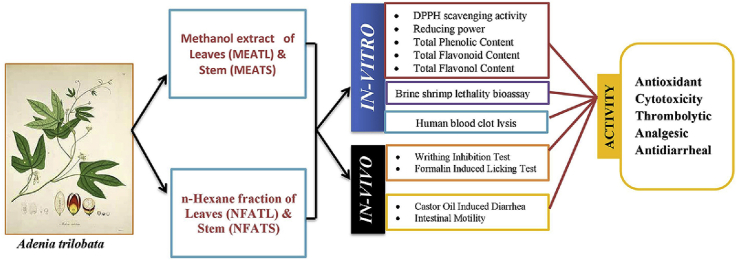
Abstract
Adenia trilobata, locally known as akandaphal in Bangladesh, has some traditional uses. Leaves and stems extracted with pure methanol (MEATL, MEATS) and fractioned by n-hexane (NFATL, NFATS), which was subjected to qualitative phytochemical analysis. The qualitative phytochemical analysis of four extracts showed the presence of secondary metabolites such as alkaloid, carbohydrate, glycosides, flavonoids, phenols, flavonol, and saponins. All four extracts of A. trilobata, exhibited a strong antioxidant activity while a moderately (MEATS = 328 μg/mL) to weakly toxic (NFATL = 616.85 μg/mL) LC50 observed in brine shrimp lethality bioassay. In thrombolytic test, MEATL (18.54 ± 2.18%; P < 0.01) and MEATS (25.58 ± 4.76%; P < 0.0001) showed significant percentage of clot lysis in human blood. The in vivo analgesic activity carried by acetic acid test and formalin test, while the antidiarrheal activity assayed by two standard methods e.g., castor oil-induced diarrhea and castor oil-induced gastrointestinal motility. Both, in vivo model, showed an extremely significant (P < 0.0001) dose-dependent manner percentage of inhibition in comparison to the control group. Present results suggested, A. trilobata could be a potential source for antioxidative, cytotoxic, thrombolytic, analgesic, antidiarrheal agents which require further study to identify the mechanism of A. trilobata.
Keywords: A. trilobata, Analgesic activity, Antidiarrheal, Antioxidant, Cytotoxic, Thrombolytic
Abbreviations: OS, oxidative stress; UV, ultra-violet; DPPH, 1,1-diphenyl, 2-picryl hydrazyl; A. trilobata, Adenia trilobata; LC50, 50% lethal concentration; IC50, 50% inhibitory concentration; FCR, Folin-Ciocalteu reagent; IP, intraperitoneal; SEM, standard error mean; ANOVA, Analysis of variance; b.w., body weight
Graphical abstract
Highlights
-
•
Detection of different plant metabolites in extracts of different plant parts.
-
•
Strong antioxidant activity was found for all four extracts.
-
•
Moderate to weakly toxic LC50 observed in brine shrimp lethality bioassay.
-
•
A significant percentage of clot lysis in human blood was perceived.
-
•
Extremely significant analgesic and antidiarrheal activity was found.
1. Introduction
The redox homeostasis plays an essential part in maintaining health and disease prevention. The imbalance of antioxidants and reactive oxygen species (ROS) is responsible for producing oxidative stress (OS) [1]. Free radicals initiate oxidative stress resulting in DNA damage and tissue damage which caused inflammation or cell death [2]. OS is associated with the prevalence of the cardiac disease, cancers, diabetes, neurodegenerative diseases, autoimmune disorders, aging, and others. The plant derives substances such as vegetables, and dietary fruits are rich in source of antioxidant. Antioxidant suggested having a significant benefit in health by reducing oxidative stress [1]. Thrombosis is a vital physiopathology which causes several atherothrombotic diseases (e.g., myocardial or cerebral infarction). The formation of a thrombus or blood clots in the artery because of the homeostatic imbalance leads to blockage of vascular organ and while recovering causes fatal significances, myocardial, or cerebral infarction, as well as death [3]. Pain is an unsavory phenomenon that comprises sensory experiences, including time, space, force, feeling, insight, and inspiration [4]. Several analgesic agents are isolated from natural sources such as morphine, aspirin [5]. Micro-organisms like Salmonella, Escherichia coli, Vibrio cholera, and Shigella are the most regular reasons for diarrhea in developing countries [6].
Biodiversity has a significant contribution to human livelihood. As per World Health Organization (WHO) reports, around 80% of the worldwide population still depends on herbal medications; today, several medicines owe their origin to medicinal plants [7]. Form the beginning of history; nature is the potential source for the drug substances. In the scientific community, the interest for new bioactive compounds from plant kingdoms is increasing day by day. In general, functional food or nutraceutical formulations could be useful in preventing several chronic diseases such as cancer, diabetes, a various inflammatory disorder, and obesity [8]. So, the international community encourages developing the naturally derived new compounds for the development of new drugs, which provide a tremendous pharmacological activity with lesser adverse effects and also less costly than available synthetic medicines [[9], [10], [11], [12]]. The cultivation of such plants, mainly if they are endemic, can be a potential income source for the developing countries. Thus, the disclosure of normal cures has additionally increased a great deal of consideration in these decades in the cosmetic sector. But, in some cases, modern science had not yet affirmed the ethnopharmacological used [13,14]. So, there is a strong need for the development of new cancer prevention agents, antinociceptive agent, and antidiarrheal agent from common natural sources for the development of novel drug products.
A. trilobata belongs to the Passifloraceae family, which is locally known as akandaphal. It is distributed in the Chittagong district of Bangladesh and also found in Andaman Is., Assam, East Himalaya, Myanmar, Pakistan, and West-Himalaya. A. trilobata has no exploratory work for human use; however, this plant utilized by the clans and nearby groups of the people for their medicinal services. A review found that the poultice of the leaves of this plant uses to treat headache, knee pain, snake bite, and stomach trouble [15,16]. In summary, there are no scientific reports on the biological activities of A. trilobata.
For these reasons, the present study figured to identify phytoconstituents and evaluate the antioxidant, cytotoxic, thrombolytic, analgesic, and antidiarrheal activities of methanol (MEATL, MEATS) and n-hexane (NFATL, NFATS) extract of A. trilobata leaves and stems.
2. Materials and methods
2.1. Chemicals
DPPH (1,1-diphenyl, 2-picryl hydrazyl), gallic acid, quercetin, sodium acetate, ferric chloride, and trichloroacetic acid obtained from Sigma Chemical Co. USA. Potassium ferricyanide, Folin-Ciocalteu reagent, aluminium chloride, sodium carbonate, and methanol purchased from Merck, Germany. Ascorbic acid purchased from SD Fine Chem. Ltd. India. Lyophilized streptokinase vial (1500000 IU), and vincristine sulfate (1 mg/vial) was purchased from Beacon Pharmaceuticals Ltd. Bangladesh.
2.2. Animals
Both sexes of Swiss albino mice weighing 25–35 gm. purchased from the Jahangirnagar University, Dhaka-1343, Bangladesh, at six-seven weeks old. The animals housed in standard conditions (room temperature 25 ± 2 °C; relative humidity 55–60%, 12 h light/dark cycle), with food pellets and water supply. The animals were adapted with the laboratory conditions for 14 days to use for the experiments. The study approved by the Institutional Animal Ethical Committee, Department of Pharmacy, International Islamic University Chittagong, Bangladesh according to governmental guidelines under the reference Pharm/PND/150/20–2019 [17].
2.3. Plant materials
Fresh leaves and stems of A. trilobata collected from the Hajarikhil Hill tract area, Chittagong, Bangladesh, in February 2019, which authenticated by Md. Anwarul Islam, Department of Botany, Jahangirnagar University, Savar, Dhaka-1342, Bangladesh under accession number Anwar-0311. After the collection of A. trilobata, it identified and confirmed by Professor Dr. Mohammed Aktar Sayeed, Department of Pharmacy, International Islamic University Chittagong, Kumira, Chittagong-4318, Bangladesh.
2.4. Preparation of methanol crude extract and n-hexane fraction
The leaves and stems dried under shade and ground for ten day's period, then dried in a mechanical drier at 60–70 °C. After drying, the leaves and stems were ground to a coarse powder and dissolved into methanol for 7 days. After that, the sediments filtered and dried in a water bath at 40–50 °C. A concentrated filtrate like the deep green color obtained after completely evaporating the solvent, which used as methanol extract (MEAT) for the experiment. Five (5 gm) of crude extract dissolved into water and methanol. The solution then was shaken well with n-hexane. The upper portion was then separated carefully by the separating funnel. The solvent was evaporated entirely with the help of the water bath and obtained n-hexane fraction (NFAT) used for the experiment.
2.5. Standardization and quality control of the extract
Methanol (MEATL, MEATS) and n-hexane (NFATL, NFATS) extract of A. trilobata leaves and stems was standardized and under-went quality control through physicochemical evaluation of crude extract, ensuring the safety and acute toxicity study in animal model [7].
2.6. Phytochemical screening
The phytochemical analysis of the methanol extract and n-hexane fraction of A. trilobata leaves and stems carried out the standard method to evaluate the alkaloid, carbohydrate, flavonoid, terpenoids, tannins, saponins, phenols, quinones, cholesterol, proteins, steroids, starch, sterols and flavonol [18,19].
2.7. Antioxidant activity
2.7.1. DPPH free radical scavenging activity
Free radical scavenging activity of methanol extract and n-hexane fraction of A. trilobata leaves and stems, were determined by the method of Braca et al. [20]. The method based on the activity of scavenging the stable free radical 1, 1-diphenyl-2-picrylhydrazyl (DPPH). Three milliliter of 0.004% DPPH solution (4 mg DPPH in 100 mL of 95% methanol) added with the different concentrations (15.625 to 500 μg/mL) of the crude extract and the n-hexane fraction. The absorbance was taken at 517 nm after 30 min by the UV spectrophotometer.
| (%) Radical scavenging = {(A0-A1)/A0} × 100 |
Where, A0 = absorbance of the control; A1 = absorbance of the extract.
Here, lower the absorbance values, the higher will be the free radical scavenging activity [21]. The IC50 (50% inhibitory concentration) was calculated as it indicated the effective concentration of the extract needed to scavenge 50% of the free radicals of DPPH.
2.7.2. Reducing power capacity
Reducing power capacity was estimated by the method described by Oyaizu (1986) [22]. One milliliter of extract taken in serially diluted concentration (31.25, 62.5, 125, 250 and 500 μg/mL) and then added 2.5 mL phosphate buffer (0.2 M; pH 6.6) and potassium ferricyanide (1% w/v), respectively and incubated at 50 °C for 20 min to complete the reaction. After incubation, 2.5 mL trichloroacetic acid (10%) was added to the mixture and then centrifuged for 10 min at 3000 RPM, and 2.5 mL of the upper layer (supernatant solution) of the solution was withdrawn and added 2.5 mL distilled water and 0.5 mL FeCl3 (0.1% w/v), respectively. Then absorbance was taken at 700 nm by the UV spectrophotometer. If the absorbance of the reaction mixture increased with the increased of the concentration, then it was indicated the increased of the reducing power capacity. As a standard ascorbic acid and as a blank solution, phosphate buffer (0.2 M, pH 6.6) used.
2.7.3. Total phenol content
The total phenol content of the extract was measured by using Folin-Ciocalteau reagent (FCR) as an oxidizing agent by the method of Singleton et al. [23]. 2.5 mL Folion-Ciocalteau reagent (FCR) (10 times diluted with water) and 2.5 mL sodium carbonate (Na2CO3) (20%) was mixed with 500 μg/mL extract. The mixture was made up to 10 mL by distilled water and incubated at 25 °C for 20 min to complete the reaction. The absorbance taken at 765 nm. The total phenol content concentration in the extract was then determined as mg of gallic acid equivalent by the equation obtained from the graph of standard gallic acid.
2.7.4. Total flavonoid content
The total flavonoid content of the extract carried out by using a standard colorimetric method of Chang et al. using quercetin as standard [24,25]. In 500 μg/mL extract, 1.5 mL methanol and 100 μL aluminum chloride (AlCl3) (10%) was mixed. 100 μL potassium acetate (1 M) and 2.8 mL distilled water added into the mixture. The mixture incubated at room temperature for 30 min to complete the reaction. Then absorbance was taken at 415 nm against a blank solution containing all the reagents except extract. A standard quercetin graph determined the total flavonoid content and expressed as mg of quercetin equivalent concentration.
2.7.5. Total flavonol content
The total flavonol content determined by adopting the method described by Kumaran and Karunakaran [26]. 500 μg/mL extract mixed with 0.5 mL AlCl3 (5%, 20 gm/L) and 1 mL sodium acetate (50 gm/L) solution. For completing the reaction, the mixture incubated for 150 min at room temperature, and then absorbance was taken at 440 nm against a blank solution containing all the reagents except the extract. Total flavonol content calculated as mg/g of quercetin equivalent by using the equation obtained from the standard quercetin graph.
2.8. Brine shrimp lethality bioassay
The brine shrimp lethality bioassay of A. trilobata observed by using simple organism Artemia salina leach (saline arrangement shrimp eggs). In the artificial seawater (3.8% NaCl solution), the shrimp eggs hatched for 48 h for maturing the shrimp called nauplii. The cytotoxicity bioassay carried on brine shrimp nauplii following the method described by Meyer et al. [27,28]. The extract was dissolved in DMSO (50 μL in 5 mL solution) to prepare the test sample with artificial seawater (3.8% NaCl in water) to obtain the serially diluted concentrations of 31.25, 62.5, 125, 250, 500 and 1000 μg/mL. Vincristine sulfate used as a positive control as the preceding method in a serial concentration dilution 0.125, 0.25, 0.5, 1, 5, and 10 μg/mL. Ten of the living nauplii applied to each of all experimental vials and control vials. Following 24 h, all vials inspected by an amplifying glass, and the number of living nauplii in each vial was observed and recorded.
| % of mortality = (N0–N1/N0) × 100 |
Where, N0 = the number of nauplii taken; N1 = the number of nauplii alive.
2.9. Thrombolytic activity
Thrombolytic activity test performed using the method described by Prasad et al. [29]. As a stock solution, lyophilized streptokinase vial (1500000 IU) mixed adequately with 5 mL sterile distilled water from which appropriate dilution made. Venous blood was withdrawn (5 mL) from healthy volunteers (n = 6) without the history of anticoagulant therapy or an oral contraceptive. Then distributed (0.5 mL/tube) to each six previously weight microcentrifuge tubes (sterilized) and incubated to form the clot at 37 °C for 45 min. After the formation of the clot, completely removed the serum without disturbing the clot and each tube reweighed for calculating the clot weight. 100 μL extract (10 mg/mL) added to each tube having the pre-weighed clot. 100 μL streptokinase and 100 μL distilled water were added separately to the positive and negative control group. Incubation was done for 90 min at 37 °C and observed clot lysis. The released fluid was removed and reweighed the tube to calculate the difference in weight after clot disruption.
| % of clot lysis = (weight of clot after remove of fluid/clot weight) × 100 |
2.10. Analgesic activity assay
2.10.1. Acetic acid induced writhing inhibition test
The analgesic activity evaluated by the acetic acid-induced writhing test [30,31]. Before starting the test, all experimental animals were unfed for 2 h. The mice separated into ten groups (n = 5). As negative control, 1% Tween-80 solution at 10 mL/kg b.w. given orally and as a positive control (Diclofenac sodium) has been given at 25 mg/kg b.w., IP. Plant extracts (MEALT, NFALT, MEATS, and NFATS) administrate with a dose of 200 and 400 mg/kg b.w. by orally using gavage, respectively. Thirty minutes after administration, 0.7% acetic acid was injected into the mice intraperitoneally and record and count the number of writhing for 20 min.
2.10.2. Formalin induced paw licking test
The analgesic activity was evaluated by the formalin-induced licking test [32] with the treatment of animals of each group (n = 5), as described in the acetic acid-induced writhing test. After 30 min of the administration, 20 μL formalin (2.5% v/V) was injected into the right hind paw just under the skin of the dorsal surface by a micro-syringe having a 26-gauge needle. The licking time of the first 5 min as early phase and then 15–30 min as late phase recorded.
2.11. Anti-diarrheal activities
2.11.1. Castor oil-induced diarrhea
Antidiarrheal activities carried by the method Nwodo and Alumanah (1991) [33]. All experimental animals unfed for 24 h before starting the test. The mice separated into ten groups (n = 5). As negative control, 1% Tween-80 solution at 10 mL/kg b.w. given orally and as a positive control (loperamide) has been given at 5 mg/kg b.w., IP. Plant extract (MEATL, NFATL, MEATS, and NFATS) has been received orally by gavage in a dose of 200 and 400 mg/kg b.w., respectively. One hour after administration, 0.5 mL castor oil has been given orally and kept them in separate cages consist of adsorbent paper beneath. The feces were counted and observed every hour till 4 h for each mouse and replaced in every 1 h. The equation calculated the level of % inhibition of defecation:
Where, A = average eradication feces number of the control group; B = average eradication feces number of the text group.
2.11.2. Castor oil-induced gastrointestinal motility
This gastrointestinal motility experiment was carried out by the method described by Mascolo et al. [34] with the treatment of animals of each group (n = 5) as described in the castor oil-induced diarrhea. One hour after administration of treatment group, animals treated with 1 mL charcoal meal (10% charcoal in 5% gum acacia) administered orally to each mouse. One hour later of charcoal administration, the animals were sacrificed. The distance travel charcoal meal from the pylorus to caecum was determined and presented as the total length of the intestine in percentage. The following formulae used to express the percentage of inhibition and Peristalsis index
2.12. Statistical analysis
Values were represented in Mean ± SEM (n = 5). a P < 0.05, b P < 0.01, c P < 0.001 and d P < 0.0001 indicated statistically significant in comparison to control group followed by unpaired t-test of one-way ANOVA (GraphPad Prism ver 7.0).
3. Results
3.1. Qualitative phytochemical screening
The qualitative phytochemical analysis of methanolic and n-hexane extract of A. trilobata leaves and stem showed the presence of alkaloid, carbohydrate, glycosides, flavonoids, phenols, flavonol, and saponins in all four extracts. In contrast, terpenoids only present in the methanolic extract of A. trilobata leaves. The phytochemical analysis in a qualitative manner summarized in Table 1.
Table 1.
Comparative phytochemical screening of A. trilobata leaves and stem.
| Qualitative Phytochemical analysis of A. trilobata | ||||
|---|---|---|---|---|
| Test Name | MEATL | NFATL | MEATS | NFATS |
| Alkaloid Mayer test |
++ | + | ++ | + |
| Carbohydrate Molisch's test |
++ | + | ++ | + |
| Glycosides | ++ | + | ++ | + |
| Flavonoids | ++ | ++ | + | ++ |
| Phenols | ++ | + | + | + |
| Flavonol | ++ | + | ++ | + |
| Terpenoids | + | – | – | – |
| Tannins | – | – | – | – |
| Saponins | + | – | + | + |
| Sterols | – | – | – | – |
| Quinones | – | – | – | – |
| Cholesterol | – | – | – | – |
| Proteins | – | – | – | – |
| Steroids | – | – | – | – |
| Starch | – | – | – | – |
Here, ‘+ +’ or ‘+’: present; ‘-‘: absent.
MEATL: Methanolic extract of A. trilobata leaves, NFATL: n-hexane fraction of A. trilobata leaves, MEATS: Methanolic extract of A. trilobata stem and NFATS: n-hexane fraction of A. trilobata stem.
3.2. Antioxidant activity
3.2.1. DPPH free radical scavenging activity
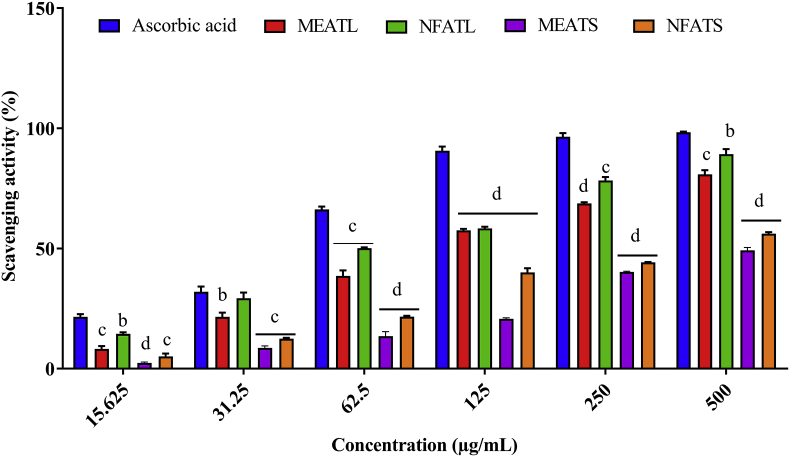
Table 2 and Fig. 1 were summarized the scavenging activity of DPPH assay and were in the following order: ascorbic acid > NFATL > MEATL > NFATS > MEATS. The antioxidant DPPH scavenging activity of A. trilobata fractions exhibited a significant (P < 0.05) manner inhibition in compared to standard drug ascorbic acid. Among all four extracts, NFATL showed the highest scavenging activity at (89.19%) at 500 μg/mL while at the same concentration ascorbic acid exhibited 98.33% scavenging activity. IC50 values calculated by the linear regression equation, whereas the IC50 values of ascorbic acid and NFATL were 36.32 and 139.65 μg/mL, respectively.
Table 2.
IC50 values with regression equation for A. trilobata fractions with reference to ascorbic acid.
| IC50 values (μg/mL) of radical scavenging | ||
|---|---|---|
| Chemicals/Plant extracts | IC50 | Regression equation |
| Ascorbic acid | 36.32 | y = 0.1374x + 45.009; R2 = 0.5688 |
| MEATL | 194.77 | y = 0.133x + 24.095; R2 = 0.7687 |
| NFATL | 139.65 | y = 0.1355x + 31.078; R2 = 0.7827 |
| MEATS | 454.09 | y = 0.095x + 6.8611; R2 = 0.9064 |
| NFATS | 372.95 | y = 0.096x + 14.197; R2 = 0.798 |
MEATL: Methanolic extract of A. trilobata leaves, NFATL: n-hexane fraction of A. trilobata leaves, MEATS: Methanolic extract of A. trilobata stem and NFATS: n-hexane fraction of A. trilobata stem.
Fig. 1.
Percentage of radical scavenging activities the DPPH assay of A. trilobata fractions and standard drug at different concentrations. Values are represented in Mean ± SEM (n = 3). b P < 0.01, c P < 0.001 and d P < 0.0001 are statistically significant in comparison to ascorbic acid followed by unpaired t-test of one-way ANOVA (GraphPad Prism 7). MEATL: Methanolic extract of A. trilobata leaves, NFATL: n-hexane fraction of A. trilobata leaves, MEATS: Methanolic extract of A. trilobata stem and NFATS: n-hexane fraction of A. trilobata stem.
3.2.2. Reducing power activity
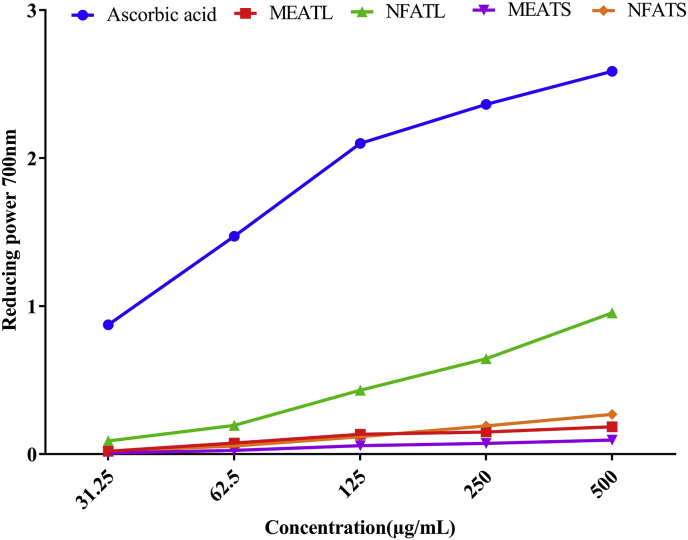
In Fig. 2, the dose-response curve for reducing power activity for A. trilobata fractions summarized (31.25–500 μg/mL). Reducing power will be increased with the increase of the concentration of the samples. The orders for reducing power activity were as followed: ascorbic acid > NFATL > NFATS > MEATL > MEATS. NFATL exhibited higher reducing power activity 0.955 at 500 μg/mL.
Fig. 2.
Reducing power of A. trilobata fractions and standard drug at different concentrations. MEATL: Methanolic extract of A. trilobata leaves, NFATL: n-hexane fraction of A. trilobata leaves, MEATS: Methanolic extract of A. trilobata stem and NFATS: n-hexane fraction of A. trilobata stem.
3.2.3. Total phenolic, flavonoid and flavonol contents
Quantitative analysis of antioxidant relevant phytochemicals total phenol content, total flavonoid content, and total flavonol content of A. trilobata (AT) fractions (500 μg/mL) summarized in Table 3 along with their regression equation. The orders for antioxidant relevant phytochemicals activity were as followed: NFATL > MEATL > NFATS > MEATS. NFATL showed the highest total phenol content (69.68 ± 0.67 mg GAE/g AT), total flavonoid content (53.69 ± 0.35 mg QE/g AT), and total flavonol content (153.26 ± 0.75 mg GAE/g AT) followed by MEATL, NFATS, and MEATS.
Table 3.
Quantitative analysis of antioxidant relevant phytochemicals total phenol content, total flavonoid content and total flavonol content of A. trilobata (AT) fractions (500 μg/mL).
| Plant extracts | Total phenol content (mg GAE/g AT) | Total flavonoid content (mg QE/g AT) | Total flavonol content (mg GAE/g AT) |
|---|---|---|---|
| MEATL | 40.19 ± 0.69 | 46.67 ± 0.23 | 118.91 ± 0.99 |
| NFATL | 69.68 ± 0.67 | 53.69 ± 0.35 | 153.26 ± 0.75 |
| MEATS | 13.69 ± 1.85 | 10.62 ± 0.55 | 26.00 ± 1.94 |
| NFATS | 23.44 ± 1.04 | 12.85 ± 0.28 | 37.19 ± 1.19 |
| Regression equation | y = 0.0039x + 0.0406 R2 = 0.9981 |
y = 0.0102x - 0.0637 R2 = 0.9693 |
y = 0.0039x + 0.0406 R2 = 0.9981 |
MEATL: Methanolic extract of A. trilobata leaves, NFATL: n-hexane fraction of A. trilobata leaves, MEATS: Methanolic extract of A. trilobata stem and NFATs: n-hexane fraction of A. trilobata stem.
Each value in the table is represented as mean ± SEM (n = 3).
3.3. Brine shrimp cytotoxicity
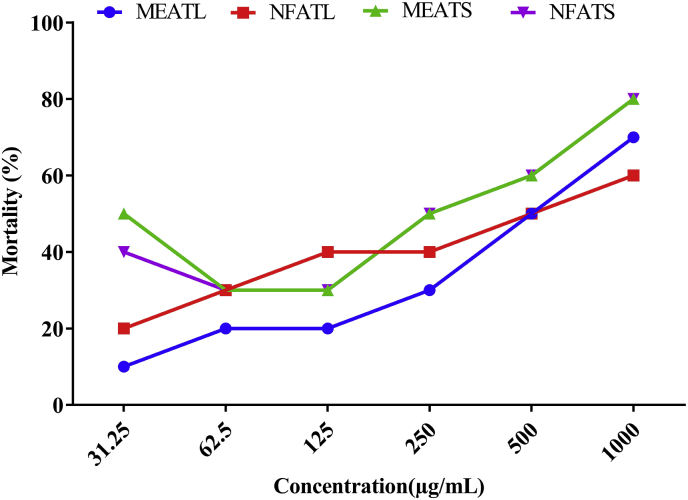
Table 4 and Fig. 3 summarized the LC50 values with the regression equation and the percentage of mortality of A. trilobata fractions, where no extract found to be toxic in comparison to positive control vincristine sulfate (2.16 μg/mL). The A. trilobata fractions exhibited moderately (MEATS = 328 μg/mL) to weakly toxic (NFATL = 616.85 μg/mL) LC50 observed.
Table 4.
LC50 values with regression equation for A. trilobata fractions with reference to vincristine sulfate.
| LC50 values (μg/mL) of Brine shrimp | ||
|---|---|---|
| Chemicals/Plant extracts | LC50 | Regression equation |
| Vincristine sulfate | 2.16 | y = 7.6315x + 33.536, R2 = 0.8585 |
| MEATL | 607.70 | y = 0.0596x + 13.781; R2 = 0.9629 |
| NFATL | 616.85 | y = 0.0346x + 28.657; R2 = 0.821 |
| MEATS | 328.02 | y = 0.0455x + 35.075; R2 = 0.7897 |
| NFATS | 362.00 | y = 0.0498x + 31.972; R2 = 0.9053 |
MEATL: Methanolic extract of A. trilobata leaves, NFATL: n-hexane fraction of A. trilobata leaves, MEATS: Methanolic extract of A. trilobata stem and NFATs: n-hexane fraction of A. trilobata stem.
Fig. 3.
Percentage of mortality of brine shrimp of A. trilobata fractions and standard drug at different concentrations. MEATL: Methanolic extract of A. trilobata leaves, NFATL: n-hexane fraction of A. trilobata leaves, MEATS: Methanolic extract of A. trilobata stem and NFATs: n-hexane fraction of A. trilobata stem.
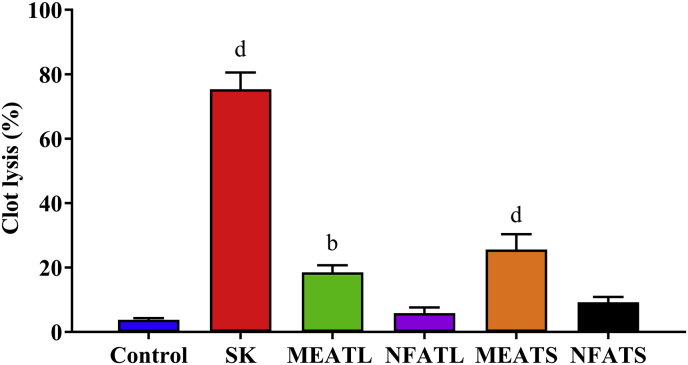
3.4. Thrombolytic activity
The thrombolytic activity of methanolic and n-hexane extract of A. trilobata leaves and stem summarized in Fig. 4. The MEATS showed the highest percentage of clot lysis (25.58 ± 4.76%, P < 0.0001) in comparison to negative control water (3.78 ± 0.49%), whereas the standard drug streptokinase exhibited 75.35 ± 5.21% (P < 0.0001). The orders for percentage of clot lysis were as followed: streptokinase > MEATS > MEATL > NFATS > NFATL > water.
Fig. 4.
Percentage of clot lysis of human blood by A. trilobata fractions and standard drug. Values are represented in Mean ± SEM (n = 6). b P < 0.01 and d P < 0.0001 are statistically significant in comparison to negative control (water) followed by unpaired t-test of one-way ANOVA (GraphPad Prism 7). SK: streptokinase, MEATL: Methanolic extract of A. trilobata leaves, NFATL: n-hexane fraction of A. trilobata leaves, MEATS: Methanolic extract of A. trilobata stem and NFATS: n-hexane fraction of A. trilobata stem.
3.5. Analgesic activity
3.5.1. Acetic acid induced writhing inhibition test
The methanolic and n-hexane extract of A. trilobata leaves stem at a dose of 200 and 400 mg/kg exhibited a significant decreased in the number of writhing. After induced of acetic acid, the NFATL (200 and 400 mg/kg) showed 20 and 17 writhing in per 20 min while the MEATL writhing (200 and 400 mg/kg) counted 23.33 and 14.33 in per 20 min; whereas the standard drug diclofenac Na (10 mg/kg) showed 12.33 writhing/20 min. The highest percentage of inhibition (57.85%) observed at MEATL (400 mg/kg), whereas Diclofenac Na showed 63.74% (Table 5).
Table 5.
Effect of A. trilobata fractions on acetic acid induced writhing response on Swiss albino mice.
| Acetic acid induced writhing inhibition test | ||
|---|---|---|
| Treatments (mg/kg) | Number of writhing | Inhibition (%) |
| Control | 34.00 ± 3.00 | – |
| Diclofenac Na 10 | 12.33 ± 0.88 d | 63.74 |
| MEATL 200 | 23.33 ± 1.76 b | 31.38 |
| MEATL 400 | 14.33 ± 0.88 c | 57.85 |
| NFATL 200 | 20.00 ± 2.00 b | 41.18 |
| NFATL 400 | 17.00 ± 1.00 c | 50.00 |
| MEATS 200 | 26.67 ± 1.45 a | 21.56 |
| MEATS 400 | 21.33 ± 0.88 b | 37.26 |
| NFATS 200 | 26.33 ± 1.45 a | 22.56 |
| NFATS 400 | 23.67 ± 1.45 b | 30.38 |
Values are represented in Mean ± SEM (n = 5). a P < 0.05, b P < 0.01, c P < 0.001 and d P < 0.0001 are statistically significant in comparison to Diclofenac Na followed by unpaired t-test of one-way ANOVA (GraphPad Prism 7).
MEATL: Methanolic extract of A. trilobata leaves, NFATL: n-hexane fraction of A. trilobata leaves, MEATS: Methanolic extract of A. trilobata stem and NFATS: n-hexane fraction of A. trilobata stem.
3.5.2. Formalin induced analgesic test
The effect of formalin-induced licking tests for analgesic activity summarized in Table 6. The extract of methanolic and n-hexane extract of A. trilobata leaves and stem at a dose of 200 and 400 mg/kg exhibited a significant depleted manner decreased in both early and late phases. But, MEATL (400 mg/kg) showed significant (P < 0.0001) percentage of inhibition in both early and late phases (60.83% and 61.65%, respectively), which were almost similar to standard drug diclofenac Na (68.68 and 63.16%).
Table 6.
The effect of A. trilobata fractions in Swiss albino mice to evaluate the analgesic activity by formalin induced licking response.
| Formalin induced licking test | ||||
|---|---|---|---|---|
| Treatment (mg/kg) | Early phase (0–5 min) | Inhibition (%) | Late phase (15–30 min) | Inhibition (%) |
| Control | 55.33 ± 4.33 | – | 44.33 ± 0.33 | – |
| Diclofenac Na10 | 17.33 ± 0.33 d | 68.68 | 16.33 ± 0.33 d | 63.16 |
| MEATL 200 | 36.67 ± 3.18 b | 33.72 | 29.33 ± 2.03 d | 33.84 |
| MEATL 400 | 21.67 ± 1.76 d | 60.83 | 17.00 ± 1.52 d | 61.65 |
| NFATL 200 | 43.00 ± 1.73 a | 22.28 | 32.33 ± 0.33 d | 27.07 |
| NFATL 400 | 27.33 ± 2.03 c | 50.61 | 19.67 ± 1.76 d | 55.63 |
| MEATS 200 | 31.67 ± 1.76 c | 42.76 | 22.00 ± 2.08 d | 50.37 |
| MEATS 400 | 27.00 ± 2.65 c | 51.20 | 25.66 ± 1.20 d | 42.0 |
| NFATS 200 | 34.33 ± 2.73 b | 37.95 | 24.67 ± 1.20 d | 44.35 |
| NFATS 400 | 28.67 ± 1.20 c | 48.18 | 26.33 ± 1.45 d | 40.60 |
Values are represented in Mean ± SEM (n = 5). a P < 0.05, b P < 0.01, c P < 0.001 and d P < 0.0001 are statistically significant in comparison to Diclofenac Na followed by unpaired t-test of one-way ANOVA (GraphPad Prism 7). MEATL: Methanolic extract of A. trilobata leaves, NFATL: n-hexane fraction of A. trilobata leaves, MEATS: Methanolic extract of A. trilobata stem and NFATS: n-hexane fraction of A. trilobata stem.
3.6. Anti-diarrheal activity
3.6.1. Castor oil-induced diarrhea in mice
The effect of castor oil-induced diarrhea in mice by A. trilobata fractions summarized in Table 7. In comparison to the negative control, the extract showed significant inhibition in both diarrhea and defecation phase in a dose-dependent manner while the MEATL 200, 400 mg/kg showed extremely significant inhibition in defecation (P < 0.0001) and diarrhea (P < 0.001) which is higher than the standard drug loperamide.
Table 7.
The effect of A. trilobata fractions on castor oil induced diarrhea in mice (feces count).
| Castor oil induced diarrhea test | ||||
|---|---|---|---|---|
| Treatment | Total number of feces | % of Inhibition of defecation | Total number of diarrheal feces | % Inhibition of diarrhea |
| Control | 14.60 ± 0.87 | – | 6.40 ± 0.81 | – |
| Loperamide 5 | 5.40 ± 0.24 d | 63.01 | 2.20 ± 0.20 c | 65.63 |
| MEATL 200 | 4.33 ± 0.33 d | 70.34 | 1.75 ± 0.14 c | 72.66 |
| MEATL 400 | 2.45 ± 0.10 d | 83.22 | 1.57 ± 0.32 c | 75.47 |
| NFATL 200 | 6.83 ± 0.36 d | 53.21 | 1.9 ± 0.15 c | 70.31 |
| NFATL 400 | 3.50 ± 0.52 d | 76.02 | 1.42 ± 0.08 c | 77.81 |
| MEATS 200 | 7.08 ± 1.08 c | 51.51 | 3.17 ± 0.3 b | 50.47 |
| MEATS 400 | 7.65 ± 0.78 c | 47.60 | 1.92 ± 0.44 c | 70.00 |
| NFATS 200 | 9.33 ± 0.88 c | 36.09 | 2.58 ± 0.22 c | 59.69 |
| NFATS 400 | 7.58 ± 0.3 c | 48.08 | 2.17 ± 0.17 c | 66.09 |
Values are represented in Mean ± SEM (n = 5). b P < 0.01, c P < 0.001 and d P < 0.0001 are statistically significant in comparison to Tween-80 (Control) followed by unpaired t-test of one-way ANOVA (GraphPad Prism 7).
MEATL: Methanolic extract of A. trilobata leaves, NFATL: n-hexane fraction of A. trilobata leaves, MEATS: Methanolic extract of A. trilobata stem and NFATS: n-hexane fraction of A. trilobata stem.
3.6.2. Castor oil induced intestinal motility
The effect of castor oil-induced intestinal motility by A. trilobata fractions summarized in Table 8. In comparison to the negative control, the extract showed a significant decrease in peristalsis index while the NFATL 400 mg/kg showed significant reduced (48.79 ± 1.94; P < 0.0001), whereas the standard drug loperamide showed 43.04 ± 2.79 (P < 0.0001) percentage of decrease. Of those, 400 mg/kg dose of NFATL exhibited the highest percentage inhibition (41.22%) of intestinal motility, whereas the standard drug loperamide (48.09%).
Table 8.
The effect of A. trilobata fractions with reference to Loperamide on intestinal motility in mice by using charcoal as a marker.
| Castor oil-induced intestinal motility test | ||||
|---|---|---|---|---|
| Treatment | Total Length of Intestine (cm) | Distance Travel by Charcoal (cm) | Peristalsis Index (%) | Inhibition (%) |
| Control | 50.33 ± 0.33 | 43.66 ± 2.91 | 86.69 ± 5.23 | – |
| Loperamide 5 | 52.66 ± 0.33 c | 22.66 ± 1.45 c | 43.04 ± 2.79 d | 48.09 |
| MEATL 200 | 53.67 ± 0.88 b | 36.00 ± 1.53 a | 67.11 ± 3.00 b | 17.56 |
| MEATL 400 | 57.33 ± 1.20 c | 29.66 ± 1.76 b | 51.71 ± 2.59 c | 32.06 |
| NFATL 200 | 54.33 ± 1.45 a | 35.00 ± 1.53 a | 67.37 ± 1.51 b | 19.85 |
| NFATL 400 | 52.66 ± 1.45 | 25.66 ± 0.88 c | 48.79 ± 1.94 d | 41.22 |
| MEATS 200 | 48.66 ± 0.67 a | 30.00 ± 1.53 b | 61.58 ± 2.29 b | 31.29 |
| MEATS 400 | 49.00 ± 0.58 | 29.67 ± 1.15 b | 60.60 ± 5.02 b | 32.06 |
| NFATS 200 | 53.00 ± 1.15 a | 31.00 ± 1.15 b | 58.50 ± 1.91 c | 29.01 |
| NFATS 400 | 52.33 ± 3.53 | 28.33 ± 1.76 b | 54.17 ± 3.53 c | 35.11 |
Values are represented in Mean ± SEM (n = 5). a P < 0.05, b P < 0.01, c P < 0.001 and d P < 0.0001 are statistically significant in comparison to Tween-80 (control) followed by unpaired t-test of one-way ANOVA (GraphPad Prism 7).
MEATL: Methanolic extract of A. trilobata leaves, NFATL: n-hexane fraction of A. trilobata leaves, MEATS: Methanolic extract of A. trilobata stem and NFATS: n-hexane fraction of A. trilobata stem.
4. Discussion
Phytochemical analysis of plant extract revealed the physiological activities as well as therapeutic activities also [35]. The phytochemical analysis of A. trilobata shows the presence of several secondary metabolites, whereas alkaloids are a particular group of secondary nitrogenous compounds which used to treat several human and animal disorder during middle age [36]. Another major phytochemical group is flavonoids, which used to treat cardiovascular diseases, cancer, and anti-inflammatory. Flavonoids usually take in dietary [37]. Presence of phenol in plants, having a contribution in physiological or biological elements of the plant [38].
Plants are an excellent source for natural antioxidants, whereas several phytochemicals have antioxidant properties. Their primary action is to ensure the protection against oxidative stress from free radicals [39,40]. Free radicals involve in several diseases like coronary infarction, atherosclerosis, neurodegenerative diseases and cancer [41]. Due to the potent antioxidant activity of polyphenolic substances (flavonoids and phenolic acids) in a biological system, are of interest [42,43]. According to literature, oxidative stress inhibited by quercetin [44] and gallic acid regulates the reactive species generation and improved a higher ratio of glutathione/oxidized glutathione [45]. Generally, the antioxidant activity was enhanced synergistically by antiradical plant phenolic substances [46]. Study reports, using of these phytochemicals substances as chemical agents (e.g., anti-inflammatory, antioxidant, anticancer), which may be useful to prevent the reactive oxygen species and antioxidant defense system [47]. In our study, all of this antioxidant relevant phytochemicals (total phenol content, total flavonoid content, and total flavonol content) of A. trilobata exhibited higher antioxidant activity in this order: NFATL > MEATL > NFATS > MEATS which also correlated with IC50 values of DPPH scavenger. At the same time, the extract also exhibited a significant decrease in free radical scavenging assay with the correlation of concentration. The brine shrimp lethality bioassay is a safe, effective, and economical method to determine the bioactivity of plant products [48]. The correlation between brine shrimp lethality bioassay and in vitro depletion of solid tumors in a human was introduced by the National Cancer Institute (NCI, USA). It is useful because it used as a pre-screening test for antitumor research [49]. LC50 and toxicity are inversely proportional, whereas the higher the toxicity, the lower will be the LC50. The value of LC50 over 1000 μg/mL considered to be non-toxic, ranging from 500 to 1000 μg/mL is weakly toxic, 100–500 μg/mL is moderately toxic while less than 100 μg/mL is considered as highly toxic [50]. In our study, no A. trilobata extracts found to be toxic in comparison to Vincristine sulfate, while a moderately (MEATS = 328 μg/mL) to weakly toxic (NFATL = 616.85 μg/mL) LC50 observed. The observed cytotoxic activity may be present of alkaloid phytochemical in the extract [51], which required further study to evaluate the cytotoxic compounds.
The available drugs in the market for thrombolytic, especially streptokinase, convert plasminogen to plasmin and increased lysis of the clot. The researcher revealed that flavonoids (plant metabolites) affect embolus and cardiovascular disease by interfering with platelet activation which is a risk factor for a cardiac disorder [52,53]. Our study suggests that the MEATS and MEATL could be a potential source to inhibit the clot formation. The current finding of A. trilobata through clot lysis in blood supports the earlier study on different plant species of the Passifloraceae family [54].
Nociception triggered or activated by mediators, which cause inflammation, edema, and diapedesis or leukocyte eruption [55]. Acetic acid-induced in mice is a behavioral analgesia mediated observation where IP administrations of acetic acid produce increase prostaglandin in the fluid of peritoneal [56]. By inhibiting the prostaglandin synthesis by any chemical substances which lower the number of writhing mentioned as analgesia. In contrast, central and peripheral antinociceptive pain is a biphasic method for formalin-induced licking test. The early phase and late phase is neurogenic and inflammatory pain response, respectively. Narcotics inhibit both phases while the non-steroidal anti-inflammatory drug (NSAID) inhibits the late phase [57]. In an acetic acid test, the extract of A. trilobata showed a significant dose-dependent manner percentage of inhibition in the nociceptive nerve, whereas the methanolic and n-hexane fraction of A. trilobata leaves showed the highest percentage of inhibition. Similarly, the administration of methanolic and n-hexane fraction of A. trilobata leaves and stem showed significant inhibition in both phases in a depleted manner, whereas the MEATL (400 mg/kg) showed the highest percentage of inhibition. The result showed significant inhibition in analgesic activity might be for the presence of alkaloids [58]. At the same time, the saponins and flavonoids phytochemicals are responsible for the anti-inflammatory properties of medicinal plants [59,60].The analgesic activity might also show because of the traditional used in pain sensation [15,16].
In antidiarrheal screening, castor oil cause water and electrolyte penetrability changes in the intestinal mucosal layers, bringing about liquid and watery luminal content that rapidly eliminate by intestines [61]. Ricinoleic acid is a natural laxative which is the active metabolite of castor oil, used in evaluating castor oil-induced diarrheal activity. Castor oil act on the small intestine to change the action of smooth muscle GI [62]. In our study, both models for antidiarrheal showed extremely significant inhibition of diarrhea. In contrast, the castor oil-induced diarrhea model exhibited extremely significant inhibition in defecation (P < 0.0001) and diarrhea (P < 0.001) by MEATL (200, 400 mg/kg), which is higher than the standard drug loperamide. Though the extremely significant activity in the castor oil-induced diarrhea model, it did not possess similar activity in castor oil-induced intestinal motility test. In contrast, the NFATL showed the maximum inhibition of intestinal motility. But, the finding of both models suggesting that the A. trilobata could be a potential source for diarrheal treatment, which proven the traditional used in stomach trouble [15,16].
5. Conclusion
The study reported that A. trilobata could be a potential source for antioxidant, cytotoxic, thrombolytic, analgesic, antidiarrheal activity due to the presence of secondary metabolites (e.g., alkaloid, flavonoid, phenol). Furthermore, studies highly recommended in identifying the mechanism of A. trilobata because there are no scientific reports related to biological activity.
Ethical approval
The study approved by the Institutional Animal Ethical Committee, Department of Pharmacy, International Islamic University Chittagong, Bangladesh according to governmental guidelines under the reference of Pharm/PND/150/20–2019.
6. Consent for publication
All authors have agreed to publish all materials belongs to this article.
7. Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This work is conducted with the individual funding of all authors.
CRediT authorship contribution statement
Niloy Barua: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing - original draft. Md Arfin Ibn Aziz: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing - original draft. Abu Montakim Tareq: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing - original draft. Mohammed Aktar Sayeed: Funding acquisition, Project administration, Resources, Supervision. Najmul Alam: Investigation, Methodology, Validation, Visualization. Nobi ul Alam: Investigation, Methodology, Validation, Visualization. Mohammad Amran Uddin: Investigation, Methodology, Validation, Visualization. Chadni Lyzu: Resources, Software, Validation, Visualization, Writing - original draft. Talha Bin Emran: Funding acquisition, Project administration, Resources, Supervision, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Authors are very much thankful to the Department of Pharmacy, International Islamic University Chittagong, Bangladesh for research facilities and other logistic supports.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2020.100772.
Contributor Information
Niloy Barua, Email: niloybaruaniloy@gmail.com.
Md Arfin Ibn Aziz, Email: arfinibnaziz151085@gmail.com.
Abu Montakim Tareq, Email: montakim0.abu@gmail.com.
Mohammed Aktar Sayeed, Email: sayeed_ustc@yahoo.com.
Najmul Alam, Email: nazmul9alam@gmail.com.
Nobi ul Alam, Email: nobiulalamshahin59@gmail.com.
Mohammad Amran Uddin, Email: imranshajal555@gmail.com.
Chadni Lyzu, Email: tithy_bmb@yahoo.com.
Talha Bin Emran, Email: talhabmb@gmail.com, talhabmb@bgctub.ac.bd.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Mendonça-Junior F.J., Scotti M.T., Nayarisseri A., Zondegoumba E.N., Scotti L. Natural bioactive products with antioxidant properties useful in neurodegenerative diseases. Oxi. Med. Cell. Longev. 2019:1–2. doi: 10.1155/2019/7151780. 7151780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Festa F., Aglitti T., Duranti G., Ricordy R., Perticone P., Cozzi R. Strong antioxidant activity of ellagic acid in mammalian cells in vitro revealed by the comet assay. Anticancer Res. 2001;21(6A):3903–3908. [PubMed] [Google Scholar]
- 3.Nicolini F.A., Nichols W.W., Mehta J.L., Saldeen T.G., Schofield R., Ross M., Player D.W., Pohl G.B., Mattsson C. Sustained reflow in dogs with coronary thrombosis with K2P, a novel mutant of tissue-plasminogen activator. J. Am. Coll. Cardiol. 1992;20(1):228–235. doi: 10.1016/0735-1097(92)90164-i. [DOI] [PubMed] [Google Scholar]
- 4.Milind P., Monu Y. Laboratory models for screening analgesics. Int. Res. J. Pharm. 2013;4:15–19. [Google Scholar]
- 5.Kumar M., Shek A., Akbar Z. A review on analgesic: from natural sources. Int. J. Pharm. Biol. Arch. 2010;1:95–100. [Google Scholar]
- 6.Tenório J.A., Dulciana S., da Silva T.M., da Silva T.G., Ramos C.S. Solanum paniculatum root extract reduces diarrhea in rats. Rev. Brasde. Farmacogn. 2016;26(3):375–378. [Google Scholar]
- 7.Sen T., Samanta S.K. Medicinal plants, human health and biodiversity: a broad review. Adv. Biochem. Eng. Biotechnol. 2015;147:59–110. doi: 10.1007/10_2014_273. [DOI] [PubMed] [Google Scholar]
- 8.Krosnick S., Porter-Utley K., Macdougal J., Jørgensen P., McDade L. New Insights into the evolution of Passiflora subgenus Decaloba (Passifloraceae): Phylogenetic relationships and morphological synapomorphies. Syst. Bot. 2013;38(3):692–713. [Google Scholar]
- 9.Okur M.E., Özbek H., Polat D.Ç., Yılmaz S., Arslan R. Hypoglycemic activity of Capparis ovata desf. var. palaestina zoh. methanol extract. Braz. J. Pharm. Sci. 2018;54(3) [Google Scholar]
- 10.Apu A.S., Muhit M.A., Tareq S.M., Pathan A.H., Jamaluddin A.T.M., Ahmed M. Antimicrobial activity and brine shrimp lethality bioassay of the leaves extract of Dillenia indica linn. J. Young Pharm. 2010;2(1):50–53. doi: 10.4103/0975-1483.62213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Traka M.H., Mithen R.F. 23 (7) Plant Cell.; 2011. Plant science and human nutrition: Challenges in assessing health-promoting properties of phytochemicals; pp. 2483–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okur M.E., Polat D.C., Ozbek H., Yilmaz S., Yoltas A., Arslan R. Evaluation of the antidiabetic property of Capparis ovata Desf. Var. Palaestina Zoh. extracts using in vivo and in vitro approaches. Endocr. Metab. Immune Disord. 2018;18(5):489–501. doi: 10.2174/1871530318666180328110524. [DOI] [PubMed] [Google Scholar]
- 13.Lall N., Kishore N. Are plants used for skin care in South Africa fully explored? J. Ethnopharmacol. 2014;153(1):61–84. doi: 10.1016/j.jep.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 14.Okur M.E., Ayla Ş., Çiçek Polat D., Günal M.Y., Yoltaş A., Biçeroğlu Ö. 70 (10) J. Pharm. Pharmacol.; 2018. Novel insight into wound healing properties of methanol extract of Capparis ovata Desf. Var. Palaestina zohary fruits; pp. 1401–1413. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh A. Survey of ethno-medicinal climbing plants in andaman and nicobar islands, India. Int. J. Pharm. Life Sci. 2014;5(7):3671–3677. [Google Scholar]
- 16.Kichu M., Malewska T., Akter K., Imchen I., Harrington D., Kohen J., Vemulpad S.R., Jamie J.F. An ethnobotanical study of medicinal plants of Chungtia village, Nagaland, India. J. Ethnopharmacol. 2015;166:5–17. doi: 10.1016/j.jep.2015.02.053. [DOI] [PubMed] [Google Scholar]
- 17.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 18.Harborne A. springer science & business media; 1998. Phytochemical Methods a Guide to Modern Techniques of Plant Analysis. [Google Scholar]
- 19.Auwal M.S., Saka S., Mairiga I.A., Sanda K.A., Shuaibu A., Ibrahim A. Preliminary phytochemical and elemental analysis of aqueous and fractionated pod extracts of Acacia nilotica (Thorn mimosa) Vet. Res. Forum. 2014;5(2):95–100. [PMC free article] [PubMed] [Google Scholar]
- 20.Braca A., DE Tommasi N., Di Bari L., Pizza C., Politi M., Morelli I. Antioxidant Principles from Bauhinia tarapotensis. J. Nat. Prod. 2001;64(7):892–895. doi: 10.1021/np0100845. [DOI] [PubMed] [Google Scholar]
- 21.Murali A., Ashok P., Madhavan V. In vitro antioxidant activity and HPTLC studies on the roots and rhizomes of Smilax zeylanica L. (Smilacaceae) Int. J. Pharm. Pharmaceut. Sci. 2011;3(1):192–195. [Google Scholar]
- 22.Ferreira I.C., Baptista P., Vilas-Boas M., Barros L. Free-radical scavenging capacity and reducing power of wild edible mushrooms from northeast Portugal: individual cap and stipe activity. Food Chem. 2007;100(4):1511–1516. [Google Scholar]
- 23.Singleton V.L., Orthofer R., Lamuela-Raventós R.M. vol. 299. Elsevier; 1999. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent; pp. 152–178. (Methods in Enzymology). [Google Scholar]
- 24.Chang C.-C., Yang M.-H., Wen H.-M., Chern J.-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002;10(3) [Google Scholar]
- 25.Pourmorad F., Hosseinimehr S., Shahabimajd N. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr. J. Biotechnol. 2006;5(11) [Google Scholar]
- 26.Kumaran A., Karunakaran R.J. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT Food Sci. Technol. 2007;40(2):344–352. [Google Scholar]
- 27.Meyer B., Ferrigni N., Putnam J., Jacobsen L., Nichols Dj, McLaughlin J.L. Brine shrimp: a convenient general bioassay for active plant constituents. Planta Med. 1982;45:31–34. 05. [PubMed] [Google Scholar]
- 28.Hossain S., Kader G., Nikkon F., Yeasmin T. Cytotoxicity of the rhizome of medicinal plants. Asian Pacific J. Trop. Biomed. 2012;2(2):125–127. doi: 10.1016/S2221-1691(11)60205-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prasad S., Kashyap R.S., Deopujari J.Y., Purohit H.J., Taori G.M., Daginawala H.F. Development of an in vitro model to study clot lysis activity of thrombolytic drugs. Thromb. J. 2006;4(1):14. doi: 10.1186/1477-9560-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taur D.J., Waghmare M.G., Bandal R.S., Patil R.Y. Antinociceptive activity of Ricinus communis L. leaves. Asian Pac. J. Trop. Biomed. 2011;1(2):139–141. doi: 10.1016/S2221-1691(11)60012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmed S., Rakib A., Islam M.A., Khanam B.H., Faiz F.B., Paul A., Chy M.N.U., Bhuiya N.M.A., Uddin M.M.N., Ullah S.A. Vol. 5. Clin. Phytosci.; 2019. In Vivo and in vitro pharmacological activities of Tacca Integrifolia rhizome and investigation of possible lead compounds against breast cancer through in silico Approaches; p. 36. [Google Scholar]
- 32.Okokon J.E., Nwafor P.A. Antiinflammatory, analgesic and antipyretic activities of ethanolic root extract of Croton zambesicus. Pak. J. Pharm. Sci. 2010;23(4):385–392. [PubMed] [Google Scholar]
- 33.Nwodo O., EJJoe Alumanah. Studies on Abrus precatorius seeds. II. Antidiarrhoeal Act. 1991;31(3):395–398. doi: 10.1016/0378-8741(91)90024-8. [DOI] [PubMed] [Google Scholar]
- 34.Mascolo N., Izzo A.A., Autore G., Barbato F., Capasso F. Nitric oxide and castor oil-induced diarrhea. J. Pharmacol. Exp. Therapeut. 1994;268(1):291–295. [PubMed] [Google Scholar]
- 35.Sofowora A. OpenURL; 1993. Medicinal plants and traditional. Medicine in Africa spectrum books Ltd, ibadan Nigeria. [Google Scholar]
- 36.Williamson E.M. Toxicology of Herbal Products. Springer; 2017. Herbal neurotoxicity: an introduction to its occurrence and causes; pp. 345–362. [Google Scholar]
- 37.Bertleff-Zieschang N., Rahim M.A., Ju Y., Braunger J.A., Suma T., Dai Y., Pan S., Cavalieri F., Caruso F. Biofunctional metal–phenolic films from dietary flavonoids. Chem. Commun. 2017;53(6):1068–1071. doi: 10.1039/c6cc08607a. [DOI] [PubMed] [Google Scholar]
- 38.Sampaio B.L., Edrada-Ebel R., Da Costa F.B. Effect of the environment on the secondary metabolic profile of Tithonia diversifolia: a model for environmental metabolomics of plants. Sci. Rep. 2016;6:29265. doi: 10.1038/srep29265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abbas Z.K., Saggu S., Sakeran M.I., Zidan N., Rehman H., Ansari A.A. Phytochemical, antioxidant and mineral composition of hydroalcoholic extract of chicory (Cichorium intybus L.) leaves. Saudi J. Biol. Sci. 2015;22(3):322–326. doi: 10.1016/j.sjbs.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rakib A., Ahmed S., Islam M.A., Haye A., Uddin S.N., Uddin M.M.N., Hossain M.K., Paul A., Emran T.B. Antipyretic and hepatoprotective potential of Tinospora crispa and investigation of possible lead compounds through in silico approaches. Food Sci. Nutr. 2020;8(1):547–556. doi: 10.1002/fsn3.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okeke M.I., Iroegbu C.U., Eze E., Okoli A., Esimone C. Evaluation of extracts of the root of Landolphia owerrience for antibacterial activity. J. Ethnopharmacol. 2001;78(2–3):119–127. doi: 10.1016/s0378-8741(01)00307-5. [DOI] [PubMed] [Google Scholar]
- 42.Blokhina O., Virolainen E., Fagerstedt K.V. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann. Bot. 2003;91 Spec:179–194. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pandey K.B., Rizvi S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009;2(5):270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang M., Swarts S.G., Yin L., Liu C., Tian Y., Cao Y., Swarts M., Yang S., Zhang S.B., Zhang K. Antioxidant properties of quercetin. Adv. Exp. Med. Biol. 2011;701:283–289. doi: 10.1007/978-1-4419-7756-4_38. [DOI] [PubMed] [Google Scholar]
- 45.Kim Y.J. Antimelanogenic and antioxidant properties of gallic acid. Biol. Pharm. Bull. 2007;30(6):1052–1055. doi: 10.1248/bpb.30.1052. [DOI] [PubMed] [Google Scholar]
- 46.Iacopini P., Baldi M., Storchi P., Sebastiani L. Catechin, epicatechin, quercetin, rutin and resveratrol in red grape: content, in vitro antioxidant activity and interactions. J. Food Compos. Anal. 2008;21(8):589–598. [Google Scholar]
- 47.Lotfi S. Protective efficacy of vanillic acid against GAMMA rays-induced hepatic damage in mice. Isot. Radiat. Res. 2011;43(1):215–222. [Google Scholar]
- 48.Rahman M.A., bin Imran T., Islam S. 3rd. Vol. 20. Elsevier; 2013. pp. 213–225. (Islam SJSjobs: Antioxidative, Antimicrobial and Cytotoxic Effects of the Phenolics of Leea Indica Leaf Extract). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson J., Goetz C., McLaughlin J., Suffness M. A blind comparison of simple bench‐top bioassays and human tumour cell cytotoxicities as antitumor prescreens. Phytochem. Anal. 1991;2(3):107–111. [Google Scholar]
- 50.Nguta J., Mbaria J., Gakuya D., Gathumbi P., Kabasa J., Kiama S. Biological screening of Kenyan medicinal plants using Artemia salina (Artemiidae) Pharmacologyonline. 2011;2:458–478. [Google Scholar]
- 51.Nobori T., Miura K., Wu D.J., Lois A., Takabayashi K., Carson D.A. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature. 1994;368(6473):753–756. doi: 10.1038/368753a0. [DOI] [PubMed] [Google Scholar]
- 52.Emran T.B., Rahman M.A., Uddin M.M.N., Rahman M.M., Uddin M.Z., Dash R., Lyzu C. Vol. 15. BMC Complement. Altern. Med.; 2015. Effects of organic extracts and their different fractions of five Bangladeshi plants on in vitro thrombolysis; p. 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rahman M.A., Sultana R., Emran T.B., Islam M.S., Rahman M.A., Chakma J.S., Rashid H.U., Hasan C.M.M. Vol. 13. BMC Complement. Altern. Med.; 2013. Effects of organic extracts of six Bangladeshi plants on in vitro thrombolysis and cytotoxicity; p. 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aziz M.A., Ahsan M., Hasan C., Masud M.M. Tocopherols, polyprenols and steroids from Passiflora edulis and bioactivities of its extractives. Dhaka Univ. J. Pharm. Sci. 2017;16(1):55–60. [Google Scholar]
- 55.Mazzon E., Esposito E., Di Paola R., Muia C., Crisafulli C., Genovese T., Caminiti R., Meli R., Bramanti P., Cuzzocrea S. Effect of tumour necrosis factor‐α receptor 1 genetic deletion on carrageenan‐induced acute inflammation: a comparison with etanercept. Clin. Exp. Immunol. 2008;153(1):136–149. doi: 10.1111/j.1365-2249.2008.03669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahmed F., Shahid I., Khatun A., Subhan N. Antidiarrhoeal and neuropharmacological activities of Avicennia officinalis Linn. Hamdard Med. 2008;51(1):18–23. [Google Scholar]
- 57.Khatun H., Majumder R., Al M., Alam E.K., Jami S.I., Alam B. Preliminary pharmacological activity of the methanolic extract of Premna integrifolia barks in rats. Avicenna J. Phytomed. 2014;4(3):215–224. [PMC free article] [PubMed] [Google Scholar]
- 58.Antherden L.M. eighth ed. Oxford University Press; London: 1969. Textbook of Pharmaceutical Chemistry; pp. 813–814. [Google Scholar]
- 59.Khalid S., Shahzad A., Basharat N., Abubakar M., Anwar P. Phytochemical screening and analysis of selected medicinal plants in gujrat. J. Phytochem. Biochem. 2018;2:108. [Google Scholar]
- 60.Dutta T., Paul A., Majumder M., Sultan R.A., Emran T.B. Pharmacological evidence for the use of Cissus assamica as a medicinal plant in the management of pain and pyrexia. Biochem. Biophys. Rep. 2020;21:100715. doi: 10.1016/j.bbrep.2019.100715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rizzo V., Clifford M.N., Brown J.E., Siracusa L., Muratore G. Effects of processing on the polyphenol and phenolic acid content and antioxidant capacity of semi‐dried cherry tomatoes (Lycopersicon esculentum M.) J. Sci. Food Agric. 2016;96(6):2040–2046. doi: 10.1002/jsfa.7315. [DOI] [PubMed] [Google Scholar]
- 62.Mathias J., Martin J., Burns T., Carlson G., Shields R. Ricinoleic acid effect on the electrical activity of the small intestine in rabbits. J. Clin. Invest. 1978;61(3):640–644. doi: 10.1172/JCI108975. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.