Abstract
Background
Surgery remains to be the main therapeutic approach for retroperitoneal sarcomas (RPS) although evidence supports that complementary radiotherapy increases local-control and survival. We present a multidisciplinary management and experience of a tertiary cancer center in the treatment of RPS and analyze current evidence of radiotherapy efficacy.
Patients and methods
We retrospectively reviewed 19 patients with primary or relapsed RPS treated between November 2009 and October 2018. Multidisciplinary approach comprised complete resection in 15 patients (79%) achieving resection R0 in 11 patients (58%), R1 in 4 patients (21%) and R2 in 2 patients (10%). Seven patients (37%) underwent a preoperative radiation (PRORT), 10 patients (53%), post-operative radiation (PORT) and 2 patients (10%), received radiotherapy exclusively. Ten patients (53%) received adjuvant chemotherapy.
Results
With a median follow-up of 24 months (2–114 months), actuarial rates of loco-regional relapse free survival (LRFS) at 1, 2 and 3 years were 77%, 77% and 67%, respectively. Actuarial rates of distant-metastases-free survival (DMFS), disease-free survival (DFS) and overall survival (OS) at 1, 2 and 3 years were 100%, 100% and 80% for DMFS; 94%, 77% and 67% for DFS and 100%, 91% and 91% for OS, respectively. Only surgical margins (negative vs. positive) showed significance for 3y-LRFS: 100% vs. 34.3%, p = 0.018. Treatment tolerance was acceptable with no acute or late toxicity higher than grade 2.
Conclusions
Complementary radiotherapy appears to be useful and well tolerated for the multidisciplinary management of RPS. Presence of positive surgical margins seems to be the most relevant prognostic factor through the follow-up.
Keywords: Retroperitoneal sarcomas, Radiation treatment, Locoregional recurrence-free survival, Overall survival
1. Introduction
Retroperitoneal soft tissue sarcomas (RPS) are comprised of a set of rare tumors with a crude incidence of 0.3 cases per 100,000 per year and with an estimated survival at 5 years of 42% according to the data of the RARECARE project in Europe.1
Optimal treatment of RPS comprises complete macroscopically surgical resection without leaving tumor cells in resection margins (R0 resection) although due to the infiltrative nature of the RPS, the frequent close vicinity to surrounding healthy structures and the habitual large tumor size, attaining R0 surgery could be difficult and local recurrence rate remains high. Unlike to what has been established for soft-tissue sarcomas (STS) arising in the extremities, the evidence of benefits from the addition of either adjuvant or neo-adjuvant radiation treatment to radical surgery in the management of RPS is still a subject of debate, although recent analysis by using large databases have shown that radiation treatment in RPS not only improves local control but also is an independent prognostic factor for overall survival in high risk RPS.2, 3, 4, 5, 6, 7, 8
We present the results of a retrospective analysis of our institutional experience through the use of pre- or postoperative radiotherapy in the multidisciplinary management of RPS, as well as a comprehensive review of the evidence published during the 21st century in relation to the effectiveness of radiation treatment for RPS and of the existence of risk factors that could recommend its use in daily clinical practice.
2. Materials and methods
Patients with histological diagnosis of RPS who received pre- or postoperative radiotherapy as part of a multidisciplinary treatment with curative intention between November 2009 and October 2018 were retrospectively analyzed.
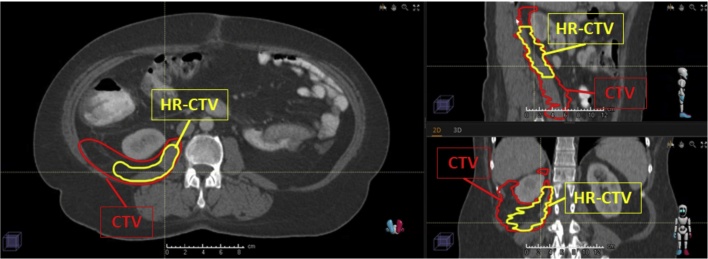
All the patients were simulated in a supine position using a vacuum-fix bag and with both arms raised above the head. CT was performed with oral and intravenous contrast and image acquisition slices of ≤3-mm. Target volumes were delineated according to internal guidelines as follows: gross tumor volume (GTV) was defined as the macroscopic tumor observed on the axial images and clinical target volume (CTV) was determined by adding 2 cm in the cephalo-caudal and radial direction to GTV. In those patients undergoing PORT, CTV included the resection bed and residual macroscopic tumor, if present, while avoiding healthy organs that have settled into the post-operative cavity. An attempt was made to define a high-risk area for recurrence (HR-CTV) in patients who had received PORT by joint assessment with a surgeon, radiologist, and radiation oncologist (Fig. 1). Planning target volume (PTV) was defined by adding 0.5–1 cm to CTV. Organs at risk (OAR) including the stomach, duodenum, small bowel, kidneys and spinal cord were contoured and classified as avoidance structures. The primary objective was to obtain a PTV coverage higher or equal to 95% with 95% of the prescription dose (PTV V95% ≥ 95%). Dose in OAR were constricted to: a maximal dose (Dmax) of 55 Gy in the stomach, duodenum or small bowel; Dmax of 45 Gy in spinal cord and median dose (Dmed) inferior to 20 Gy in both kidneys unless close/directly involved.
Fig. 1.
PORT volume definition: red contour represents primary clinical target volume (CTV), a larger area at risk that will receive a moderate radiation dose, whereas yellow contour represents high risk posterior margin (HR-CTV) that will simultaneously receive a higher total dose. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Elekta's XiO (Elekta Instrument AB Stockholm, Sweden) planning system was used until 2014. Afterwards, dosimetry plans were generated by using RayStation (RaySearch Laboratories, Stockholm, Sweden). Fourteen patients (74%) underwent IMRT/VMAT with IGRT, whereas in 5 patients (26%) conformal 3D-plans were used. Conventional 2 Gy fractionation was used in 9 patients (48%), moderate hypofractionation (2.4–3 Gy/fraction) in 5 patients (26%) and extreme fractionation (≥4 Gy/fraction) in 5 patients (26%). Globally, more than half of patients underwent hypo-fractionated radiotherapy. Due to differences related to fractionations used in our series we calculated biologically effective dose (BED) and biologically equivalent doses in a 2 Gy fraction (EQD2 Gy) according to linear-quadratic formalism in order to facilitate further comparisons between different radiation schedules. BED refers to the biological effect of any radiotherapy treatment, taking into account changes in dose-per-fraction or dose rate, total dose and overall treatment time,9 but biologically effective is not the same as biologically equivalent, leading to the concept of EQD2 Gy to compare treatments administered with different doses and fractions. The main parameters of this model, α and β, represent the intrinsic radio-sensitivity and have been defined for both, normal tumors and normal tissues. Although the α/β ratio for soft-tissue sarcoma is still unknown, a value below 10 (mostly, α/β ≈ 4 Gy) has been suggested to favor a high fractionation sensitivity.10, 11 Assuming α/β value of 4 for sarcoma cells corresponding to BED and EQD2 values for a conventional treatment of 25 fractions at 2 Gy/day would be 75 Gy and 50 Gy, respectively. In our series, median administered EQD2 Gy dose is 64 Gy (range 50–75 Gy) and median BED is 94 Gy (range 75–120 Gy), representing a moderate total dose escalation over the 50–50.4 Gy traditionally used.
3. Statistical evaluation
Follow-up was considered from the end of the entire treatment to the date of the last evaluation. Disease free survival (DFS) was estimated from the last day of EBRT or the day of surgery until locoregional or distant relapse. Loco-regional relapse free survival (LRFS) and distant metastases free survival (DMFS) were estimated at the time of first event. Patients dying from intercurrent disease without evidence of tumor were censored at the date of death. Overall survival (OS) was defined as the time interval between treatment and the date of death, whatever the cause, or the date of last follow-up. Statistical analysis was performed using SYSTAT, version 20.0 (SPSS, Chicago, IL). Actuarial LRFS, DMFS, DFS and OS were calculated using the Kaplan–Meier method. A log-rank test was used for comparison between survival curves and a Chi-square test was used for comparisons between groups. A level of p < 0.05 was considered statistically significant.12
Acute and late complications were scored according to the CTCAE v5.0 scale proposed by the National Cancer Institute.13 Acute toxicity was defined as the adverse effects registered in the patients from the first day of EBRT until 3 months after its finalization. Late toxicity was defined as adverse effects directly attributable to EBRT observed from 3 months to date of last follow-up. The toxicities reported included only those attributable to local treatments applied. Toxicities related to chemotherapy or other systemic treatments are not included.
4. Results
A total of 19 patients, 10 women (53%) and 9 men (47%), with a median age of 54 years (37–68 years) were included. Fourteen patients (74%) underwent radiation therapy at the time of the first diagnosis while 5 patients (26%) were treated for locoregional recurrence of RPS after surgery and chemotherapy. Seven patients (37%) were asymptomatic by the time of initial diagnosis and diagnosis of retroperitoneal tumor was established after abdominal ultrasound for suspected fatty liver disease because of fortuitous identification of elevated serum aminotransferase levels or after low-dose CT scan in heavy smokers for lung cancer screening. Five patients (26.5%) consulted for abdominal pain, 5 patients (26.5%) for increases in abdominal circumference, 1 patient (5%) for weight loss and 1 patient for deep venous thrombosis.
The size of the tumor was defined using the largest diameter in computed tomography (CT) and/or magnetic resonance images (MRI). The tumor's maximum diameter ranged between 3.2 cm and 32 cm (median 12 cm).
The most common histological subtype was liposarcoma (53%), followed by leiomyosarcoma (21%) and pleomorphic undifferentiated sarcoma (16%). Other histologies including synovial sarcoma and myofibroblastic sarcoma accounted for remaining 10%. Histological grade according to the Fédération Nationale des Centres de Lutte Contre Le Cancer (FNCLCC) histological grading system was grade 1 in 7 tumors (37%), grade 2 in 7 cases (37%) and grade 3 in 5 cases (26%).
Tumor staging according to the AJCC 8th edition (2016) was: stage Ib in 5 cases (26.5%), stage II in 1 case (5%), stage IIIa in 4 cases (21%), stage IIIb in 7 cases (37%), and stage IV in 2 cases (10.5%). One of the two patients with initial oligometastatic presentation had liver involvement while the others had concomitant liver and pulmonary disease.
Complete characteristics of analyzed patients are detailed in Table 1.
Table 1.
Complete characteristics of analyzed patients.
| Gender | Female: 10 (53%) |
| Male: 9 (47%) | |
| Age (median) | 55 years old (36–69 years old) |
| Presentation | Primary: 15 (79%) |
| Relapsed: 4 (21%) | |
| Size (median) | 12 cm (3.2–32) |
| Histological subtype | Liposarcoma: 10 (53%) |
| Leiomyosarcoma: 4 (21%) | |
| Pleomorphic undifferentiated: 3 (16%) | |
| Other: 2 (10%) | |
| Histological grade | Grade 1: 7 (37%) |
| Grade 2: 7 (37%) | |
| Grade 3: 5 (26%) | |
| Surgical margins | Negative: 11 (58%) |
| Positive: 8 (42%) | |
| Chemotherapy | Yes: 10 (53%) |
| No: 9 (47%) | |
Seventeen patients (89.5%) underwent surgical resection with radical intention of the tumor and involved surrounding tissues. In the remaining 2 patients, only a biopsy procedure was performed. Complete resection was achieved in 15 out of the 17 operated patients (79%). According to a microscopic exam, 11 out of the 17 operated patients (65%) were classified as macroscopically and microscopically free margin (R0 resection), 4 patients (23%) as R1 resection (margin microscopically positive) and 2 patients (12%) as R2 resection (margin macro- and microscopically positive).
Seven patients out of the 19 analyzed (37%) underwent preoperative radiotherapy (PRORT), 10 patients (53%) postoperative radiotherapy (PORT) and 2 patients (10%) received exclusive external beam radiation therapy (EBRT). No patient underwent intraoperative radiation treatment (IORT). Median interval of time between PRORT and surgery was 11 weeks (range 6–16 weeks) and between surgery and PORT, 11.5 weeks (range 11–15 weeks). Ten patients (53%) received systemic poli-chemotherapy based upon doxorubicin in combination with ifosfamide, gemcitabine, docetaxel, eribulin or olaratumab. Systemic chemotherapy was administered at the discretion of the medical oncologist and in the presence of high-risk features for distant tumor spread (high risk, unfavorable histology, large size or recurrent tumor).
With a median follow-up of 24 months (2–114 months), 13 patients (68%) are alive without tumor, 4 patients (21%) are alive with tumor and 2 patients (10%) are dead: 1 patient because of tumor progression and 1 patient because of a post-operative infection.
Five patients (26%) developed local recurrence during the follow-up period, with a median time to recurrence of 21 months (range 12–84 months). Actuarial rates of LRFS at 1, 2 and 3 years were 77%, 77% and 67%, respectively. Two patients were re-operated, achieving complete R0 resections in both cases and being free of disease at 4 and 12 months after second surgical procedure and 2 other patients underwent re-irradiation up to a total dose of 50 Gy in 10 fractions of 5 Gy. After second radiotherapy course, one patient died as a result of a post-operative infection (intercurrent death) while the other patient is alive without evidence of disease 15 months after re-irradiation. Finally, 1 patient with local relapse started treatment with trabectedin having received 21 cycles with stabilization of the disease without developing a new recurrence or distant metastasis.
Two patients (10%) developed distant metastases on follow-up. Actuarial rates of DMFS at 1, 2 and 3-years were 100%, 100% and 80%, respectively. Both patients underwent PRORT for undifferentiated pleomorphic sarcoma and malignant inflammatory myofibroblastic tumor, and subsequently complete resection (R0) in one case and R1 resection (microscopically affected margins) in the other. Both patients received systemic chemotherapy after surgery.
Finally, actuarial rates at 1, 2 and 3 years of DFS and OS were 94%, 77% and 67% for DFS and 100%, 91% and 91% for OS, respectively.
Univariate analysis of risk factors for LRFS, DFS and OS included sex, age, and primary vs. relapsed RPS at diagnoses, tumor size, histology, surgical margins status, systemic treatment and tumor grade. It should be noted that in order to facilitate comparisons between groups, we decided to consider the median age and tumor size values for the univariate analysis. We did not find any factor significantly related to OS, and only surgical margins (negative vs. positive) showed significance for 3-year LRFS: 100% vs. 34.3%, p = 0.018 (Table 2). Otherwise, different histology from liposarcoma, leiomyosarcoma or pleomorphic sarcoma was associated with lower 3-year DMFS (0% vs. 100%, p = 0.039), although this data should be taken cautiously due to the small number of patients analyzed. Likewise, chemotherapy administration appeared significantly related to 3-year LRFS and 3-year DFS (35% vs. 100%, p = 0.021 and 35% vs. 100%, p = 0.005, respectively), although this fact could only be reflecting a more aggressive phenotype. Due to few significant factors identified on univariate analysis, the low number of patients and the still short follow-up, multivariate analysis was not performed. Sixteen patients (84%) presented any degree of radiotherapy related acute toxicity: grade 1 diarrhea in 9 patients (47%); grade 1 nausea/vomiting in 9 patients (47%); and transient grade 1 abdominal pain and grade 1 dysuria in 1 patient (5%), respectively. No cases of late toxicity directly attributable to radiation therapy have been observed. One patient died because of immediate surgical complications.
Table 2.
Univariate analysis of risk factors for locoregional free survival, DFS and OS.
| 3y-LRFS (%) | 3y-DFS (%) | 3y-OS (%) | ||
|---|---|---|---|---|
| Gender | F | 55.6 | 55.6 | 83.3 |
| M | 80 | 100 | 100 | |
| p | 0.37 | 0.21 | 0.95 | |
| Age (median y-o) | <54 | 53.6 | 53.6 | 100 |
| ≥54 | 80 | 80 | 80 | |
| p | 0.35 | 0.94 | 0.70 | |
| Primary vs. relapse | Primary | 77.9 | 77.9 | 100 |
| Relapsed | 50 | 50 | 75 | |
| p | 0.49 | 0.88 | 0.10 | |
| Tumor size (median, cm) | ≤12 | 80 | 80 | 100 |
| >12 | 58.3 | 58.3 | 83.3 | |
| p | 0.40 | 0.31 | 0.28 | |
| Histology | LP | 74.1 | 74.1 | 83.3 |
| Non-LP | 60 | 60 | 100 | |
| p | 0.9 | 0.43 | 0.28 | |
| Surgical resection margins | Negative | 100 | 100 | 100 |
| Positive | 34.3 | 34.3 | 80 | |
| P | 0.018 | 0.097 | 0.13 | |
| Chemotherapy | YES | 35 | 35 | 80 |
| NO | 100 | 100 | 100 | |
| p | 0.021 | 0.005 | 0.65 | |
| Histological grade | G1 | 100 | 75 | 100 |
| G2 | 50 | 50 | 75 | |
| G3 | 37.5 | 37.5 | 100 | |
| p | 0.15 | 0.15 | 0.41 | |
Italic values represent level of statitistical significance according to Log-rank test performed.
Bold values only remark those values reaching statistical significance at log-rank test (< 0.05).
5. Discussion
RPS are a group of rare and heterogeneous tumors arising from mesenchymal tissue representing less than 1% of all malignant retroperitoneal neoplasms. Histologically, there are 3 predominant subtypes: liposarcoma (26–64.5%), leiomyosarcoma (13.2–31%) and pleomorphic undifferentiated sarcoma (7–27%). The most frequent RPS subtypes are well-differentiated liposarcoma and de-differentiated liposarcoma, representing nearly 70% of all new cases, and leiomyosarcoma (LMS) (15%).14, 15, 16, 17 RPS have a worse prognosis than limb STS due to the combination of different factors. On the one hand, RPS present as large lesions due to their slow and insidious growth, being greater than 20 cm in more than 50% of the cases, and approximately half of them are of high grade histological subtype. On the other hand, their location in close proximity to vital organs and structures and, in many cases, infiltrating them, reduces the possibility of a complete surgical resection with free margins decreasing the chances of survival, unlike in the case of limb STS.18
Surgery is the first curative option in RPS regardless of its size, histological subtype or location. The objective of the surgery is to achieve a complete macroscopical resection of the tumor but due to the characteristics of the RPS it is sometimes difficult to reach tumor-free surgical margins and sometimes greater resections are associated to unjustified morbidity. The multi-institutional analysis led by Gronchi et al. including more than 1000 patients with RPS showed a significant increase in locoregional recurrence (HR 2.81, p < 0.001) and a reduction in overall survival (HR 2.36, p 0 0.001) in patients with R2 resection versus a R0/R1 resection.19
Complete surgical resection of the tumor, as well as the structures and surrounding organs infiltrated by the tumor, leads to local control rates between 40% and 59% of patients (Table 3).5, 15, 20, 21, 22, 23, 24 However, due to poor rates of locoregional control, some European groups have advocated the performance of a more extensive surgery, the so-called liberal en bloc or compartmental resection that, using the STS surgery of extremities as a model, advocates for a complete removal of the tumor, the affected organs and also the healthy surroundings and unaffected organs including the colon, kidney or psoas muscle. Bonvalot et al. analyzed the impact of a large compartmental resection in 120 patients with RPS observing that en bloc resection is a significant variable, predicting a 3.29-fold lower rate of abdominal recurrence compared to simple complete resection.25 Likewise, Gronchi et al. observed a 5-year local failure rate of 29% in 152 patients with RPS treated with extensive surgery vs. 48% in 136 patients treated with a more conventional surgery.26 A joint analysis of both series including 249 patients treated with compartmental upfront surgery and a median follow-up of 37 months observed an actuarial rate of local recurrence at 5 years of 22.3%.27 However, these studies were both criticized for their retrospective nature, the bias in the patient selection and the low impact of a more aggressive treatment in the local control and overall survival rates despite associating higher surgical morbid-mortality rates.28, 29 Contrary to limb STS, in which the main cause of death is distant metastatic dissemination of the disease, local progression is the main cause of death for RPS. That is why ensuring local control appears to be a priority objective in the treatment of RPS. The combination of radiotherapy and surgery, in different sequences, has been considered as the most adequate therapeutic strategy to obtain adequate local control rates and, subsequently, increase overall and cause-specific survival. The majority of the evidence supporting a combined approach comes from retrospective studies and from the analysis of large population databases, since there are no controlled randomized trials with a sufficient number of patients and follow-up. The American College of Surgeons Oncology Group proposed a randomized phase III trial (ACOSOG Z9031) in RPS based on radical surgery with or without PRORT radiotherapy. Unfortunately, it was prematurely closed due to low recruitment without being able to provide evidence.30 The first results of the phase III randomized study STRASS (Surgery With or Without Radiation Therapy in Untreated Non-metastatic Retroperitoneal Sarcoma) with PRORT radiotherapy at a dose of 50.4 Gy and radical en bloc resection performed by the European Organization for Research and Treatment of Cancer (EORTC) have been presented at the 2019 annual meeting of the American Society of Clinical Oncology (ASCO). According to presented data based on 248 evaluable patients, there seems to be no benefit in 3-year abdominal recurrence free survival with the use of PRORT radiotherapy (66.4% with PRORT radiotherapy vs. 58.7% with surgery alone, p = 0.3) although an apparent benefit exists in the subgroup of patients with histological diagnosis of liposarcoma (71.6% with radiotherapy vs. 60.4% with surgery alone, p = 0.049), but a longer follow-up is needed to definitively evaluate these results.31
Table 3.
Results of patients with RPS treated with exclusive surgery.
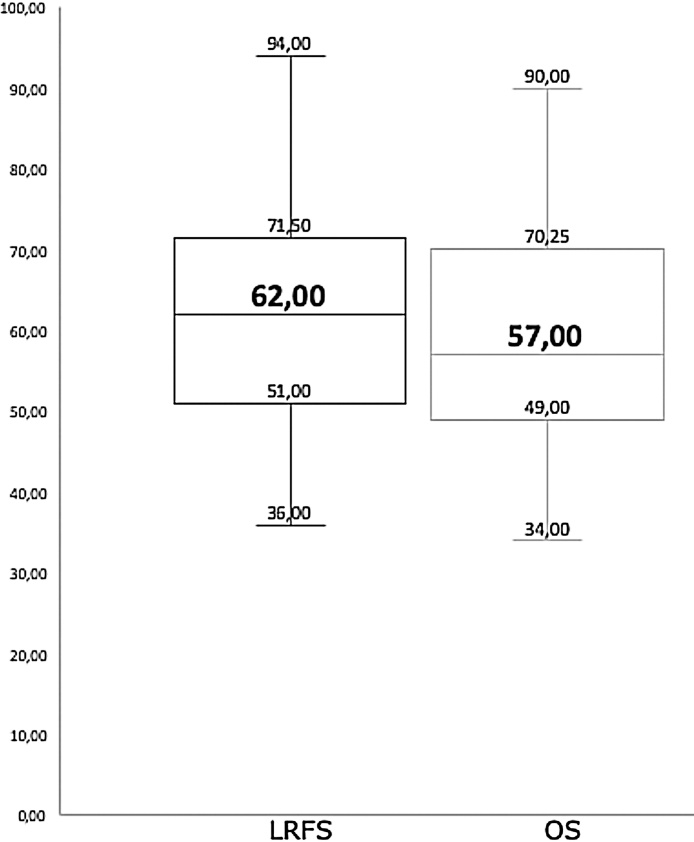
Large databases analyses, with all the limitations associated with their retrospective nature, have nonetheless pointed to a benefit of the combination of radiotherapy and surgery for the treatment of RPS. The analysis conducted by Nussbaum et al. in the National Cancer Data Base (NCDB) included 9608 patients with RPS who were treated through exclusive surgery (6290 patients) or combined surgery and radiotherapy (563 PRORT patients and 2215 PORT patients). The results showed that the addition of radiotherapy, both PRORT (HR = 0.70) and PORT (HR = 0.78), was associated with a significant improvement in overall survival versus exclusive surgery.8 Conversely, on the data collected on the basis of Surveillance, Epidemiology, and End Results (SEER), Tseng et al. published the results of the analysis performed on 1350 patients diagnosed with RPS and treated by surgery followed by adjuvant radiotherapy in 24% of them. The authors found that postoperative radiotherapy did not have an impact leading to a better overall survival.32 However, more recently and using also SEER database, Bates et al. analyzed 480 patients diagnosed with high-grade RPS treated by surgery, of whom 144 (30.0%) received PORT. Radiotherapy improved median OS compared to those patients who did not receive it (HR = 0.79, p = 0.023).33 Most evidence of the efficacy of radiotherapy in the treatment of RPS comes from non-randomized studies. Table 4 shows results from 43 studies either retrospective (27 studies), prospective (15 studies) or randomized (1 study) involving 4730 patients with RPS published in the 21st century and using radiotherapy as part of the multidisciplinary treatment.4, 19, 20, 23, 25, 31, 34, 40, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76 Those studies that did not specify the characteristics of the radiation treatment, both in sequence and in total dose, fractions and volumes of treatment, have been intentionally excluded. Liposarcoma is the most frequent histology treated, accounting for nearly half of treated patients, followed by leiomyosarcoma and malignant fibrous histiocytoma. Complete macroscopic resection, a concept that includes R0 and R1 resections, was achieved in a median of 83% of patients (49–100%). Seventy-nine percent of included patients underwent radiation treatment, with a median EBRT dose, either delivered with PRORT or PORT, of 50 Gy (45–59.4 Gy). Additionally, 21 studies used intraoperative radiotherapy, with electrons in 16 studies and brachytherapy in 5 studies, as a boost either before or after EBRT. Median number of patients receiving systemic chemotherapy in the different studies is 58% (range 0–100%). Despite a great heterogeneity in the number of patients included, follow-up time, surgical approach, modality and fractionation scheme of radiotherapy and systemic treatments between the published studies, and with a follow-up between 10 and 106 months, the median OS for all the studies was 57% and median LRFS, of those studies that specified it, was 62% (range 36–89%) (Fig. 2). In spite of the relatively low number of patients and short follow-up, results observed in our series are comparable with previous published experiences both in OS and LRFS.
Table 4.
Clinical experiences with radiotherapy in RPS in the 21st century.
| Author | N | MFU | Histology | Type of study | R0/R1 resection (%) | Radiotherapy (median dose) | Chemotherapy (%) | LRFS | DFS | OS | Prognostic factors for: |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LRFS | OS | |||||||||||
| Stoeckle (2001)20 | 145 | 47 | LP 30% | Retrospective | 65 | PORT 56% (50 Gy) | 35 | 52% 5y | 29% 5y | 49% 5y | Grade (low vs. high) Radiotherapy (given vs. not given) |
Margins (positive vs. negative) |
| LM 23% | Grade (low vs. high) | |||||||||||
| SP 17% | Size | |||||||||||
| Other 30% | Histological subtype (liposarcoma vs. no liposarcoma) | |||||||||||
| Gieschen (2001)43 | 37 | 38 | LP 22% | Retrospective | 79 | PRORT 100% (45 Gy) | 0 | 72% 5y | 50% 5y | 56% 5y | None | Radiotherapy (given vs. not given) |
| LM 19% | PRORT + IORT 54% (45 Gy + 10–20 Gy) | |||||||||||
| MFH 16% | ||||||||||||
| Other 43% | ||||||||||||
| Gilbeau (2002)4 | 45 | 63 | LP 58% | Prospective | 96 | PORT 62% (49 Gy) | 11 | 40% 5y | NR | 60% 5y | Margins (positive vs. negative) | Margins (positive vs. negative) |
| LM 18% | PORT + IORT 31% | |||||||||||
| Other 24% | IORT 7% (15 Gy) | |||||||||||
| Petersen (2002)44 | 87 | 42 | LP 32% | Retrospective | 83 | PRORT + IORT 60% | 11.5 | 59% 5y | 29% 5y | 48% 5y | Margins (positive vs. negative) | Size |
| LM 29% | PORT + IORT 14% | Radiotherapy (given vs. not given) | ||||||||||
| Other 39% | IORT 12% | |||||||||||
| PRORT + IORT + PORT 14% | ||||||||||||
| (EBRT 47.6 Gy; IORT 15 Gy) | ||||||||||||
| Youssef (2002)45 | 60 | 36 | LP 17% | Retrospective | 75 | PORT 73% (52.2 Gy) | 71% 5y | 53% 5y | 56% 5y | Margins (positive vs. negative) | Margins (positive vs. negative) | |
| LM 20% | IOBT + PORT 27% (16 Gy + 42 Gy) | 0 | Female sex (vs. male) | Female sex (vs. male) | ||||||||
| MFH 27% | ||||||||||||
| Other 36% | ||||||||||||
| Jones (2002)46 T | 55 | 19 | LP 69% | Prospective | 84 | PRORT 33% (45 Gy) | 0 | NR | NR | 73% 2y | None | Grade (low vs. high) |
| LM 15% | PRORT + BT 42% (45 Gy + 25 Gy) | Primary presentation (vs. recurrence) | ||||||||||
| MFH 9% | ||||||||||||
| Other 7% | ||||||||||||
| Bobin (2003)47 | 24 | 53 | LP 50% | Retrospective | 92 | PRORT + IORT 29% (45-50 Gy + 15 Gy) | 0 | NR | 28% 3y | 56% 3y | NS | NS |
| LM 12.5% | IORT + PORT 62.5% (15 Gy + 45–50 Gy) | |||||||||||
| MFH 8% | ||||||||||||
| Zlotecki (2005)48 | 40 | 34 | LP 37.5% | Retrospective | 85 | PRORT 62.5% (50 Gy) | 17.5 | 65% 5y | NR | 69% 5y | Margins (positive vs. negative) | Margins (positive vs. negative) |
| LM 15% | PORT 37.5% (50 Gy) | Size | Grade (low vs. high) | |||||||||
| Other 47.5% | Radiotherapy (given vs. not given) | |||||||||||
| Krempien (2006)49 | 67 | 30 | LP 51% | Prospective | 82 | PORT + IORT 67% (45GY + 15 Gy) | 0 | 40% 5y | 28% 5y | 62% 5y | Margins (positive vs. negative) | Margins (positive vs. negative) |
| LM 15% | IORT 32% (15 Gy) | Grade (low vs. high) | ||||||||||
| MFH 10% | Primary presentation (vs. recurrence) | |||||||||||
| Other 24 | ||||||||||||
| Pawlik (2006)50 | 72 | 40 | LP 40% | Prospective | 95 | PRORT 75% (45 Gy) | 49 | 60% 5y | 46% 5y | 50% 5y | None | None |
| LM 26% | IORT 39% (15 Gy) | |||||||||||
| MFH 15% | POBT 21% (25 Gy) | |||||||||||
| Other 18% | ||||||||||||
| Tzeng (2006)37 | 16 | 28 | LP 25% | Prospective | 88 | PRORT (57.5 Gy) | 0 | NR | 80% 2y | NR | NS | NS |
| LM 25% | ||||||||||||
| Feng (2007)51 | 88 | 24 | LP 20% | Retrospective | 72 | PRORT 20% | 55 | 51% 5y | 30% 5y | 34% 5y | Margins (positive vs. negative) | Margins (positive vs. negative) |
| LM 35% | PORT 60% | Female sex (vs. male) | Grade (low vs. high) | |||||||||
| MFH 17% | Both 5% | Radiotherapy (given vs. not given) | Female sex (vs. male) | |||||||||
| Other 28% | Definitive 15% | |||||||||||
| (56.4 Gy) | ||||||||||||
| Ballo (2007)52 | 83 | 47 | LP 29% | Retrospective | 52 | PRORT 60% (50 Gy) | 47 | 40% 10y | 67% 10y | DSS 44% 10y | Margins (positive vs. negative) | Grade (low vs. high) |
| LM 19% | PORT 40% (55 Gy) | Primary presentation (vs. recurrence) | ||||||||||
| MFH 21% | PRE/PORT + IORT 22% (50 Gy + 15 Gy) | Age | ||||||||||
| Other 31% | ||||||||||||
| Bossi (2007)38 | 18 | 27 | LP 72.2% | Prospective | 89 | PRORT (50 Gy) | 0 | 94% 1y | 94.4% 1y | NR | NS | NS |
| White (2007)53 | 38 | 57 | LP 50% | Prospective | 96 | PRORT 66% | 0 | 80% 5y | 80% 5y | 74% 5y | Grade (low vs. high) | Grade (low vs. high) |
| LM 10.5% | PORT 3% | Radiotherapy (given vs. not given) | ||||||||||
| MFH 5% | Exclusive RT 29% | |||||||||||
| Other 34.5% | ||||||||||||
| Zagar (2008)54 | 31 | 22 | LP 32% | Retrospective | 79 | PRORT + IORT 61% (59.4 Gy + 11 Gy) | 0 | 77% 2y | NR | 70% 2y | NS | NS |
| LM 48% | IORT + PORT39% (11 Gy + 59.4 Gy) | |||||||||||
| MFH 10% | ||||||||||||
| Other 10% | IMRT 32% | |||||||||||
| Bonvalot (2009)25 | 382 | 52 | LP 50% | Retrospective | 73 | PORT 29% (45 Gy) | 38 | 49% 5y | NR | 57% 5y | Margins (positive vs. negative) | Margins (positive vs. negative)) |
| LM 18% | PRORT 3% (45 Gy) | Grade (low vs. high) | Grade (low vs. high) | |||||||||
| MFH 9% | PRE/POR + IORT 5% (45 Gy + 15 Gy) | Histological subtype | ||||||||||
| Other 23% | ||||||||||||
| Lehnert (2009)23 | 110 | 89 | LP 54% | Prospective | 90 | IORT + PORT 34.5% (15 Gy + 44 Gy) | 8 | 40% 5y | NR | DSS 49% 5y | Grade (low vs. high) | Margins (positive vs. negative) |
| LM 23% | Primary presentation (vs. recurrence) | Grade (low vs. high) | ||||||||||
| Other 23% | ||||||||||||
| Gholami (2009)55 | 41 | 10 | LP 54% | Retrospective | 93 | PRORT 2% | 20% | NR | NR | 46% 5y | None | Margins (positive vs. negative) |
| LM 17% | IORT 19.5% | Grade (low vs. high) | ||||||||||
| Other 29% | IORT + PORT 12% | |||||||||||
| PORT 15% | ||||||||||||
| IORT (12.5 Gy); EBRT (40–50 Gy) | ||||||||||||
| Dziewirski (2010)56 | 70 | 20 | LP 51% | Retrospective | 100 | IOBT 31% (20 Gy) | 3 | 51% 5y | NR | 55% 5y | Primary presentation (vs. recurrence) | Grade (low vs. high) |
| LM 14% | IOBT + PORT 34% (20 Gy + 50 Gy) | Radiotherapy (given vs. not given) | Histological subtype (liposarcoma vs. no liposarcoma) | |||||||||
| Other 45% | Primary presentation (vs. recurrence) | |||||||||||
| Sampath (2010)57 | 261 | 59 | LP 47% | Retrospective | 49 | PRORT 1.2% | 14 | 69% 5y | NR | 57% 5y | Margins (negative vs. positive) | Margins (negative vs. positive) |
| LM 31% | PORT 19.5% | Grade (low vs. high) (low vs. high) | Grade (low vs. high) | |||||||||
| Other 22% | (50.4 Gy) | Radiotherapy (given vs. not given) (given vs. not given) | Histological subtype (liposarcoma vs. no-liposarcoma) | |||||||||
| Donhaue (2010)58 | 55 | 68 | LP 20% | Retrospective | 82 | PRORT 56% | 100 | NR | NR | DSS 47% 5y | None | Age |
| LM 31% | PORT 44% | |||||||||||
| MFH 7% | (50 Gy) | |||||||||||
| Other 42% | ||||||||||||
| Yoon (2010)59 | 20 | 33 | LP 50% | Prospective | 90 | PRORT 71% (50 Gy) | 15 | 82% 3y | 87% 3y | 87% 3y | Primary presentation (vs. recurrence) | None |
| LM 21.4% | IORT 43% (11 Gy) | |||||||||||
| Other 28.6% | PORT 21% (50 Gy) | |||||||||||
| IMPT: 35.7%; IMRT 39.3%; both 7% | ||||||||||||
| Lee (2011)60 | 40 | 41 | LP 42.5% | Retrospective | 77.5 | PORT 100% (55.9 Gy) | 30 | 62% 5y | 31.5% 5y | 52% 5y | NS | NS |
| LM 27.5% | ||||||||||||
| MFH 17.5% | ||||||||||||
| Others 12.5% | ||||||||||||
| Fuks (2012)61 | 50 | 55 | LP 50% | Retrospective | 42 | PORT 56% (45 Gy) | 30 | 61% crude rate | NR | 46% 5y | Margins (positive vs. negative) | Margins (positive vs. negative) |
| LM 32% | ||||||||||||
| Other 18% | ||||||||||||
| Paryani (2012)62 | 58 | 29 | LP 38% | Retrospective | 76 | PRORT 72% (50.4 Gy) | 0 | 62% 5y | NR | 49% 5y | Margins (positive vs. negative) | Margins (positive vs. negative) |
| LM 22% | PORT 28% (49.6 Gy) | Size | Size | |||||||||
| McBride (2013)63 | 33 | 33 | LP 48% | Retrospective | 50 | PRORT 70% (50 Gy) | 30 | NR | 45.3% 5y | 63.5% 5y | Multifocality | None |
| LM 36% | PRORT + IOBT 30% (77.5 Gy) | |||||||||||
| De Wever (2013)64 | 29 | 84 | LP 100% | Prospective | 100 | PRORT 100% (50 Gy) | 0 | NR | 79% 3y | 70% 3y | None | Histological subtype (liposarcoma vs. no liposarcoma) |
| Alford (2013)65 | 24 | 28 | LP 50% | Retrospective | 75 | PRORT 100% (45–50.4 Gy) | NR | 68.5% 5y | 49% 5y | 54% 5y | NS | NS |
| LM 17% | ||||||||||||
| Sweeting (2013)66 | 18 | 43 | LP 50% | Prospective | 89 | PRORT 94% (45 Gy) | 0 | 36% 5y | NR | 72% 5y | NS | NS |
| LM 28% | IORT 100% (12.5 Gy) | |||||||||||
| MFH 11% | ||||||||||||
| Other 11% | ||||||||||||
| Le Pechoux (2013)67 | 110 | 49 | LP 58% | Retrospective | 97 | PORT 44% (50.4 Gy) | 37 | NR | NR | 74% 5y | Radiotherapy (given vs. not given) | None |
| LM 14% | ||||||||||||
| Other 28% | ||||||||||||
| Stucky (2014)68 | 37 | 45 | LP 68% | Retrospective | 84 | PRORT + IORT 59% (45 Gy + 12.5 Gy) | 24 | 89% 5y | 89% 5y | 60% 5y | Radiotherapy (given vs. not given) | None |
| LM 13% | ||||||||||||
| El-Bared (2014)69 | 21 | 22 | LP 62% | Retrospective | 66 | PRORT 100% (50 Gy) | 24 | 41% 5y | 41% 5y | 51% 5y | Margins (positive vs. negative) | Grade (low vs. high) |
| LM 19% | Histological subtype (liposarcoma vs. no liposarcoma) | Female sex (vs. male) | ||||||||||
| Size | ||||||||||||
| Primary presentation)vs. recurrence) | ||||||||||||
| Smith (2014)70 | 40 | 106 | LP 70% | Prospective | 78 | PRORT 100% (45 Gy) | 0 | NR | 69% 5y | 70% 5y | Grade (low vs. high) | Primary presentation (vs. recurrence) |
| LM 12.5% | PRORT + BT 48% (45 Gy + 20 Gy) | Primary presentation (vs. recurrence) | ||||||||||
| Roeder (2014)40 | 27 | 33 | LP 70% | Prospective | 99 | PRORT 93% + IORT 85% (50 Gy + 12 Gy) | 0 | 72% 5y | NR | 74% 5y | Primary presentation (vs. recurrence) | None |
| LM 30% | ||||||||||||
| Trovik (2014)71 | 97 | 55 | LP 62% | Prospective | 91 | PRORT 12% | 15.5 | 55% 5y | NR | 60% 5y | Margins (positive vs. negative) | Grade (low vs. high) |
| LM 29% | PORT 38% | Grade (low vs. high) | Size | |||||||||
| Other 9% | (50 Gy) | Size | Age | |||||||||
| Radiotherapy (given vs. not given) | Radiotherapy (given vs. not given) | |||||||||||
| Bishop (2015)72 | 121 | 100 | LP 35% | Retrospective | 48 | PRORT 73% (50.4 Gy) | 64 | 56% 5y | NR | 57% 5y | Margins (positive vs. negative | Margins (positive vs. negative)) |
| LM 23% | PORT 27% (55 Gy) | Primary presentation (vs. recurrence) | Grade (low vs. high) | |||||||||
| MFH 28% | ||||||||||||
| Other 14% | ||||||||||||
| Gronchi (2016)19 | 1007 | 58 | LP 63% | Retrospective | 95.3 | PRORT/PORT 32% | 18 | 65% 10y | NR | 46% 10y | Margins (positive vs. negative) | Margins (positive vs. negative) |
| LM 19% | Grade (low vs. high) | Grade (low vs. high) | ||||||||||
| Others 18% | Histological subtype (liposarcoma vs. no liposarcoma) | Size | ||||||||||
| Size | Primary presentation (vs. recurrence) | |||||||||||
| Age | Age | |||||||||||
| Radiotherapy (given vs. not given) | Multifocality | |||||||||||
| Multifocality | ||||||||||||
| Abdelfath (2016)73 | 131 | NR | LP 38% | Retrospective | 80 | PRORT 5% | 28 | NR | NR | MST 49 months | Margins (positive vs. negative) | Margins (positive vs. negative) |
| LM 40% | PRORT + IORT 3% | Histological subtype (liposarcoma vs. no liposarcoma) | Grade (low vs. high) | |||||||||
| Other | PORT 11.5% | Radiotherapy (given vs. not given) | Age | |||||||||
| 22% | RTE EXCLUSIVA 2% | Female sex (vs. male) | ||||||||||
| 40–50.4 Gy | Radiotherapy (given vs. not given) | |||||||||||
| Lee (2016)34 | 77 | 36 | LP 83% | Retrospective | 62 | PORT 42% (54 Gy) | 8 | 57% 3y | NR | 82% 3y | Grade (low vs. high) | None |
| Histological subtype (liposarcoma vs. no liposarcoma) | ||||||||||||
| Cosper (2017)74 | 30 | 36 | LP 33% | Retrospective | 80 | PRORT 37% (55 Gy) | 30 | NR | NR | 50% 5y | Margins (positive vs. negative)) | None |
| LM 33% | PORT 63% (60.4 Gy) | |||||||||||
| Other 9% | ||||||||||||
| IMRT | ||||||||||||
| Kim (2018)75 | 80 | 37 | LP 52.5% | Retrospective | 86 | PORT 47.5% (54 Gy) | 29 | 48% 5y | NR | 71% 5y | Radiotherapy (given vs. not given) | None |
| LM 22.5% | ||||||||||||
| PS 11.2% | ||||||||||||
| Others 13.8% | ||||||||||||
| Haas (2019)76 | 234 | 27 | WDLP | Retrospective | 96 | PRORT 14%/PORT 4.5%/Both 1% | 5 | 78% 5y | NR | 90% 5y | Margins (positive vs. negative) | Age |
| 242 | 27 | DDLP Grade (low vs. high) 1–2 | 95.5 | PRORT 27.5%/PORT 6%/Both 1% | 15 | 58% 5y | 66.5% 5y | Size | ||||
| 131 | 22 | DDLP Grade (low vs. high) 3 | 88.5 | PRORT 18%/PORT 13%/Both 4% | 22 | 64% 5y | 37% 5y | Multifocality | ||||
| (All groups 50 Gy) | ||||||||||||
| STRASS (EORTC 62092) Bonvalot (2019)31 | 248 | NR | LP 74.5% | RCT: | NR | PRORT (50.4 Gy) | NR | @3y: | NR | NR | NS | NS |
| PRORTRT + Surgery | 66.4% | |||||||||||
| vs. | vs. | |||||||||||
| Surgery alone | 58.7% (p = 0.3) | |||||||||||
| (LP 71.6% vs. 60.4%, p = 0.049) | ||||||||||||
| Current series | 19 | 15 | LP 53% | Retrospective | 88 | PRORT 37% | 53 | 67% 3y | 67% 3y | 91% 3y | Margins (positive vs. negative) | None |
| LM 21% | PORT 53% | |||||||||||
| Other 26% | Exclusive 10% | |||||||||||
| (62 Gy) | ||||||||||||
MFU: median follow-up (months); LRFS: locoregional relapse free survival; OS: overall survival; DFS: disease free survival; DSS: disease specific survival; LM: leiomyosarcoma; LP: liposarcoma; MFH: malignant fibrous histiocytoma; IORT: intraoperative radiotherapy; IOBT: intraoperative brachytherapy; PRORT: pre-operative radiotherapy; PORT: postoperative Radiotherapy; SIB: simultaneous integrated boost: LRF: locoregional failure; NR: not reported; NS: not specified; IMPT: intensity modulated proton therapy.
Fig. 2.
Boxplot side-by-side comparison of locoregional relapse free survival (LRFS) (Left) and overall survival (OS) (Right) median rates in the 43publications on retroperitoneal sarcomas. Rectangular box denotes interquartile range. Thick line in the box denotes median values.
Many of the published studies also seek to identify prognostic factors for RPS. Table 4 shows the factors identified in each of the studies for both local control and survival. The prognostic factors most frequently related to increased risk of local failure are, in descending order of importance, the involvement of surgical margins, lack of complementary radiotherapy, high histological grade and recurrent or large tumors and histological subtype other than liposarcoma. In the same way, the absence of free margins, the non-liposarcoma histological subtype, the high histological grade or the large tumor size have been associated with a worse overall survival. Use of radiation treatment is one of the main prognostic factors for local control and survival in most of series, although no unanimous consensus exists regarding the optimal timing of administration. From a theoretical point of view there may be some benefits in the PRORT administration. First, it facilitates the administration of the treatment, since the tumor itself displaces vital healthy structures away from the volume of irradiation minimizing the dose in them; second, radiotherapy applied directly on the tumor could thicken the pseudo capsule that usually surrounds the RPS making it more acellular and, thus, facilitating its resection by minimizing the risk of local recurrence; third, PRORT could reduce the risk of tumor microembolism during surgical manipulation. Finally, from the radiobiological point of view, the effectiveness of radiotherapy would be greater in a tissue not subject to hypoxic conditions or where an accelerated repopulation mechanism has initiated as it could happen after surgical manipulation.34 On the other hand, postoperative radiotherapy allows resectable tumors to be removed safely by upfront surgery, preventing the risk of growth or spread during treatment that would render them inoperable, while facilitating a definitive analysis about the nature of the tumor, its histology, grade and surgical margins, which could allow adjusting the volume and final irradiation dose. As previously mentioned, only two randomized studies (ACOSOG Z-9031 and STRASS) evaluated the administration of PRORT radiotherapy versus exclusive surgery, although without conclusive results.30, 31 Intraoperative radiation therapy (IORT) has been used in 21 studies to maximize dose to tumor bed/dose to normal tissue ratio. Nowadays, IORT could be delivered with electrons or with high dose rate (HDR) brachytherapy. IORT has specific advantages in the retroperitoneum: potential advantage of improving the therapeutic ratio with a single high dose capable of sterilizing the microscopic disease responsible for tumor repopulation, which occurs during the surgery-to-radiotherapy interval, while decreasing the risk of radiation-induced toxicity in dose-limiting normal tissues, which can be displaced or protected during IORT. IORT can be delivered alone or with additional EBRT before or after surgery shortening the overall radiation treatment time.35 As with extremity STS, there is a clear relationship between total dose and tumor control. Because of the close presence of sensitive structures such as kidneys, duodenum, small bowel or spinal cord, the recommended dose usually is 50–50.4 Gy in 25–28 daily fractions of 1.8–2 Gy, which can be completed with a boost after PRORT with intraoperative radiotherapy, brachytherapy or PORT, especially in the case of R1/R2 resections. Fein et al.36 showed that the administration of doses higher than 55.2 Gy was associated with a greater probability of local control. In the same way Lewis et al. found a significant association between local failure rates and doses <55 Gy.3 However, both groups agreed on the difficulty of administering a higher dose in retroperitoneum for the surrounding structures. In recent years, some groups have been investigating the possibility of a selective increase in the dose of PRORT radiotherapy in those areas considered in the initial evaluation by the surgeon, radiologist and radiation oncologist as unlikely to be able to achieve a resection with free margins. Thus, the works of Tzeng et al. and Bossi et al. propose, in addition to treating the entire retroperitoneal tumor at a moderate dose (45–50 Gy), an escalation of the PRORT dose to the part of the tumor that is considered at risk, typically the region of the tumor that adheres to the posterior abdominal wall, the vertebral bodies and the great vessels.37, 38 The increasing generalization in the use of IMRT/VMAT radiotherapy techniques with IGRT replacing 3D conformal radiotherapy allows a better distribution of the dose with a reduction in the dose in healthy tissues making a moderate dose-escalation in the tumor possible, usually recommended above 50–50.4 Gy. Different techniques are being studied to achieve this dose escalation. The option of an integrated simultaneous boost allows the delivery of a higher dose in those regions that are considered to be at higher risk for recurrence while maintaining a lower dose in areas of lower risk. The multicenter trial NCT01659203 led by the Massachusetts General Hospital seeks to determine the efficacy of increasing the dose in risk areas to 63 Gy in 28 fractions while moderate risk volume receives 50.4 Gy in 28 fractions, using intensity-modulated techniques with both protons and photons.39 Likewise, Roeder et al. are currently investigating PRORT dose-escalated neoadjuvant IMRT and IORT in patients with retroperitoneal soft tissue sarcoma with doses of 45–50 Gy to PTV and 50–56 Gy to GTV in 25 fractions, followed by surgery and IORT (10–12 Gy).40 Another way to proceed with a moderate dose escalation in RPS is to take advantage of the radiobiological characteristics of these tumors and modify the dose administered per fraction by using schemes with moderate hypofractionation. In our series, and assuming at α/β value of 4 for sarcoma cells, the median dose was an accumulated EQD2 for tumor of 62 Gy, and up to 75–80 Gy in selected cases, representing a moderate total dose escalation over the 50–50.4 Gy traditionally used.10, 11 New radiation therapy approaches, which also include the use of proton and heavy-particle beams are currently under investigation and have shown good tolerance and response rates in STS.41, 42
Finally, the use of systemic chemotherapy in the treatment of RPS is a very controversial aspect. The rarity of these tumors means that the number of them included in the analysis of the effectiveness of chemotherapy schedules is very low. Also, the enormous heterogeneity existing in RPS makes it difficult to evaluate the efficacy in different histological subtypes or in different histological grades. Most evidence comes from the extrapolation of what is observed in limb STS, suggesting a possible benefit in tumors of high histological grade or unfavorable subtypes such as LMS or DDL. Even so, it has not been possible to demonstrate a clear benefit in local control or survival with the use of conventional chemotherapy.77, 78 An ulterior attempt failed to demonstrate the effect of chemotherapy directed to a histological subtype, such as trabectedin for liposarcoma or leiomyosarcoma, eribulin or palbociclib for liposarcomas or pazopanib in non-lipomatous.79 Concurrent administration of chemotherapy and radiotherapy searching for a synergistic effect, has been recently tested showing promising results in limb STS.80
We are fully aware of the limitations of our study. This is a retrospective analysis, with a limited number of patients and with a not too long follow-up. On the other hand, it has not been possible to have a comparative group with whom to evaluate differences in prognosis and final outcomes, so it is not possible to establish firm and definitive conclusions. Despite all this, we believe that the results observed in our series compare well with what has been published in recent years and may contribute to increasing the evidence of the role of radiotherapy in the multidisciplinary treatment of RPS.
6. Conclusion
Despite the above-mentioned limitations, our experience suggests probable safety and usefulness of radiotherapy in the multidisciplinary management of RPS. Likewise, and in spite of the absence of sufficient randomized studies and the difficulty in carrying them out, evidence from modern prospective and retrospective series of patients supports the use of radiotherapy for RPS, especially in the presence of poor prognostic factors (i.e.: affected margins, high grade, large size). Nevertheless, although significant progress has been observed, the long-term local control, overall survival and disease-free survival rates need to be improved. Radiotherapy dose-escalation and hypofractionated radiation schedules as well as new chemo-radiotherapy combinations are therapeutic alternatives that could offer attractive possibilities and are worth exploring in future clinical trials.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Stiller C.A., Trama A., Serraino D. Descriptive epidemiology of sarcomas in Europe: report from the RARECARE project. Eur J Cancer. 2013;49:684–695. doi: 10.1016/j.ejca.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Mohindra P., Neuman H.B., Kozak K.R. The role of radiation in retroperitoneal sarcomas. Curr Treat Options Oncol. 2013;14:425–441. doi: 10.1007/s11864-013-0236-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis J.J., Leung D., Woodruff J.M., Brennan M.F. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg. 1998;228:355–365. doi: 10.1097/00000658-199809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilbeau L., Kantor G., Stoeckle E. Surgical resection and radiotherapy for primary retroperitoneal soft tissue sarcoma. Radiother Oncol. 2002;65:137–143. doi: 10.1016/s0167-8140(02)00283-9. [DOI] [PubMed] [Google Scholar]
- 5.Nathan H., Raut C.P., Thornton K. Predictors of survival after resection of retroperitoneal sarcoma: a population-based analysis and critical appraisal of the AJCC staging system. Ann Surg. 2009;250:970–976. doi: 10.1097/SLA.0b013e3181b25183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heslin M.J., Lewis J.J., Nadler E. Prognostic factors associated with long-term survival for retroperitoneal sarcoma: implications for management. J Clin Oncol. 1997;15:2832–2839. doi: 10.1200/JCO.199715.8.2832. [DOI] [PubMed] [Google Scholar]
- 7.Toulmonde M., Bonvalot S., Meeus P. Retroperitoneal sarcomas: patterns of care at diagnosis, prognostic factors and focus on main histological subtypes: a multicenter analysis of the French Sarcoma Group. Ann Oncol Off J Eur Soc Med Oncol. 2014;25:735–742. doi: 10.1093/annonc/mdt577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nussbaum D.P., Rushing C.N., Lane W.O. Preoperative or postoperative radiotherapy versus surgery alone for retroperitoneal sarcoma: a case-control, propensity score-matched analysis of a nationwide clinical oncology database. Lancet Oncol. 2016;17:966–975. doi: 10.1016/S1470-2045(16)30050-X. [DOI] [PubMed] [Google Scholar]
- 9.Fowler J.F. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62:679–694. doi: 10.1259/0007-1285-62-740-679. [DOI] [PubMed] [Google Scholar]
- 10.Haas R.L.M., Miah A.B., LePechoux C. Preoperative radiotherapy for extremity soft tissue sarcoma; past, present and future perspectives on dose fractionation regimens and combined modality strategies. Radiother Oncol. 2016;119:14–21. doi: 10.1016/j.radonc.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Leeuwen C.M., Oei A.L., Crezee J. The alfa and beta of tumours: a review of parameters of the linear-quadratic model, derived from clinical radiotherapy studies. Radiat Oncol. 2018;13:96. doi: 10.1186/s13014-018-1040-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplan E.L.M.P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 13.Services H. CTCAE; 2017. Common Terminology Criteria for Adverse Events. [Google Scholar]
- 14.Jaques D.P., Coit D.G., Hajdu S., Brennan M.F. management of primary and recurrent soft-tissue sarcoma of the retroperitoneum. Ann Surg. 1989:51–59. doi: 10.1097/00000658-199007000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strauss D.C., Hayes A.J., Thway K. Surgical management of primary retroperitoneal sarcoma. Br J Surg. 2010;97:698–706. doi: 10.1002/bjs.6994. [DOI] [PubMed] [Google Scholar]
- 16.van Dalen T., Hoekstra H.J., van Geel A.N. Locoregional recurrence of retroperitoneal soft tissue sarcoma: second chance of cure for selected patients. Eur J Surg Oncol. 2001;27:564–568. doi: 10.1053/ejso.2001.1166. [DOI] [PubMed] [Google Scholar]
- 17.Gronchi A., Casali P.G., Fiore M. Retroperitoneal soft tissue sarcomas: patterns of recurrence in 167 patients treated at a single institution. Cancer. 2004;100:2448–2455. doi: 10.1002/cncr.20269. [DOI] [PubMed] [Google Scholar]
- 18.Gemici K., Buldu I., Acar T. Management of patients with retroperitoneal tumors and a review of the literature. World J Surg Oncol. 2015;13:143. doi: 10.1186/s12957-015-0548-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gronchi A., Strauss D.C., Miceli R. Variability in patterns of recurrence after resection of primary retroperitoneal sarcoma (RPS): a report on 1007 patients from the Multi-institutional Collaborative RPS Working Group. Ann Surg. 2016;263:1002–1009. doi: 10.1097/SLA.0000000000001447. [DOI] [PubMed] [Google Scholar]
- 20.Stoeckle E., Coindre J.M., Bonvalot S. Prognostic factors in retroperitoneal sarcoma: a multivariate analysis of a series of 165 patients of the French Cancer Center Federation Sarcoma Group. Cancer. 2001;92:359–368. doi: 10.1002/1097-0142(20010715)92:2<359::aid-cncr1331>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 21.Ferrario T., Karakousis C.P. Retroperitoneal sarcomas: grade and survival. Arch Surg. 2003;138:248–251. doi: 10.1001/archsurg.138.3.248. [DOI] [PubMed] [Google Scholar]
- 22.Hassan I., Park S.Z., Donohue J.H. Operative management of primary retroperitoneal sarcomas: a reappraisal of an institutional experience. Ann Surg. 2004;239:244–250. doi: 10.1097/01.sla.0000108670.31446.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehnert T., Cardona S., Hinz U. Primary and locally recurrent retroperitoneal soft-tissue sarcoma: local control and survival. Eur J Surg Oncol. 2009;35:986–993. doi: 10.1016/j.ejso.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Nishimura J., Morii E., Takahashi T. Abdominal soft tissue sarcoma: a multicenter retrospective study. Int J Clin Oncol. 2010;15:399–405. doi: 10.1007/s10147-010-0075-4. [DOI] [PubMed] [Google Scholar]
- 25.Bonvalot S., Rivoire M., Castaing M. Primary retroperitoneal sarcomas: a multivariate analysis of surgical factors associated with local control. J Clin Oncol. 2009;27:31–37. doi: 10.1200/JCO.200818.0802. [DOI] [PubMed] [Google Scholar]
- 26.Gronchi A., Lo Vullo S., Fiore M. Aggressive surgical policies in a retrospectively reviewed single-institution case series of retroperitoneal soft tissue sarcoma patients. J Clin Oncol. 2009;27:24–30. doi: 10.1200/JCO.200817.8871. [DOI] [PubMed] [Google Scholar]
- 27.Bonvalot S., Miceli R., Berselli M. Aggressive surgery in retroperitoneal soft tissue sarcoma carried out at high-volume centers is safe and is associated with improved local control. Ann Surg Oncol. 2010;17:1507–1514. doi: 10.1245/s10434-010-1057-5. [DOI] [PubMed] [Google Scholar]
- 28.MacNeill A.J., Fiore M. Surgical morbidity in retroperitoneal sarcoma resection. J Surg Oncol. 2018;117:56–61. doi: 10.1002/jso.24902. [DOI] [PubMed] [Google Scholar]
- 29.Pisters P.W.T. Resection of some – but not all – clinically uninvolved adjacent viscera as part of surgery for retroperitoneal soft tissue sarcomas. J Clin Oncol. 2009;27:6–8. doi: 10.1200/JCO.200818.7138. [DOI] [PubMed] [Google Scholar]
- 30.Surgery With or Without Radiation Therapy in Treating Patients With Primary Soft Tissue Sarcoma of the Retroperitoneum or Pelvis. Alliance for Clinical Trials in Oncology ClinicalTrials.gov Identifier: NCT00091351. Available from: https://clinicaltrials.gov/ct2/show/NCT00091351.
- 31.Bonvalot S., Gronchi A., Le Pechoux C. STRASS (EORTC 62092): a phase III randomized study of preoperative radiotherapy plus surgery versus surgery alone for patients with retroperitoneal sarcoma. J Clin Oncol. 2019;37:11001. doi: 10.1200/JCO.201937.15_suppl.11001. [DOI] [Google Scholar]
- 32.Tseng W.H., Martinez S.R., Do L., Tamurian R.M., Borys D., Canter R.J. Lack of survival benefit following adjuvant radiation in patients with retroperitoneal sarcoma: a SEER analysis. J Surg Res. 2011;168:e173–e180. doi: 10.1016/j.jss.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Bates J.E., Dhakal S., Mazloom A., Constine L.S. The benefit of adjuvant radiotherapy in high-grade nonmetastatic retroperitoneal soft tissue sarcoma: a SEER analysis. Am J Clin Oncol. 2018;41:274–279. doi: 10.1097/COC.0000000000000259. [DOI] [PubMed] [Google Scholar]
- 34.Lee H.S., Yu I.J., Lim D.H., Kim S.J. Retroperitoneal liposarcoma: the role of adjuvant radiation therapy and the prognostic factors. Radiat Oncol J. 2016;34:216–222. doi: 10.3857/roj.2016.0185.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calvo F.A., Meirino R.M., Orecchia R. Intraoperative radiation therapy first part: rationale and techniques. Crit Rev Oncol Hematol. 2006;59:106–115. doi: 10.1016/j.critrevonc.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Fein D.A., Corn B.W., Lanciano R.M., Herbert S.H., Hoffman J.P., Coia L.R. Management of retroperitoneal sarcomas: does dose escalation impact on locoregional control? Int J Radiat Oncol Biol Phys. 1995;31:129–134. doi: 10.1016/0360-3016(94)E0302.-Z. [DOI] [PubMed] [Google Scholar]
- 37.Tzeng C-WD, Fiveash J.B., Popple R.A. Preoperative radiation therapy with selective dose escalation to the margin at risk for retroperitoneal sarcoma. Cancer. 2006;107:371–379. doi: 10.1002/cncr.2200.5. [DOI] [PubMed] [Google Scholar]
- 38.Bossi A., De Wever I., Van Limbergen E., Vanstraelen B. Intensity modulated radiation-therapy for preoperative posterior abdominal wall irradiation of retroperitoneal liposarcomas. Int J Radiat Oncol Biol Phys. 2007;67:164–170. doi: 10.1016/j.ijrobp.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 39.DeLaney T.F., Chen Y.-L., Baldini E.H. Phase 1 trial of preoperative image guided intensity modulated proton radiation therapy with simultaneously integrated boost to the high risk margin for retroperitoneal sarcomas. Adv Radiat Oncol. 2017;2:85–93. doi: 10.1016/j.adro.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roeder F., Ulrich A., Habl G. Clinical phase I/II trial to investigate preoperative dose-escalated intensity-modulated radiation therapy (IMRT) and intraoperative radiation therapy (IORT) in patients with retroperitoneal soft tissue sarcoma: interim analysis. BMC Cancer. 2014;14:617. doi: 10.1186/1471-2407-14-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamada T., Tsujii H., Tsuji H. Efficacy and safety of carbon ion radiotherapy in bone and soft tissue sarcomas. J Clin Oncol. 2002;20:4466–4471. doi: 10.1200/JCO.200210.050. [DOI] [PubMed] [Google Scholar]
- 42.Serizawa I., Kagei K., Kamada T. Carbon ion radiotherapy for unresectable retroperitoneal sarcomas. Int J Radiat Oncol Biol Phys. 2009;75:1105–1110. doi: 10.1016/j.ijrobp.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 43.Gieschen H.L., Spiro I.J., Suit H.D. Long-term results of intraoperative electron beam radiotherapy for primary and recurrent retroperitoneal soft tissue sarcoma. Int J Radiat Oncol Biol Phys. 2001;50:127–131. doi: 10.1016/s0360-3016(00)0158.9-3. [DOI] [PubMed] [Google Scholar]
- 44.Petersen I.A., Haddock M.G., Donohue J.H. Use of intraoperative electron beam radiotherapy in the management of retroperitoneal soft tissue sarcomas. Int J Radiat Oncol Biol Phys. 2002;52:469–475. doi: 10.1016/s0360-3016(01)0259.5-0. [DOI] [PubMed] [Google Scholar]
- 45.Youssef E., Fontanesi J., Mott M. Long-term outcome of combined modality therapy in retroperitoneal and deep-trunk soft-tissue sarcoma: analysis of prognostic factors. Int J Radiat Oncol Biol Phys. 2002;54:514–519. doi: 10.1016/s0360-3016(02)0294.2-5. [DOI] [PubMed] [Google Scholar]
- 46.Jones J.J., Catton C.N., O'Sullivan B. Initial results of a trial of preoperative external-beam radiation therapy and postoperative brachytherapy for retroperitoneal sarcoma. Ann Surg Oncol. 2002;9:346–354. doi: 10.1007/bf.02573869. [DOI] [PubMed] [Google Scholar]
- 47.Bobin J.Y., Al-Lawati T., Granero L.E. Surgical management of retroperitoneal sarcomas associated with external and intraoperative electron beam radiotherapy. Eur J Surg Oncol. 2003;29:676–681. doi: 10.1016/s0748-7983(03)0013.9-2. [DOI] [PubMed] [Google Scholar]
- 48.Zlotecki R.A., Katz T.S., Morris C.G., Lind D.S., Hochwald S.N. Adjuvant radiation therapy for resectable retroperitoneal soft tissue sarcoma: the University of Florida experience. Am J Clin Oncol. 2005;28:310–316. doi: 10.1097/01.coc.0000158441.9645.5.31. [DOI] [PubMed] [Google Scholar]
- 49.Krempien R., Roeder F., Oertel S. Intraoperative electron-beam therapy for primary and recurrent retroperitoneal soft-tissue sarcoma. Int J Radiat Oncol Biol Phys. 2006;65:773–779. doi: 10.1016/j.ijrobp.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 50.Pawlik T.M., Pisters P.W.T., Mikula L. Long-term results of two prospective trials of preoperative external beam radiotherapy for localized intermediate- or high-grade retroperitoneal soft tissue sarcoma. Ann Surg Oncol. 2006;13:508–517. doi: 10.1245/ASO.200605.035. [DOI] [PubMed] [Google Scholar]
- 51.Feng M., Murphy J., Griffith K.A. Long-term outcomes after radiotherapy for retroperitoneal and deep truncal sarcoma. Int J Radiat Oncol Biol Phys. 2007;69:103–110. doi: 10.1016/j.ijrobp.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 52.Ballo M.T., Zagars G.K., Pollock R.E. Retroperitoneal soft tissue sarcoma: an analysis of radiation and surgical treatment. Int J Radiat Oncol Biol Phys. 2007;67:158–163. doi: 10.1016/j.ijrobp.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 53.White J.S., Biberdorf D., DiFrancesco L.M., Kurien E., Temple W. Use of tissue expanders and pre-operative external beam radiotherapy in the treatment of retroperitoneal sarcoma. Ann Surg Oncol. 2007;14:583–590. doi: 10.1245/s10434-006-9139-0. [DOI] [PubMed] [Google Scholar]
- 54.Zagar T.M., Shenk R.R., Kim J.A. Radiation therapy in addition to gross total resection of retroperitoneal sarcoma results in prolonged survival: results from a single institutional study. J Oncol. 2008;2008:824036. doi: 10.1155/2008/8240.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gholami S., Jacobs C.D., Kapp D.S., Parast L.M., Norton J.A. The value of surgery for retroperitoneal sarcoma. Sarcoma. 2009;2009:605840. doi: 10.1155/2009/6058.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dziewirski W., Rutkowski P., Nowecki Z.I. Surgery combined with brachytherapy in patients with retroperitoneal sarcomas. J Contemp Brachyther. 2010;2:14–23. doi: 10.5114/jcb.2010.1371.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sampath S., Hitchcock Y.J., Shrieve D.C., Randall R.L., Schultheiss T.E., Wong J.Y.C. Radiotherapy and extent of surgical resection in retroperitoneal soft-tissue sarcoma: multi-institutional analysis of 261 patients. J Surg Oncol. 2010;101:345–350. doi: 10.1002/jso.2147.4. [DOI] [PubMed] [Google Scholar]
- 58.Donahue T.R., Kattan M.W., Nelson S.D., Tap W.D., Eilber F.R., Eilber F.C. Evaluation of neoadjuvant therapy and histopathologic response in primary, high-grade retroperitoneal sarcomas using the sarcoma nomogram. Cancer. 2010;116:3883–3891. doi: 10.1002/cncr.2527.1. [DOI] [PubMed] [Google Scholar]
- 59.Yoon S.S., Chen Y.-L., Kirsch D.G. Proton-beam, intensity-modulated, and/or intraoperative electron radiation therapy combined with aggressive anterior surgical resection for retroperitoneal sarcomas. Ann Surg Oncol. 2010;17:1515–1529. doi: 10.1245/s10434-010-0935-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee H.J., Song S.Y., Kwon T.-W. Treatment outcome of postoperative radiotherapy for retroperitoneal sarcoma. Radiat Oncol J. 2011;29:260–268. doi: 10.3857/roj.2011.29.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fuks D., Verhaeghe J.-L., Marchal F. Surgery and postoperative radiation therapy in primary retroperitoneal sarcomas: experience of the cancer centre Alexis-Vautrin. Cancer Radiother. 2012;16:194–200. doi: 10.1016/j.canrad.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 62.Paryani N.N., Zlotecki R.A., Swanson E.L. Multimodality local therapy for retroperitoneal sarcoma. Int J Radiat Oncol Biol Phys. 2012;82:1128–1134. doi: 10.1016/j.ijrobp.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 63.McBride S.M., Raut C.P., Lapidus M. Locoregional recurrence after preoperative radiation therapy for retroperitoneal sarcoma: adverse impact of multifocal disease and potential implications of dose escalation. Ann Surg Oncol. 2013;20:2140–2147. doi: 10.1245/s10434-013-2868-y. [DOI] [PubMed] [Google Scholar]
- 64.De Wever I., Laenen A., Van Limbergen E. Pre-operative irradiation for retroperitoneal liposarcoma: results of a pilot study. Acta Chir Belg. 2013;113:315–321. [PubMed] [Google Scholar]
- 65.Alford S., Choong P., Chander S., Henderson M., Powell G., Ngan S. Outcomes of preoperative radiotherapy and resection of retroperitoneal sarcoma. ANZ J Surg. 2013;83:336–341. doi: 10.1111/j.1445-2197.2012.0621.1.x. [DOI] [PubMed] [Google Scholar]
- 66.Sweeting R.S., Deal A.M., Llaguna O.H. Intraoperative electron radiation therapy as an important treatment modality in retroperitoneal sarcoma. J Surg Res. 2013;185:245–249. doi: 10.1016/j.jss.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Le Pechoux C., Musat E., Baey C. Should adjuvant radiotherapy be administered in addition to front-line aggressive surgery (FAS) in patients with primary retroperitoneal sarcoma? Ann Oncol Off J Eur Soc Med Oncol. 2013;24:832–837. doi: 10.1093/annonc/mds516. [DOI] [PubMed] [Google Scholar]
- 68.Stucky C-CH, Wasif N., Ashman J.B., Pockaj B.A., Gunderson L.L., Gray R.J. Excellent local control with preoperative radiation therapy, surgical resection, and intra-operative electron radiation therapy for retroperitoneal sarcoma. J Surg Oncol. 2014;109:798–803. doi: 10.1002/jso.2357.6. [DOI] [PubMed] [Google Scholar]
- 69.El-Bared N., Taussky D., Mehiri S., Patocskai E., Roberge D., Donath D. Preoperative intensity modulated radiation therapy for retroperitoneal sarcoma. Technol Cancer Res Treat. 2014;13:211–216. doi: 10.7785/tcrt.2012.5003.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smith M.J.F., Ridgway P.F., Catton C.N. Combined management of retroperitoneal sarcoma with dose intensification radiotherapy and resection: long-term results of a prospective trial. Radiother Oncol. 2014;110:165–171. doi: 10.1016/j.radonc.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 71.Trovik L.H., Ovrebo K., Almquist M. Adjuvant radiotherapy in retroperitoneal sarcomas. A Scandinavian Sarcoma Group study of 97 patients. Acta Oncol. 2014;53:1165–1172. doi: 10.3109/0284186X.2014.921723. [DOI] [PubMed] [Google Scholar]
- 72.Bishop A.J., Zagars G.K., Torres K.E. Combined modality management of retroperitoneal sarcomas: a single-institution series of 121 patients. Int J Radiat Oncol Biol Phys. 2015;93:158–165. doi: 10.1016/j.ijrobp.2015.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abdelfatah E., Guzzetta A.A., Nagarajan N. Long-term outcomes in treatment of retroperitoneal sarcomas: a 15 year single-institution evaluation of prognostic features. J Surg Oncol. 2016;114:56–64. doi: 10.1002/jso.2425.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cosper P.F., Olsen J., DeWees T. Intensity modulated radiation therapy and surgery for Management of Retroperitoneal Sarcomas: a single-institution experience. Radiat Oncol. 2017;12:198. doi: 10.1186/s13014-017-0920-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim H.J., Koom W.S., Cho J., Kim H.S., Suh C.O. Efficacy of Postoperative Radiotherapy Using Modern Techniques in Patients with Retroperitoneal Soft Tissue Sarcoma. Yonsei Med J. 2018;59:1049–1056. doi: 10.3349/ymj.2018.59.9.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haas R.L.M., Bonvalot S., Miceli R. Radiotherapy for retroperitoneal liposarcoma: a report from the Transatlantic Retroperitoneal Sarcoma Working Group. Cancer. 2019;125:1290–1300. doi: 10.1002/cncr.3192.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Woll P.J., Reichardt P., Le Cesne A. EORTC Soft Tissue and Bone Sarcoma Group and the NCIC Clinical Trials Group Sarcoma Disease Site Committee, Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): a multicentre randomised controlled trial. Lancet Oncol. 2012;13:1045–1054. doi: 10.1016/S1470-2045(12)70346-7. [DOI] [PubMed] [Google Scholar]
- 78.Le Cesne A., Ouali M., Leahy M.G. Doxorubicin-based adjuvant chemotherapy in soft tissue sarcoma: pooled analysis of two STBSG-EORTC phase III clinical trials. Ann Oncol. 2014;25:2425–2432. doi: 10.1093/annonc/mdu460. [DOI] [PubMed] [Google Scholar]
- 79.Gronchi A., Ferrari S., Quagliuolo V. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): an international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol. 2017;18:812–822. doi: 10.1016/S1470-2045(17)30334-0. [DOI] [PubMed] [Google Scholar]
- 80.Gronchi A., Hindi N., Cruz J. Trabectedin and RAdiotherapy in Soft Tissue Sarcoma (TRASTS): results of a phase I study in Myxoid Liposarcoma from Spanish (GEIS), Italian (ISG), French (FSG) Sarcoma Groups. EClinicalMedicine. 2019;9:35–43. doi: 10.1016/j.eclinm.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]