Abstract
Introduction
Sarcoidosis is a granulomatous systemic disease that becomes chronic in approximately one third of affected patients resulting in quality of life and functional impairment. Immunosuppressive drugs other than steroids represent alternative therapeutic options, but side effects like liver and bone marrow toxicity or increased susceptibility to infections limit their use. Pathophysiological studies in sarcoidosis patients demonstrate altered regulatory T-cell functions with a reduced expression of CTLA-4 (CD152) and prolonged inflammation. Therefore, interfering with CTLA-4 using abatacept might be a therapeutic option in sarcoidosis similar to rheumatoid arthritis therapy.
Methods/design
This is a multicenter prospective open-labeled single arm phase II study addressing the safety of abatacept in sarcoidosis patients. 30 patients with chronic sarcoidosis requiring immunosuppressive therapy beyond 5 mg prednisolone equivalent will be treated with abatacept in combination with corticosteroids for one year in two centers.
The primary endpoint is the number and characterization of severe infectious complications under treatment with abatacept.
Secondary endpoints are the rate of all infections, patient-related outcomes (assessed by questionnaires), lung function and immunological parameters including alveolar inflammation assessed by bronchoaveolar lavage.
Discussion
This is the first trial of abatacept in patients with sarcoidosis. It is hypothesized that administration of abatacept is safe in patients with chronic sarcoidosis and can limit ongoing inflammation. Patients’ wellbeing is assessed by established questionnaires. Immunological work-up will highlight the effect of abatacept on inflammatory pathways in sarcoidosis.
Trial registration
The trial has been registered at the German Clinical Trial Registry (Deutsches Register Klinischer Studien, DRKS) with the identity number DRKS00011660.
Keywords: Chronic sarcoidosis, Therapy, Abatacept, Regulatory T-cells, Patient-reported outcome, King's sarcoidosis questionnaire
Abbreviations: BAL, bronchoalveolar lavage; CMV, cytomegaly-virus; EBV, Epstein-Barr-Virus; FVC, forced vital capacity; GHS, general health score; IFN-γ, Interferon-γ; IL, interleukin; KSQ, King's sarcoidosis questionnaire; TLC, total lung capacity; TNF, tumor-necrosis factor; TReg, regulatory T-cells; 18FDG-PET-CT, 18Fluor-Desoxy-Glucose positron-emission tomography combined with computer tomography
1. Introduction
Sarcoidosis is a rare chronic granulomatous disorder of unknown origin [1,2] characterized by granuloma formation especially within the lungs and regional lymphnodes. Granulomtous reaction is supposed to derive from an exaggerated immune cell reaction involving a Th1-driven alveolar inflammation [[3], [4], [5]].
Therapy in sarcoidosis aims to dampen this Th1-driven inflammation. Corticosteroids are the mainstay in sarcoidosis therapy [6,7]. Based on their efficacy in other Th1- driven diseases, immunosuppressive drugs are used for sarcoidosis with their efficacy described in case series and retrospective analyses [8,9]. TNF-binding antibodies have been used successfully in several cases of steroid-resistant sarcoidosis as a target therapy based on the pivotal role of TNF for sarcoidosis [10,11], a subsequent prospective trial could only demonstrate a small increase in FVC without corresponding melioration of patient-reported outcome parameters [12] However, subsequent in-depth analyses revealed that underlying pathophysiological differences influence response to TNF-neutralizing therapy [13,14] pointing out that a priori characterization of patients may help to predict therapeutic response.
Besides the Th1-driven proinflammatory milieu, functionally impaired regulatory T-cells (Tregs) are described in sarcoidosis that insufficiently dampen ongoing T-cell driven inflammation [[15], [16], [17]]. Restoration of Tregs’ function by inhaled vasoactive intestinal peptide led to clinical improvement [17] and therefore Tregs may represent a promising therapeutic target in sarcoidosis [4,18,19].
T-cell activation requires two signals by the T-cell receptor (activated by MHC) and by CD28 (activated by CD80/CD86). The co-stimulatory effect of CD80/CD86 can be modulated by cytotoxic T-lymphocyte antigen-4 (CTLA-4), an inhibitory type 1 transmembrane receptor constitutively expressed on regulatory T-cells and induced in activated T-cells [[20], [21], [22], [23]]. CTLA-4 outcompetes CD28 for its binding to CD80/D86 expressed by antigen presenting cells resulting in a reduced activation of conventional T cells [24,25]. In CTLA-4 knock out mice, Tregs lack their suppressive activity leading to a breakdown of peripheral tolerance and the outbreak of autoimmune diseases [26,27]. CTLA-4 polymorphisms have been associated with autoimmune diseases in humans [28]. Heterozygous CTLA-4 mutations resulting in impaired expression or function of CLTA-4 were described in patients with severe immune dysregulation syndromes [[29], [30], [31]]. In these patients insufficient CTLA-4 function results in a sustained T-cell activation with organ infiltration and granuloma formation by these activated T-cells. Further observations emphasize the role of CTLA-4 malfunctioning in sarcoidosis. Decreased CTLA-4 expression could be detected on regulatory T-cells in sarcoidosis patients [32] and blocking CTLA-4 to breach immunotolerance in cancer therapy can lead to granulomatous disease mimicking sarcoidosis [[33], [34], [35]].
Abatacept, a CTLA-4–Ig fusion protein, can be pharmaceutically used because abatacept itself can capture CD80/CD86, thereby interfering with T-cell activation [36,37].
Therefore abatacept represents a potential therapy for sarcoidosis. Abatacept has been approved for therapy of rheumatoid and psoriatic arthritis [[38], [39], [40]], both of which are mainly Th1-driven autoimmune diseases.
This prospective open-labeled single-arm trial intends to assess the safety of abatacept in chronic, steroid-refractory sarcoidosis with lung function, patient-related outcome parameters and immunological parameters as secondary endpoints.
Immunological work-up will further allow a deeper insight in pathophysiological alterations of individuals affected by sarcoidosis and their impact for abatacept treatment.
2. Methods and analysis
2.1. Study design
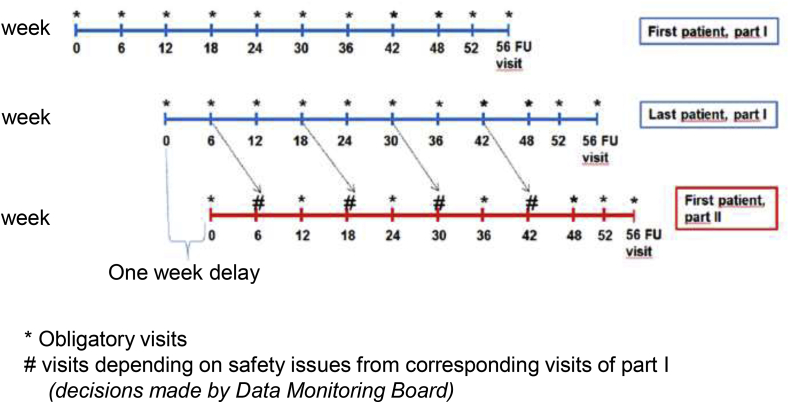
This is a multicenter prospective open-labeled single-arm phase II study. 30 individuals are planned to receive abatacept at two sites, (i) Medical Center – University of Freiburg, and (ii) Medical School Hannover, University of Hannover. The primary objective is to assess the safety of abatacept, because it is the first time applied to sarcoidosis patients at all. To assure and sufficiently assess the safety of the treated individuals, the first six patients are scheduled to be visited at a six-week interval. An independent data monitoring committee (see also below) evaluates safety data every twelve weeks starting after the sixth patient has completed the six-week visit. Based on the committee's advice, visit intervals will be scheduled for the following participants (six-week interval or twelve-week interval). Fig. 1 shows a graphic time table. Fig. 2 shows the study flow chart.
Fig. 1.
Schedule of patient inclusion.
Patients will be included in two phases. The first six patients (blue time lines) will be included in part I and will have visits every six weeks to address especially safety aspects. After the sixth patient has been included a one week delay is planned prior to inclusion of patient No. 7 (first patient of part II, red time line). Patients of part II will have visits every twelve weeks. The Data Monitoring Board can advise to increase or reduce visit frequency based on safety data obtained from the patients included in part I. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
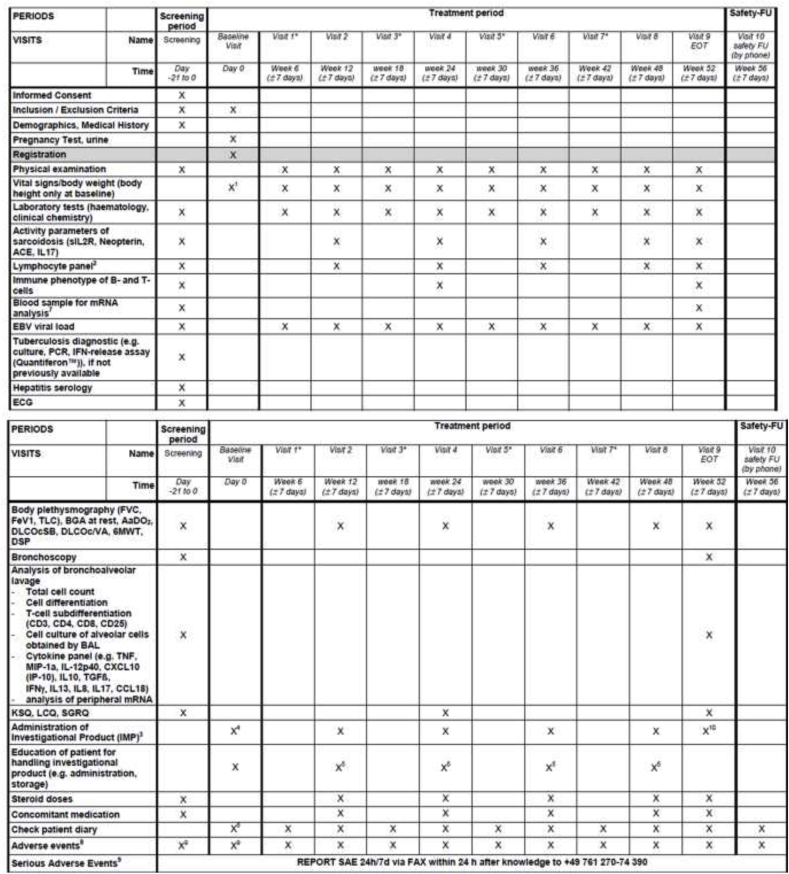
Fig. 2.
Visit schedule and assessment
Fig. 2 highlights the visit schedule for all patients including the screening period, treatment period and follow-up visit. Footnotes are the following:
* visits 1, 3, 5 and 7 are required for part I- group, for part II-group the necessity for these visits will be determined by results from part I-group and advises of the DMC.
1 only at baseline inclusive height
2 CD4, CD8, B and NK cell numbers
3 125 mg/ml syringe weekly; sc injection at clinic and dispensing of investigational product for administration at home (once weekly)
4 administration of first dose of trial medication and subsequent observation for at least 30 min after administration
5 re-education, if needed
6 dispensing of patient diary and education on completion
7 special tubes for mRNA collection
8 documentation period AE/reporting period SAE from 1st application of IMP until 30 days after last application of IMP
9before 1st application of IMP just documentation and reporting of SAE 10 last sc injection at clinic; NO dispensing of investigational product for administration at home (once weekly).
2.2. Primary and secondary objectives and endpoints
Primary endpoint is the safety of abatacept in sarcoidosis patients. This endpoint was chosen because there has not been any study or case report on abatacept treatment in sarcoidosis. Safety is measured as the number of severe infectious complications (defined as any opportunistic infection, any infection requiring hospitalization or intravenous administration of antibiotics, and EBV infections) during treatment period.
Key secondary objectives include the evaluation of safety and efficacy of abatacept in sarcoidosis patients, as well as the description of patient reported outcomes. As main safety-related secondary outcome parameter, the number and rate of all infectious complications will be analysed and compared to infectious complications within the year before study inclusion. Non-infectious complications of abatacept treatment will be monitored as (severe) adverse reaction and graded according to the current “Common Terminology Criteria for Adverse Events” (CTCAE).
Additional secondary endpoints will focus on therapy-induced changes of clinical, lung-functional, immunological and patient-reported outcomes.
Primary and secondary endpoints of the trial will be compared to corresponding parameters within the year prior to study inclusion.
Furthermore, the steroid dose and need of steroid pulse to control sarcoidosis flares will be documented during the trial period.
Additionally the study will allow an in-depth analysis of patients’ immunological characteristics. This will allow identifying patients who can benefit the most of abatacept treatment. Several previous studies have shown that individual characteristics of sarcoidosis patients influence treatment success.
Table 1 presents the study endpoints in detail.
Table 1.
Primary and secondary endpoints of the study with detailed explanation and arguments for choosing these endpoints.
| Endpoint | Explanation | comment | |
|---|---|---|---|
| Primary | Safety assessment of abatacept in patients with chronic sarcoidosis depending on immunosuppressive regime, measured as number of infectious complications during treatment period | Number of infectious complications during treatment period of one year:
|
Immunosuppression harbors the risk of especially opportunistic infections. Therefore, infections were chosen as primary endpoint to assess safety. The above-mentioned definitions of monitored infections avoid the false-positive detection of infections or detection of minor infections like seasonal flue. |
| Secondary | Safety assessment of abatacept | - Rate of infections during treatment period compared to rate of similar infections in included patients within a one year period prior to study inclusion (where available)
|
Individuals included in this study suffer from chronic sarcoidosis and received immunosuppressive therapies beforehand. Therefore, the complications of abatacept treatment must be compared to previous complications. Therefore, infections and other therapy-related complications will be retrospectively analysed for the year before study enrolment and compared to events during abatacept treatment. |
| Assessment of patient reported outcome parameters during treatment with abatacept | Change in KSQ (King's sarcoidosis questionnaire) St. George Respiratory Questionnaire (SGRQ) Leicester Cough Questionnaire (LCQ) |
Patient-related outcomes are increasingly recognized as relevant endpoints in clinical studies. Therefore, three questionnaires were included in the study to measure patient-related outcome as change during treatment. KSQ: This questionnaire comprises many issues that affect well-being of patients suffering from sarcoidosis including fatigue, excertional dyspnea, medication side effects and the influence of all these parameters on activities of daily life. A change of 6 points are considered clinically significant [42,61]. St. George Respiratory Questionnaire: This questionnaire is the most frequently used tool to assess patients' respiratory symptoms. Even though it has never been evaluated for sarcoidosis, it is often used and requested. The minimal important difference for interstitial lung diseases is considered to be 6 points change [62]. Leicester Cough Questionnaire: The Leicester Cough Questionnaire is a very recent questionnaire focusing on the symptom of cough, which often affects patients with sarcoidosis. Cough can hardly be treated and often leads to physical and psychical impairment of patients. The minimal important difference is 1.3 points [63]. |
|
| Efficacy assessment of treatment with Abatacept, measured as pulmonary function at regular visits (12, 24 36, 48 and 52 weeks) | TLC FVC DLCO SB DLCO/VA pO2 at rest and after 6MWT AaDO2 DSP |
Sarcoidosis is mostly affecting the lung and lung functional impairments represent one major indication for therapy. TLC: The Total lung capacity reflects best changes of the lung volume because it measures vital capacity as well as residual volume. A change of 10% is considered to be clinical relevant FVC: The Forced Vital Capacity has been the most important lung function parameter in several studies for interstitial lung diseases. A change of 10% from baseline has been used in most studies. DLCOcSB: Diffusing capacity single breath (DLCOcSB) has been considered to be a good parameter for assessing sarcoidosis' impact on lung function and identifying patients at risk for progession. A 15% change is considered significant. DLCOc/VA: DLCOc/VA mirrors the diffusion capacity for oxygen and is considered a very sensitive marker for interstitial abnormalities in lung diseases and sarcoidosis. pO2at rest and after 6MWT: This parameter allows to monitor the oxygenation under rest and under exertion. Deterioration is defined as 50 m reduction of walking distance or as a decrease of >5 mmHg compared to baseline in the 6 min walk test. Distance saturation product (DSP): DSP is measured after 6MWT. It is calculated by the product of the walking distance and the oxygen saturation after the walk divided by 100. Therefore, this product integrates both components of the 6MWT, that is distance and saturation. AaDO2– alveolar arterial pO2difference: AaDO2 is calculated by the alveolar air equation and is a subtle maker of O2-diffusion impairment. |
|
| Laboratory parameters | sIL2R Neopterin ACE IL17 Total white blood cell count B- and T-cell count Characterization of T- and B-cell subtypes mRNA measurement |
Laboratory parameters have been used to monitor inflammatory activity of sarcoidosis, even though all markers have limitations. sIL2R: This parameter reflects inflammatory processes has been found in chronic sarcoidosis. It can be used for monitoring disease activity in sarcoidosis. Neopterin: This parameter reflects inflammatory processes as have been found in chronic sarcoidosis. It can be used for monitoring disease activity in sarcoidosis. ACE: ACE is in part produced by granulomas and can therefore be used to monitor granuloma burden in sarcoidosis. IL17: Interfering with CTLA is supposed to influence Th17-T-cells and thereby IL17 might be used to assess the influence of Abatacept on TH17 T-cells. mRNA measurement: With the use of Abatacept, we expect alterations of surface molecules on immune cells (e.g. T-cells, monocytes, macrophages). These alterations might be explained e.g. by (i) Abatacept-mediated internalization of surface molecules or by (ii) transcriptional/translational changes induced by Abatacept. The analysis of mRNA will help to dissect the underlying molecular mechanism. B- and T-cell count, characterization of T- and B-cell subtypes: As mentioned above, Abatacept is supposed to influence T-cell response in chronic sarcoidosis. Therefore, change of T-cell subtypes may reflect the biological response to Abatacept. |
|
| Bronchoalveolar lavage parameters | Total cell count/100 ml lavage fluid Percentage of differential bronchoalveolar cells Subtypes of T-cells in bronchoalveolar lavage Surface molecules as markers of cell activation, e.g. HLA-DR, CD57 etc. Released cytokines by BAL cells TNF MIP-1a IL-12p40 CXCL10 (IP-10) IL10 TGFβ IFNγ, IL13 IL8 IL17 CCL18 mRNA measurement |
BAL fluid and cell analysis of alveolar cells have been proven to deepen the understanding of alveolar inflammation as ongoing in sarcoidosis. Therefore, participants of this study will undergo bronchoscopy and BAL before starting abatacept and after one year of treatment. As abatacept influences T-cell/APC interaction, we expect changes in T-cell subtypes and lower inflammatory response of alveolar cells. Total cell count/100 ml lavage fluid: Total cell number is increased in most inflammatory processes affecting the alveolar compartment and is therefore an unspecific general marker of alveolar inflammation. Percentage of differential bronchoalveolar cells: As mentioned above, sarcoidosis is characterized by a lymphocytic alveolitis. Therefore, it can be expected that the percentage of lymphocytes decreases under successful treatment. Additional information can be drawn from the percentage of neutrophils because they predict a more aggressive course of sarcoidosis. Under Abatacept treatment, a reduction of neutrophils as sign of less inflammatory activity can be expected. Subtypes of T-cells in bronchoalveolar lavage: Abatacept influences T-cell subtype and it can be expected that the T-cell subtype in the alveolar compartment is changed after abatacept treatment. Expression of surface molecules as surrogates of cell activation: Several surface molecules on immune cells indicate their activation state (e.g. HLA-DR, CD57) and thereby might serve as markers of therapy success under Abatacept treatment. Cytokines produced by alveolar cells: Cells obtained by the bronchoalveolar lavage will be cultivated under suitable conditions and released cytokines in the supernatants will be measured, e.g. TNF, MIP-1a, IL-12p40, CXCL10 (IP-10), IL10, TGFβ, IFNγ IL13, IL8, IL17 and CCL18. mRNA measurement: With the use of Abatacept, we expect alterations of surface molecules on immune cells (e.g. T-cells, monocytes, macrophages). These alterations might be explained e.g. by (i) Abatacept-mediated internalization of surface molecules or by Refs. [64] (ii) transcriptional/translational changes induced by Abatacept. The analysis of mRNA will help to dissect the underlying molecular mechanism. |
|
| Need of therapy escalation/modification | Daily steroid dose Need for steroid pulses (see below) Need for therapy modification (see below) |
The two main goals of sarcoidosis treatment are (i) control of inflammatory activity that damages organs' function and [64] (ii) to achieve disease control by a minimum or no corticosteroid treatment. Therefore, the steroid doses used to control disease are a good surrogate for the therapeutic effect of Abatacept Daily steroid dose: Daily steroid doses will be monitored and compared to the steroid doses in the year before Abatacept treatment was started. Need for steroid pulses: Number of steroid pulses to control disease flairs and the dosage used will be monitored and compared to the dosage before. Need for therapy modification: Therapy modification (e.g. need of therapy interruption, therapy escalation) will be monitored. |
2.3. Participants and inclusion/exclusion criteria
Participants of the trial must have been diagnosed with sarcoidosis according to current guidelines [3], and must be in need of immunosuppressive therapy beyond 5 mg prednisolone equivalent per day. Individuals may be included if corticosteroid dose cannot be reduced to levels of 5 mg prednisolone equivalent or less because of persistent or recalcitrant sarcoidosis symptoms (including increasing symptom burden, increase in inflammatory conditions, deterioration of organ malfunctioning, lung functional impairment). Additionally, individuals on combination therapy (e.g. azathioprine and corticosteroids) may be enrolled if combination therapy has to be stopped because of lacking efficacy or side effects. The decision to escalate or change therapy relies on the physicians’ appraisal, taking into consideration that sarcoidosis treatment integrates multiple aspects rather than clear cut-off values. However, patients must have sarcoidosis-associated symptoms (documented by a KSQ –GHS < 80) [41,42] and signs of inflammatory activity (increased neopterine or sIL2R [43]) and an indication to immunosuppressive therapy because of pulmonary involvement.
Besides individuals with severe comorbidities not related to sarcoidosis, severe mental disease or (potential) contraindications against abatacept are excluded.
Table 2, Table 3 summarize in detail inclusion and exclusion criteria.
Table 2.
Inclusion criteria with explanation.
| Criterion | Explanation |
|---|---|
| Signed written informed consent | Formal requirement to participate in clinical trials |
| Ability to understand the nature, significance and consequences of the study and to comply with them | |
| Age 18 years or above; male of female patients | |
| Diagnosis of sarcoidosis according to current applicable ATS/WASOG guidelines | There is no unique diagnostic test to confirm the diagnosis of sarcoidosis. Rather, sarcoidosis is diagnosed epicritically by integrating clinical, radiological, serological and histological findings. For this study, the sarcoidosis is diagnosed according to ATS recommendations. The diagnosis of participants of this study will be verified by critically reviewing previous examinations and addition of indicated further examinations (e.g. ruling out other granulomatous diseases). |
Immunosuppressive therapy (≥5 mg prednisolone equivalent per day or any additional immunsuppressive therapy independently of the steroid dose) within the last 3 months prior screening for pulmonary involvement as defined by one of the following
|
Abatacept might represent a second-line agent for treating sarcoidosis given for steroid-refractory sarcoidosis. Therefore, a steroid dose of ≥5 mg prednisolone equivalent for longer than three months or an additional immunosuppressive agent are required for study inclusion. Indication for immunosuppression must be given by pulmonary involvement. |
| King's Sarcoidosis Questionnaire (KSQ), GHS module: Score<80 | KSQ questionnaire integrates patient-experienced disease burden. As sarcoidosis-associated symptoms are a major indication for treatment, we intend to include symptomatic patients within this trial defined by a decreased KSQ score. |
Need for therapy escalation beyond 5 mg prednisolone equivalent according to the physician's appraisal, e.g.:
|
Indication for escalation or change of immunosuppression in sarcoidosis treatment is mostly a clinical decision integrating therapy-associated side effects, disease control, inflammatory conditions or hints for disease progression. The term “physician's appraisal” summarizes all these conditions influencing the clinical decision to change or increase immunosuppressive therapy. |
| Elevated sIL2 receptor levels and/or elevated Neopterin as sign of T-cell activation within 6 months prior to registration | sIL2R and Neopterin are laboratory parameters that indicate ongoing inflammation in sarcoidosis patients. |
Table 3.
Exclusion criteria with explanation.
| Criterion | Explanation |
|---|---|
| Severe lung functional impairment according to the treating physician interfering substantially with participation in the trial | Lung function and exercise testing are part of the study protocol and the endpoints. Therefore patients with severe lung functional impairment are excluded. |
| End stage fibrotic lung disease without expected improvement to immunosuppressive therapy as judged by the treating physician. | Sarcoidosis can lead to end stage fibrotic lung disease and the impact of immunosuppression for this condition is a matter of debate. |
| Concomitant lung disease (e.g. COPD, asthma) that interferes with clinical assessment as judged by the treating physician | Sarcoidosis can coincide with other lung diseases (e.g. COPD, emphysema) whose symptoms may be of more clinical importance for the patients than sarcoidosis-associated symptoms. Therefore these patients are excluded from the study. |
| Concurrent immunosuppressive therapy other than corticosteroids and impossibility to allow a sufficient wash-out phase. | Concurrent immunosuppressive therapy may influence the therapeutic effect of abatacept and increase susceptibility to infections. Therefore, patients with concomitant immunosuppression (besides corticosteroids) are excluded. A sufficient wash-out phase (>two weeks for DMARD, > 2 months for biologicals) is required. |
| For biologicals a wash-out phase of two months is mandatory. | |
| Previous treatment with abatacept | Abatacept is approved for different autoimmune diseases. Patients being treated with abatacept previously might have had side effects or insufficient response to treatment. Therefore, they are excluded from the study. |
| Treatment with another investigational drug within 4 weeks or 5 half-lives prior to first application of trial medication. | This rules out unexpected medical interactions or interference between different study protocols. |
| Simultaneous participation in other interventional clinical trials | |
| Serious uncontrolled concomitant diseases not caused by sarcoidosis. Examples are cardiovascular (e.g. severe uncontrolled hypertension, instable angina pectoris, severe ischemic heart disease), central nervous system (e.g. stroke, dementia), hepatic, renal, endocrine (e.g. uncontrolled diabetes), gastrointestinal (e.g. complicated diverticulitis, Crohn's disease, colitis ulcerosa) | Patients suffering from severe other medical conditions are excluded from the study because a therapeutic benefit may be difficult to judge and abatacept treatment might interfere with these other diseases. |
| History of or active psychiatric disease (including known history of or current active abuse of drugs, chemicals or alcohol) interfering with the safe participation in the study. | |
| History of or current primary or secondary immunodeficiency that could not be attributable to treatment-related immunodeficiency | Abatacept dampens immune response and may therefore aggravate an underlying immunodeficiency. Furthermore, common variable immune defect might mimic sarcoidosis and therefore CVID patients are not suffering from sarcoidosis. |
| Recurrent or active current bacterial, viral or fungal infection (excluding fungal infections of the nails), for example but not limited to active hepatitis B and C, typical or atypical mycobacteriosis or herpes zoster infections. | All these conditions are relative contraindications for treatment with abatacept because of the risk of aggravation. These conditions are relative or absolute contraindications against the use of abatacept. |
| Known malignancy or high clinical suspicion on malignant disease | |
| Lymphoma within the last five years | |
| Contraindications against treatment with Abatacept | |
| Simultaneous application of live vaccines | |
| Pregnancy indicated by positive urine pregnancy test | There are no data available that address the safety of abatacept in pregnant or breast-feeding women. |
| Breast-feeding patients | |
| Fertile patients refusing to use safe contraceptive methods during the study |
2.4. Patient recruitment
Patients will be recruited from prevalent patients of the out-patient ward. Additionally patient organizations will be informed about the trial.
2.5. Informed consent
Patients will be informed about the clinical trial if their medical conditions seem to allow participation. An experienced physician will explain the purpose and course of the trial to the patient and provide him or her with a detailed information sheet. If the patient is interested in taking part in the study, he or she can contact the study center (or will be contacted by the study center, if desired by the patient) after a sufficient time period (in general at least one week). An additional appointment will be made with the patient to answer questions and discuss concerns before the patient can voluntarily sign informed consent.
2.6. Concomitant medication
Abatacept and corticosteroids are the only allowed immunosuppressive drugs during the entire study period. Although data from other diseases suggest sufficient safety of abatacept in combination with other immunosuppressive drugs (e.g. Methotrexate [44,45]), combination therapy with other immunosuppressive drugs in this small proof-of-concept study increases variability of clinical data and therefore impairs the interpretation of results.
For all immunosuppressive drugs a suitable wash-out period is mandatory before including patients in the study.
Corticosteroids are dosed according to patients' requirements and the physician's appraisal. A target dose of less than 5 mg prednisolone equivalent per day should be achieved in each participant. Steroid dosage and need for steroid increase are monitored. There is no restriction to other drugs besides the above-mentioned immunosuppressive drugs.
2.7. Sarcoidosis flares
Flares of sarcoidosis can be treated with an increase of corticosteroid dose. In case of insufficient response and need for further treatment escalation (e.g. treatment with infliximab), the patient has to stop abatacept treatment and cannot continue participating the study.
2.8. Drug administration and monitoring
Abatacept is administered in a dosage of 125 mg subcutaneously weekly by the patients themselves. Before first application, all participants receive education/administration in subcutaneous injection technique and accurate injection will be controlled by experienced staff during each study visit.
Abatacept will be provided in prefilled syringes that will be stored at 2–8 °C. Cool packages and instructions on storage will be given to the patients to assure continuous cooling. Drug administration as well as (especially local) side reactions will be documented by the patient in a diary that is monitored at each study visit.
Empty syringes and not used trial medication will be collected, counted and documented.
2.9. Premature termination of trial treatment
The patient can have his/her study treatment terminated prematurely at any time, without having to give reasons. Furthermore, the study participation may be increased risks for the patients’ trial drug administration may be discontinued under one or more of the following conditions:
-
•
Adverse events (including intercurrent illnesses) which preclude further treatment with the investigational medicinal product
-
•
Premature termination of the trial treatment is considered to be medically indicated
-
•
Continuation of the trial treatment is unacceptable when the risks outweigh the benefits
-
•
Significant progress of sarcoidosis despite adequate treatment with trial medication and steroid comedication according to the physician's appraisal
-
•
Pregnancy
-
•
Significant violations of the trial protocol or lack of compliance on the part of the patient (e.g. taking prohibited medication)
-
•
Logistical reasons (e.g. patient changes his/her doctor or hospital or moves to another location)
If a patient discontinues trial participation, the patient will be offered to complete follow-up visits as depicted in Fig. 2. All available data and results will be documented.
2.10. Adverse events and serious adverse events
Adverse events have to be documented in the CRF starting from the first application of trial medication – in case of serious adverse events, starting from signature of informed consent – and until 30 days after last application of trial medication.
Established guidelines and definitions, standard operating procedures as well as applicable laws and regulations fill be followed in the documentation and reporting of adverse events.
2.11. Data confidentiality and data management
Information about trial patients will be kept confidential and managed under the applicable laws and regulations. The data collected during the study will be stored and evaluated in a pseudonymised form. Data management system is in compliance with good clinical practice. All relevant data will be documented in this data management system. Collected and stored data are protected by built-in security features to prevent unauthorized access to confidential participant information. Access to the system will be controlled by individually assigned user identification codes and passwords. Access to the data will be limited to authorized and trained staff.
The investigators, the trial statistician and the data management staff have access to the full trial dataset without restrictions.
2.12. Statistical analysis
The determination of the sample size is based on feasibility considerations. Sarcoidosis is a rare disease and especially patients with a chronic progressive disease requiring a therapy beyond corticosteroids are a minor group within sarcoidosis patients. Therefore, it is intended to include 30 patients, which should allow addressing the primary endpoint of safety.
As a simplified approach to the analysis of the primary endpoint, the number of severe infectious complications during the treatment period, we consider the width of a two sided 95% confidence interval for the expected number of complications, based on the normal approximation of the Poisson distribution. The Poisson distribution is characterized by equality of the expected value and the variance. With this prerequisite, we can determine the size of a two-sided 95% confidence interval. If e.g. 4 infectious complications can be expected and the sample size is 30, a two-sided 95.0% confidence interval for a single mean will extend 0.72 from the observed mean. If 9 infectious complications can be expected, a two-sided 95.0% confidence interval for a single mean will extend 1.07 from the observed mean.
The primary analysis of the number of severe infections under therapy will be performed in terms of an annual incidence rate (i.e. counts of infectious complications per year per patient), thus taking into account that the times under observation may vary. The mean annual incidence rate and a two-sided 95% confidence interval based on the normal distribution approximation will be presented.
The number of severe infectious complications will be compared between the 12 months prior to treatment where available and the 12 months during treatment by using a Poisson model with patients as random effects and time phase as fixed effect and taking individual differences in risk times into account. The difference will be described by the annual incidence in the two phases, the incidence ratio, and a 95% confidence interval.
All analyses will be based on all patients recruited for the study for whom treatment with abatacept was started.
2.13. Safety and data monitoring committee
To assure safety of trial participants the first six patients will be followed up on a six-week interval with a special focus on infectious complications (including EBV reactivation). An independent data monitoring committee with members experienced in biological therapies and clinical trials supervises the safety data described above on a regular basis. The first meeting is held after the sixth patient has completed the six-week study visit and the following meetings are prescheduled every twelve weeks. All available safety data are presented to the data monitoring committee that can advise on continuing or stopping the study or on extending intervals of study visits from 6 to 12 weeks.
2.14. Quality assurance
Clinical monitoring will be conducted on a regular basis by the Clinical Trials Unit of the Medical Center – University of Freiburg as a continuous measure of quality assurance. Audits and inspections by regulatory authorities may be conducted at any time.
2.15. Protocol version
The study was started with the protocol version V02 (date 02.06.2017). Subsequently two amendments were required (amendment 01, 13.12.2017 and amendment 03, 17.12.2018). Therefore the current protocol version is V04 (13.12.2018) including amendments 01 and 03. Amendments related to clarifying inclusion criteria (Immunosuppressive therapy ( ≥ 5 mg prednisolone equivalent per day)) and to adding two laboratory parameter (calcium and albumin). All study protocols and amendments were positively evaluated by the Ethics Committee Freiburg, University of Freiburg). All amendments did not conflict with previous inclusion of participants.
All further amendments will be documented, communicated to the funder, the sponsor and the trial centers. Amendments will be reported in resulting publications.
2.16. Trial registration
The trial has been registered at the German Clinical Trial Registry (Deutsche Register Klinischer Studien, DRKS) with the identity number DRKS00011660.
2.17. Ethics and funding
The ABASARC trial was approved by the Ethics Committee of the University of Freiburg (vote no. 163/17 (FF-MC)). Additionally, the trial was approved by the German national competent authority (“Paul-Ehrlich-Institut”, Federal Institute for Vaccines and Biomedicines, Langen, Germany). Part of the trial protocol is a patient informed consent form, describing the trial, the tested medicinal product including potential risks, and alternative treatment options.
The sponsor of the study is the Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg.
The study is an investigator-initiated trial funded by Bristol-Myers Squibb (BMS, New York, USA). BMS has no influence on the study protocol and the chosen endpoints. Obtained safety data will be reported directly to BMS and health care authorities.
BMS has no influence on the study protocol or on data collection, analysis, interpretation and publication of results.
3. Discussion
The ABASARC trial described herein focusses on three aims, which are (i) assessing the safety and efficacy of abatacept in sarcoidosis patients, (ii) exploring different endpoints that might prove suitable for further trials and (iii) characterization of the patient collective.
Even though there are several different drugs used to treat corticosteroid-dependent sarcoidosis [2,46,47], abatacept may represent a novel and unique therapeutic option for two main reasons: First, as outlined before, there are several hints that CTLA-4 expression [15,32,33,35,[48], [49], [50]] and function are dysregulated in sarcoidosis and therefore abatacept restore this defect as a target therapy. Second, its side effects differ substantially from drugs like methotrexate, azathioprine or mycophenolate mofetile especially concerning bone marrow and liver toxicity.
Inclusion criteria for this trial (i.e. starting an immunosuppressive treatment with abatacept) are based on the daily clinical practice. Immunosuppressive therapy beyond corticosteroids in sarcoidosis is not well established and varies between different centers based on expected side effects and local experiences, also local recommendations to change immunosuppressive therapy may be handled differentially [51,52]. Generally, deterioration of organ function, reduced quality of life or side effects of established therapies (e.g. diabetes or weight gain induced by steroids) are considered indications for therapy adaptation [46]. Therefore, the physicians’ appraisal for starting or changing an immunosuppressive therapy in sarcoidosis patients represents a main inclusion criteria for patients who are affected by (i) an organ manifestation requiring immunosuppression, (ii) having sarcoidosis-associated complaints assessed by KSQ and (iii) inflammatory activity of sarcoidosis measured by sIL2R or neopterin.
The first aim of the study, assessing the safety of abatacept treatment is assessed addressed as primary endpoint as the number of severe infections during treatment with abatacept and as secondary endpoint addressing the rate of severe infections, non-severe infections and non-infectious complications of abatacept therapy. Recent studies in other indications (especially rheumatoid arthritis) have shown that infections are the most important severe side effects of abatacept treatment even though infections and cardiac side effects are not increased compared to e.g. anti TNF antibodies [53,54]. Therefore, an open-labeled trial design was chosen which allows a better assessment of infectious complications occurring during treatment. To closely assess safety, the first six patients included in the trial will be monitored closely in six week-intervals. Their safety data will be monitored by an independent data safety board. As patients with an immunosuppressive therapy are at an increased risk of infections, the infectious complications occurring during the trial will be compared to infections retrospectively collected for the year prior to study participation. This will allow estimating whether abatacept markedly increases the risk of infections.
Second, secondary endpoints explore different surrogates of an effective treatment for sarcoidosis that cover markers of inflammation, lung function and patient-reported outcome parameters. Therefore, three questionnaires assessing patients' well-being were included as secondary endpoints. In particular, the King's Sarcoidosis Questionnaire that covers different health domains affected by sarcoidosis, e.g. pulmonary health, general well-being (including fatigue) or concerns about medication. This questionnaire has rarely been investigated in clinical drug studies and may prove a suitable endpoint because it has been specifically designed to capture sarcoidosis-associated symptoms and hence is clearly different from several other questionnaires typically used in sarcoidosis studies. Because KSQ has rarely been used in studies, we decided to additionally use SGRQ as an additional questionnaire. Even though validated for COPD, but not for sarcoidosis, it has been used widely in different studies investigating different interstitial lung diseases [[55], [56], [57]]. Thereby the combination of a generally used questionnaire (SGRQ) with a very disease-specific questionnaire (KSQ) may cover a broad range of patient-related outcomes.
Therapeutic interventions in sarcoidosis have been undertaken basing on pathophysiological observations and analogies to other Th1-driven inflammatory diseases. Nevertheless, to date there are no randomized controlled trials demonstrating the efficacy of a single drug intervention in sarcoidosis patients. Obstacles for a meaningful clinical study are due to the study design and the chosen endpoint on the one hand and to the heterogeneity of sarcoidosis patients and their unpredictable disease course on the other hand. For example, infliximab failed to achieve a clinically meaningful gain in FVC in sarcoidosis patients [12]. However, in a subgroup of patients with a high TNF-level, lung function improvement was more pronounced by infliximab treatment [13]. Additionally, extrapulmonary manifestations improved in the treatment group [58]. These observations indicate that meaningful clinical trials in sarcoidosis will need a well-chosen endpoint and requires inclusion of well characterized patients.
Therefore, the third aim of the ABASARC trial focusses on the identification of patients, who benefit most of abatacept treatment.
Before starting and after terminating abatacept treatment, the study protocol requires an intense clinical and immunological assessment of the recruited patients including bronchoalveolar lavage.
The use of BAL in a clinical study and changes in alveolar cell composition as a secondary outcome parameter is uncommon in clinical sarcoidosis studies. On the one hand, BAL procedure may vary between different centers, therefore a similar protocol of BAL procedure and analysis is used that is established in both centers [59]. On the other hand, alveolar inflammation may also be monitored by use of 18FDG-PET-CT, which has been used in some trials [60]. The use of BAL and its immunological work-up offers the benefit to gain insight in the pathophysiology of sarcoidosis and changes induced by abatacept. The interaction of T-cells, regulatory T-cells and antigen-presenting cells is influenced by abatacept enabling regulatory T-cell response to dampen the inflammatory response. Therefore, the immunological assessment will focus on these pathways by analyzing T-cell subpopulations in blood and BAL samples.
In summary, the immunological assessment of the alveolar and blood compartment and their changes induced by abatacept treatment together with clinical data may allow the identification of patients who experience the greatest benefit of abatacept therapy.
Ethics approval and consent to participate
The study has been approved by the local Ethics Committee Freiburg (University of Freiburg, Germany) with the reference number 163/17 (FF-MC). A subsequent version of the study protocol received a favorable opinion from the Ethics Committee Freiburg in 5 February 2019.
Consent for publication
Study results will be published in a suitable peer-review journal. BMS has no influence on data collection, data interpretation or data publication.
Availability of data and material
Aggregate data and analyses of data will be published. Participant-level data and materials may be made available to the extent permitted by data protection legislation after the study is completed and published.
Funding
The study is funded by Bristol-Myers Squibb. The funder of the study had no influence on the study design and trial protocol. The funder has no influence on data collection, management, analysis and their publication.
Authors' contributions
BCF, BG, GZ and JMQ designed the trial. BCF, ICR, AU, GI and FS wrote the study protocol and additional documents.
All authors wrote the manuscript.
Declaration of competing interest
The study has been funded by Bristol-Myers Squibb (see also below: funding).
Additional to this funding the authors declare the following competing interests: BG receives support through the Deutsche Forschungsgemeinschaft (DFG) under Germany's Excellence Strategy (CIBSS – EXC-2189 – Project ID 390939984, and RESIST – EXC 2155 – Project ID 39087428); through the E-rare programme of the EU, managed by the DFG, grant code GR1617/14–1/iPAD; and through the "Netzwerke Seltener Erkrankungen” of the German Ministery of Education and Research (BMBF), grant code: GAIN_01GM1910A. BCF reports personal fees and non-financial support from Actelion, Astra Zeneca, Boehringer Ingelheim and Roche. ICR reports personal fees from Roche and non-financial support from Grifols. GZ reports grants for the Deutsche Forschungsgemeinschaft (DFG). JMQ reports personal fees from CSL Behring, Roche and Relief Therapeutics. The other authors do not have any discloures.
Acknowledgement
We acknowledge the support of Bibiane Söllner and Veronika Frommberger in planning the study. We also acknowledge the support of Stefanie Hahn und Doris Wilde.
Contributor Information
Björn C. Frye, Email: bjoern.christian.frye@uniklinik-freiburg.de.
Ina Caroline Rump, Email: ina.caroline.rump@uniklinik-freiburg.de.
Annette Uhlmann, Email: annette.uhlmann@uniklinik-freiburg.de.
Fabian Schubach, Email: fabian.schubach@uniklinik-freiburg.de.
Gabriele Ihorst, Email: gabriele.ihorst@uniklinik-freiburg.de.
Bodo Grimbacher, Email: bodo.grimbacher@uniklinik-freiburg.de.
Gernot Zissel, Email: gernot.zissel@uniklinik-freiburg.de.
Joachim Müller Quernheim, Email: joachim.mueller-quernheim@uniklinik-freiburg.de.
References
- 1.Valeyre D. Sarcoidosis. Lancet. 2014;383(9923):1155–1167. doi: 10.1016/S0140-6736(13)60680-7. [DOI] [PubMed] [Google Scholar]
- 2.Grunewald J. Sarcoidosis. Nat. Rev. Dis. Prim. 2019;5(1):45. doi: 10.1038/s41572-019-0096-x. [DOI] [PubMed] [Google Scholar]
- 3.Statement on sarcoidosis. Joint statement of the American thoracic society (ATS), the European respiratory society (ERS) and the world association of sarcoidosis and other granulomatous disorders (WASOG) adopted by the ATS board of directors and by the ERS executive committee. Am. J. Respir. Crit. Care Med. 1999;160(2):736–755. doi: 10.1164/ajrccm.160.2.ats4-99. February 1999. [DOI] [PubMed] [Google Scholar]
- 4.Zissel G. Cellular activation in the immune response of sarcoidosis. Semin. Respir. Crit. Care Med. 2014;35(3):307–315. doi: 10.1055/s-0034-1376861. [DOI] [PubMed] [Google Scholar]
- 5.Zissel G., Prasse A., Muller-Quernheim J. Immunologic response of sarcoidosis. Semin. Respir. Crit. Care Med. 2010;31(4):390–403. doi: 10.1055/s-0030-1262208. [DOI] [PubMed] [Google Scholar]
- 6.Paramothayan N.S., Lasserson T.J., Jones P.W. Corticosteroids for pulmonary sarcoidosis. Cochrane Database Syst. Rev. 2005;(2):CD001114. doi: 10.1002/14651858.CD001114.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paramothayan S., Jones P.W. Corticosteroid therapy in pulmonary sarcoidosis: a systematic review. J. Am. Med. Assoc. 2002;287(10):1301–1307. doi: 10.1001/jama.287.10.1301. [DOI] [PubMed] [Google Scholar]
- 8.Paramothayan S., Lasserson T. Treatments for pulmonary sarcoidosis. Respir. Med. 2008;102(1):1–9. doi: 10.1016/j.rmed.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Paramothayan S., Lasserson T.J., Walters E.H. Immunosuppressive and cytotoxic therapy for pulmonary sarcoidosis. Cochrane Database Syst. Rev. 2006;(3):CD003536. doi: 10.1002/14651858.CD003536. [DOI] [PubMed] [Google Scholar]
- 10.Antoniu S.A. Targeting the TNF-alpha pathway in sarcoidosis. Expert Opin. Ther. Targets. 2010;14(1):21–29. doi: 10.1517/14728220903449244. [DOI] [PubMed] [Google Scholar]
- 11.Baughman R.P., Lower E.E., Drent M. Inhibitors of tumor necrosis factor (TNF) in sarcoidosis: who, what, and how to use them. Sarcoidosis Vasc. Diffuse Lung Dis. 2008;25(2):76–89. [PubMed] [Google Scholar]
- 12.Baughman R.P. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am. J. Respir. Crit. Care Med. 2006;174(7):795–802. doi: 10.1164/rccm.200603-402OC. [DOI] [PubMed] [Google Scholar]
- 13.Loza M.J. Inflammatory profile and response to anti-tumor necrosis factor therapy in patients with chronic pulmonary sarcoidosis. Clin. Vaccine Immunol. 2011;18(6):931–939. doi: 10.1128/CVI.00337-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wijnen P.A. Association of the TNF-alpha G-308A polymorphism with TNF-inhibitor response in sarcoidosis. Eur. Respir. J. 2014;43(6):1730–1739. doi: 10.1183/09031936.00169413. [DOI] [PubMed] [Google Scholar]
- 15.Broos C.E. Impaired survival of regulatory T cells in pulmonary sarcoidosis. Respir. Res. 2015;16:108. doi: 10.1186/s12931-015-0265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyara M. The immune paradox of sarcoidosis and regulatory T cells. J. Exp. Med. 2006;203(2):359–370. doi: 10.1084/jem.20050648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prasse A. Inhaled vasoactive intestinal peptide exerts immunoregulatory effects in sarcoidosis. Am. J. Respir. Crit. Care Med. 2010;182(4):540–548. doi: 10.1164/rccm.200909-1451OC. [DOI] [PubMed] [Google Scholar]
- 18.Zissel G., Muller-Quernheim J. Cellular players in the immunopathogenesis of sarcoidosis. Clin. Chest Med. 2015;36(4):549–560. doi: 10.1016/j.ccm.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Zissel G., Prasse A., Muller-Quernheim J. Sarcoidosis--immunopathogenetic concepts. Semin. Respir. Crit. Care Med. 2007;28(1):3–14. doi: 10.1055/s-2007-970329. [DOI] [PubMed] [Google Scholar]
- 20.Chambers C.A., Allison J.P. CTLA-4--the costimulatory molecule that doesn't: regulation of T-cell responses by inhibition. Cold Spring Harbor Symp. Quant. Biol. 1999;64:303–312. doi: 10.1101/sqb.1999.64.303. [DOI] [PubMed] [Google Scholar]
- 21.Chambers C.A. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu. Rev. Immunol. 2001;19:565–594. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 22.Coyle A.J., Gutierrez-Ramos J.C. The expanding B7 superfamily: increasing complexity in costimulatory signals regulating T cell function. Nat. Immunol. 2001;2(3):203–209. doi: 10.1038/85251. [DOI] [PubMed] [Google Scholar]
- 23.Sansom D.M. CD28, CTLA-4 and their ligands: who does what and to whom? Immunology. 2000;101(2):169–177. doi: 10.1046/j.1365-2567.2000.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qureshi O.S. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332(6029):600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soskic B. A transendocytosis perspective on the CD28/CTLA-4 pathway. Adv. Immunol. 2014;124:95–136. doi: 10.1016/B978-0-12-800147-9.00004-2. [DOI] [PubMed] [Google Scholar]
- 26.Tai X. Induction of autoimmune disease in CTLA-4-/- mice depends on a specific CD28 motif that is required for in vivo costimulation. Proc. Natl. Acad. Sci. U. S. A. 2007;104(34):13756–13761. doi: 10.1073/pnas.0706509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waterhouse P. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270(5238):985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 28.Ueda H. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423(6939):506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 29.Kuehn H.S. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science. 2014;345(6204):1623–1627. doi: 10.1126/science.1255904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schubert D. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat. Med. 2014;20(12):1410–1416. doi: 10.1038/nm.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeissig S. Early-onset Crohn’s disease and autoimmunity associated with a variant in CTLA-4. Gut. 2014;64(12):1889–-189. doi: 10.1136/gutjnl-2014-308541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Broos C.E. Decreased cytotoxic T-lymphocyte antigen 4 expression on regulatory T cells and Th17 cells in sarcoidosis: double trouble? Am. J. Respir. Crit. Care Med. 2015;192(6):763–765. doi: 10.1164/rccm.201503-0635LE. [DOI] [PubMed] [Google Scholar]
- 33.Bertrand A. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13:211. doi: 10.1186/s12916-015-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chopra A. Drug-induced sarcoidosis-like reactions. Chest. 2018;154(3):664–677. doi: 10.1016/j.chest.2018.03.056. [DOI] [PubMed] [Google Scholar]
- 35.Reddy S.B. Sarcoidosis following anti-PD-1 and anti-CTLA-4 therapy for metastatic melanoma. J. Immunother. 2017;40(8):307–311. doi: 10.1097/CJI.0000000000000181. [DOI] [PubMed] [Google Scholar]
- 36.Dumont F.J. Technology evaluation: abatacept, bristol-myers Squibb. Curr. Opin. Mol. Therapeut. 2004;6(3):318–330. [PubMed] [Google Scholar]
- 37.Ruderman E.M., Pope R.M. Drug Insight: abatacept for the treatment of rheumatoid arthritis. Nat. Clin. Pract. Rheumatol. 2006;2(12):654–660. doi: 10.1038/ncprheum0345. [DOI] [PubMed] [Google Scholar]
- 38.Genovese M.C. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N. Engl. J. Med. 2005;353(11):1114–1123. doi: 10.1056/NEJMoa050524. [DOI] [PubMed] [Google Scholar]
- 39.Genovese M.C. Efficacy and safety of the selective co-stimulation modulator abatacept following 2 years of treatment in patients with rheumatoid arthritis and an inadequate response to anti-tumour necrosis factor therapy. Ann. Rheum. Dis. 2008;67(4):547–554. doi: 10.1136/ard.2007.074773. [DOI] [PubMed] [Google Scholar]
- 40.Mease P. Abatacept in the treatment of patients with psoriatic arthritis: results of a six-month, multicenter, randomized, double-blind, placebo-controlled, phase II trial. Arthritis Rheum. 2011;63(4):939–948. doi: 10.1002/art.30176. [DOI] [PubMed] [Google Scholar]
- 41.Farin E. Translation and psychometric properties of the King's Sarcoidosis Questionnaire (KSQ) in German language. Health Qual. Life Outcome. 2019;17(1):62. doi: 10.1186/s12955-019-1131-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel A.S. The development and validation of the King's Sarcoidosis Questionnaire for the assessment of health status. Thorax. 2013;68(1):57–65. doi: 10.1136/thoraxjnl-2012-201962. [DOI] [PubMed] [Google Scholar]
- 43.Muller-Quernheim J. [Monitoring sarcoidosis therapy with immunopathologic parameters] Pneumologie. 1994;48(2):47–49. [PubMed] [Google Scholar]
- 44.Alten R. Abatacept used in combination with non-methotrexate disease-modifying antirheumatic drugs: a descriptive analysis of data from interventional trials and the real-world setting. Arthritis Res. Ther. 2018;20(1):1. doi: 10.1186/s13075-017-1488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bykerk V.P. On-drug and drug-free remission by baseline symptom duration: abatacept with methotrexate in patients with early rheumatoid arthritis. Rheumatol. Int. 2018;38:2225–2231. doi: 10.1007/s00296-018-4173-3. [DOI] [PubMed] [Google Scholar]
- 46.Rahaghi F.F. Delphi consensus recommendations for a treatment algorithm in pulmonary sarcoidosis. Eur. Respir. Rev. 2020;29(155) doi: 10.1183/16000617.0146-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schupp J.C. Sarcoidosis: drugs under investigation. Semin. Respir. Crit. Care Med. 2017;38(4):532–537. doi: 10.1055/s-0037-1603768. [DOI] [PubMed] [Google Scholar]
- 48.Broos C.E., Hendriks R.W., Kool M. T-cell immunology in sarcoidosis: disruption of a delicate balance between helper and regulatory T-cells. Curr. Opin. Pulm. Med. 2016;22(5):476–483. doi: 10.1097/MCP.0000000000000303. [DOI] [PubMed] [Google Scholar]
- 49.Prasse A., Muller-Quernheim J. Non-invasive biomarkers in pulmonary fibrosis. Respirology. 2009;14(6):788–795. doi: 10.1111/j.1440-1843.2009.01600.x. [DOI] [PubMed] [Google Scholar]
- 50.Ueda K. The impact of antibiotics on prognosis of metastatic renal cell carcinoma in Japanese patients treated with immune checkpoint inhibitors. Anticancer Res. 2019;39(11):6265–6271. doi: 10.21873/anticanres.13836. [DOI] [PubMed] [Google Scholar]
- 51.Vorselaars A.D. Cytotoxic agents in sarcoidosis: which one should we choose? Curr. Opin. Pulm. Med. 2014;20(5):479–487. doi: 10.1097/MCP.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 52.Vorselaars A.D. Methotrexate vs azathioprine in second-line therapy of sarcoidosis. Chest. 2013;144(3):805–812. doi: 10.1378/chest.12-1728. [DOI] [PubMed] [Google Scholar]
- 53.Chen S.K. Risk of hospitalized infection and initiation of abatacept versus TNF inhibitors among patients with rheumatoid arthritis: a propensity score-matched cohort study. Arthritis Care Res. (Hoboken) 2018;72(1):9–17. doi: 10.1002/acr.23824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Generali E. Risk of hospitalization for heart failure in rheumatoid arthritis patients treated with etanercept and abatacept. Rheumatol. Int. 2019;39(2):239–243. doi: 10.1007/s00296-018-4196-9. [DOI] [PubMed] [Google Scholar]
- 55.Distler O., Gahlemann M., Maher T.M. Nintedanib for systemic sclerosis-associated interstitial lung disease. Reply. N. Engl. J. Med. 2019;381(16):1596–1597. doi: 10.1056/NEJMc1910735. [DOI] [PubMed] [Google Scholar]
- 56.Flaherty K.R. Nintedanib in progressive fibrosing interstitial lung diseases. N. Engl. J. Med. 2019;381(18):1718–1727. doi: 10.1056/NEJMoa1908681. [DOI] [PubMed] [Google Scholar]
- 57.Richeldi L. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014;370(22):2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 58.Judson M.A. Efficacy of infliximab in extrapulmonary sarcoidosis: results from a randomised trial. Eur. Respir. J. 2008;31(6):1189–1196. doi: 10.1183/09031936.00051907. [DOI] [PubMed] [Google Scholar]
- 59.Frye B.C. The value of bronchoalveolar lavage for discrimination between healthy and diseased individuals. J. Intern. Med. 2019;287(1):54–65. doi: 10.1111/joim.12973. [DOI] [PubMed] [Google Scholar]
- 60.Vorselaars A.D. Effectiveness of infliximab in refractory FDG PET-positive sarcoidosis. Eur. Respir. J. 2015;46(1):175–185. doi: 10.1183/09031936.00227014. [DOI] [PubMed] [Google Scholar]
- 61.Birring S.S. Development of a symptom specific health status measure for patients with chronic cough: leicester Cough Questionnaire (LCQ) Thorax. 2003;58(4):339–343. doi: 10.1136/thorax.58.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Swigris J.J. The SF-36 and SGRQ: validity and first look at minimum important differences in IPF. Respir. Med. 2010;104(2):296–304. doi: 10.1016/j.rmed.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raj A.A., Pavord D.I., Birring S.S. Clinical cough IV:what is the minimal important difference for the Leicester Cough Questionnaire? Handb. Exp. Pharmacol. 2009;(187):311–320. doi: 10.1007/978-3-540-79842-2_16. [DOI] [PubMed] [Google Scholar]
- 64.Shino M.Y. Sarcoidosis-associated pulmonary hypertension and lung transplantation for sarcoidosis. Semin. Respir. Crit. Care Med. 2014;35(3):362–371. doi: 10.1055/s-0034-1376863. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Aggregate data and analyses of data will be published. Participant-level data and materials may be made available to the extent permitted by data protection legislation after the study is completed and published.