Abstract
Purpose
Drug utilization studies have proven to be an effective tool in the evaluation of rational drug use in different health care systems, including oncology. The drug utilization studies were used in many institutes to ensure the safe, effective and appropriate use of drugs being prescribed. The main aim of this study was to assess the utilization pattern of anticancer drugs in breast cancer patients.
Method
A retrospective cross-sectional observational study was carried out at King Saud University Medical City (KSUMC) for 1 year from January 2016 to December 2016. All female patients diagnosed with breast cancer during this year were included in the study.
Results
A total of 101 patients were included in this study. Most patients received an average of three anticancer drugs. The most commonly prescribed medication was fluorouracil, epirubicin, and cyclophosphamide (FEC) regimen, which was used in 81% of patients. Combinations of FEC + docetaxel and FEC + docetaxel + trastuzumab were received by43% and 23% of patients, respectively. Docetaxel was the most commonly used drug in neoadjuvant setting, whereas letrozole and trastuzumab were prescribed more frequently in hormonal and targeted therapies, respectively. The total drug expenditure on anticancer therapy was approximately 3.8 million Saudi Riyals (S.R), with adjuvant therapy constituting over half of the total spending. In neoadjuvant settings, the spending cost for hormonal therapy was the highest. The condition of most breast cancer patients was improved during the study period, whereas only 29% of the included patients progressed.
Conclusion
FEC was the most common regimen used in this study, consistent with the National Comprehensive Cancer Network (NCCN) guideline recommendation. Our results indicated that adherence to a clinical guideline and recommended medication regimens improved patient outcomes. Our finding indicate how analyzing drug utilization pattern could benefit institutions in managing inventory and efficiently using health care resources.
Keywords: Drug utilization review, Oncology, Breast cancer, Anticancer drugs
1. Introduction
Breast cancer is defined as uncontrolled growth of malignant cells in mammary epithelial tissues (Waks and Winer, 2019). Globally, breast cancer is the most frequently occurring cancer in women, and its incidence considerably increases with age (Waks and Winer, 2019). According to the latest report from the International Agency for Research on Cancer at World Health Organization (WHO), breast cancer is the fifth leading cause of cancer-related death, causing an overall of 627,000 deaths among women in 2018 (Rindi, 2018).
In Saudi Arabia, breast cancer ranked first among females, it is accounted for almost 16.7% of all cancer and 30.1% of all newly diagnosed cases among women according to the last reported data from the Saudi Cancer Registry (Cancer Incidence Report Saudi Arabia, 2015). The age-standardized incidence rate for breat cancer in 2014 was 22.7/100,000 for female population. The number of breast cancer has been increase among Saudi women over the last decades with the incidence rates ranged between three to eight confirmed cases of breast cancer for every 1000 patients (Asiri, 2020). The breast cancer cases where projected to continue to rise rapidly given the aging population (Ahmed et al., 2019) and reach 118,337 cases in 2025 and over 200,000 cases in 2050 (Alattas, 2019)
It has been reported that breast cancer is one of the leading causes of mortality among Saudi women, and it was estimated to cause more than 18% of total deaths annually (WHO, 2014).
Multiple factors are associated with an increased risk of breast cancer, including family history, obesity, consumption of processed foods, physical inactivity, delayed childbearing, having fewer children, earlier age at menarche, and shorter duration of breastfeeding (Lakhani et al., 2002, Lukong, 2017). Management of patients with breast cancer has been substantially advanced over the past years, which positively affects patient care and their quality of life. Anticancer drugs have been well-documented to improve patients’ outcome, although these drugs are associated with toxicity; thus, rational use of these medications is imperative. Rational use of drugs may be defined as receiving medications with appropriate indication and regimen, and at the lowest cost (Introduction to drug utilization research., 2003). Nevertheless, irrational use of drugs may lead to negative consequences, including ineffective treatment, unnecessary prescription of drugs, development of resistance to chemotherapeutic agents, adverse effects, and economic burden on both patients and hospitals. One approach to ensure rational drug use is drug utilization evaluation (DUE). DUE is as a systemic evaluation of drug usage that will help ensure rational use of medications at the individual patient level, particularly for medical, social, and economic consequences (Introduction to drug utilization research., 2003). DUE have been used globally to support universal access to medicines. Analysis of medicines utilization and expenditure can inform healthcare decision maker about the overall medication utilization by gender, comorbidities and age group. It also can inform about which medication were contributing to highest budget spending. Additionally, it can be useful to evaluate whether medicine been over or under prescribe and whether it aligns with the treatment protocol and guideline and also to see the impact of policy change on the use of medicine. Action is often following the DUE and can include change listing of the drug or restriction it used, and may rise a need for more awareness and educational program (Organization, 2018).
In Saudi Arabia, there is limited evidence of the pattern of anticancer drug use for breast cancer patients, and of the relationship between drug use and clinical outcome. DUE may help us observe the prescribing pattern of chemotherapeutic agents for breast cancer, and early signals of irrational drug use, thus providing intervention tools to improve drug use and realizing continuous improvement of treatment quality.
The primary objective of this study was to conduct a DUE of the anticancer drugs used in the treatment of breast cancer in King Saud University Medical City (KSUMC), a tertiary care teaching hospital in Riyadh, Saudi Arabia. The secondary objectives were to assess the prescribing pattern of anticancer drugs and estimate the total drug costs. The findings of this study may help decision makers to optimally use medical resources and improve the hospital formulary management.
2. Methods
2.1. Study design
A retrospective cross-sectional study was carried out at KSUMC. The study was conducted over a period of one year from 01/01/2016 to 31/12/2016. All female patients diagnosed with breast cancer were identified from the electronic medical records (EHR) of KSUMC.
2.2. Ethical consideration
This study was approved by the Institutional Ethics Committee of KSUMC (IRB # E-17-2328).
2.3. Study population
Female patients aged ≥18 years diagnosed with breast cancer as first occurring primary cancer type who received at least one treatment were included in this study. Female patients aged <18 years who were diagnosed with breast cancer and underwent surgery and radiotherapy were excluded.
2.4. Data collection
Patients’ data were retrieved from the EHR database. The extracted information included demographic data (age, height, weight, body surface Index, body mass index, marital status, family history of breast cancer, and patient’s history of other types of cancer), patient’s comorbidities, medications, date of breast cancer diagnosis, breast cancer stage, chemotherapeutic regimens, type of cancer treatment (adjuvant/metastasis), chemotherapeutic medications (drugs, doses, route of administration, and duration of treatment).
2.5. Statistical analysis
An Excel-based tool (Microsoft® Excel; Microsoft, USA Version 2018) was used for systematic data sampling and analysis.
Descriptive statistics (i.e., means and frequencies) were conducted to analyze patients’ demographic characteristics, clinical variables, study outcomes, and other variables. DUR was calculated based on individual drug and medication classes. The unit of measurement were included dispensed drug counts, volume and costs. The total drug spending was calculated as a sum of all drugs utilized during study period. The study results were presented in percentages and visualized in tables, pie charts, and bar graphs.
3. Results
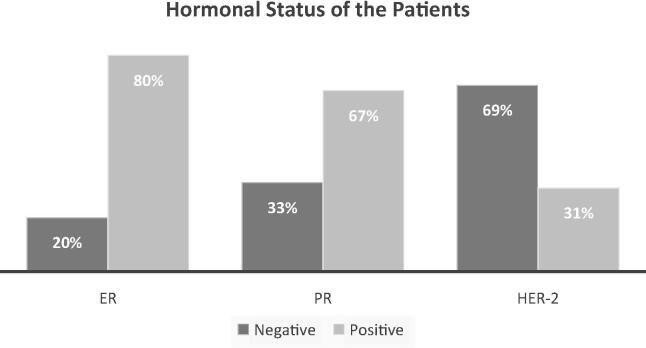
The records of a total of 122 patients were reviewed in the EHR database. Of these patients, 101 patients met our inclusion criteria. Subjects were excluded if they had other types of cancer or underwent surgery or radiation therapy. Patient characteristics are described in Table 1. The mean age was 53 years, and 75% of patients were married. More than 60% of the included patients were diagnosed at the early stages of breast cancer, i.e. stage I and II. The estrogen receptor (ER) and progesterone receptor (PR) were positive in 80% and 67% of the patients, respectively. While, HER-2 was overexpressed in 31% of the patients (Fig. 1). Utilization of anticancer drug as adjuvant and Neoadjuvant therapy in the treatment of patient with breast cancer were described in Table 2.
Table 1.
Patients Characteristics.
| Variable | n (%) |
|---|---|
| Age (mean, SD) | 53.3 years (±13.8) |
| Weight (mean, SD) | 72.8 kg (±14.7) |
| Marital status | |
| Married | 77 (76.24) |
| Single | 15 (14.85) |
| Other | 9 (8.91) |
| Menopausal status | |
| Post | 55 (54.46) |
| Pre | 36 (35.64) |
| NA | 10 (9.9) |
| Family history of breast cancer | 22 (22.22) |
| Patient’s history of other types of cancer | 6 (6.06) |
| Breast cancer stage | |
| Stage I | 27 (26.7) |
| Stage II | 35 (34.6) |
| Stage III | 10 (9.9) |
| Stage IV | 19 (18.8) |
| NA | 10 (9.9) |
NA, not available.
Fig. 1.
Estrogen receptor (ER), progesterone receptor (PR), and HER-2 overexpression status in the study population.
Table 2.
Utilization of Anticancer Medications in Neoadjuvant and Adjuvant Settings.
| Anticancer therapy | Agent | Patient count | Percentage | Anticancer therapy | Agent | Patient count | Percentage |
|---|---|---|---|---|---|---|---|
| Neoadjuvant Setting | Adjuvant setting | ||||||
| Chemotherapy | Docetaxel | 43 | 21% | Chemotherapy | Cyclophosphamide | 20 | 13% |
| Cyclophosphamide | 39 | 19% | Docetaxel | 16 | 10% | ||
| Epirubicin | 37 | 18% | Fluorouracil | 14 | 9% | ||
| Fluorouracil | 37 | 18% | Epirubicin | 13 | 8% | ||
| Carboplatin | 2 | 1% | Doxorubicin | 3 | 2% | ||
| Doxorubicin | 2 | 1% | Cisplatin | 2 | 1% | ||
| Paclitaxel | 2 | 1% | Vinorelbine | 2 | 1% | ||
| Capecitabine | 1 | 0% | |||||
| Cisplatin | 1 | 0% | |||||
| Vinorelbine | 1 | 0% | |||||
| Hormonal therapy | Letrozole | 10 | 5% | Hormonal therapy | Letrozole | 26 | 17% |
| Tamoxifen | 7 | 3% | Tamoxifen | 22 | 14% | ||
| Fulvestrant | 4 | 2% | Fulvestrant | 1 | 1% | ||
| Goserelin | 1 | 0% | Goserelin | 1 | 1% | ||
| Trastuzumab | 20 | 10% | |||||
| Targeted therapy | Pertuzumab | 1 | 0% | Targeted therapy | Trastuzumab | 16 | 10% |
| Bevacizumab | 1 | 0% | |||||
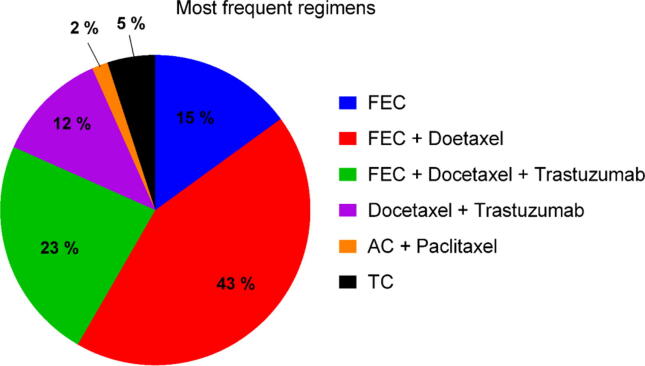
The average number of anticancer drugs received per patient was three. The most commonly prescribed regimen, which was used in 81% of patients, was a combination of fluorouracil, epirubicin, and cyclophosphamide (FEC), which was used with or without docetaxel and trastuzumab in HER-2 positive patients. Combinations of FEC + docetaxel and FEC + docetaxel + trastuzumab were received by 43% and 23% of patients, respectively. The rest 34% of the patients received other regimens, such as docetaxel + trastuzumab, taxane and cyclophosphamide, or anthracycline and cyclophosphamide + paclitaxel (Fig. 2).
Fig. 2.
Various anticancer regimens used in breast cancer patients. AC, anthracycline and cyclophosphamide; FEC, fluorouracil, epirubicin, and cyclophosphamide; TC, taxane and cyclophosphamide.
3.1. Anticancer drug spending
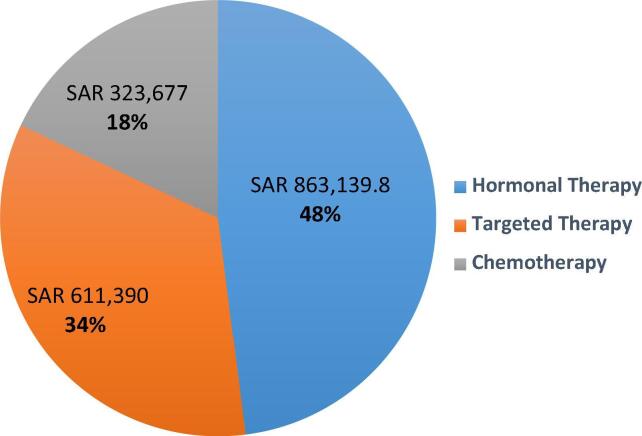
Our sudy indicate that the annual drug expenditure on anticancer therapy was approximately 3.8 million Saudi Riyals. Total spending on adjuvant therapy was 2.1 milllion which accounted for 53% of the total spending. Docetaxel was the most commonly used drug in neoadjuvant setting, with 18% of the patients receiving docetaxel. Letrozole and trastuzumab were the most commonly prescribed drugs in hormonal and targeted therapies, respectively Table 2. In neoadjuvant settings, hormonal therapy showed the highest spending cost (48%), followed by targeted therapy (34%) and chemotherapy (18%) [Fig. 3]. Moreover, although fulvestrant was prescribed only to 2% of patients, it accounted for 44% of the total neoadjuvant drug spending.
Fig. 3.
Anticancer medication spending by group in neoadjuvant setting (total spending = SAR 1.8 million).
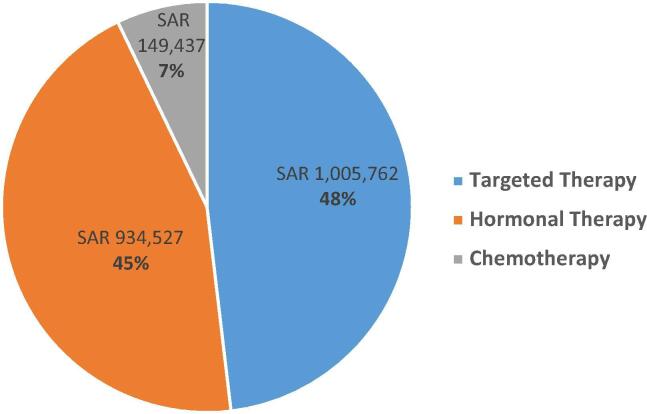
In adjuvant settings, cyclophosphamide and trastuzumab were the most commonly used drugs in chemotherapy and targeted therapy. Targeted therapy showed the highest spending cost (48%), followed by hormonal therapy (45%). Only 7% of the total spending cost was for chemotherapy [Fig. 4].
Fig. 4.
Anticancer medication spending by group in adjuvant setting (total spending SAR 2.09 million).
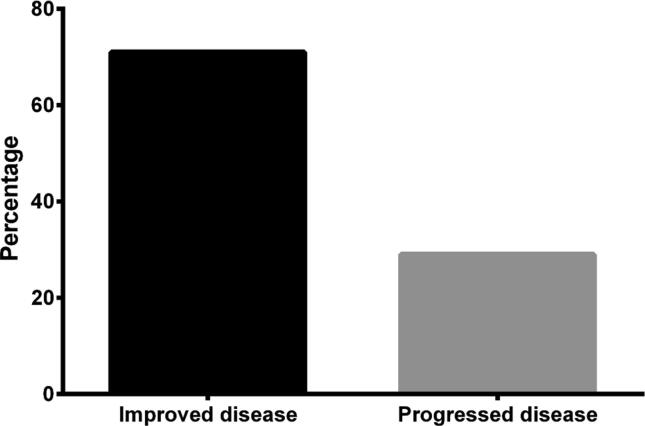
The frequencies of breast cancer progression and improvement were examined, and the results are shown in Fig. 5. A total of 71% of the included patients showed improvement of breast cancer, whereas the other 29% progressed.
Fig. 5.
Breast cancer treatment outcomes.
4. Discussion
Breast cancer, one of the most common neoplasms in women, is a leading cause of cancer-related deaths in our region and worldwide, and responsible for a large portion of medical expenditure for cancer patients (Bray, 2012). Breast cancer incidence increases proportionally with age (WHO, 2014, Saudi Cancer Registry. Cancer incidence reports., 2015). The average age of the participant of this study was 53.3 years, which was slightly higher than the range of 40 to 50 years reported in previous studies (Maxwell Ogochukwu Adibe and Ma’aji, 2019, Vinodkumar Mugada and Munagala, 2016). Most participants in this study had stage 1 or 2 breast cancer, and this finding eliminated the possibility of late presentation for diagnosis. However, further investigation is required to reveal the real reason for this difference. Treatment of patients with breast cancer required extensive use of medications and resources. Irrational use of drugs is a common problem in many health care systems, potentially leading to poor patient outcomes and increased treatment costs and resources. WHO promotes drug utilization studies in every health care system to assess and assure rational drug use (Dukes and Organization, 1993, Bachhav and Kshirsagar, 2015). DUE is considered an important tool in health care systems, as it can be used to provide information to identify drug use problems, ensure standard drug therapy, monitor drug expenses, assess drug effectiveness (Introduction to drug utilization research., 2003).
Several studies have evaluated the rational use of anticancer medications. Adebayo A. Onitilo, et al conducted a retrospective study including 2907 women aged ≥18 years diagnosed with invasive breast cancer from four institutions across the United States to examine the utilization of neoadjuvant chemotherapy (NAC) (Onitilo, 2013). The aim was to assess NAC use and its association with pre-operatively identified clinical factors, surgical approach, and breast conservation therapy failure (Onitilo, 2013). Of these patients, 111 out of 2907 (3.8%) patients were treated with NAC (Onitilo, 2013). Younger age, pre-operatively known positive nodal status, and increasing clinical tumor size were found to be associated with NAC use (Onitilo, 2013). NAC use in this study was infrequent, which might be underutilized in eligible patients desiring breast conservation, and varied among institutions (Onitilo, 2013). Accordingly, the principal aim of our current study is to examine the pattern and expenditure of anticancer drugs in Saudi Arabia and compare our results with those of other studies in the literature.
In the present study, the average number of anticancer medications prescribed per patient were three and the most frequently used regimen was FEC, consistent with the current NCCN guidelines (REF), which recommend FEC as a first line treatment for breast cancer. This number of medication was also in agreement with that reported in a study by Dave DJ, et al, but higher than that in other studies, which showed an average of 1.93–1.97 anticancer drug prescribed per patient (Maxwell Ogochukwu Adibe and Ma’aji, 2019, Vinodkumar Mugada and Munagala, 2016, Dave et al., 2014). In addition, this study reported that FEC regimen was used in 80% of the total regimen prescribed, similar to a previous study (Dave et al., 2014). This regimen has been studied for its efficacy and safety, and was found to provide good survival benefit (Dave et al., 2014, Moura Silva et al., 2006). Many DUEs conducted for breast cancer were limited to specific anticancer treatments, i.e. chemotherapy, specific breast cancer stage, or specific type of neoadjuvant therapy (Onitilo, 2013, Kulkarni et al., 2014). As a wide range of chemotherapeutic agents have been extensively used to treat cancer at different stages, this study was designed to evaluate all anticancer therapies: chemotherapy, hormonal therapy, and targeted therapy for breast cancer patients. In addition, adjuvant and neoadjuvant therapies have been evaluated in patients with different breast cancer stages.
The most commonly prescribed anticancer drug in our study was chemotherapy in neoadjuvant setting, with docetaxel (21%), cyclophosphamide (19%), 5-Fluorouracil (18%), and epirubicin (18%) as the most frequently used anticancer drugs. In addition, hormonal therapies such as letrozole (17%) and tamoxifen (14%) were the most common anticancer drugs in adjuvant setting. Different studies showed different prescribing patterns of anticancer drugs. Cisplatin followed by 5-fluorouracil has been reported as the most utilized anticancer treatment in two different studies, (Maxwell Ogochukwu Adibe and Ma’aji, 2019, Vinodkumar Mugada and Munagala, 2016) whereas carboplatin followed by paclitaxel was the most utilized regimen in other studies (Kulkarni et al., 2014). The difference in drug utilization between the studies may be attributed to the used management guidelines, cancer stage, and patient status.
The high incidence of cancer is associated with increasing total health care cost. The Saudi government provides treatment free of charge for cancer patients in public hospitals. Hence, expenditure analysis is highly demanded to ensure good utilization of an institution’s budget. The total annual spending on anticancer drugs was 3.8 million. In this study, analysis of cost distribution of different anticancer drug categories showed that hormonal therapy represented more than half of the total annual spending (51%), but showed minimal cost distribution in other studies (Kumar et al., 2018, Jakupi et al., 2018). Trastuzumab alone in all settings accounted for 41.5% of the total annual cost, representing the highest cost among the drugs. This finding was consistent with that of another study (Kumar et al., 2018, Jakupi et al., 2018). In neoadjuvant setting, hormonal therapy accounted for 50% of the total cost, whereas targeted therapy accounted for 48%. Comparing the cost distribution of international drug locally and internationally may result in differences due to population; availability of products, biosimilars and generics; and clinical guidelines (Kumar et al., 2018).
This study provided a guide on the effectiveness of regimens, as the condition of many patients was improved and only few (21%) progressed. Moreover, with the availability of generics and biosimilars in the near future, medical costs may be much lower. This finding contributed to encourage health care providers to adhere to clinical guidelines.
5. Study limitation
One of the limitations of this study was that the expenditure analysis was conducted using the prices available at the Saudi Food and Drug Authority website, which are often higher than the actual prices. However, this information is impossible to reveal to public. Missing data from the electronic records were another limitation of this retrospective study. In addition, this was a retrospective study conducted in single center which may affect the generalizability of this study to other institutes.
6. Conclusion
FEC was the most common regimen received by women with breast cancer in this study, consistent with the guideline recommendation. This finding indicated that adherence to clinical guideline improved patient outcomes and lowered the progression of the disease. Moreover, the findings of this study emphasized that utilization and expenditure analyses should be conducted from time to time to manage inventory in hospital pharmacies.
7. Source of funding
King Saud University (Riyadh, Saudi Arabia) research supporting project number (RSP-2019-76).
Declaration of Competing Interest
The authors declare no professional affiliation, financial interest, or conflict with the subject matter or information discussed in this manuscript.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmed A.E. Trends and Projections of Breast Cancer in Saudi Arabia: A National Incidence Rates by Gender, Age, Nationality, and Years (1999–2014) Arabia. 2019;12(4) [Google Scholar]
- Alattas M.T. Cancer Control Priorities and Challenges in Saudi Arabia: A Preliminary projection of Cancer Burden. Lung. 2019;6:1–70. [PubMed] [Google Scholar]
- Asiri S. Incidence Rates of Breast Cancer by Age and Tumor Characteristics Among Saudi Women: Recent Trends. Cureus. 2020:12(1). doi: 10.7759/cureus.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachhav S.S., Kshirsagar N.A. Systematic review of drug utilization studies & the use of the drug classification system in the WHO-SEARO Region. Indian J. Med. Res. 2015;142(2):120. doi: 10.4103/0971-5916.164223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F. Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. Lancet Oncol. 2012;13(8):790–801. doi: 10.1016/S1470-2045(12)70211-5. [DOI] [PubMed] [Google Scholar]
- Cancer Incidence Report Saudi Arabia 2015. Saudi Cancer Registry, 2018.
- Dave D.J., Pillai A., Shah D.V., Agarwal S., Goel A. An Analysis of Utilization Pattern of Anticancer Drugs in Diagnosed Cases of Carcinoma in A Tertiary Care Teaching Hospital. Int. J. Basic Appl. Med. Sci. 2014;4(1):251–259. [Google Scholar]
- Dukes M.N.G., Organization W.H. Regional Office for Europe; 1993. Drug utilization studies: methods and uses: World Health Organization. [Google Scholar]
- Introduction to drug utilization research. 2003.
- Jakupi A. Utilization and expenditure of anti-cancer medicines in Kosovo: findings and implications. PharmacoEconomics-open. 2018;2(4):423–432. doi: 10.1007/s41669-017-0066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni M.D., Hussaini S.A., Padwal S.L., Khandelwal P.N., Doifode S.M., More P.P. Drug utilization review of anticancer drugs in cancer outpatient department of the Government Medical College, Aurangabad. Int. J. Basic Clin. Pharmacol. 2014;3(5):879–883. [Google Scholar]
- Kumar B.S., Maria S., Shejila C.H., Udaykumar P. Drug Utilization Review and Cost Analysis of Anticancer Drugs Used in a Tertiary Care Teaching Hospital. Indian J. Pharmaceutical Sci. 2018;80(4):686–693. [Google Scholar]
- Lakhani S.R. The pathology of familial breast cancer: predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2. J. Clin. Oncol. 2002;20(9):2310–2318. doi: 10.1200/JCO.2002.09.023. [DOI] [PubMed] [Google Scholar]
- Lukong K.E. Understanding breast cancer - The long and winding road. BBA Clin. 2017;7:64–77. doi: 10.1016/j.bbacli.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell Ogochukwu Adibe D.O.A., Ma’aji Hadiza Usman. Anticancer drugs utilization for initiation phase of breast and cervical cancers chemotherapies in a Nigerian tertiary hospital. J. Appl. Pharmaceutical Sci. 2019;9(3):111–116. [Google Scholar]
- Moura Silva F., Tosello C., Laloni M.T., Andrade C.M., Bertozzi A., Vernaglia P., Correa F.M., Moraes R.P., Goes J.S. Goes FEC 60 adjuvant chemotherapy (AdCT) in breast cancer (BC) patients (Pts): 10-year follow-up results. J. Clin. Oncol. 2006;24(18_suppl) [Google Scholar]
- Onitilo A.A. Utilization of neoadjuvant chemotherapy varies in the treatment of women with invasive breast cancer. PLoS ONE. 2013;8(12):e84535. doi: 10.1371/journal.pone.0084535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization, W.H., 2018. Methods to analyse medicine utilization and expenditure to support pharmaceutical policy implementation.
- Rindi G. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod. Pathol. 2018;31(12):1770. doi: 10.1038/s41379-018-0110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudi Cancer Registry. Cancer incidence reports., 2015. Saudi Arabia.
- Vinodkumar Mugada A.P., Munagala Mounika. Drug Utilization Evaluation of Anticancer Drugs in a Tertiary Care Teaching Hospital: a Descriptive Observational Study. J. Appl. Pharmaceutical Sci. 2016;6(10):98–101. [Google Scholar]
- Waks A.G., Winer E.P. Breast Cancer Treatment: A Review. JAMA. 2019;321(3):288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- WHO . WHO; 2014. Cancer Country Profile. [Google Scholar]