Abstract
Amino acids are attractive metabolites for the pharmaceutical and food industry field. On one hand, the construction of microbial cell factories for large-scale production aims to satisfy the demand for amino acids as bulk biochemical. On the other hand, amino acids enhance flavor formation in fermented foods. Concerning the latter, flavor formation in dairy products, such as cheese is associated with the presence of lactic acid bacteria (LAB). In particular, Lactococcus lactis, one of the most important LAB, is used as a starter culture in fermented foods. The proteolytic activity of some L. lactis strains results in peptides and amino acids, which are flavor compounds or flavor precursors. However, it is still a challenge to isolate bacterial cells with enhanced amino acid production and secretion activity. In this work, we developed a growth-based sensor strain to detect the essential amino acids isoleucine, leucine, valine, histidine and methionine. Amino acids are metabolites that can be secreted by some bacteria. Therefore, our biosensor allowed us to identify wild-type L. lactis strains that naturally secrete amino acids, by using co-cultures of the biosensor strain with potential amino acid producing strains. Subsequently, we used this biosensor in combination with a droplet-based screening approach, and isolated three mutated L. lactis IPLA838 strains with 5–10 fold increased amino acid-secretion compared to the wild type. Genome re-sequencing revealed mutations in genes encoding proteins that participate in peptide uptake and peptide degradation. We argue that an unbalance in the regulation of amino acid levels as a result of these gene mutations may drive the accumulation and secretion of these amino acids. This biosensing system tackles the problem of selection for overproduction of secreted molecules, which requires the coupling of the product to the producing cell in the droplets.
Keywords: Amino acids, Lactococcus lactis, Biosensor, Droplet-technology, FACS, EMS
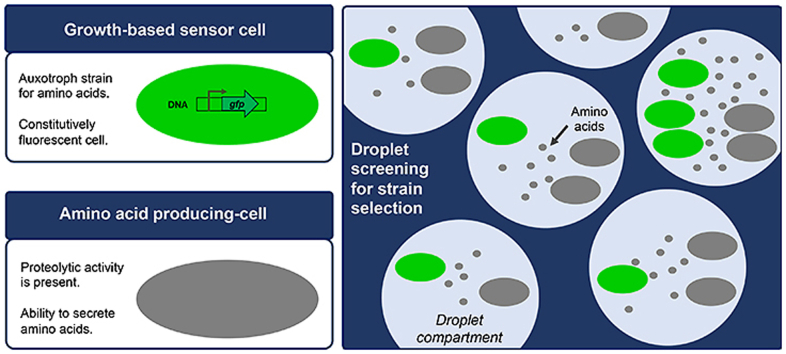
Graphical abstract
The development of a fluorescent sensor cell, able to grow only in the presence of essential amino acids, allows selection of strains with an improved capacity of amino acid production and secretion, by a droplet-based screening platform.
Highlights
-
•
An amino acid-responsive biosensor was developed for fluorescent-based screening.
-
•
Production of amino acids in wild-type Lactococcus lactis strains was improved after selection of EMS-mutants using high-throughput droplet-based screening technology.
-
•
Mutations in genes encoding proteins of the proteolytic system of L. lactis improve amino acid secretion. For instance glutamate secretion was enhanced 10-fold and leucine up to 30-fold.
-
•
The identification of target genes is important to improve amino acid production in other lactic acid bacteria (LAB).
1. Introduction
Bacteria produce a plethora of extracellular compounds during growth as well as in stationary phase (Pinu et al., 2018). Some of these compounds are attractive metabolites in industrial microbiology, for instance the large-scale production of amino acids that find application as flavoring agents, as feed additives, as artificial sweeteners, and for pharmaceutical purposes (D’Este et al., 2018). Recently, an increasing demand for amino acids has led to the application of new technologies towards the development of amino acid-producing microbial cells (Hirasawa and Shimizu, 2016). Industrially, the secretion of amino acids by some bacteria has an economic relevance for biotechnological fermentation procedures, as it simplifies the extraction and purification of these metabolites. For instance, the first described glutamate-secreting bacterium Corynebacterium glutamicum has been used in engineering approaches to increase the production of glutamate, lysine and other flavor active amino acids at a large-scale (Georgi et al., 2005).
Besides their production as bulk biochemicals by fermentative procedures, amino acids are relevant precursors of flavor compounds in dairy fermentations (D’Este et al., 2018; Lee and Wendisch, 2017). Therefore, the selection of bacterial strains that naturally (e.g. through mutations) overproduce and secrete amino acids is relevant in food industry as well. However, the diffusion of these compounds into the environment where these strains grow, is a limiting factor to achieve this selection (Chen et al., 2018). To facilitate the selection process of bacterial strains that show higher production levels of secreted compounds, approaches where producers are co-cultivated with a biosensor in microdroplets have been developed (van Tatenhove-Pel et al., 2020). In principle, biosensors translate the presence of metabolites into a fluorescence signal, and facilitate the screening process by their combination with high-throughput approaches (Lim et al., 2015). Amino acid biosensors can overcome the problem of quantifying the amount of free amino acids in complex (food) matrices, and also provide the possibility of continuous monitoring of the amino acid concentration during fermentation processes (Bertels et al., 2012).
In dairy fermentations, the starter cultures produce amino acids that enhance the flavor of food products (Ayad et al., 1999; Centeno et al., 2002). Lactic acid bacteria (LAB) have been extensively used in manufacturing processes to obtain fermented foods due to their contribution to the aroma and texture of the products . Lactococcus lactis is one of the best-known LAB, and it has become a paradigm in terms of understanding proteolysis and peptide utilization (Liu et al., 2010; Song et al., 2017). This bacterium is fastidious in nutrient requirements. For instance, most L. lactis strains are auxotrophic for several amino acids: isoleucine, leucine, valine, glutamic acid, histidine and methionine (Adamberg et al., 2012; Teusink et al., 2011; Hernandez-Valdes et al., 2020). In fact, a limiting factor in cheese production is the low growth rate as a result of the small amounts of free essential amino acids in milk (Ziadi et al., 2010). Thus, since casein is the most abundant protein in milk, an efficient proteolytic system is required to breakdown casein molecules into peptides, which intracellularly undergo further degradation to provide L. lactis with essential amino acids during growth in milk (Savijoki et al., 2006).
The formation of flavor compounds is dependent on the proteolytic system of LAB (Smit et al., 2005). The casein degradation starts by the activity of the extracellular proteinase PrtP, which produces oligopeptides of 4 to 30 amino acid residues. Next, the casein-derived peptides are imported into the cell via the oligopeptide transport system Opp, and the di- and tripeptide transport systems DtpP and DtpT. Inside the cell, the imported peptides are degraded by aminopeptidases (PepN, PepX, and PepC) and endopeptidases (PepO and PepF), giving rise to free amino acids. Lastly, some of the free amino acids contribute directly, or only after chemical or enzymatic conversion, to the formation of flavor and aroma compounds such as aldehydes, alcohols and esters (Doeven et al., 2005; Sanz et al., 2001; Ziadi et al., 2010).
Based on cheese trials and sensory panels, the contribution of amino acids to flavor formation has been described (Centeno et al., 2002; De Palencia et al., 2004; Gutiérrez-Méndez et al., 2008). Alcohol, aldehydes and esters are mainly derived from branched-chain amino acids (valine, leucine and isoleucine), whereas sulphur aroma is enriched by the presence of methionine and cysteine, and floral/fruity notes are produced by aromatic amino acids (tyrosine, tryptophan and phenylalanine) (Helinck et al., 2004; Seefeldt and Weimer, 2000; Smit et al., 2009). Notably, sensory analysis revealed that glutamate is the main source of umami taste in Cheddar and Swiss cheese, and the intensity of umami taste increases as more free glutamic acid is produced during cheese ripening (Drake et al., 2007; Yamaguchi and Ninomiya, 2000).
In this study, we developed a growth-based biosensor to detect secreted amino acids (Leu, Ile, Val, Met, Glu, His) by using a L. lactis strain that lacks functional peptide transport systems. Thus, the L. lactis biosensor only grows in the presence of free amino acids, and essentially it can be applied to select overproducer cells of any other bacteria able to provide the biosensor with the essential amino acids. We used L. lactis strains as producer cells to benchmark the performance of our biosensor. A first screening for amino acid secretion, based on the growth of the fluorescent biosensor revealed that some wild-type L. lactis strains are naturally able to secrete the essential amino acids. To identify mutants of strain IPLA838 with increased amino acid secretion, we performed droplet-based high-throughput screening, which allowed us to isolate non-GMO bacterial strains with the desired phenotype and potentially higher flavor-forming capacity.
2. Material and methods
2.1. Bacterial strains and plasmids
The bacterial strains used in this study are listed in Table S1. L. lactis cells were grown as standing cultures at 30 °C in M17 broth (Difco™ BD, NJ, USA) or in standard chemically defined medium (CDM) (Goel et al., 2012), supplemented with glucose or lactose (Sigma-Aldrich, MO, USA) at a concentration of 0.5% (w/v). CDM contained 49.6 mM NaCl, 20.1 mM Na2HPO4, 20.2 mM KH2PO4, 9.7 μM (±)-α-lipoic acid, 2.10 μM D-pantothenic acid, 8.12 μM nicotinic acid, 0.41 μM biotin, 4.91 μM pyridoxal hydrochloride, 4.86 μM pyridoxine hydrochloride, 2.96 μM thiamine hydrochloride, 0.24 μM (NH4)6Mo7O24, CaCl2 20.4 μM, 1.07 μM CoSO4, 1.20 μM CuSO4, 1.04 μM ZnSO4, 20.12 μM FeCl3, 1.46 mM L-alanine, 1.40 mM L-arginine, 0.61 mM L-asparagine, 1.03 mM L-aspartic acid, 0.35 mM L-cysteine, 0.66 mM L-glutamic acid, 0.66 mM L-glutamine, 0.39 mM glycine, 0.16 mM L-histidine, 0.63 mM L-isoleucine, 0.89 mM L-leucine, 1.02 mM L-lysine, 0.27 mM L-methionine, 0.39 mM L-phenylalanine, 3.58 mM L-proline, 1.64 mM L-serine, 0.57 mM L-threonine, 0.18 mM L-tryptophan, 2.76 mM L-tyrosine and 0.73 mM L-valine.
Table 1.
Contents of essential amino acids in culture supernatants of wild-type strains.
| Sample | Glu | His | Val | Met | Ile | Leu | |
|---|---|---|---|---|---|---|---|
| CDM-casein | B | ND | ND | 0.8 ± 0.1 | ND | ND | 1.1 ± 0.1 |
| SK11 | A | 14.2 ± 0.2 | ND | 1.1 ± 0.1 | ND | 0.7 ± 0.1 | 0.7 ± 0.1 |
| B | 39.7 ± 0.8 | 5.8 ± 0.3 | 1.7 ± 0.1 | ND | 2.2 ± 0.3 | 0.7 ± 0.1 | |
| NZ9000pLP712 | A | 36.6 ± 1.0 | 4.4 ± 0.5 | 5.5 ± 0.5 | 5.2 ± 0.9 | 3.6 ± 0.4 | 2.9 ± 0.2 |
| B | 60.8 ± 0.5 | 9.9 ± 0.2 | 10.1 ± 0.4 | ND | 3.0 ± 0.1 | 10.0 ± 0.2 | |
| MG610 | A | 3.3 ± 0.2 | ND | ND | ND | 0.9 ± 0.1 | ND |
| B | 5.3 ± 0.1 | ND | ND | ND | 0.7 ± 0.1 | ND | |
| Wg2 | A | 15.0 ± 0.2 | 2.6 ± 0.1 | 36.0 ± 0.7 | 6.6 ± 0.4 | 11.7 ± 0.4 | 18.2 ± 0.1 |
| B | 29.3 ± 0.7 | 4.6 ± 0.1 | 67.9 ± 1.3 | 11.7 ± 0.4 | 23.5 ± 0.2 | 35.0 ± 0.2 | |
| NCDO176 | A | 41.1 ± 0.1 | 5.9 ± 0.1 | 39.9 ± 0.3 | 11.5 ± 0.4 | 6.4 ± 0.3 | 20.8 ± 0.4 |
| B | 59.2 ± 0.4 | 8.0 ± 0.2 | 56.0 ± 0.2 | 15.2 ± 0.1 | 10.2 ± 0.2 | 38.3 ± 0.9 | |
| WW4 | A | 228.1 ± 1.9 | 11.6 ± 0.4 | 145.8 ± 0.6 | 52.8 ± 0.9 | 30.7 ± 0.8 | 124.5 ± 1.4 |
| B | 262.0 ± 1.7 | 16.2 ± 0.2 | 152.0 ± 1.1 | 52.9 ± 0.4 | 28.8 ± 0.4 | 162.2 ± 1.3 | |
| IPLA838 | A | 11.0 ± 0.3 | 3.9 ± 0.1 | 1.6 ± 0.1 | 1.5 ± 0.1 | 0.6 ± 0.1 | 2.0 ± 0.1 |
| B | 21.1 ± 0.4 | 6.3 ± 0.1 | 3.8 ± 0.2 | 3.2 ± 0.1 | 0.4 ± 0.2 | 2.8 ± 0.1 | |
| aa solution | 991.3 ± 1.5 | 144.4 ± 1.1 | 642.0 ± 2.6 | 211.8 ± 1.3 | 531.1 ± 0.3 | 790.0 ± 0.9 | |
Values indicate concentration (μM). Two sampling points are shown: A-end of lag growth phase, and B- mid exponential growth phase (see Fig. S4). After the strains were grown in CDM-casein medium, the concentrations of amino acids in the culture supernatants were measured using a HPLC assay coupled with fluorescence detection. Strains with positive and negative amino acid secretion capacity are shown. A positive (standard amino acid solution) and negative (CDM-casein) control are shown. Amino acids below the detection limit of the HPLC assay are indicated as ND. Quantifications were performed in duplicates, and average values are shown. Error is SD.
E. coli DH5α (Life Technologies, Gaithersburg, MD, USA) strains were used to isolate the plasmids listed in Table S1. Cells were grown at 37 °C in Luria-Bertani broth or Luria-Bertani agar 1.5% (w/v) (Difco™ BD, NJ, USA).
2.2. Media and culture conditions
The CDM-casein medium used in this study was prepared based on the chemically defined medium recipe, but without the amino acid solution, resulting in medium named CDM-aa in this study. CDM-casein contains casein according to Hammarsten 1% (w/v) (Merck & Co., NJ, USA), and was supplemented with glucose or lactose (Sigma-Aldrich, MO, USA) at a concentration of 0.5% (w/v).
M17-agar plates were prepared by adding agar 1.5% (w/v) and glucose (GM17) or lactose (LM17) to M17. When necessary, the culture medium was supplemented with erythromycin (Sigma-Aldrich, MO, USA) 5 μg mL−1 or chloramphenicol (Sigma-Aldrich, MO, USA) 5 μg mL−1.
For microscopy experiments and plate-reader assays, L. lactis overnight cultures were grown in the standard CDM, i.e. with amino acid solution, supplemented with glucose 0.5% (w/v) and the corresponding antibiotic. Cells were collected by centrifugation from exponential growth cultures (optical density of 0.3 at 600 nm) and washed three times with phosphate-buffered saline (PBS) solution (pH 7.2) containing: KH2PO4 15.44 μM, NaCl 1.55 mM and Na2HPO4 27.09 μM.
Subsequently, for co-cultivation experiments, washed cells of both strains were adjusted to optical density of 0.5 at 600 nm and mixed in a 1:10 ratio (producer strain:GFPsensor). The CDM-casein medium was used to perform the co-cultivation experiments. The mixture of cells was used to perform time-lapse experiments or plate-reader assays.
2.3. General DNA manipulation techniques
Procedures for DNA manipulations (gel electrophoresis and transformation) were performed as described by Sambrook and Russell (Sambrook and Russell, 2001). PCR reactions were performed in an Eppendorf thermal cycler (Eppendorf, Hamburg, Germany) with L. lactis chromosomal DNA as template, using Phusion polymerase (Thermo Fisher Scientific Inc., MA, USA). Oligonucleotides (Table S2) were purchased from Biolegio (Nijmegen, The Netherlands). Plasmid DNA and PCR products were isolated and cleaned-up with a high-pure plasmid isolation kit (Roche Applied Science, Mannheim, Germany), according to the protocol of the manufacturer. Subsequent sequencing (Macrogen, Amsterdam, The Netherlands) was used to verify the constructs.
2.4. Construction of the GFPsensor strain
We used the L. lactis AG500 strain (Kunji et al., 1995). The vector pSEUDO::Pusp45-sfgfp(Bs) (Overkamp et al., 2013) was introduced into competent cells of L. lactis AG500 by electroporation (at 2.5 kV, 25 μF, 200 Ohm). The vector was integrated into the silent llmg_pseudo10 locus by a single-crossover integration as described previously (Overkamp et al., 2013). Transformants were selected on M17-agar plates supplemented with sucrose, glucose and erythromycin 5 μg mL−1, yielding the L. lactis GFPsensor strain.
2.5. DNA sequencing
The mutated IPLA838 cells (MUTproducers) selected by FACS were isolated on M17-agar plates. A single colony of each strain was grown as standing culture in 5 mL of M17 broth, supplemented with 0.5% (w/v) lactose, and incubated overnight at 30 °C. Cells from the three cultures were collected by centrifugation at 10,000 rpm for 3 min in a Microfuge 16 centrifuge (Beckman Coulter, Woerden, The Netherlands). Genomic DNA was isolated with a GenElute bacterial genome DNA kit (Sigma-Aldrich, Munich, Germany) according to the manufacturer’s instructions.
The genomes of all different colonies were paired-end sequenced at the Beijing Genomics Institute (BGI, Copenhagen N, Denmark) on a BGISEQ-500 platform. A total of 5 million paired-end reads (150 bp) were generated. FastQC version 0.11.589 was used to examine the quality of the reads. Identification of mutations was performed with Breseq (Deatherage and Barrick, 2014), using the complete genome of Lactococcus lactis subsp. lactis IPLA838, as a reference sequence.
2.6. Time-lapse microscope experiments
Washed cells were transferred to a solidified thin layer of CDM-casein with high-resolution agarose 1.5% (w/v) (Sigma-Aldrich, MO, USA). A standard microscope slide was prepared with a 65 μL Gene Frame AB-0577 (1.5 × 1.6 cm) (Thermo Fisher Scientific Inc., MA, USA). A 30 μL volume of heated CDM-casein agar was set in the middle of the frame and covered with another microscope slide to create a homogeneous surface after cooling. The upper microscope slide was removed and 1 μL of bacterial cells were spotted on the agar. The frame was sealed with a standard microscope coverslip.
Microscopy observations and time-lapse recordings were performed with a temperature-controlled (Cube and box incubation system Life Imaging Services) DeltaVision (Applied Precision, Washington, USA) IX7I microscope (Olympus, PA, USA), at 30 °C. Images were obtained with a CoolSNAP HQ2 camera (Princeton Instruments, NJ, USA) at X60 or X100 magnification. 300-W xenon light source, bright-field objective and GFP filter set (filter from Chroma, excitation 470/40 nm and emission 525/50 nm). Snapshots in bright-field and GFP-channel were taken every 10 min for 20 h with 10% APLLC while LED light and a 0.05 s exposure for bright-field, or 100% xenon light and 0.8 s of exposure for GFP-signal detection. The raw data was stored using softWoRx 3.6.0 (Applied precision) and analyzed using ImageJ software (Schindelin et al., 2012).
2.7. Plate reader assays
Cultures of L. lactis were grown and prepared as described in Method 2.2. For growth curves, L. lactis cells were diluted 1:50 in CDM-casein, containing glucose or lactose 0.5% (w/v). Growth was recorded in 0.2 mL cultures in 96-well micro-titer plates and monitored by using a micro-titer plate reader VarioSkan (Thermo Fisher Scientific Inc., MA, USA). Growth was recorded with measurements of the optical density at 600 nm (OD600) and the GFPsensor signal (excitation 485 nm and emission 535 nm) was recorded every 10 min for 24 h. Both signals were corrected for the background value of the medium used for growth. The GFPsensor signals are shown in relative fluorescence units to the growth (RFU/OD600).
2.8. Quantification of amino acids content by HPLC
2.8.1. Sample preparation
Each bacterial strain was inoculated in 10 mL of CDM-casein and grown at 30 °C. Following growth (see supplementary figure S3B), 5 mL of each culture was harvested by centrifugation at two points: A (OD600 = 0.3) and B (OD600 = 1.5). The supernatants were transferred into a clean tube, filtered through nitrocellulose Whatman filters (0.45 μm and 0.2 μm) and stored at 4 °C for subsequent HPLC analysis.
Derivatization of standard amino acids and samples with o-phtalaldehyde (OPA) and 9-fluorenylmethyloxycarbonyl (FMOC) reagent solutions was set to be carried out automatically in the HPLC autosampler. Briefly, the derivatization was performed with a programmable automatic injector by mixing 1 μL of sample (or standard solution) with 2.5 μL of borate buffer pH = 10.4. After 0.2 min, 0.5 μL of OPA is added and mixed. Subsequently, 0.4 μL of FMOC added and mixed, followed by mixing 32 μL of solvent A (10 mM Na2HPO4 and 10 mM Na2B2O7, pH 8), and the final injection of the whole mixture.
2.8.2. HPLC conditions
HPLC amino acid analysis was performed on an Agilent 1100 HPLC binary system (Agilent, Santa Clara, USA) equipped with an 1100 Fluorescence detector (FLD) and a Gemini C18 column (2 × 250 mm, 5 μm, Phenomenex, Torrance, USA). Borate buffer (0.4 M H3BO3) was used, and the mobile phases consisted of Solvent A (10 mM Na2HPO4 and 10 mM Na2B2O7, pH 8.2) and Solvent B (mixture of 45:45:10 acetonitrile/methanol/water). An aliquot of 1 μL derivatized sample (ad described above) was injected into the HPLC column equilibrated with Solvent A. The elution was carried out at a flow rate of 0.5 ml/min with the following program: from 0 to 0.5 min in 2% Solvent B, from 0.5 to 20 min gradient step to reach 57% Solvent B, from 20 to 20.1 min gradient step 57–100% solvent B, 20.1 to 23.5 min 100% solvent B, 23.5 min to 23.6 min from 100% to 2% solvent B, and at 25 min ended.
The fluorescence detector (FLD) was set to Ex = 340 nm Em = 450 nm for all OPA derivatives and Ex = 266 Em = 305 nm for the FMOC derivatives eluting at the end of the chromatogram.
2.8.3. Amino acids quantification
Quantifications of amino acids were performed based on a five point calibration line between 5 and 500 μM. Data analysis was performed by using the Chemstation software to quantify the amino acids. The concentrations of the amino acids were obtained by measuring the FLD peak areas.
2.9. Chemical mutagenesis
Ethyl methanesulfonate (EMS) at a final concentration of 25 mM was added to a 2 mL overnight bacterial culture of the IPLA838 strain. After 3 h h of exposure to EMS, the cells were washed, 500 μL of them were stored at −80 °C (sample 1) and the rest of the cells were diluted in GM17 for overnight recovery (1:100 dilution). From the recovered cells, 500 μL were stored (sample 2), and the rest of cells were exposed to EMS as described above, washed and 500 μL of them were stored at −80 °C (sample 3). The rest of the cells were recovered in GM17 overnight (1:100 dilution). 500 μL of the recovered cells (sample 4) were mixed with 500 μL of all the other three samples (sample 1, 2 and 3), resulting in the mutagenized IPLA838 sample used to produce agarose droplets.
2.10. Agarose-based droplet technique
2.10.1. Sample preparation
Ultra-low gelling temperature agarose (Sigma-Aldrich) 1.15% (w/v) was added to CDM-casein for agarose droplet generation. Cells were prepared as described in Methods 2.2, the cells were washed and transferred to the aqueous phase (CDM-casein with low-gelling agarose 1.15% (w/v). Assuming that OD600 = 1 corresponds to 109 cells mL−1, the concentration of producer cells was set to 2.6 × 107 cells mL−1 and GFPsensor cells was set to 2.7 × 108 cells mL−1. Both cells were mixed, collected by centrifugation, and resuspended in the warm agarose solution (30 °C).
2.10.2. Droplet formation
The agarose solution and oil solution (HFE-7500 fluorinated oil supplemented with 0.2% (v/v) pico-surf 1; Sphere Fluidics, Cambridge, UK) were mixed. Droplets were generated using a high-performance dispersing instrument (Ultra Turrax® T25, IKA). The resulted agarose droplets were incubated on ice for gelation. After solidification, the agarose beads were incubated for 20 h at 30 °C. After incubation, the beads were incubated on ice for 30 min. The emulsion was destabilized by addition of an equal volume of 20% (v/v) perfluoro-1-octanol (PFO). Droplets formation resulted in droplets with different size. Therefore, two rounds of filtration were performed (30 μm and 50 μm, CellTrics® Systems), yielding agarose beads of size between 30 – 50 μm that can be sorted by fluorescence-activated cell sorting (FACS). The encapsulation of cells in droplets is dictated by the Poisson distribution, and it estimates the number of cells present in each droplet (Collins et al., 2015; Sinha et al., 2019). Considering that the agarose beads have an average diameter of 40 μm, each bead contains an average of 9 GFPsensor cells. Additionally, ~36.6% beads contain one producer cell, ~16.3% beads contain 2 producer cells, ~40.9% beads not containing any cells, and ~6.2% beads containing 2 or more producer cells.
2.11. Agarose beads sorting
The agarose beads were washed and resuspended in PBS. The analysis/sorting of the beads was performed with a MoFlo XDP sorter (Beckman Coulter), using a nozzle of 150 μm. The GFP-signal at single-cell level was recorded in 30,000 events using a 488 nm argon laser. The FlowJo software was used for data analysis (https://www.flowjo.com/). The agarose beads showing a fluorescence signal higher than the wild-type sample were recovered by fluorescence-activated cell sorting (FACS). The single-beads sorting was performed in 96-well plates. Then, the selected L. lactis producer cells were recovered by incubation in M17 supplemented with lactose 0.5% (w/v), a growth condition where only producer cells grow.
2.12. Statistics
Statistical analyses were performed using Prism 6.01 (GraphPad software https://www.graphpad.com/). All experiments were repeated at least three times. All replicates are biological replicates.
2.13. Bioinformatics
Alignments and sequences identities were determined by using Clustal Omega using the full-length protein sequences (Sievers et al., 2011). Identification of mutations was performed with Breseq version 0.32.1 (Deatherage and Barrick, 2014).
3. Results
3.1. A whole-cell biosensor for selection of amino acid-secreting strains
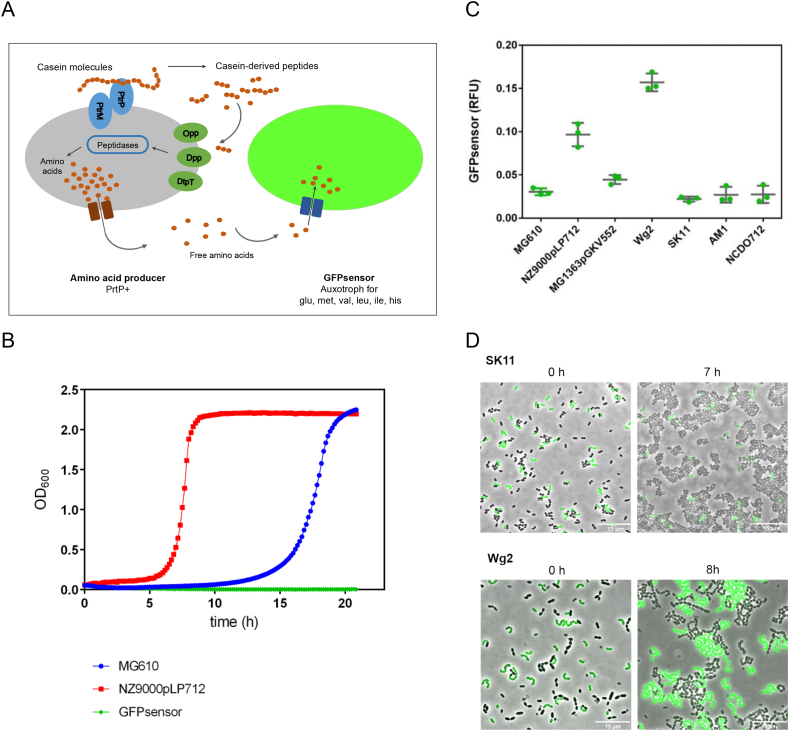
L. lactis MG1363 is auxotrophic for the amino acids leucine, isoleucine, valine, methionine, histidine and glutamic acid, as most lactic acid bacteria (Chopin, 1993; Flahaut et al., 2013). We constructed a L. lactis GFPsensor strain, which exhibits a constitutive GFP expression through the culture, and it is incapable of growth on peptides because it lacks functional peptide transporters (see Methods 2.4 and Fig. S1). The sensor cells, deficient of all peptide transport systems, are fully dependent on the uptake of free essential amino acids from their environment to grow. We aimed to test the performance of the constructed biosensor by monitoring its growth and fluorescence when it is co-cultivated with bacteria that may secrete amino acids. Accordingly, we used L. lactis wild-type strains to investigate whether the capacity to secrete the essential amino acids is present in these bacteria. Fig. 1A describes our strategy based on co-cultivation of two bacterial cells, the GFPsensor and a potential amino acid producer. The amino acid producer is a proteinase positive (PrtP+) strain that is able to grow in a medium containing casein as nitrogen source. In contrast, the GFPsensor is unable to import casein-derived peptides, but can take up the essential amino acids that might be secreted by the producer. Consequently, the growth of GFPsensor cells by measurements of GFP expression is used as an indicator of the level of amino acids secreted by wild-type cells. Thus, the lower the amounts of produced essential amino acid, the lower the GFPsensor fluorescence signal (RFU).
Fig. 1.
A whole-cell sensor system to detect secreted amino acids. (A) An amino acid producer cell bears the PrtP proteinase to breakdown casein molecules, which provides the cell with casein-derived peptides. These peptides are intracellularly further degraded by peptidases, which results in free amino acids. The GFPsensor cell lacks the oligopeptide peptide transport systems and therefore, it is unable to grow unless the amino acid producer secretes essential amino acids to the environment. (B) Growth curves of PrtP + strains (MG610 and NZ9000pLP712, in blue and red, respectively) performed with CDM-casein. Both PrtP + strains are able to degrade casein and grow, in contrast to the GFPsensor (green line), which is unable to grow. (C) The fluorescence signal of the GFPsensor strain (y-axis) indicates its growth when co-cultivated with potential producer strains (x-axis). The growth of the GFPsensor indicates secretion of essential amino acids (Leu, Iso, Val, Met, His and Glu) by the producer L. lactis strains. Fluorescence measurements were recorded by plate reader assays; three samples per strain are shown. (D) Snapshots of time-lapse experiments. The GFPsensor cells are co-cultivated with the SK11 strain (top images), and co-cultivated with the Wg2 strain (bottom images). Overlays of fluorescence-channel and bright-field are shown. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Two proteinase positive (PrtP+) strains were selected to perform amino acid secretion experiments, the MG610 and NZ9000pLP712 strains (see Table S1). Initially, we tested the growth of these strains in chemically defined medium (CDM) supplemented with casein 1% (w/v) (CDM-casein). Fig. 1B shows that both PrtP + strains are able to grow under these conditions, but the GFPsensor strains do not grow (Fig. 1B). Next, we co-cultivated the GFPsensor with each of the two PrtP + strains, in order to test their capacity to secrete amino acids. The sensor is unable to grow in the presence of any of the PrtP + strains (Fig. S2). These results suggest that the PrtP + strains do not secrete enough amounts of the essential amino acids. In agreement with this finding, previous studies on the catalytic activity of PrtP demonstrated that this proteinase degrades casein into oligopeptides, and only traces of free phenylalanine were detected (Kunji et al., 1995; Mierau et al., 1997). Remarkably, our data supports the idea that the free amino acids released by casein degradation via PrtP do not reach the minimum amounts of the essential amino acids to sustain the growth of the GFPsensor. Thus, other different factors than the presence of the proteinase genes might result in the amino acid secretion capacity of certain strains.
3.2. Benchmarking of the growth-based amino acid sensor
Since no evidence of amino acid secretion by the PrtP + strains (MG610 and NZ9000pLP712) was found, we aimed to investigate whether this capacity is present in other L. lactis strains. We tested the growth of the GFPsensor when co-cultivated with different strains of a collection of different wild-type L. lactis strains (see Table S1). Fig. 1C and 1D show that the GFPsensor grows in co-cultivation with the L. lactis Wg2 strain, whereas it is unable to grow in co-cultivation with other strains, such as the L. lactis SK11 strain. This result indicates that, while Wg2 secretes at least the six essential amino acids, SK11 lacks this property. Importantly, there are minor differences in the amino acid sequences of the proteinases from different strains, i.e. the PrtP amino acid sequences of the strains Wg2 and SK11 are 98% identical (Mierau et al., 1997). Although NZ9000pLP712 shows a GFPsensor signal higher than other strains, the time-lapse experiment (Fig. S2) shows a poor growth of the GFPsensor. This observation might be explained by very low levels of amino acids secretion that still are detected by plate reader measurements. Correspondingly, based on these findings, we aimed to use the GFPsensor as a detection tool for identifying amino acid secretion in other potential amino acid producers.
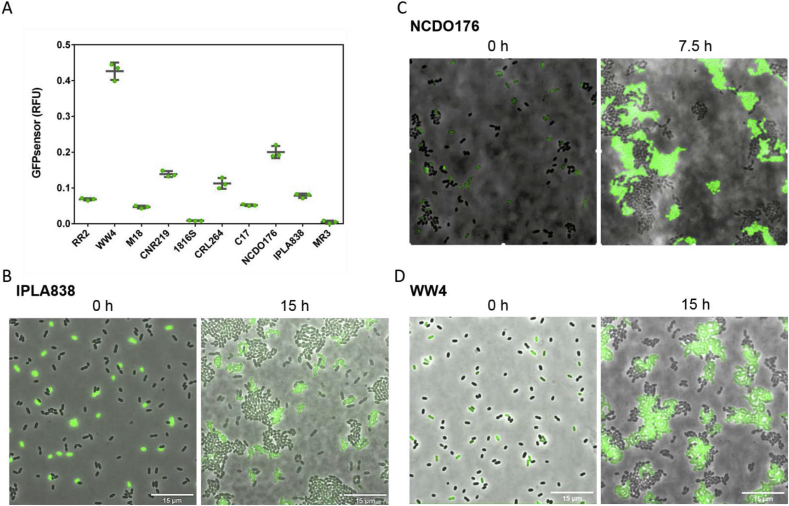
Strains of Lactococcus lactis subsp. lactis biovar diacetylactis are a component of mixed cultures used in the dairy industry (Dhaisne et al., 2013). Besides the well-known citrate utilization by this bacterium, which results in production of carbon dioxide and diacetyl (Siezen et al., 2011), we investigated whether some L. lactis biovar diacetylactis strains are also able to secrete amino acids at a relatively high level. A total of eight L. lactis biovar diacetylactis from different sources were collected (see Table S1). The presence of the prtP gene in these strains was assessed by PCR amplification (Fig. S3). Next, the PrtP + L. lactis biovar diacetylactis strains were screened for amino acid secretion by co-cultivation with the GFPsensor. Fig. 2 shows that the growth of the GFPsensor is highly promoted by co-cultivation with the WW4 and NCDO176 strains, and to a lesser extent by co-cultivation with others such as CNRZ190 and CRL264. The observations by fluorescence microscopy of the co-cultivation experiments (Fig. 2B, 2C and 2D) are in agreement with the GFPsensor values of plate-reader assays in Fig. 2A (see Fig. S4), for instance the low fluorescence value for the co-cultivation with the IPLA838 strain is observed as a low number of GFPsensor cells in Fig. 2B.
Fig. 2.
Screening ofLactococcus lactissubsp.lactisbiovardiacetylactis strains for amino acids secretion. (A) GFPsensor fluorescence signal (y-axis) when the GFPsensor strain is co-cultivated with different producer strains (x-axis), the higher the GFPsensor fluorescence, the higher the number of GFPsensor cells. (B, C, D) Representative snapshots of co-cultivation of the GFPsensor with three different producer strains by fluoresce microscopy. The three strains are able to grow, but IPLA838 (B) promotes poor growth of the GFPsensor, NCDO176 (C) promotes better growth of the GFPsensor, and the presence of WW4 (D) highly promotes the growth of the GFPsensor. Overlays of the fluorescence-channel and the bright-field are shown.
3.3. L. lactis cells secrete the amino acids into their environment
As noted above, bacteria produce and secrete many metabolites into their culture media, including amino acids (Krämer, 1994; Pinu et al., 2018). Since the report of glutamate secretion by Corynebacterium glutamicum in 1957 (Kinoshita et al., 1957), many other amino acid producers have been described (Hirasawa and Shimizu, 2016; Lee and Wendisch, 2017). Recent studies about cross-feeding interactions in bacteria have also identified a contact-dependent amino acid exchange between donor and recipient cells (D’Souza et al., 2018; Mee et al., 2014; Shitut et al., 2019). Therefore, we addressed the question whether the observed growth of the GFPsensor strain, when obtaining essential amino acids from producer strains, is contact-dependent. To this end, supernatants of monocultures of L. lactis NCDO176 (amino acid producer) and MG610 (non-amino acid producer) were collected after the strains were grown in CDM-casein (Fig. S5 and Methods). The GFPsensor is able to grow in CDM-aa (without amino acids) supplemented with the supernatant of the NCDO176 strain, but it is unable to grow when the CDM-aa is supplemented with the supernatant of the non-amino acid producer (Fig. S6). These results revealed that the amino acids are secreted into the culture supernatant in a non-contact dependent way.
To further confirm our findings, we quantified the amino acids content in the supernatant of cultures of both, amino acid producers and non-amino acid producers by an HPCL assay (see Methods 2.8). Table 1 shows that the amino acid producers indeed secrete the essential-amino acids. Moreover, the profiles of amino acid contents in the culture supernatants are consistent with the GFPsensor signal values observed in Fig. 1, Fig. 2A. The lowest amino acid contents correspond to non-amino acid producers such as the SK11 strain, and the highest amino acid contents are observed in the culture supernatant of the WW4 strain. Taken together, these results show that the amino acid secretion capacity is present in L. lactis producer strains, and it is not dependent on the contact to the GFPsensor strain.
3.4. Enhanced amino acid secretion by L. lactis
A diverse range of amino acid secretion profiles is observed by different lactococcal strains (see Table 1). Consequently, we attempted to obtain strains with high-yield amino acid production, and aimed to improve a strain with a relatively low amino acid secretion profile. Although classical approaches, e.g. random mutagenesis, have been used for a long time as a common technique to improve microorganisms (Adrio and Demain, 2006; Derkx et al., 2014), the selection of mutants with improved secretion of a compound of interest is a difficult task due to diffusion of the compound into the environment where the bacteria grow (Kaminski et al., 2016; van Tatenhove-Pel et al., 2020). Recent technologies have tackled this challenge by usage of compartmentalization techniques, where the metabolite of interest is confined in a delimited area (Chen et al., 2017; Terekhov et al., 2017).
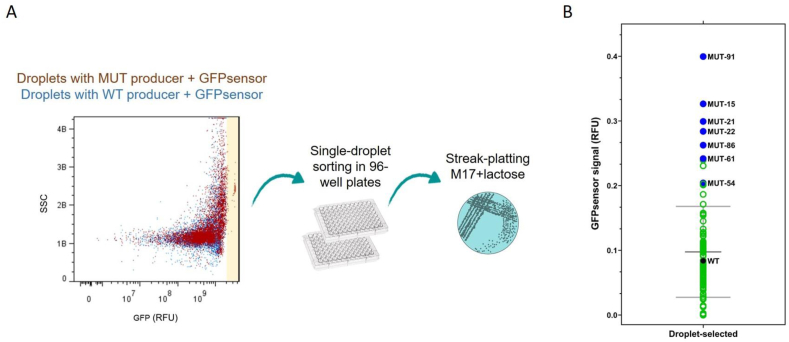
We employed an agarose-based droplet technology to co-cultivate the GFPsensor with mutated cells of the amino acid-producer strain in order to select for (non-GMO) strains with improved-amino acid secretion. Since WW4 highly promotes the GFPsensor growth, indicating that it secretes already a high concentration of essential amino acids, the chances to distinguish enhanced secretion by WW4 are lower than using a strain with relatively low amino acid secretion capacity. The L. lactis IPLA838 strain was selected as amino acid producer to perform the droplet-based high-throughput screening because of its relatively low levels of amino acid secretion (Fig. 2A, Table 1). To achieve this aim, strain IPLA838 was subjected to chemical mutagenesis, resulting in a mutated population (designated as MUTproducers). Encapsulation of MUTproducer-GFPsensor cells was performed in aqueous droplets in oil (see Fig. S7 and Methods 2.10). After incubation for 18 h at 30 °C, we aimed to recover the droplets with the highest number of GFPsensor cells, i.e. the droplets where the MUTproducer cells yielded the highest amounts of essential amino acids. Fig. 3A shows the workflow to obtain the droplets of interest. We analyzed 30,000 droplets. Droplets showing a fluorescence signal above the threshold of droplets with co-cultivated GFPsensor and wild-type amino acid-producer cells were recovered by fluorescence-activated cell sorting (FACS, see Fig. S8). Although droplets in different sizes (30–50 μm) were obtained (polydispersity), the droplets with high fluorescence, i.e. droplets containing candidate overproducers, show similar values of side-scattered light (SSC), an indication of the inner complexity of the droplets, in the flow cytometric analysis (See Fig. S8D). This observation implies that the higher GFP signals are not necessarily a consequence of polydispersity, and therefore the enrichment of overproducers is feasible with a first round of FACS screening. Single-droplet sorting was performed in 96-well plates. Subsequently, the MUTproducer cells of each plate well (one droplet in each well of the 96-well plate) were streak-platted on one M17-agar plate. Single colonies were isolated on M17-agar plates with lactose 0.5% (w/v), a carbon source that only MUTproducer cells are able to utilize.
Fig. 3.
Screening strategy to selectL. lactisstrains with enhanced amino acid secretion. (A) GFPsensor fluorescence signal (RFU; x-axis) and side-scattered light measurement (SSC; y-axis) of droplets containing mutated cells (MUT) and GFPsensor cells (dark red) and droplets containing wild-type cells (WT) and GFPsensor cells (blue). The sorted droplets that showed fluorescence signal above the threshold (droplets containing WT cells) are highlighted in a light yellow. The single-droplet sorting was performed in 96-well plate and single colonies were obtained by streak-platting method in M17 agar plates containing lactose as carbon source. (B) Screening for amino acid secretion by 103 MUT strains by co-cultivation with the GFPsensor. The GFPsensor fluorescence signal (y-axis) when it is co-cultivated with each MUT strain is shown. The wild-type strain (WT) is indicated (black dot), and strains that highly promote the growth of the GFPsensor are highlighted (blue dots). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Next, 103 MUTproducer strains were randomly selected from 103 single colonies (one colony per M17-agar plate) for further characterization. The capacity of amino acid secretion by the selected MUTproducers was assessed by co-cultivation with the GFPsensor in the plate-reader assay as described above. Fig. 3B shows the GFPsensor signal in co-cultivation with each MUTproducer strain, i.e. it shows the amino acid secretion capacity of each MUTproducer strain. Importantly, a random selection of MUT producers, i.e. without using our droplet-method, reduces the chances to enrich amino acid overproducers (Fig. S9). Thus, we demonstrate that the use of a combination of droplet-technology with FACS, allows to efficiently isolate overproducers.
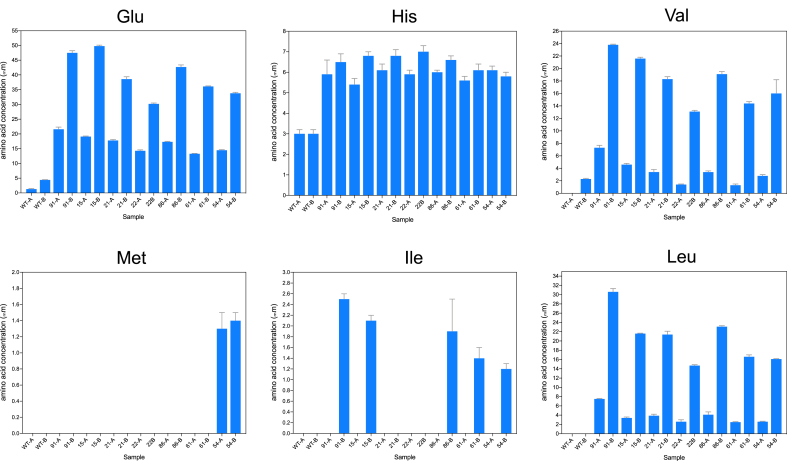
Seven L. lactis MUTproducer strains with the highest enhanced-amino acid secretion were selected to quantify the amino acids contents in culture supernatants. Fig. 4 shows that the enhancement of amino acid secretion favored mostly glutamic acid in all strains, with improvements up to ~10 fold (strains MUT-91 and MUT-15) compared to the wildtype, followed by leucine with ~30 fold higher concentrations in strain MUT-91, valine secretion improved ~10 fold in strain MUT-91 and MUT-15. And to a lesser extent, histidine secretion was doubled. Minor enhancements of isoleucine and methionine production were observed.
Fig. 4.
Contents of essential amino acids in culture supernatants of MUT strains. Values indicate concentration (μM), two sampling points are shown: A-end of lag growth phase, and B- mid exponential growth phase (see Fig. S4). After the strains were grown in CDM-casein medium, the concentrations of amino acids in the culture supernatants were measured using a HPLC assay coupled with fluorescence detection. The samples of the wildtype correspond to the first two bars in each plot (A and B samples). Quantifications were performed in duplicates, and average values are shown. Error is shown as SD. Exact concentrations for all the samples are shown in Table S6.
3.5. Identification of point mutations
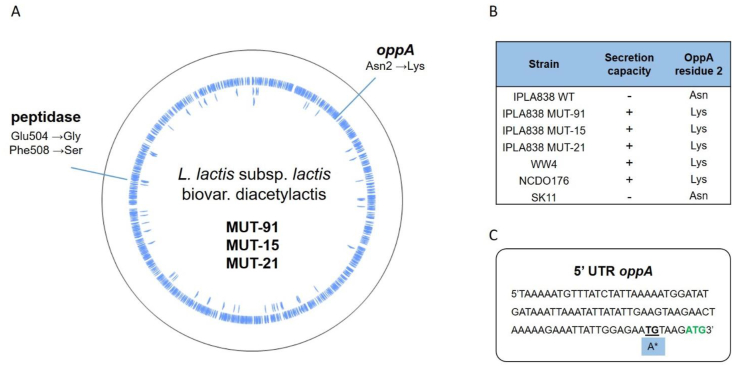
The genomic DNA of the three MUTproducer strains with the highest GFPsensor signal values in Fig. 3B (MUT-91, MUT-15 and MUT-21) was sequenced in order to identify the mutations in genes that confer enhanced amino acid secretion by L. lactis (see Methods). The DNA sequencing data reveal that the three strains have several mutations in common (see Table S3). In Fig. 5A two relevant mutations are shown, a double amino acid substitution in the IPLA838_00548 gene encoding a peptidase and one amino acid substitution in the oppA gene that encodes the oligopeptide-binding protein.
Fig. 5.
Identified mutations in the genomic DNA sequences of three MUT strains with the highest amino acid secretion. (A) Amino acid substitutions related to the proteolytic system of L. lactis are shown: two mutations F508S (TTT→TCT) and E504G (GAA→GGA) occurred in a gene encoding a peptidase (IPLA838_00548), and one mutation N2K (AAC→AAA) occurred in the oppA gene. (B) Relationship between the amino acid secretion capacity of different strains and the second residue of the OppA amino acid sequence. (C) Identified mutations in the 5′ UTR of the oppA gene, a transition (T→A) and the one nucleotide deletion (ΔG) is indicated with an asterisk (∗).
As described above, our findings show that the sole presence of the proteinase PrtP does not result in amino acid secretion (Fig. 1c, Fig. S2). Since there are several PrtP proteinase types, based on their amino acid sequences (Exterkate et al., 1993), we investigated whether the amino acid secretion capacity is related to a specific proteinase type. The amino acid sequences of the proteinase PrtP of strains with and without amino acid secretion capacity were analyzed and no relationship between this property and proteinase types or bacterial subspecies is observed (Table S4, Table S5).
Remarkably, the mutations in the IPLA838_00548 and oppA genes suggest that the secretion capacity by L. lactis is related to the peptide uptake system and the enzymes responsible to further degrade peptides into amino acids. We suggest that an imbalance or defect in peptides uptake or degradation, results in accumulation of free amino acids, which the cell needs to remove by secretion. Moreover, we analyzed the OppA protein sequences in both, positive and negative amino acid-secreting strains, and observed a relationship between the mutation Asn2→Lys that occurred in the IPLA838 strains and the amino acid secretion capacity (Fig. 5B). For instance, the positive amino acid-secreting strains MUT-91, MUT-15 and MUT21 have a lysine at position 2 of the OppA amino acid sequence as other positive amino acid-secreting strains such as WW4 and NCDO176. In contrast to the IPLA838 with low amino acid-secretion capacity, which has an asparagine at position 2 of the OppA amino acid sequence, as well in the OppA amino acid sequence of the negative amino acid-secreting strain SK11. In addition, Fig. 5C shows that other mutations observed in the three sequenced MUT strains are in the 5’ UTR region of the oppA gene, one nucleotide substitution (T→A) and one nucleotide deletion (ΔG). We speculate that these mutations contribute to the amino acid production capacity, although an extra analysis of the contribution of these mutations in the oppA expression is required.
4. Discussion
In this work we have developed and characterized a biosensor for detection of the amino acids leucine, isoleucine, valine, methionine, histidine and glutamic acid. Essentially, our biosensor might find application in the amino acids market because it can be employed to select for L. lactis strains that overproduce these amino acids. Another application is to develop this sensor further into a semi quantitative biosensor to detect amino acids in complex (food) matrices, which is currently a difficult task that depends on analytical techniques and tedious sample preparation methods (Bertels et al., 2012). But most relevant in this study, growth-based biosensors are useful tools for screening wild-type strains based on their ability to secrete metabolites (Bertels et al., 2012). In this respect, LAB strains with increased production of flavor-promoting amino acids are an attractive target (Le Bars and Yvon, 2007; Yvon and Rijnen, 2001). Hence, we characterized a small collection of L. lactis biovar diacetylactis strains based on their ability to secrete amino acids, and the GFPsensor enabled us to identify wild-type strains with high-yield amino acid secretion.
Droplet-based technologies have become a powerful strategy to improve the production of secreted metabolites (Chou et al., 2015; van Tatenhove-Pel et al., 2020). Based on the co-cultivation of mutated amino acid producing strains with the GFPsensor in agarose droplets, we could identify variants with increased secretion of mainly glutamate, leucine and valine. Moreover, we observe that when the GFPsensor grows in co-cultivation with either WW4 or MUT-91, it provides similar fluorescence signals, indicating similar growth. These observations support the idea that the free amino acids released by MUT-91, despite being lower than that of WW4, reach the minimum amount of essential amino acids to sustain the growth of the GFPsensor. In addition, we cannot exclude the possibility that by providing the minimum or higher concentrations of glutamic acid, leucine and valine, such as the secretion by MUT-91, once these amino acids are taken up, intracellular conversion of these three amino acids into other (essential) amino acids or chemical compounds benefits the GFPsensor growth.
It is important to note that when the GFPsensor meets the required or higher concentrations of the essential amino acids, the culture reaches similar cell densities. Since WW4 highly promotes the GFPsensor growth, i.e. secretes essential amino acids at high concentrations, the chances to distinguish enhanced secretion by WW4 are lower than when using a strain with relatively low levels of amino acid secretion such as IPLA838. Therefore, a limitation of our droplet-based screening is the selection of wild-type L. lactis strains with amino acid secretion at similar or higher yields to the WW4 strain. Further work on the GFPsensor to increase its amino acid requirements might tackle this limitation. For instance, a decrease in amino acid affinity by the GFPsensor would result in higher amino acid requirements for growth. Thus, an increased dynamic range might facilitate the selection of wild-type L. lactis strains with higher-titers of amino acids. Alternatively, one can adjust the ratio between the producer and sensor strain in the droplets when high production is expected.
In Gouda and Cheddar type cheese, the starter cultures contain L. lactis subsp. lactis and L. lactis subsp. cremoris, not only for milk acidification, but also for the formation of cheese flavor compounds through the production of peptides and amino acids, which are converted to volatile compounds (Marcelino, 2013). Sensory studies support the relationship of free amino acid content and flavor formation (Singh et al., 2003). Glutamate is largely responsible for the umami taste in cheeses, whereas branched-chain amino acids (leucine, valine and isoleucine) are the major precursors of aroma compounds (Drake et al., 2007; Smit et al., 2009). Therefore, we hypothesize that our isolated strains with increased concentrations of these amino acids might have a positive effect on flavor formation. Mutations in the peptide uptake and peptide degradation process, i.e. the gene encoding the oligopeptide transport system (oppA) and the IPLA838_00548 peptidase, suggest these genes as targets to improve amino acid secretion.
Food fermentations rely on a mixture of microbes to obtain a product with desired properties (Cárcoba et al., 2000; Kieronczyk et al., 2003). Mixed-culture fermentations are environments where microbes establish bacterial interactions such as metabolite exchange and the food products are a result of tasks performed by more than one microbe (Bachmann et al., 2017). Thus, our selected (non-GMO) strains with high-yield amino acid production are candidates to participate in consortia of microbes in fermenting food products. Since previous studies have shown that proteolytic L. lactis strains (PrtP+) supply peptides to non-proteolytic strains (PrtP-) (Sieuwerts et al., 2008), we propose that the secretion of essential amino acids might also exert a positive effect, for instance by stimulation the growth of other microbes in consortia.
CRediT authorship contribution statement
Jhonatan A. Hernandez-Valdes: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing - original draft. Myrthe aan de Stegge: Investigation, Data curation, Methodology, Validation, Writing - review & editing. Jos Hermans: Methodology, Validation, Writing - review & editing. Johan Teunis: Methodology, Validation, Writing - review & editing. Rinke J. van Tatenhove-Pel: Methodology, Writing - review & editing. Bas Teusink: Funding acquisition, Writing - review & editing. Herwig Bachmann: Funding acquisition, Writing - review & editing. Oscar P. Kuipers: Conceptualization, Funding acquisition, Supervision, Writing - review & editing.
Declaration of competing interest
We confirm that there are no conflicts of interest associated with this publication.
Acknowledgments
We thank Anne de Jong (Department of Molecular Genetics, University of Groningen) for his help to analyze the genome sequencing data. J.A.H.V., R.J.v.T, O.P.K, B.T. and H.B were financed by the Netherlands Organisation for Scientific Research, as part of the research program TTW with project number 13858.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mec.2020.e00133.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Adamberg K., Seiman A., Vilu R. Increased biomass yield of Lactococcus lactis by reduced overconsumption of amino acids and increased catalytic activities of enzymes. PloS One. 2012 doi: 10.1371/journal.pone.0048223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrio J.L., Demain A.L. Genetic improvementof processes yielding microbial products. FEMS Microbiol. Rev. 2006 doi: 10.1111/j.1574-6976.2005.00009.x. [DOI] [PubMed] [Google Scholar]
- Ayad E.H.E., Verheul A., De Jong C., Wouters J.T.M., Smit G. Flavour forming abilities and amino acid requirements of Lactococcus lactis strains isolated from artisanal and non-dairy origin. Int. Dairy J. 1999 doi: 10.1016/S0958-6946(99)00140-5. [DOI] [Google Scholar]
- Bachmann H., Molenaar D., Branco Dos Santos F., Teusink B. Experimental evolution and the adjustment of metabolic strategies in lactic acid bacteria. FEMS Microbiol. Rev. 2017 doi: 10.1093/femsre/fux024. [DOI] [PubMed] [Google Scholar]
- Bertels F., Merker H., Kost C. Design and characterization of auxotrophy-based amino acid biosensors. PloS One. 2012 doi: 10.1371/journal.pone.0041349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárcoba R., Delgado T., Rodríguez A. Comparative performance of a mixed strain starter in cow’s milk, Ewe’s milk and mixtures of these milks. Eur. Food Res. Technol. 2000 doi: 10.1007/s002170000157. [DOI] [Google Scholar]
- Centeno J.A., Tomillo F.J., Fernández-García E., Gaya P., Nuñez M. Effect of wild strains of Lactococcus lactis on the volatile profile and the sensory characteristics of ewes’ raw milk cheese. J. Dairy Sci. 2002 doi: 10.3168/jds.S0022-0302(02)74404-4. [DOI] [PubMed] [Google Scholar]
- Chen J., Vestergaard M., Jensen T.G., Shen J., Dufva M., Solem C., Jensena P.R. Finding the needle in the haystack-the use of microfluidic droplet technology to identify vitamin-secreting lactic acid bacteria. mBio. 2017 doi: 10.1128/mBio.00526-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Vestergaard M., Shen J., Solem C., Dufva M., Jensen P.R. Droplet-based microfluidics as a future tool for strain improvement in lactic acid bacteria. FEMS Microbiol. Lett. 2018 doi: 10.1093/femsle/fny258. [DOI] [PubMed] [Google Scholar]
- Chopin A. Organization and regulation of genes for amino acid biosynthesis in lactic acid bacteria. FEMS Microbiol. Rev. 1993 doi: 10.1016/0168-6445(93)90056-F. [DOI] [PubMed] [Google Scholar]
- Chou W.L., Lee P.Y., Yang C.L., Huang W.Y., Lin Y.S. Recent advances in applications of droplet microfluidics. Micromachines. 2015 doi: 10.3390/mi6091249. [DOI] [Google Scholar]
- Collins D.J., Neild A., deMello A., Liu A.Q., Ai Y. The Poisson distribution and beyond: methods for microfluidic droplet production and single cell encapsulation. Lab Chip. 2015 doi: 10.1039/c5lc00614g. [DOI] [PubMed] [Google Scholar]
- D’Este M., Alvarado-Morales M., Angelidaki I. Amino acids production focusing on fermentation technologies – a review. Biotechnol. Adv. 2018 doi: 10.1016/j.biotechadv.2017.09.001. [DOI] [PubMed] [Google Scholar]
- D’Souza G., Shitut S., Preussger D., Yousif G., Waschina S., Kost C. Ecology and evolution of metabolic cross-feeding interactions in bacteria. Nat. Prod. Rep. 2018 doi: 10.1039/c8np00009c. [DOI] [PubMed] [Google Scholar]
- De Palencia P.F., De La Plaza M., Mohedano M.L., Martínez-Cuesta M.C., Requena T., López P., Peláez C. Enhancement of 2-methylbutanal formation in cheese by using a fluorescently tagged Lacticin 3147 producing Lactococcus lactis strain. Int. J. Food Microbiol. 2004 doi: 10.1016/j.ijfoodmicro.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Deatherage D.E., Barrick J.E. Identification of mutations in laboratory-evolved microbes from next-generation sequencing data using breseq. Methods Mol. Biol. 2014 doi: 10.1007/978-1-4939-0554-6_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkx P.M.F., Janzen T., Sørensen K.I., Christensen J.E., Stuer-Lauridsen B., Johansen E. The art of strain improvement of industrial lactic acid bacteria without the use of recombinant DNA technology. Microb. Cell Factories. 2014 doi: 10.1186/1475-2859-13-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaisne A., Guellerin M., Laroute V., Laguerre S., Cocaign-Bousquet M., Bourgeois P. Le, Loubiere P. Genotypic and phenotypic analysis of dairy lactococcus lactis biodiversity in milk: volatile organic compounds as discriminating markers. Appl. Environ. Microbiol. 2013 doi: 10.1128/AEM.01018-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doeven M.K., Kok J., Poolman B. Specificity and selectivity determinants of peptide transport in Lactococcus lactis and other microorganisms. Mol. Microbiol. 2005 doi: 10.1111/j.1365-2958.2005.04698.x. [DOI] [PubMed] [Google Scholar]
- Drake S.L., Carunchia Whetstine M.E., Drake M.A., Courtney P., Fligner K., Jenkins J., Pruitt C. Sources of umami taste in Cheddar and Swiss cheeses. J. Food Sci. 2007 doi: 10.1111/j.1750-3841.2007.00402.x. [DOI] [PubMed] [Google Scholar]
- Exterkate F.A., Alting A.C., Bruinenberg P.G. Diversity of cell envelope proteinase specificity among strains of Lactococcus lactis and its relationship to charge characteristics of the substrate-binding region. Appl. Environ. Microbiol. 1993;59:3640–3647. doi: 10.1128/aem.00247-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flahaut N.A.L., Wiersma A., Van De Bunt B., Martens D.E., Schaap P.J., Sijtsma L., Dos Santos V.A.M., De Vos W.M. Genome-scale metabolic model for Lactococcus lactis MG1363 and its application to the analysis of flavor formation. Appl. Microbiol. Biotechnol. 2013 doi: 10.1007/s00253-013-5140-2. [DOI] [PubMed] [Google Scholar]
- Georgi T., Rittmann D., Wendisch V.F. Lysine and glutamate production by Corynebacterium glutamicum on glucose, fructose and sucrose: roles of malic enzyme and fructose-1,6-bisphosphatase. Metab. Eng. 2005 doi: 10.1016/j.ymben.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Goel A., Santos F., de Vos W.M., Teusink B., Molenaar D. Standardized assay medium to measure Lactococcus lactis enzyme activities while mimicking intracellular conditions. Appl. Environ. Microbiol. 2012 doi: 10.1128/aem.05276-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Méndez N., Vallejo-Cordoba B., González-Córdova A.F., Nevárez-Moorillón G.V., Rivera-Chavira B. Evaluation of aroma generation of Lactococcus lactis with an electronic nose and sensory analysis. J. Dairy Sci. 2008 doi: 10.3168/jds.2007-0193. [DOI] [PubMed] [Google Scholar]
- Helinck S., Le Bars D., Moreau D., Yvon M. Ability of thermophilic lactic acid bacteria to produce aroma compounds from amino acids. Appl. Environ. Microbiol. 2004 doi: 10.1128/AEM.70.7.3855-3861.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Valdes Jhonatan, van Gestel Jordi, Kuipers Oscar. A riboswitch gives rise to multi-generational phenotypic heterogeneity in an auxotrophic bacterium. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-15017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa T., Shimizu H. Recent advances in amino acid production by microbial cells. Curr. Opin. Biotechnol. 2016 doi: 10.1016/j.copbio.2016.04.017. [DOI] [PubMed] [Google Scholar]
- Kaminski T.S., Scheler O., Garstecki P. Droplet microfluidics for microbiology: techniques, applications and challenges. Lab Chip. 2016 doi: 10.1039/c6lc00367b. [DOI] [PubMed] [Google Scholar]
- Kieronczyk A., Skeie S., Langsrud T., Yvon M. Cooperation between Lactococcus lactis and nonstarter lactobacilli in the formation of cheese aroma from amino acids. Appl. Environ. Microbiol. 2003 doi: 10.1128/AEM.69.2.734-739.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita S., Udaka S., Shimono M. Studies on the amino acid fermentation Part I. Production of L-glutamic acid by various Microorganisms∗. J. Gen. Appl. Microbiol. 1957 doi: 10.2323/jgam.3.193. [DOI] [PubMed] [Google Scholar]
- Krämer R. Secretion of amino acids by bacteria: physiology and mechanism. FEMS Microbiol. Rev. 1994 doi: 10.1016/0168-6445(94)90102-3. [DOI] [Google Scholar]
- Kunji E.R.S., Hagting A., De Vries C.J., Juillard V., Haandrikman A.J., Poolman B., Konings W.N. Transport of β-casein-derived peptides by the oligopeptide transport system is a crucial step in the proteolytic pathway of Lactococcus lactis. J. Biol. Chem. 1995 doi: 10.1074/jbc.270.4.1569. [DOI] [PubMed] [Google Scholar]
- Le Bars D., Yvon M. Formation of diacetyl and acetoin by Lactococcus lactis via aspartate catabolism. J. Appl. Microbiol. 2007 doi: 10.1111/j.1365-2672.2007.03539.x. 0, 070915215109007-??? [DOI] [PubMed] [Google Scholar]
- Lee J.H., Wendisch V.F. Production of amino acids – genetic and metabolic engineering approaches. Bioresour. Technol. 2017 doi: 10.1016/j.biortech.2017.05.065. [DOI] [PubMed] [Google Scholar]
- Lim J.W., Ha D., Lee J., Lee S.K., Kim T. Review of micro/nanotechnologies for microbial biosensors. Front. Bioeng. Biotechnol. 2015 doi: 10.3389/fbioe.2015.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Bayjanov J.R., Renckens B., Nauta A., Siezen R.J. The proteolytic system of lactic acid bacteria revisited: a genomic comparison. BMC Genom. 2010;11:5–8. doi: 10.1186/1471-2164-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelino J. Lactic Acid Bacteria - R & D for Food, Health and Livestock Purposes. 2013. Lactic acid bacteria as starter-cultures for cheese processing: past, present and future developments. [DOI] [Google Scholar]
- Mee M.T., Collins J.J., Church G.M., Wang H.H. Syntrophic exchange in synthetic microbial communities. Proc. Natl. Acad. Sci. 2014 doi: 10.1073/pnas.1405641111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mierau I., Venema G., Kok J., Kunji E.R.S. Casein and peptide degradation in lactic acid bacteria. Biotechnol. Genet. Eng. Rev. 1997 doi: 10.1080/02648725.1997.10647945. [DOI] [PubMed] [Google Scholar]
- Overkamp W., Beilharz K., Weme R.D.O., Solopova A., Karsens H., Kovács Á.T., Kok J., Kuipers O.P., Veening J.W. Benchmarking various green fluorescent protein variants in Bacillus subtilis, Streptococcus pneumoniae, and Lactococcus lactis for live cell imaging. Appl. Environ. Microbiol. 2013 doi: 10.1128/AEM.02033-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinu F.R., Granucci N., Daniell J., Han T.L., Carneiro S., Rocha I., Nielsen J., Villas-Boas S.G. Metabolite secretion in microorganisms: the theory of metabolic overflow put to the test. Metabolomics. 2018 doi: 10.1007/s11306-018-1339-7. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Russell D. third ed. Cold Spring Harbor, N.Y. :Cold Spring Harbor Laboratory Press; 2001. Molecular Cloning: A Laboratory Manual. [Google Scholar]
- Sanz Y., Lanfermeijer F.C., Renault P., Bolotin A., Konings W.N., Poolman B. Genetic and functional characterization of dpp genes encoding a dipeptide transport system in Lactococcus lactis. Arch. Microbiol. 2001 doi: 10.1007/s002030100270. [DOI] [PubMed] [Google Scholar]
- Savijoki K., Ingmer H., Varmanen P. Proteolytic systems of lactic acid bacteria. Appl. Microbiol. Biotechnol. 2006 doi: 10.1007/s00253-006-0427-1. [DOI] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., Tinevez J.Y., White D.J., Hartenstein V., Eliceiri K., Tomancak P., Cardona A. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012 doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seefeldt K.E., Weimer B.C. Diversity of sulfur compound production in lactic acid bacteria. J. Dairy Sci. 2000 doi: 10.3168/jds.S0022-0302(00)75168-X. [DOI] [PubMed] [Google Scholar]
- Shitut S., Ahsendorf T., Pande S., Egbert M., Kost C. Nanotube-mediated cross-feeding couples the metabolism of interacting bacterial cells. Environ. Microbiol. 2019 doi: 10.1111/1462-2920.14539. [DOI] [PubMed] [Google Scholar]
- Sieuwerts S., De Bok F.A.M., Hugenholtz J., Van Hylckama Vlieg J.E.T. Unraveling microbial interactions in food fermentations: from classical to genomics approaches. Appl. Environ. Microbiol. 2008 doi: 10.1128/AEM.00113-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers F., Wilm A., Dineen D., Gibson T.J., Karplus K., Li W., Lopez R., McWilliam H., Remmert M., Söding J., Thompson J.D., Higgins D.G. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011 doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siezen R.J., Bayjanov J.R., Felis G.E., van der Sijde M.R., Starrenburg M., Molenaar D., Wels M., van Hijum S.A.F.T., van Hylckama Vlieg J.E.T. Genome-scale diversity and niche adaptation analysis of Lactococcus lactis by comparative genome hybridization using multi-strain arrays. Microb. Biotechnol. 2011 doi: 10.1111/j.1751-7915.2011.00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh T.K., Drake M.A., Cadwallader K.R. Flavor of cheddar cheese: a chemical and sensory perspective. Compr. Rev. Food Sci. Food Saf. 2003 doi: 10.1111/j.1541-4337.2003.tb00021.x. [DOI] [PubMed] [Google Scholar]
- Sinha N., Subedi N., Wimmers F., Soennichsen M., Tel J. A pipette-tip based method for seeding cells to droplet microfluidic platforms. J. Vis. Exp. 2019 doi: 10.3791/57848. [DOI] [PubMed] [Google Scholar]
- Smit B.A., Engels W.J.M., Smit G. Branched chain aldehydes: production and breakdown pathways and relevance for flavour in foods. Appl. Microbiol. Biotechnol. 2009 doi: 10.1007/s00253-008-1758-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit G., Smit B.A., Engels W.J.M. Flavour formation by lactic acid bacteria and biochemical flavour profiling of cheese products. FEMS Microbiol. Rev. 2005 doi: 10.1016/j.femsre.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Song A.A.L., In L.L.A., Lim S.H.E., Rahim R.A. A review on Lactococcus lactis: from food to factory. Microb. Cell Factories. 2017 doi: 10.1186/s12934-017-0669-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terekhov S.S., Smirnov I.V., Stepanova A.V., Bobik T.V., Mokrushina Y.A., Ponomarenko N.A., Belogurov A.A., Rubtsova M.P., Kartseva O.V., Gomzikova M.O., Moskovtsev A.A., Bukatin A.S., Dubina M.V., Kostryukova E.S., Babenko V.V., Vakhitova M.T., Manolov A.I., Malakhova M.V., Kornienko M.A., Tyakht A.V., Vanyushkina A.A., Ilina E.N., Masson P., Gabibov A.G., Altman S. Microfluidic droplet platform for ultrahigh-throughput single-cell screening of biodiversity. Proc. Natl. Acad. Sci. U.S.A. 2017 doi: 10.1073/pnas.1621226114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teusink B., Bachmann H., Molenaar D. Systems biology of lactic acid bacteria: a critical review. Microb. Cell Factories. 2011;10:S11. doi: 10.1186/1475-2859-10-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tatenhove-Pel R.J., Hernandez-Valdes J.A., Teusink B., Kuipers O.P., Fischlechner M., Bachmann H. Microdroplet screening and selection for improved microbial production of extracellular compounds. Curr. Opin. Biotechnol. 2020 doi: 10.1016/j.copbio.2019.10.007. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S., Ninomiya K. Umami and food palatability. J. Nutr. 2000 doi: 10.1093/jn/130.4.921s. [DOI] [PubMed] [Google Scholar]
- Yvon M., Rijnen L. Cheese flavour formation by amino acid catabolism. Int. Dairy J. 2001 doi: 10.1016/S0958-6946(01)00049-8. [DOI] [Google Scholar]
- Ziadi M., Bergot G., Courtin P., Chambellon E., Hamdi M., Yvon M. Amino acid catabolism by Lactococcus lactis during milk fermentation. Int. Dairy J. 2010 doi: 10.1016/j.idairyj.2009.07.004. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.