Abstract
Bladder cancer (BC) is a relatively common tumor, with a male preponderance. High-grade muscle invasive bladder cancer (MIBC) has a very high incidence of pelvic lymph node metastasis at presentation. Involvement of the retro-crural lymph nodes, although has been described in other pelvic tumors, is very uncommon for BC. Cryoablation in the retro-crural region is extremely challenging due to the proximity to the critical structures like inferior venacava and aorta and has not been extensively reported. We describe a 56-year old male patient with MIBC who underwent extensive treatments including radical cystoprostatectomy, chemoradiation and immunotherapy, ultimately with localized disease in the retro-crural region. Single session cryoablation of these lymph nodes was performed with a curative intent yielding a positive response that has persisted for more than 2 years.
Keywords: Transitional cell cancer (TCC); Cryoablation; Retro-crural; Muscle invasive bladder cancer, Positron emission tomography
Introduction
Bladder cancer (BC) is the fourth most prevalent malignancy in the United states [1]. Majority of them are transitional cell cancers (TCC) [1]. The likelihood of nodal metastasis increases with the depth of invasion and hence radical cystectomy with pelvic lymph node dissection is the recommended treatment for muscle invasive bladder cancer (MIBC) [2,3]. The obturator and the internal iliac lymph nodes are most commonly involved. Involvement of retro crural lymph nodes, in TCC has not been previously reported. Patients with distant metastatic disease are usually treated with systemic therapy with a palliative intent. In this case report, we describe a case of bladder TCC metastatic to the retro-crural nodes refractory to systemic therapy, successfully treated with cryoablation yielding a long-term sustained response.
Case report
A workup for asymptomatic hematuria in a 56-year-old male patient with cystoscopy and transurethral resection of bladder tumor (TURBT) revealed high grade muscle invasive TCC. A staging positron emission tomography (PET) scan at the time of initial diagnosis, showed 18-Fluoro deoxy glucose (18FDG) avid retroperitoneal (RP) and pelvic lymph nodes. Based on this his clinical staging was T2N2M0 (stage IV). Following this, he received 6 cycles of neoadjuvant chemotherapy with a combination of methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) and the follow-up PET scan showed good response with decreased activity and size of the RP and pelvic lymph nodes. Four months after initiating neoadjuvant chemotherapy, the patient was discussed at tumor board and considered a good candidate for radical cystoprostatectomy, bilateral pelvic/RP lymph node dissection and creation of an ileal conduit. Three-months after the surgery the follow up PET, showed 18FDG avid, enlarged RP lymph nodes, suggestive of disease recurrence. Patient was treated with mitomycin + 5 Flurouracil and concurrent radiation. A dose of 4500 cGy in 25 fractions to the pelvis to include the pelvic and RP lymph nodes was followed by a boost to the PET positive lymph nodes resulting in a final dose of 5400 cGy in 30-33 fractions. The PET scan obtained four months after the final fraction of radiation treatment showed complete response to chemoradiation. Follow-up PET in 4 months, unfortunately showed enlarged 18FDG avid retro-crural lymph nodes. This was considered as first line treatment failure and the patient started on immunotherapy (pembrolizumab). However, despite immunotherapy, there was stable persistent 18F-FDG activity in the enlarged retro-crural lymph nodes (Fig. 1). The lymph nodes measured 8 & 12 mm in short axis dimensions and demonstrated standardize uptake values of 7.4 and 7.2, respectively (Fig. 1). This was the only site of recurrent/residual disease nine months after initiating immunotherapy. He was discussed at multi-disciplinary tumor board and referred to interventional radiology for cryoablation of this stable metastatic nodal disease.
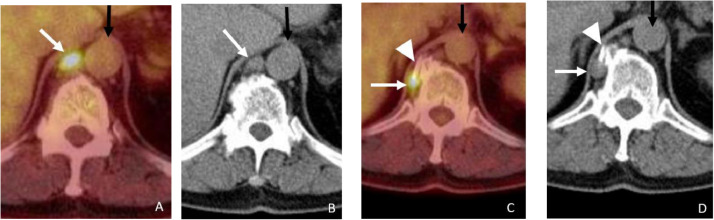
Fig. 1.
Precryoablation PET/CT. The axial fused PET/CT images (A and C) and the corresponding CT images (C and D) showing enlarged 18FDG avid lymph nodes in the retro-crural region (white arrows). The more superior lymph node (A and B) is very close to the right lateral margin of the Aorta (black arrows) and the more inferior lymph node (C and D) is on the right side of the vertebral body adjacent to an osteophyte.
The procedure was performed in the prone position with conscious sedation. Two PCS-24/RS-24 (Healthcare. Inc, Austin, TX, USA) cryoprobes were used for the ablation. The probes were advanced under Computed tomography (CT) guidance into each of the lymph nodes and positioned appropriately to ensure complete coverage of the lymph nodes (Fig. 2). Two 12-minute freeze separated by a 5-minute thaw cycle were performed. Intraprocedural CT imaging confirmed that both the nodes were included in the resulting ice ball, without involving the adjacent vital structures (Fig. 2). Following the ablation, the probes were withdrawn, and no complications were noted on the post procedure CT. The patient was discharged same day after appropriate recovery from sedation.
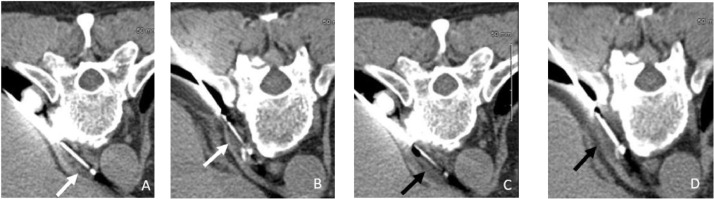
Fig. 2.
Prone position axial CT of the superior (A) and inferior (B) lymph nodes with the cryoprobes in place (white arrows). Prone position axial CT images of the superior (C) and inferior (D) lymph nodes after the 12-minute freeze phase showing the ice ball encompassing the lymph nodes. The loss of margins and fluid like appearance of the lymph nodes (black arrows) is due to the low density of the ice ball.
Follow up PET scan 6 months after the cryoablation, revealed resolution of the 18FDG avidity and significant decrease in size of both the lymph nodes, (Fig. 3) suggesting a complete response to treatment. The positive response to cryoablation has lasted for more than 2 years, at the time of writing this manuscript, as confirmed by follow up PET scans obtained at 6-month intervals from the time of the procedure. No additional sites of recurrence have been noted either.
Fig. 3.
Follow-up PET/CT 6 months after cryoablation. Axial PET/CT fused images (A and C) and corresponding CT images (B and D) showing no 18FDG activity in the retro-crural region. The superior lymph node (white arrow in A and B) is barely visualized and the inferior lymph node (white arrow in C and D) is not seen suggesting good response to treatment. Note the osteophyte at the level of the inferior lymph node serves as a bony landmark to identify the location of the lymph node prior to cryoablation (as seen in Fig. 1C and D).
Discussion
BC is the 9th most common cancer worldwide, 4th most common cancer in the United States and the 7th most common cancer in men [1,4] Majority of the patients are men with a mean age 73 years at diagnosis [4]. Risk factors for BC include, male sex, older age, cigarette smoking, white race, exposure to certain chemicals, pelvic radiation, chronic bladder infection/irritation [5]. TCC is the most common type of BC, accounting for approximately 90% of cases [6]. Painless hematuria is the most common presenting symptom of BC [7]. Patients with advanced disease may present with symptoms related to metastatic involvement. The most common sites for metastasis are lymph nodes, bone, lung, liver, and peritoneum [8,9]. Adrenal gland metastasis is rarely seen [10]. Cystoscopy combined with CT urography is commonly employed in the diagnosis and work up of patients with BC [1,7]. PET/CT is used for overall staging of the disease given its high specificity to identify lymph node metastasis [11], [12], [13]. Patients with abnormal findings on cystoscopy usually undergo TURBT, which removes the visible tumor, provides information needed for definitive diagnosis, grading, and assess the depth of invasion [14,15]. Treatment of BC depends on the pathologic extent of disease at the time of TURBT and on subsequent staging according to the tumor-node-metastasis classification system. Most tumors are nonmuscle-invasive bladder cancers. The primary treatment for nonmuscle-invasive bladder cancers is TURBT, followed by instillation of Bacillus Calmette–Guérin or intravesical chemotherapy [1,16]. However for MIBC, more aggressive treatments in the form of cisplatin-based neoadjuvant chemotherapy followed by radical cystectomy with bilateral pelvic lymphadenectomy are strongly recommended [17], [18], [19], [20]. Extensive lymph node dissection has been shown to improve progression-free and overall survival [21,22]. However, in patients with metastatic disease at presentation chemotherapy is the preferred treatment option [23,24]. Immunotherapy is approved as a second-line treatment for metastatic BC in patients who fail or cannot tolerate chemotherapy [25,26]. Chemoradiotherapy is recommended for recurrent or persistent disease in MIBC [1].
Lymph node spread occurs commonly with high-grade MIBC [27,28]. Major draining sites are the regional obturator and internal iliac lymphnodes [29], [30], [31], [32]. Though retro-crural lymph node metastasis has been described for other malignancies like ovarian cancer, to our knowledge we did not find reports of BC metastasis to this lymph node network [33], [34], [35].
Cryoablation has been safely and effectively used in the definitive treatment of primary lung, liver, prostate, and renal cancers [36], [37], [38], [39], [40]. The biggest advantage of cryoablation over surgery is that it is minimally invasive, making it an attractive option for patients with several co-morbidities which would render them inoperable. Cryoablation achieves cellular damage in two phases: a directly cytotoxic freezing phase followed by a thawing phase. The freezing cycle induces cellular dehydration and ice formation which disorganizes cellular structures and function, which are then completely disrupted by the intracellular water flux that occurs during the thawing phase [41], [42], [43].
Percutaneous ablation in general is an underutilized tool in management of recurrent/ metastatic disease. As with surgery, cryoablation when properly performed can destroy tumor cells, thereby eliminate localized disease, and improve overall survival as in our patient who is disease free 2 years into his cryoablation procedure. The retro-crural area is close to critical structures like inferior venacava and aorta, which makes cryoablation of lesions in this area more challenging than elsewhere, and perhaps the reason why there are not many reports of ablations performed in this zone. This along with the long-lasting positive response to cryoablation of lymph nodes in an area which is unusual for spread of BC, make this case worthy of report.
Contributorship statement
Study conception (AB, JM, JH, RD), Data collection (JM, AB), Manuscript writing (JM, AB, JH,RD), Critical revision (AB,JM), Final approval (AA, JH, RD, JM, AB).
Data sharing
Data used in this study are not shared publicly.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Acknowledgments: None.
Conflicts of interest: The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at 10.1016/j.radcr.2020.05.022.
Appendix A. Supplementary materials
Supplementary Raw Research Data. This is open data under the CC BY license http://creativecommons.org/licenses/by/4.0/
References
- 1.Clark PE, Agarwal N, Biagioli MC, Eisenberger MA, Greenberg RE, Herr HW. Bladder cancer: clinical practice guidelines in oncology. JNCCN J Natl Compr Cancer Netw. 2013;11(4):446–475. doi: 10.6004/jnccn.2013.0059. [DOI] [PubMed] [Google Scholar]
- 2.Gakis G, Efstathiou J, Lerner SP, Cookson MS, Keegan KA, Guru KA. ICUD-EAU international consultation on bladder cancer 2012: radical cystectomy and bladder preservation for muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2013;63(1):45–57. doi: 10.1016/j.eururo.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Schuchardt P, Yasin J, Davis RM, Thimmappa N, Bhat AP. Pelvic trauma. Contemp Diagnostic Radiol. 2019;42(21):1–6. doi: 10.1097/01.CDR.0000582600.38333.f4. [DOI] [Google Scholar]
- 4.Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2017;71(1):96–108. doi: 10.1016/j.eururo.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Cumberbatch MGK, Cox A, Teare D, Catto JWF. Contemporary occupational carcinogen exposure and bladder cancer. JAMA Oncol. 2015;1(9):1282–1290. doi: 10.1001/jamaoncol.2015.3209. [DOI] [PubMed] [Google Scholar]
- 6.Eble JN, Sauter G, Epstein JI, Sesterhenn IA. World Health Organization classification of tumours. Pathol Genet Tumours Urinary Syst Male Genital Organs. 2004 [Google Scholar]
- 7.Davis R, Jones JS, Barocas DA, Castle EP, Lang EK, Leveillee RJ. Diagnosis, evaluation and follow-up of asymptomatic microhematuria (AMH) in adults: AUA guideline. J Urol. 2012;188(6 suppl.):2473–2481. doi: 10.1016/j.juro.2012.09.078. [DOI] [PubMed] [Google Scholar]
- 8.Shinagare AB, Ramaiya NH, Jagannathan JP, Fennessy FM, Taplin ME, Van Den Abbeele AD. Metastatic pattern of bladder cancer: correlation with the characteristics of the primary tumor. Am J Roentgenol. 2011;196(1):117–122. doi: 10.2214/AJR.10.5036. [DOI] [PubMed] [Google Scholar]
- 9.Verma M, Yarlagadda B, Hendrani A, Bhat AP, Kumar S. Simplified rapid protocol for assessing the thoracic aortic dimensions and pathology with noncontrast MR angiography. Int J Angiol. 2019;28(2):130–136. doi: 10.1055/s-0039-1688473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kabeel K, Marjara J, Bhat R, Gaballah AH, Abdelaziz A, Bhat AP. Spontaneous hemorrhage of an adrenal myelolipoma treated with transarterial embolization: a case report. Radiol Case Rep. 2020;15(7):961–965. doi: 10.1016/j.radcr.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeong IG, Hong S, You D, Hong JH, Ahn H, Kim CS. FDG PET–CT for lymph node staging of bladder cancer: a prospective study of patients with extended pelvic lymphadenectomy. Ann Surg Oncol. 2015;22(9):3150–3156. doi: 10.1245/s10434-015-4369-7. [DOI] [PubMed] [Google Scholar]
- 12.Soubra A, Hayward D, Dahm P, Goldfarb R, Froehlich J, Jha G. The diagnostic accuracy of 18F-fluorodeoxyglucose positron emission tomography and computed tomography in staging bladder cancer: a single-institution study and a systematic review with meta-analysis. World J Urol. 2016;34(9):1229–1237. doi: 10.1007/s00345-016-1772-z. [DOI] [PubMed] [Google Scholar]
- 13.Bhat A, Davis R, Bryan W. A rare case of bleeding duodenal varices from superior mesenteric vein obstruction -treated with transhepatic recanalization and stent placement. Indian J Radiol Imaging. 2019;29(3):313. doi: 10.4103/ijri.ijri_21_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall MC, Chang SS, Dalbagni G, Pruthi RS, Seigne JD, Skinner EC. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol. 2007;178(6):2314–2330. doi: 10.1016/j.juro.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Bhat AP, Pimpalwar A, Dyke PC. Ultrasonography and X-Ray guided drain placement to evacuate a pneumopericardium/pneumomediastinum in a 1-day-old infant. Indian J Radiol Imaging. 2019;29(1):94–97. doi: 10.4103/ijri.IJRI_447_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel PJ, Hieb RA, Bhat AP. Percutaneous revascularization of chronic total occlusions. Tech Vasc Interv Radiol. 2010;13(1):23–36. doi: 10.1053/j.tvir.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Hollenbeck BK, Dunn RL, Ye Z, Hollingsworth JM, Skolarus TA, Kim SP. Delays in diagnosis and bladder cancer mortality. Cancer. 2010;116(22):5235–5242. doi: 10.1002/cncr.25310. [DOI] [PubMed] [Google Scholar]
- 18.Gore JL, Lai J, Setodji CM, Litwin MS, Saigal CS. Mortality increases when radical cystectomy is delayed more than 12 weeks: results from a surveillance, epidemiology, and end results-medicare analysis. Cancer. 2009;115(5):988–996. doi: 10.1002/cncr.24052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang SS, Bochner BH, Chou R, Dreicer R, Kamat AM, Lerner SP. Treatment of non-metastatic muscle-invasive bladder cancer: AUA/ASCO/ASTRO/SUO guideline. J Urol. 2017;198(3):552–559. doi: 10.1016/j.juro.2017.04.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhat P, Sridhar P, Sreenivasan N, Kalyanpur A. Ct diagnosis of epiploic appendagitis-a case report. Indian J Radiol Imaging. 2006;16(4):447. doi: 10.4103/0971-3026.32242. [DOI] [Google Scholar]
- 21.Stimson CJ, Chang SS, Barocas DA, Humphrey JE, Patel SG, Clark PE. Early and late perioperative outcomes following radical cystectomy: 90-day readmissions, morbidity and mortality in a contemporary series. J Urol. 2010;184(4):1296–1300. doi: 10.1016/j.juro.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Sreenivasan N, Kalyanpur A, Bhat A, Sridhar P, Singh J. CT diagnosis of cecal diverticulitis. Indian J Radiol Imaging. 2006;16(4):451. doi: 10.4103/0971-3026.32244. [DOI] [Google Scholar]
- 23.Leow JJ, Martin-Doyle W, Rajagopal PS, Patel CG, Anderson EM, Rothman AT. Adjuvant chemotherapy for invasive bladder cancer: a 2013 updated systematic review and meta-analysis of randomized trials. Eur Urol. 2014;66(1):42–54. doi: 10.1016/j.eururo.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 24.Ghouri MA, Gupta N, Bhat AP, Thimmappa ND, Saboo SS, Khandelwal A. CT and MR imaging of the upper extremity vasculature: pearls, pitfalls, and challenges. Cardiovasc Diagn Ther. 2019;9(S1):S152–S173. doi: 10.21037/cdt.2018.09.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghatalia P, Zibelman M, Geynisman DM, Plimack E. Approved checkpoint inhibitors in bladder cancer: which drug should be used when. Ther Adv Med Oncol. 2018;10 doi: 10.1177/1758835918788310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konala VM, Adapa S, Aronow WS. Immunotherapy in bladder cancer. Am J Ther. February 2019;1 doi: 10.1097/mjt.0000000000000934. [DOI] [PubMed] [Google Scholar]
- 27.Shankar PR, Barkmeier D, Hadjiiski L, Cohan RH. A pictorial review of bladder cancer nodal metastases. Transl Androl Urol. 2018;7(5):804–813. doi: 10.21037/tau.2018.08.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thimmappa N, Bhat AP, Bishop K, Nagpal P, Prince MR, Saboo SS. Preoperative cross-sectional mapping for deep inferior epigastric and profunda artery perforator flaps. Cardiovasc Diagn Ther. 2019;9(S1):S131–S142. doi: 10.21037/cdt.2018.10.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roth B, Wissmeyer MP, Zehnder P, Birkhäuser FD, Thalmann GN, Krause TM. A new multimodality technique accurately maps the primary lymphatic landing sites of the bladder. Eur Urol. 2010;57(2):205–211. doi: 10.1016/j.eururo.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 30.Hedgire SS, Pargaonkar VK, Elmi A, Harisinghani AM, Harisinghani MG. Pelvic nodal imaging. Radiol Clin North Am. 2012;50(6):1111–1125. doi: 10.1016/j.rcl.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Paño B, Sebastià C, Buñesch L, Mestres J, Salvador R, Macías NG. Pathways of lymphatic spread in male urogenital pelvic malignancies. Radiographics. 2011;31(1) doi: 10.1148/rg.311105072. [DOI] [PubMed] [Google Scholar]
- 32.Schuchardt PA, Yasin JT, Davis RM, Tewari SO, Bhat AP. The role of an IVC filter retrieval clinic-a single center retrospective analysis. Indian J Radiol Imaging. 2019;29(4):391–396. doi: 10.4103/ijri.IJRI_258_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Im HJ, il Kim Y, Paeng JC, Chung JK, Kang SB, Lee DS. Retrocrural lymph node metastasis disclosed by 18F-FDG PET/CT: a predictor of supra-diaphragmatic spread in ovarian cancer. Nucl Med Mol Imaging (2010) 2012;46(1):41–47. doi: 10.1007/s13139-011-0115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McMahon CJ, Rofsky NM, Pedrosa I. Lymphatic metastases from pelvic tumors: anatomic classification, characterization, and staging. Radiology. 2010;254(1):31–46. doi: 10.1148/radiol.2541090361. [DOI] [PubMed] [Google Scholar]
- 35.Bhat AP, Schuchardt PA, Bhat R, Davis RM, Singh S. Metastatic appendiceal cancer treated with Yttrium 90 radioembolization and systemic chemotherapy: a case report. World J Radiol. 2019;11(13):116–125. doi: 10.4329/wjr.v11.i9.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maria T, Georgiades C. Percutaneous cryoablation for renal cell carcinoma. J Kidney Cancer VHL. 2015;2(3):105. doi: 10.15586/jkcvhl.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheen AJ, Poston GJ, Sherlock DJ. Cryotherapeutic ablation of liver tumours. Br J Surg. 2002;89(11):1396–1401. doi: 10.1046/j.1365-2168.2002.02292.x. [DOI] [PubMed] [Google Scholar]
- 38.Littrup PJ, Freeman-Gibb L, Andea A, White M, Amerikia KC, Bouwman D. Cryotherapy for breast fibroadenomas. Radiology. 2005;234(1):63–72. doi: 10.1148/radiol.2341030931. [DOI] [PubMed] [Google Scholar]
- 39.Mouraviev V, Polascik TJ. Update on cryotherapy for prostate cancer in 2006. Curr Opin Urol. 2006;16(3):152–156. doi: 10.1097/01.mou.0000193393.54598.9f. [DOI] [PubMed] [Google Scholar]
- 40.Senne J, Davis R, Yasin J, Brimmo O, Evenski A, Bhat A. Computed tomography guided radio-frequency ablation of osteoid osteomas in atypical locations. Indian J Radiol Imaging. 2019;29(3):253. doi: 10.4103/ijri.ijri_259_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baust JG, Gage AA, Bjerklund Johansen TE, Baust JM. Mechanisms of cryoablation: clinical consequences on malignant tumors. Cryobiology. 2014;68(1):1–11. doi: 10.1016/j.cryobiol.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erinjeri JP, Clark TWI. Cryoablation: mechanism of action and devices. J Vasc Interv Radiol. 2010;21(suppl. 8):S187. doi: 10.1016/j.jvir.2009.12.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yasin J, Thimmappa N, Kaifi JT, Avella DM, Davis R, Tewari SO. CT-guided cryoablation for post-thoracotomy pain syndrome: A retrospective analysis. Diagnostic Interv Radiol. 2020;26(1):53–57. doi: 10.5152/dir.2019.19179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Raw Research Data. This is open data under the CC BY license http://creativecommons.org/licenses/by/4.0/