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wittmann G., Hrabovszky E., Lechan R.M. Distinct glutamatergic and GABAergic subsets of hypothalamic pro-opiomelanocortin neurons revealed by in situ hybridization in male rats and mice. The Journal of Comparative Neurology. 2013;521(14):3287–3302. doi: 10.1002/cne.23350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taniguchi H., He M., Wu P., Kim S., Paik R., Sugino K. A resource of cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71(6):995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madisen L., Zwingman T.A., Sunkin S.M., Oh S.W., Zariwala H.A., Gu H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nature Neuroscience. 2010;13(1):133–140. doi: 10.1038/nn.2467. nn.2467 [pii] 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Metzger D., Chambon P. Site- and time-specific gene targeting in the mouse. Methods. 2001;24(1):71–80. doi: 10.1006/meth.2001.1159. S1046-2023(01)91159-4 [pii] [DOI] [PubMed] [Google Scholar]
- 44.Alsina R., Trotta M., Bumaschny V.F. Hypothalamic proopiomelanocortin is necessary for normal glucose homeostasis in female mice. Frontiers in Endocrinology. 2018;9:554. doi: 10.3389/fendo.2018.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. S1046-2023(01)91262-9 [pii] [DOI] [PubMed] [Google Scholar]
- 46.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T. Fiji: an open-source platform for biological-image analysis. Nature Methods. 2012 doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hauptmann G. 2015. In situ hybridization methods. [Google Scholar]
- 48.Burke L.K., Doslikova B., D'Agostino G., Greenwald-Yarnell M., Georgescu T., Chianese R. Sex difference in physical activity, energy expenditure and obesity driven by a subpopulation of hypothalamic POMC neurons. Molecular Metabolism. 2016;5(3):245–252. doi: 10.1016/j.molmet.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lam D.D., Attard C.A., Mercer A.J., Myers M.G., Rubinstein M., Low M.J. Conditional expression of Pomc in the Lepr-positive subpopulation of POMC neurons is sufficient for normal energy homeostasis and metabolism. Endocrinology. 2015;156(4):1292–1302. doi: 10.1210/en.2014-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lam D.D., de Souza F.S.J., Nasif S., Yamashita M., López-Leal R., Otero-Corchon V. Partially redundant enhancers cooperatively maintain mammalian Pomc expression above a critical functional threshold. PLoS Genetics. 2015;11(2):1–21. doi: 10.1371/journal.pgen.1004935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhan C., Zhou J., Feng Q., Zhang J. -e., Lin S., Bao J. Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. Journal of Neuroscience. 2013 doi: 10.1523/jneurosci.2742-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dennison C.S., King C.M., Dicken M.S., Hentges S.T. Age-dependent changes in amino acid phenotype and the role of glutamate release from hypothalamic proopiomelanocortin neurons. Journal of Comparative Neurology. 2016;524(6):1222–1235. doi: 10.1002/cne.23900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones G.L., Wittmann G., Yokosawa E.B., Yu H., Mercer A.J., Lechan R.M. Selective restoration of Pomc expression in glutamatergic POMC neurons: evidence for a dynamic hypothalamic neurotransmitter network. Eneuro. 2019 doi: 10.1523/eneuro.0400-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen P., Williams S.M., Grove K.L., Smith M.S. Melanocortin 4 receptor-mediated hyperphagia and activation of neuropeptide Y expression in the dorsomedial hypothalamus during lactation. Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2004;24(22):5091–5100. doi: 10.1523/JNEUROSCI.0588-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim M.S., Rossi M., Abusnana S., Sunter D., Morgan D.G.A., Small C.J. Hypothalamic localization of the feeding effect of agouti-related peptide and α-melanocyte-stimulating hormone. Diabetes. 2000 doi: 10.2337/diabetes.49.2.177. [DOI] [PubMed] [Google Scholar]
- 56.Baldini G., Phelan K.D. The melanocortin pathway and control of appetite-progress and therapeutic implications. Journal of Endocrinology. 2019 doi: 10.1530/JOE-18-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paxinos G., Franklin K.B.J. 2nd ed. 2001. The mouse brain in stereotaxic coordinates. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.