Highlights
-
•
The SOX9 expression increased in tumor tissues and peripheral blood of malignant and benign bone tumors.
-
•
The protein level of SOX9 is enhanced in malignant bone tumor tissues.
-
•
SOX9 over-expression correlated with tumor severity, grade, invasion feature, poor response to therapy, and recurrence.
Abbreviations: MSC, multipotent stem cells; SOX9, SRY-Box Transcription Factor 9; CSC, cancer stem cell; PBMC, peripheral blood mononuclear cell; GCT, giant cell tumor; FBS, fasting blood sugar; LDL, low-density lipoprotein; WBC, white blood cells; HB, memoglobin; RBC, red blood cell; CPP, C - reactive protein test; ESR, erythrocyte sedimentation rate; PBS, phosphate-buffered saline; OCT, optimal cutting temperature; DAB, 3, 3′-diaminobenzidine; PMSF, phenylmethylsulfonyl fluoride; PVDF, polyvinylidene difluoride; SEM, standard error mean; FOXO1, Forkhead Box O1; FOXO3, Forkhead Box O3
Keywords: Bone cancer, SOX9, CSC marker, Malignant bone tumors, Benign bone tumors
Abstract
Purpose
The status of the local and circulating SOX9, a master regulator of the tumor fate, and its relevance to tumor types, severity, invasion feature, response to therapy, and chemotherapy treatment were surveyed in bone cancer in the current study.
Methods
The SOX9 expression level was evaluated in tissue and peripheral blood mononuclear cells from patients with different types of malignant and benign bone tumors also tumor margin tissues using Real-Time PCR. The protein level of SOX9 was assessed using immunohistochemistry and western blot analysis. Also, the correlations of the SOX9 expression level with the patient’s clinical and pathological features were considered.
Results
The remarkable overexpression of SOX9 was detected in bone tumors compared to tumor margin tissues (P < 0.0001). Malignant bone tumors revealed a higher expression of SOX9 compared to benign tumors (P < 0.0001) while osteosarcoma tumors showed higher expression levels compared to Ewing sarcoma, and chondrosarcoma. Overexpression of SOX9 was observed in high grade, metastatic, recurrent tumors also tumors with poor response to therapy. Besides, the patients under the chemotherapy treatment demonstrated higher levels of SOX9 compared to the rest of malignant tumors (P = 0.02). The simultaneous up-regulation of circulating SOX9 in the patients with bone cancer was observed compared to healthy individuals (P < 0.0001) accompanying with overexpression of SOX9 in malignant tumors compared to benign tumors (P < 0.0001). The circulating SOX9 expression was up-regulated in the patients with malignant bone tumors who receive chemotherapy treatment also patients with high grade, metastatic, recurrent tumors. The protein level of SOX9 was in line with our data on the SOX9 gene expression.
Conclusion
The simultaneous overexpression of local and circulating SOX9 in bone cancer besides its positive correlation with tumor severity, malignancy, size, and chemotherapy may deserve receiving more attention in bone cancer diagnosis and therapy.
1. Introduction
Bone tumors cover a wide range of clinical conditions, which impose a burden of morbidity on the patients [1]. The uncontrolled proliferation of bone cells might emerge from the existence of multipotent stem cells (MSC) and they can cause bone tumor progression leading to bone fracture, healthy bone tissue destruction, skeletal complications, pain, and peripheral inflammation [2]. Also, various histological types of bone tumors make them challenging to be handled by clinicians, so they lead to remarkable failure in tumor removal and management [2], [3]. Recently, thanks to the improvements in imaging methods, endoscopic techniques, immune-based assays, gene analysis, surgical approaches, and new tumor markers detection; early tumor identification is facilitated [4], [5], [6]. However, the necessity of early diagnosis and tumor screening, as well as the unknown cause of bone cell dis-regulation and aberrant proliferation, provoked researchers to characterize the molecular mechanisms underlying the bone tumor initiation and progression [7], [8], [9], [10]. SOX9 (SRY-box transcription factor 9) is proposed as a novel cancer stem cell (CSCs) marker with the ability in maintaining cells in the undifferentiated status, which causes their re-newel and differentiation [11], [12]. Moreover, SOX9 is a multi-functional transcription factor, which plays a critical role in maintaining stem cell features, cell growth, and differentiation [13]. The effective role of SOX9 in tissue organogenesis like chondrogenesis might be due to its correlation with the downstream signaling pathways like Wnt and Notch, which spotted SOX9 as a potent biomarker affecting tumor cell fate [14], [15], [16]. The SOX9 tendency to be elevated during tumor genesis has been reported in some malignancies such as esophageal, prostate, and pancreatic cancers [17], [18]. Interestingly, it was shown that the cartilage formation was enhanced following the simultaneous delivery of morphogenetic protein 2, which is considered as a regulator of osteogenesis and chondrogenesis and SOX9 expression in dedifferentiated chondrocytes [19]. Also, it was shown that SOX9 can regulate the collagen transcription level in response to the 17β-estradiol, and cause chondrogenesis [20], [14], [21]. Despite evidence on the effective role of SOX9, as a regulator in chondrogenesis and collagen expression [22], the relevance of SOX9 in bone tumor genesis has yet to be clarified. Regarding the potent role of SOX9 in tissue regeneration and organogenesis, and also its correlation with cellular signaling pathways affecting cell growth, proliferation, and death; the current study aimed to evaluate the status of SOX9 in diverse bone tumor and healthy bone tissues at both gene and protein levels beside assessing the statement of SOX9 in peripheral blood mononuclear cells of the patients with various types of bone tumors, to provide evidence regarding the status of local and circulating SOX9 in bone cancer.
2. Material and method
2.1. Patients and sample collection
The number of 150 bone tumor tissues, 150 tumor margins, and 150 blood samples of the same patients with bone tumor as well as 60 healthy blood samples were enrolled in the current study with local ethical approval and informed consent. The project was approved ethically by the ethics committee of vice president of research at Iran University of Medical Sciences (IR.IUMS.REC.1397.1283). The tumor and tumor margin samples were collected from patients who were subjected to surgery at the Shafa Orthopedic Hospital. Following surgical resection, fresh tissues were kept at −80 for later evaluation. To evaluate the circulating SOX9 gene expression level, the total amount of 6 ml peripheral blood was taken from the patients and healthy age and sex-matched controls and applied for the peripheral blood mononuclear cell (PBMC) separation. The clinic- pathological features of the patients with malignant bone tumors are summarized in Table 1 and the variables are presented as the number of patients and percentages. Also, the statistical differences between malignant bone tumors and the variables are shown in Table 1. Briefly, the equal number of most prevalent types of malignant bone tumors including osteosarcoma, Ewing's Sarcoma, and chondrosarcoma were enrolled in the study. As it is shown in Table 1, 44% of patients with osteosarcoma and 56% of patients with Ewing's Sarcoma were under 20 years of age while all of the chondrosarcoma patients were above 20. In this survey, 48%, 32%, and 40% of the patients were male in osteosarcoma, Ewing's Sarcoma, and chondrosarcoma, subsequently.
Table 1.
The clinic- pathological features of patients with malignant bone tumors.
| Demographic features | Groups | Osteosarcoma (N = 25) |
Ewing's Sarcoma (N = 25) |
Chondrosarcoma (N = 25) |
P-Value |
|---|---|---|---|---|---|
| Age | ≤20 >20 |
11(44%) 14(56%) |
14(56%) 11(44%) |
0 25(100%) |
<0.001 |
| Gender | Male Female |
12(48%) 13(52%) |
8(32%) 17(68%) |
10(40%) 15(60%) |
0.352 |
| Tumor grade* | Low (grade I/II) High (grade III) |
3(15%) 17(85%) |
5(25%) 15(75%) |
12(57.14%) 9(42.85%) |
0.011 |
| Tumor size* (cm) | ≤8 >8 |
5(25%) 15(75%) |
9(45%) 11(55%) |
13(61.9%) 8(38.1%) |
0.059 |
| Metastasis | Yes No |
9(36%) 16(64%) |
9(36%) 16(64%) |
7(28%) 18(72%) |
0.787 |
| Chemotherapy | Positive Negative |
17(68%) 8(32%) |
14(56%) 11(44%) |
0 25(100%) |
<0.001 |
| Huvos grade* | Grade 1/2 Grade 3/4 |
12(66.66%) 6(33.33%) |
9(64.28%) 5(35.71%) |
0 0 |
0.959 |
| Tumor recurrence | Yes No |
7(28%) 18(72%) |
6(24%) 19(76%) |
0 0 |
0.747 |
* Indicated the number of patients with available information.
Additionally, 85%, 75%, and 42.85% of the patients had high-grade tumors in osteosarcoma, Ewing's Sarcoma, and chondrosarcoma, subsequently. Also, the tumor size in 75%, 55%, and 38.1% of the patients was over 8 cm in osteosarcoma, Ewing's Sarcoma, and chondrosarcoma, subsequently. As a matter of invasive feature of tumors, 36% of osteosarcoma, 36% of Ewing's Sarcoma, and 28% of chondrosarcoma tumors were metastatic. Moreover, amongst patients with malignant bone tumors, 68% of patients with osteosarcoma and 56% of patients with Ewing's Sarcoma received chemotherapy treatment before the surgery and none of our patients with osteochondroma were under chemotherapy regimen prior to the surgery. The osteosarcoma patients received the standard combination of doxorubicin, cisplatin, and methotrexate as chemotherapy approaches while Ewing's Sarcoma patients received the combination of vincristine, cyclophosphamide, and doxorubicin. The tumor necrosis over 95% was used as a cutoff value to determine the histological response to the chemotherapy treatment based on the Huvos system grading. Accordingly, grade 1/2 shows little or partial response to the chemotherapy (low rate of tumor necrosis) while grade 3/4 shows good response to the chemotherapy (over 95% tumor necrosis) [23]. In the current study, 66.66% of patients with osteosarcoma and 64.28% of patients with Ewing's Sarcoma showed poor response to chemotherapy treatment. Moreover, 28% of osteosarcoma tumors and 24% of Ewing's Sarcoma tumors were recurrent. Moreover, the clinical features of patients with benign bone tumors are summarized in Table 2. Among the 75 patients with benign bone tumors, 28% of patients with osteochondroma, 36% of patients with Giant-cell tumor and 52% of patients with exostosis were under 20 years of age. Among the enrolled patients with benign bone tumors, 76% of osteochondroma, 76% of Giant cell Tumor, and 64% of exostosis patients were male. As it is indicated in Table 2, most of the patients had tumors less than 8 cm in size. Additionally, the biochemical features of the patients are summarized in Table3, which shows over 80% of the patients were normal as a point of FBS, SGPT, SGOT, LDL, WBC, and HB, also approximately around 70% of the patients were at the normal range of RBC, CPP, and ESR
Table 2.
The clinic-pathological features of patients with benign bone tumors.
| Demographic features | Groups | Osteochondroma (N = 25) | Giant cell Tumor (N = 25) | Exostosis (N = 25) | P-value |
|---|---|---|---|---|---|
| Age | ≤20 >20 |
7(28%) 18(72%) |
9(36%) 16(64%) |
13(52%) 12(48%) |
0.21 |
| Gender | Male Female |
16(76%) 9(24%) |
16(76%) 9(24%) |
16(64%) 9(36%) |
1.00 |
| Tumor size* (cm) | ≤8 >8 |
17(85%) 3(15% |
18(90%) 2(10%) |
19(90.47%) 2(9.5%) |
0.83 |
* Indicated the number of patients with available information.
Table 3.
The biochemical features of patients with bone tumors.
| Parameters | Range | Numbers | (%) | std ± Mean |
|---|---|---|---|---|
| Fasting Blood Sugar (mg/dl) | Normal: 70–140 High: 140≥ |
126 24 |
84% 16% |
1.53 ± 97.80 3.18 ± 155.2 |
| SGPT | Normal: Male < 41, Female < 31 High: Male 41≥ Female 31≥ |
133 11 6 |
88.66% 7.33% 4% |
0.53 ± 14.77 9.07 ± 63.73 4.91 ± 50.67 |
| SGOT (mg/dl) | Normal: Male < 38 Female < 32 High: Male 38≥, Female32≥ |
130 12 8 |
86.66% 8% 5.33% |
0.41 ± 19.29 5.27 ± 55.83 1.08 ± 4640.75 |
| LDL | Normal range: <140 High: 480> |
132 18 |
88% 12% |
4.10 ± 91.76 6.30 ± 161.6 |
| WBC (*1000/mm3) | Normal range: 4–10 High: 10≥ |
128 22 |
85.33% 14.66% |
0.13 ± 7.24 0.43 ± 12.75 |
| HB |
Normal range: 14–18 Low: 14≤ |
110 40 |
73.33% 26.66% |
0.15 ± 14.71 0.26 ± 10.12 |
| RBC (*1000/mm3) | Normal range: 4.5–6.2 Low: 4.5≤ |
106 44 |
70.66% 29.33% |
0.45 ± 4.92 2.5 ± 3.4 |
| CRP | Negative Positive |
110 40 |
73.33% 26.66% |
|
| ESR (mm/h) | Normal range: Male: <15, Female:<20 High: Male 15≥ High: Female 20≥ |
100 27 23 |
66.66% 18% 15.33% |
0.50 ± 7.70 8.5 ± 72.25 7.8 ± 70.75 |
2.2. Peripheral blood mononuclear cell (PBMC) separation
The density gradient centrifugation by Ficoll-Hypaque (Sigma Chemical Co, St Louis, MO, USA) was applied for PBMC separation from the collected blood of patients and healthy subjects. Following separation, cells were washed and purified using phosphate-buffered saline (PBS) and counted by hemocytometer and the same number of cells was used for further evaluation.
2.3. RNA extraction, cDNA synthesis, and Real-Time PCR
To measure the gene expression level of SOX9, the tumor and tumor margin tissues, as well as PBMCs, were used for RNA extraction by Trizol (Invitrogen, Grand Island, USA) based on the manufacturer’s instructions. The quantity and quality of the extracted RNA of each sample were evaluated using a Nanodrop spectrophotometer (Nanodrop Technologies). The amount of 1 µg of extracted RNA was applied for cDNA synthesis using PrimeScript First Strand cDNA Synthesis Kit Takara, Japan) according to the manufacturer’s instructions. The synthesized cDNA of each sample was used for Real-Time PCR reaction using SYBR Premix Ex Taq II (Takara, Japan) which was performed in Applied Biosystems Step One Plus, Real-time system (Applied Biosystems, USA). The designed primers which were used are listed in Table 4 and the running program was as 1 cycle at 95 °C for 5 min following 40 cycles at 95 °C for 5 s, 55 °C for 20 s, and 60 °C for 35 s. To normalize the SOX9 gene expression, the beta-actin expression level was assessed as a housekeeping gene and the comparative CT (2−ΔCt) method was applied for analysis.
Table 4.
Primers used for qRT-PCR assessment of gene expressions.
| Gene | Primers | Primer sequence | Tm |
|---|---|---|---|
| SOX-9 | Forward Reverse |
5′– GCTTCTCGCTCTCGTTCAGA-3′ 5′-CAACGGCTCCAGCAAGAACA −3′ |
58 |
| Beta-Actin | Forward Reverse |
5-GAT CTC CTT CTG CAT CCT GT-3′ 5′-TGG GCA TCC ACG AAA CTA C- 3′ |
57 |
2.4. Immunohistochemistry
To evaluate the protein expression level of SOX9 in tumor and normal tissues, samples were evaluated via immunohistochemistry. Briefly, following tissue fixation in 4% paraformaldehyde and incubation in 20% sucrose, the frozen tissue blocks were prepared using Optimal Cutting Temperature (OCT) embedding medium during incubation in −27 °C. The 10 nm tissue sections of samples were prepared using cryotome and the washing buffer, H2O2 solution, Triton 5%, and blocking buffer were used subsequently prior to antibody staining. The dilution of 1:400 of a monoclonal antibody of SOX9 (Cell Signaling, the Netherlands) was applied following proper incubation and the anti-rabbit IgG HRP-conjugated secondary antibody (Cell Signaling, the Netherlands) was used with 3, 3′-diaminobenzidine (DAB, Dako) in an appropriate incubating time to visualize the antibody binding. The ethanol and xylolin were used for final-dehydration and subsequent mounting. The semi-quantitative method was applied for slide scoring by pathologists and based on the staining intensity the scores were assigned as 0 (absent), +1 (weak), +2 (moderate), or +3 (strong) staining.
2.5. Protein extraction and western blot analysis
The western blot analysis was applied to further evaluate the status of the SOX9 protein level in tumor and normal bone tissues. In this regard, the approximate amount of 5 mg of tissue powder was added to the lysis buffer mixture containing Phosphatase Inhibitor, Protease Inhibitor, and phenylmethylsulfonyl fluoride (PMSF). Following proper vortex, ice incubation, and centrifugation, the supernatants were subjected to protein concentration assay separately using BCA Protein Assay Kit (TaKaRa Bio Inc, Japan). Notably, the bovine serum albumin was applied as the standard. The 12% SDS-polyacrylamide gel electrophoresis was used for total protein separation by transferring to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA). The Tris-buffered Saline containing 5% skim milk and 0.1% Tween 20 were utilized for membrane blockage for 4 h at room temperature. The membranes were incubated overnight with SOX9 (Cell Signaling, the Netherlands) and GAPDH (Cell Signaling, the Netherlands) primary antibodies at 4 °C following incubation at room temperature with HRP-conjugated secondary antibody. The Amersham ECL detection reagent (Amersham Biosciences, UK) was used for the immunodetection of the proteins. To quantify the protein level, the intensity of each protein bands was measured from the scanned film by densitometric analysis using Image J software (NIH, Bethesda, MD, USA) [24]. The ratio of the SOX9 protein level to the GAPDH intensity was reported as a final result of protein quantification for each patient.
2.6. Statistical analysis
The comparative CT (2−ΔCt) method was used for gene expression analysis and the differences between groups were assessed statistically using the t-test and one-way analysis of variance (ANOVA). Based on the Kolmogorov-Smirnov analysis, data in benign and malignant groups distributed normally. The correlation of SOX9 expression level with the age, tumor size, tissue gene expression, PBMC gene expression, and protein level was assessed using the Pearson correlation coefficient test. The chi-square test was used to analyze the statistical differences between bone tumors and the variables (age, gender, tumor grade, tumor size, metastasis, chemotherapy status, response to therapy and tumor recurrence). The regression analysis was applied to determine the value of the variables in predicting SOX9 expression level in tumors. The Graph Pad Prism version 6 (Graph Pad Software, San Diego California) and Statistical Package for Social Science (SPSS v.16) were used for calculation of all statistics. P values <0.05 (two-sided) were considered statistically significant.
3. Results
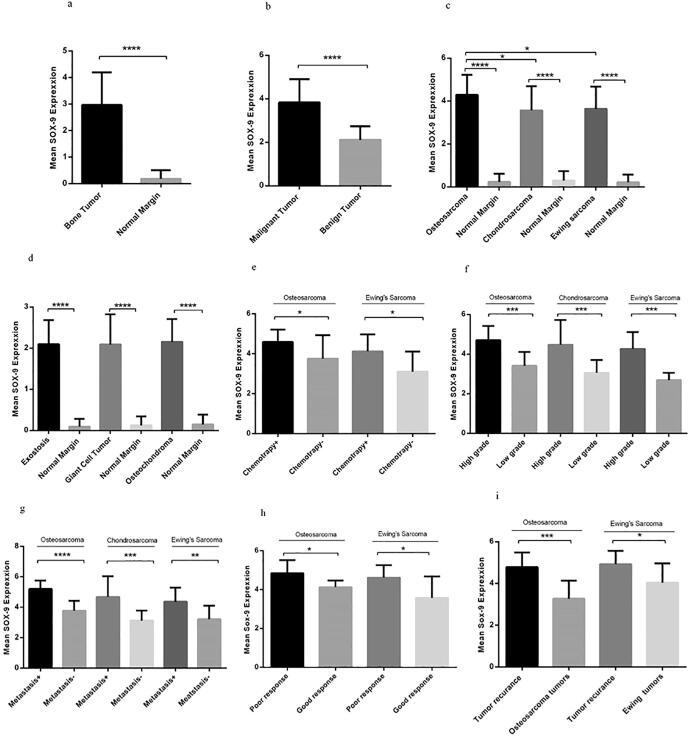
3.1. The SOX9 expression level in different types of primary bone tumors
Based on our data, the expression level of SOX9 was elevated significantly in bone tumors compared to normal bone tissues (P < 0.0001) (Fig. 1a). The mean and standard error mean (SEM) of SOX9 mRNA level was 2.973 ± 0.1005 and 0.1858 ± 0.02 in tumor and margin groups, respectively which reveals the remarkable elevation of SOX9 expression in tumor tissues. Moreover, regarding the possible involvement of SOX9 in tumor severity, the expression level of SOX9 was detected in malignant and benign bone tumors separately and it was revealed that SOX9 expression was increased significantly in malignant bone tumors (P = 0.0001) compared to benign tumors (Fig. 1b). Among the malignant tumors, the osteosarcoma, Ewing's Sarcoma, and chondrosarcoma tumor tissues were included in the survey and our data showed considerable overexpression of SOX9 in each malignant tumor group compared to their matched normal margins (P = 0.0001). Accordingly, the mean and the SEM of the SOX9 mRNA level were 4.29 ± 0.22, 3.56 ± 0.29, and 3.63 ± 021 in osteosarcoma, chondrosarcoma, and Ewing’s Sarcoma, respectively (Fig. 1c). The approximate 1.20- and a 1.18-fold increase was observed in the SOX9 expression level in osteosarcoma compared to chondrosarcoma and Ewing’s Sarcoma, respectively. Despite the overexpression of SOX9 in benign bone tumors (osteochondroma, Giant cell Tumor, and exostosis) compared to each group-matched normal margin (P = 0.0001), no difference in the expression level of SOX9 was observed between benign tumor groups (Fig. 1d). The SOX9 mRNA level was 4.59 ± 0.15 and 3.75 ± 0.39 in tumor tissue of osteosarcoma and Ewing’s Sarcoma of patients received chemotherapy regimen, respectively, which revealed the approximate of 1.22- and 1.32-fold increase compared to the patients with no history of chemotherapy in each group (Fig. 1e). Notably, high-grade tumors referred to those tumors in which their cells are moderate or poorly differentiated compared to healthy bone cells. Accordingly, the mean mRNA level of SOX9 in high-grade tumors of osteosarcoma, chondrosarcoma and Ewing’s Sarcoma was 4.70 ± 0.17, 4.47 ± 0.41 and 4.26 ± 0.21, respectively, which showed 1.37, 1.46- and a 1.58-fold increase compared to the low-grade tumors in each group, respectively (Fig. 1f). The significant overexpression of SOX9 was detected in metastatic tumors of osteosarcoma (5.21 ± 0.18), chondrosarcoma (4.68 ± 051), and Ewing’s Sarcoma (4.36 ± 0.30) which showed 1.38, 1.49- and a 1.35-fold increase compared to non-metastatic tumors in each group, respectively (Fig. 1g). Based on our data, osteosarcoma tumors with poor response to chemotherapy expressed a higher level of SOX9 (4.85 ± 0.19) compared to tumors with good response (4.13 ± 0.13) (P = 0.02), also Ewing’s Sarcoma tumors with poor response to chemotherapy expressed 4.62 ± 0.21 mRNA level of SOX9 compared to tumors with good response (3.5 ± 0.49) which was statistically significant (P = 0.04) (Fig. 1h). The overexpression of SOX9 was observed in recurrent osteosarcoma tumors (4.78 ± 0.28) and recurrent Ewing’s Sarcoma (4.92 ± 0.23) compared to the non-recurrent tumors (4.92 ± 0.23) (Fig. 1i)
Fig. 1.
The SOX9 mRNA expression in bone tumors. The SOX9 expression level was evaluated in different bone tumor tissues and its expression level was elevated in a bone tumor (a) versus normal bone tissues also malignant bone tumors versus benign tumors (b). The SOX9 expression evaluated in different types of malignant bone tumors compared to tumor margins in each group (c) and benign bone tumors normal paired tissue in each group (d). SOX9 overexpression observed in chemotherapy received patients (e), high-grade tumors (f), metastatic tumors in each malignant group (g), tumors with poor response to chemotherapy (h), and recurrent tumors (i). The Statistical differences between groups are shown as asterisk (*= P < 0.05, **= P < 0.01, ***= P < 0.001, ****= P < 0.0001).
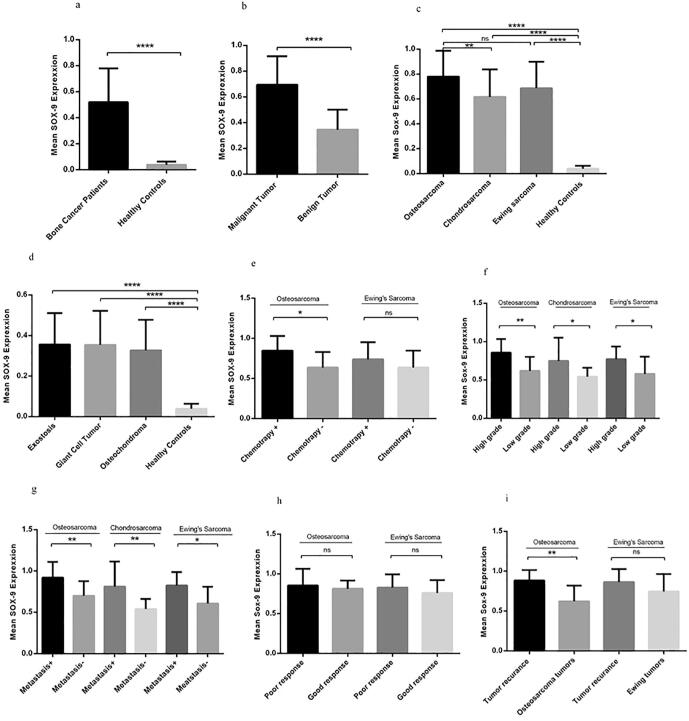
3.2. The circulating level of SOX9 expression in patients with bone tumors
Considering the feasibility and preference of circulating biomarkers in efficient and early diagnosis, the expression pattern of SOX9 was evaluated in peripheral blood of patients with bone cancer. Based on our data, the expression level of SOX9 was significantly elevated in the PBMC of patients with a bone tumor compared to healthy controls (P = 0.0001) (Fig. 2a). It was revealed that the mean and SEM of the SOX9 mRNA level was 0.519 ± 0.02 and 0.03 ± 0.0043 in patients compared to controls. Apparently, the mRNA level of SOX9 was reduced in peripheral blood of patients compared to their tumor tissues however its up-regulation was considerable in patients in comparison to healthy subjects. Additionally, the expression level of SOX9 was enhanced in patients with malignant bone tumors compared to patients with benign bone tumors (P = 0.0001) (Fig. 2b) with the SOX9 mRNA level mean and SEM of 0.694 ± 0.025 and 0.34 ± 0.01, respectively. The PBMC of malignant bone tumors showed significant elevation in SOX9 expression level compared to the PBMC of healthy controls (P = 0.0001) and the SOX9 level was significantly overexpressed in patients with osteosarcoma (0.78 ± 0.05) compared to patients with chondrosarcoma (0.61 ± 0.06) (Fig. 2c). In accordance with tumor tissues, the PBMC of benign bone cancers showed no statistical difference between osteochondroma, Giant cell Tumor, and 64% exostosis while considerable expression of SOX9 was observed in benign bone cancers compared to the PBMC of healthy subjects (P = 0.0001) (Fig. 2d). The mRNA level of SOX9 in PBMC of patients with osteosarcoma who received chemotherapy regimen was 0.84 ± 0.004 compared to the patients with no history of chemotherapy (0.63 ± 0.06) which showed 1.33 fold increase in SOX9 mRNA level (P = 0.014) while the difference was not significant for Ewing’s Sarcoma (Fig. 2e). Based on our data, the circulating level of SOX9 was increased in osteosarcoma patients with high-grade tumors (0.85 ± 0.04) (P = 0.005), in chondrosarcoma patients (0.74 ± 0.10) (P = 0.02) and patients with Ewing’s Sarcoma (0.77 ± 0.04) (P = 0.02) (Fig. 2f). The mRNA level of SOX9 in PBMC of the patients with metastatic osteosarcoma (0.92 ± 0.06), chondrosarcoma (0.81 ± 0.11) and Ewing’s Sarcoma (0.82 ± 0.05) showed significant elevation compared to nonmetastatic groups (Fig. 2g) while the SOX9 level revealed no significant difference in malignant bone cancers with poor or good response (Fig. 2h). Notably, the PBMC of the patients with recurrent osteosarcoma showed significant overexpression of SOX9 compared to the rest of osteosarcoma patients (P = 0.02) while Ewing’s Sarcoma patients revealed no difference in SOX9 expression level in recurrent versus non-recurrent tumors (Fig. 2I).
Fig. 2.
The SOX9 expression in peripheral blood of patients and healthy subjects. The expression level of SOX9 was increased in PBMCs of patients with bone cancer compared to healthy subjects (a) also patients with malignant bone tumors compared to benign tumors (b). The overexpression of SOX9 is shown in different types of malignant bone tumors compared to healthy subjects (c), also different types of benign bone tumors (d), SOX9 expression elevated in osteosarcoma patients receiving chemotherapy regimen (e), also the SOX9 overexpression observed in malignant high-grade tumors (f) and malignant metastatic tumors in each group (g). The SOX9 expression in PBMC showed no difference in patients with poor or good response to therapy (h), patients with recurrent tumors overexpressed circulating SOX9 (i). The Statistical differences between groups are shown as (*= P < 0.05, **= P < 0.01, ****= P < 0.0001).
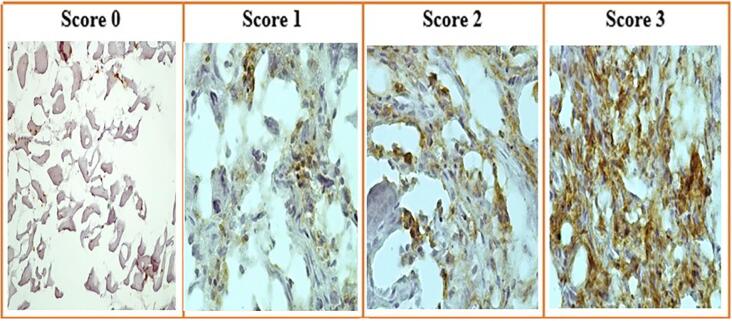
3.3. The overexpression of SOX9 protein in bone tumor tissues
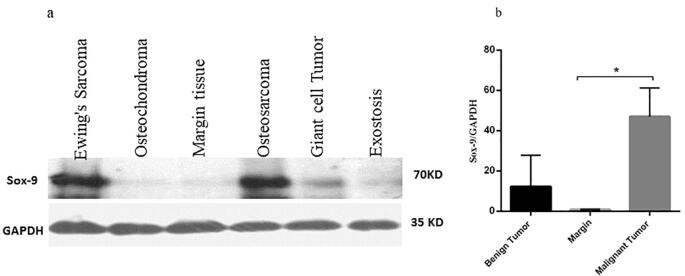
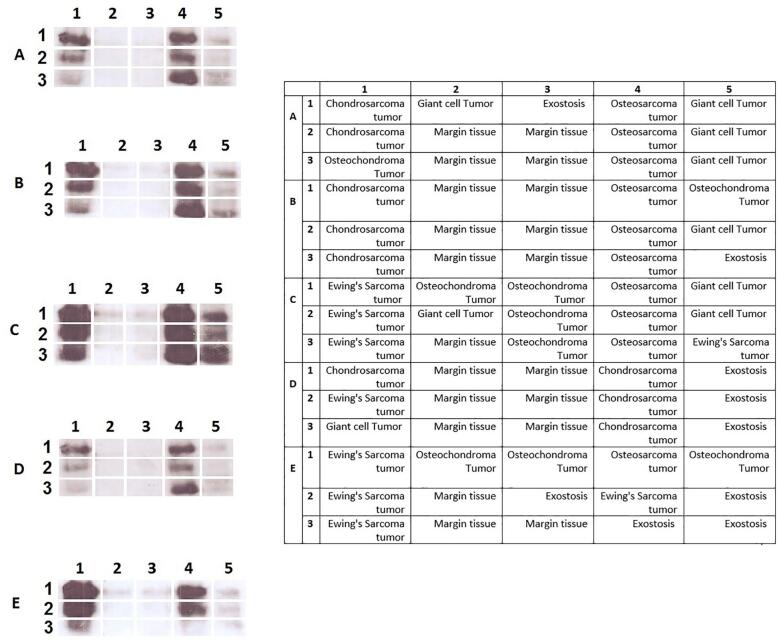
According to delineate the expression pattern of SOX9 in bone tumors, it is critical to determine that the overexpression of SOX9 in tumor tissues is accompanied by an elevation of its protein level. To this aim, the protein level of SOX9 was assessed in tumor and normal tissues using immunohistochemistry to determine both localization and expression levels of SOX9. Based on our data, 12% of osteosarcoma tumors showed no expression of SOX9 while 16%, 32%, and 40% of osteosarcoma tumors revealed a weak, moderate, and strong level of SOX9, respectively (Table 5). Also, 20%, 28%, and 28% of Ewing's Sarcoma tumors showed a weak, moderate, and strong level of SOX9, respectively while 24% of these tumors had no expression. The representative images of SOX9 immunohistochemistry staining in bone tumors are illustrated in Fig. 3. Based on our data, 40% of chondrosarcoma tumors showed weak expression while 20% and 16% of tumors showed moderate and strong SOX9 expression, respectively. Also, 40% of the patients with the history of chemotherapy regimen showed moderate expression of SOX9 while 23% of patients revealed no expression. The strong expression of SOX9 was not observed in benign bone cancers while 36%, 20%, and 24% of osteochondroma, Giant cell Tumor, and exostosis tumors showed a moderate level of SOX9, respectively. Moreover, the approximate of 70.83% of metastatic tumors showed a strong expression of SOX9. Additionally, the SOX9 protein evaluation was detected via western blot analysis to clarify the amount and total SOX9 protein expression in tissues. The western blot analysis was performed on 29 malignant tumors (10 osteosarcoma, 9 Giant-cell Tumor, 10 chondrosarcomas), 26 benign tumors (9 Giant-cell Tumor, 9 osteochondroma, 8 exostosis) and 20 margin tissues and the representative of the protein bands are illustrated in Fig. 4a. Our data revealed that malignant tumors significantly overexpress SOX9 protein compared to tumor margins (P = 0.04) (Fig. 4b). The up-regulation of SOX9 was detected in malignant tumors compared to benign tumors although it was not statistically significant. Based on our data, the expression of SOX9 in benign bone tumors was not remarkable compared to normal bone tissues (Fig. 4b).
Table 5.
The immunohistochemistry results of Sox-9 protein evaluation in patients with bone cancer.
| Scores | Osteosarcoma | Ewing's Sarcoma | Chondrosarcoma | Chemotherapy received patients |
|---|---|---|---|---|
| Negative | 12% | 24% | 24% | 23% |
| Positive (1+) | 16% | 20% | 40% | 16% |
| Positive (2+) | 32% | 28% | 20% | 40% |
| Positive (3+) | 40% | 28% | 16% | 21% |
| Scores | Osteochondroma | Giant cell Tumor | Exostosis | Metastatic patients |
| Negative | 48% | 60% | 56% | 0 |
| Positive (1+) | 16% | 20% | 20% | 4.16% |
| Positive (2+) | 36% | 20% | 24% | 25% |
| Positive (3+) | 0 | 0 | 0 | 70.83% |
Fig. 3.
The SOX9 protein assessment via immunohistochemistry. The SOX9 protein level was evaluated in different bone tumor tissues and the SOX9 expression level was indicated as weak expression (a), moderate expression (b), and strong expression (c).
Fig. 4.
The total SOX9 protein level enhanced in malignant bone tumors. The protein level of SOX9 was measured in malignant and benign bone tumors also non-cancerous bone tissues. The overexpression of SOX9 protein was detected in malignant bone tumors (a). The relative SOX9 level of expression was significantly elevated in malignant tumors compared to normal tissues (b). The SOX9 protein size was 70 KD which was normalized with internal control GAPDH (35KD).
3.4. Association of SOX9 expression with the demographic features of the bone cancer patients
As it is illustrated in Table 6, in malignant bone cancers, the SOX9 expression in tumor tissues and SOX9 protein levels by immunohistochemistry showed a significant correlation with the patient's age while SOX9 expression in PBMC was not correlated with age. In malignant bone cancers, the SOX9 gene expression in tumor tissues, PBMC, and its protein level was correlated with tumor size, significantly. Also, in malignant bone cancers, the SOX9 expression in tumor tissue of the patients was significantly correlated with the SOX9 level in PBMC and the SOX9 protein level of the same patients also the SOX9 gene expression in PBMC of the patients was correlated with the protein level of SOX9 in the same patients. In benign bone cancer, no correlation of SOX9 expression in tumor tissue, PBMC, and protein level with age was observed. However; the Sox-9 expression in benign bone cancers was significantly correlated with tumor size in benign tumor tissue, PBMC, and protein level. The same as malignant bone cancers, SOX9 gene expression in tumor tissues of benign bone cancers was correlated with its protein expression and expression in PBMC of the same patients also SOX9 expression in PBMC of benign bone cancers was correlated significantly with SOX9 protein level in the same patients (Table 6). The regression analysis of the common variables in both benign and malignant bone cancers revealed that the tumor size and the SOX9 expression level in PBMC of the patients also the protein level of SOX9 in tumor tissues were significantly valuable in predicting SOX9 expression level in tumor tissues. Also, the impact of tumor malignancy on the SOX9 gene expression level in tumor tissue was considerable and nearly significant (P = 0.051) (Table 7).
Table 6.
The correlation of Sox-9 expression with patient’s age, tumor size, gene expression level in tumor tissue, PBMC and protein expression level.
| Variable | Malignant bone cancer |
Benign bone cancer |
||||
|---|---|---|---|---|---|---|
| Tumor gene expression | PBMC gene expression | Protein expression | Tumor gene expression | PBMC gene expression | Protein expression | |
| Age | ||||||
| Correlation | 0.244* | 0.128 | 0.237* | −0.135 | −0.083 | −0.079 |
| P value | 0.035 | 0.274 | 0.041 | 0.249 | 0.480 | 0.502 |
| Tumor size | ||||||
| Correlation | 0.691** | 0.640** | 0.711** | 0.678** | 0.653** | 0.623** |
| P value | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| Tumor gene expression | ||||||
| Correlation | 0.834** | 0.793** | 0.814** | 0.702** | ||
| P value | 1 | 0.0001 | 0.0001 | 1 | 0.0001 | 0.0001 |
| PBMC gene expression | ||||||
| Correlation | 0.834** | 0.717** | 0.814** | 0.866** | ||
| P value | 0.0001 | 1 | 0.0001 | 0.0001 | 1 | 0.0001 |
*Correlation is significant at the 0.05 level (2-tailed).
**Correlation is significant at the 0.01 level (2-tailed).
Table 7.
The regression model of Sox-9 tumor expression in patients with bone cancer.
| Variable (predictors) | Standardized Coefficients (Beta) | t | P value |
|---|---|---|---|
| Tumor type | 0.083 | 1.209 | 0.229 |
| Tumor malignancy | 0.146 | 1.972 | 0.051* |
| Tumor size | 0.137 | 2.553 | 0.012** |
| Patient age | 0.049 | 1.382 | 0.170 |
| Patient gender | −0.007 | −0.201 | 0.841 |
| Sox-9 expression in PBMC | 0.473 | 6.696 | 0.0001**** |
| Sox-9 protein level | 0.201 | 3.288 | 0.001** |
4. Discussion
Despite recent improvements regarding primary bone tumor diagnosis and therapeutic strategies, nonspecific symptoms beside late patient referring and early detection failing are still prominent drawbacks causing cancer-induced morbidity and mortality [2], [25]. More recently, SOX9 gained attention, as a multi-functional transcription factor, by considering its role in regulating cell growth, development, and differentiation, which might lead to tissue organogenesis and tissue direction to specific lineage [11], [18]. Considering the pushing role of SOX9 in tumor formation and development, in the current study, its local and circulating expression pattern was perused in different types of bone tumors. The SOX9 gene overexpression level was detected in bone tumors compared to normal paired bone tissues. In line with our data, the up-regulation of SOX9 was reported in other malignancies such as esophageal, prostate, and pancreatic cancers [17], [18], [21]. Interestingly, the SOX9 overexpression was observed in bone malignant tumors compared to benign bone tumors. In accordance with our data, it was demonstrated that SOX9 expression increased in osteosarcoma tissues at both protein and gene levels, which was correlated with tumor advance stage and invasive features [26]. In support of this evidence, our data revealed that the SOX9 overexpression in malignant bone tumors was correlated with tumor size, high grade, invasive feature, tumor recurrence, and poor response. Interestingly, no previous study provided evidence on the status of SOX9 in different types of bone tumors. To clarify whether the SOX9 expression pattern might be affected by tumor type; the tumor tissues of the most prevalent malignant (osteosarcoma, Ewing sarcoma, and chondrosarcoma), as well as the most prevalent benign (Exostosis, Giant Cell tumor (GCT), and osteochondroma) bone tumors, were included in our survey, and based on our data, despite SOX9 overexpression in tumors tissues, no specific differences were observed regarding SOX9 expression in different types of benign bone tumors both in tumor tissues and PBMCs of the patients. Also, it was reported that SOX9 is involved in mesenchymal cells into chondrocytes differentiation; therefore, it might have a distinguishable expression in primitive small cell malignancies likewise mesenchymal chondrosarcoma [27]. Our data revealed that among small cell malignancies such as chondrosarcoma, Ewing sarcoma, and partly osteosarcoma; the expression level of SOX9 has increased significantly compared to normal bone tissues and benign bone tumors, while the SOX9 expression was increased more in osteosarcoma compared to the other malignant bone tumors. Based on our data, the SOX9 expression level enhanced in local recurrent tumors as well as tumors with high grades and metastatic tumors compared to the rest of malignant tumors, which indicated the possible impact of SOX9 in tumor invasive behavior. Tumor recurrence occurs due to the activation and/or re-activation of remaining tumor cells following a period of remission and stands as a challenging concern for both treatment and diagnosis. Regarding bone cancer, tumor recurrence might rate up to 65%, which is considerable and also problematic for the patients, and determining the underlying mechanism should be considered as a priority [28]. The effective role of SOX9 in tumor severity was observed in other tissues. Accordingly, it was shown that SOX9 was overexpressed in human esophageal squamous cell cancer which was correlated with tumor severity also SOX9 stimulated cell proliferation and tumorigenicity, possibly through affecting phosphorylated FOXO1, FOXO3, and Akt [21]. Our data compared the possible effects of chemotherapy treatment on the expression of SOX9 and therefore revealed that SOX9 was overexpressed in malignant tumor tissues of the patients under chemotherapy regimen compared to those malignant tumors without chemotherapy history. However; the overexpression of SOX9 in chemotherapy-received patients was remarkable in tumor tissues not in PBMCs. It was elucidated that tumor mass is a heterogeneous population of cells composed of CSC subpopulation [29]. Several studies reported that chemotherapy treatment is applied as a therapeutic choice to shrink the tumor and destroy cancer cells that might lead to CSC enrichment as well as inducing CSC activation and reconstruction [30], [31]. Interestingly, the promoting effect of chemotherapy on CSC make-up and renewal might increase tumor cell capability of the division [32]. Regarding the fact that SOX9 is considered as a CSC marker and tumor mass composed of the CSC population, the higher SOX9 expression in bone tumor tissues is rational and explainable [11]. Accordingly, due to the effective role of SOX9, as a master regulator in tumor fate, it might be considered as an effective or responsible factor for tumor response to the treatments and can also be proposed for further evaluations in chemotherapy agent design. A growing body of evidence supports the efficiency of peripheral blood as a possible circulating source of biomarkers to get insight into the less invasive diagnostic approaches [30], [33]. The behavior of immune cells in pathological conditions like cancer is influenced by tumor cells which cause functional changes in immune cells and subsequent immune system response towards tumor retrieval or proliferation [34]. Therefore, cancer-related gene expression profiling of PBMCs might reflect the presence of tumor cells/cancer stem cells in blood, and also uncover the functional alteration of immune cells regarding tumor formation and development. Up to the best of our knowledge, no previous study has been performed to evaluate the simultaneous expression pattern of SOX9 in blood and tumor tissues of various bone tumors. The SOX9 expression assessment in PBMCs of the patients revealed its overexpression in patients with bone tumors compared to healthy subjects. Also, the patients with malignant tumors expressed higher levels of SOX9 compared to the rest of the patients, which indicates its possible diagnostic value to distinguish bone cancer as a point of severity. The expression level of circulating SOX9 showed significant elevation in osteosarcoma patients compared to the other malignant tumors also overexpression was observed in high grade, metastatic and recurrent tumors in osteosarcoma, Ewing's Sarcoma and chondrosarcoma while showed no considerable differences among different types of benign bone tumors. Based on our data, it seems that local SOX9 might be more effective on bone tumor diagnosis as well as tumor formation underlying mechanism. The lower level of SOX9 mRNA in peripheral blood compared to local tumor tissue can be rationally influenced by the number of undifferentiated cells and also cancer stem cells present in tumors compared to blood [32]. Also, only a small fraction of the CSC population might release into the bloodstream, which makes it difficult for detection and evaluation [35]. Based on the fact that mRNA expression might be affected by several genetic and epigenetic factors, mRNA expression might not always end up in the protein translation [36]. Therefore, in the current study, the SOX9 assessment was evaluated at both mRNA and protein levels to present a more valuable and relevant outcome. Based on our data, the SOX9 mRNA and protein elucidated consistency and the simultaneous overexpression of SOX9 at both mRNA and protein levels in bone tumors, and also malignant tumors delineated a promising picture of SOX9 as a diagnostic marker in bone cancer. Altogether, considering the simultaneous assessment of SOX9 expression in tumor tissues beside PBMCs of the patients, our data provide a more efficient and accessible picture of SOX9 expression pattern in bone cancer and further pieces of evidence to utilize SOX9 as a diagnostic marker; however, further mechanistic studies are required to unravel the exact involvement of SOX9 in bone tumor pathogenesis.
Acknowledgments
Acknowledgement
This work was financially supported by Iran University of Medical Sciences (Grant Number: 97-4-75-13584).
Conflicts of interest
The authors declare that there is no conflict of interest.
Ethics approval and consent to participate
The project was ethically approved by the ethics committee of Vice president of research of Iran University of Medical Sciences with ethics committee code: IR.IUMS.REC.1397.1283. All participants were informed before surgery and following the informed consent, they were included in the study. The signed consent form for each patient is available.
Author contributions
AH carried out the experiments and performed sample collection, AM provided patients samples and contributed to clinical interpretation, VS contributed to data analysis and writing manuscript, KHJ and MB provided tumor and normal tissues, PB contributed to immunohistochemistry interpretation, SF contributed to clinical interpretation, ZR contributed to data analysis, NKH, ZA, MI and FH carried out the experiments, MTY designed the study, performed data analysis and wrote the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jbo.2020.100300.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary figure 1.
References
- 1.Johnson R.W., Suva L.J. Hallmarks of bone metastasis. Calcif. Tissue Int. 2018;102(2):141–151. doi: 10.1007/s00223-017-0362-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown H.K., Schiavone K., Gouin F., Heymann M.F., Heymann D. Biology of bone sarcomas and new therapeutic developments. Calcif. Tissue Int. 2018;102(2):174–195. doi: 10.1007/s00223-017-0372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piccioli A., Maccauro G., Spinelli M.S., Biagini R., Rossi B. Bone metastases of unknown origin: epidemiology and principles of management. J. Orthopaedics Traumatol. 2015;16(2):81–86. doi: 10.1007/s10195-015-0344-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Oronzo S., Brown J., Coleman R. The value of biomarkers in bone metastasis. Eur. J. Cancer Care. 2017;26(6) doi: 10.1111/ecc.12725. [DOI] [PubMed] [Google Scholar]
- 5.Zhu X.C., Zhang J.L., Ge C.T., Yu Y.Y., Wang P., Yuan T.F., Fu C.Y. Advances in cancer pain from bone metastasis. Drug Design Develop. Ther. 2015;9:4239–4245. doi: 10.2147/DDDT.S87568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miwa S., Yamamoto N., Hayashi K., Takeuchi A., Igarashi K., Tsuchiya H. Therapeutic targets for bone and soft-tissue sarcomas. Int. J. Mol. Sci. 2019;20(1) doi: 10.3390/ijms20010170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsuzuki S., Park S.H., Eber M.R., Peters C.M., Shiozawa Y. Skeletal complications in cancer patients with bone metastases. Int. J. Urol. 2016;23(10):825–832. doi: 10.1111/iju.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hutanu D., Popescu R., Stefanescu H., Pirtea L., Candea A., Sarau C., Boruga O., Mehdi L., Ciuca I., Tanasescu S. The molecular genetic expression as a novel biomarker in the evaluation and monitoring of patients with osteosarcoma-subtype bone cancer disease. Biochem. Genet. 2017;55(4):291–299. doi: 10.1007/s10528-017-9801-1. [DOI] [PubMed] [Google Scholar]
- 9.Kim Y.H., Goh T.S., Lee C.S., Oh S.O., Kim J.I., Jeung S.H., Pak K. Prognostic value of microRNAs in osteosarcoma: a meta-analysis. Oncotarget. 2017;8(5):8726–8737. doi: 10.18632/oncotarget.14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawrence D.A. Transforming growth factor-beta: a general review. Eur. Cytokine Netw. 1996;7(3):363–374. [PubMed] [Google Scholar]
- 11.Kawai T., Yasuchika K., Ishii T., Miyauchi Y., Kojima H., Yamaoka R., Katayama H., Yoshitoshi E.Y., Ogiso S., Kita S., Yasuda K., Fukumitsu K., Komori J., Hatano E., Kawaguchi Y., Uemoto S. SOX9 is a novel cancer stem cell marker surrogated by osteopontin in human hepatocellular carcinoma. Sci. Rep. 2016;6:30489. doi: 10.1038/srep30489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirzaei A., Madjd Z., Kadijani A.A., Alinaghi S., Akbari A., Tavoosidana G. Cancer stem cell’s potential clinical implications. Iran. J. Cancer Prevent. 2017;10(1) [Google Scholar]
- 13.Kadaja M., Keyes B.E., Lin M., Pasolli H.A., Genander M., Polak L., Stokes N., Zheng D., Fuchs E. SOX9: a stem cell transcriptional regulator of secreted niche signaling factors. Genes Dev. 2014;28(4):328–341. doi: 10.1101/gad.233247.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawaguchi Y. Sox9 and programming of liver and pancreatic progenitors. J. Clin. Investig. 2013;123(5):1881–1886. doi: 10.1172/JCI66022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu C.F., Angelozzi M., Haseeb A., Lefebvre V. SOX9 is dispensable for the initiation of epigenetic remodeling and the activation of marker genes at the onset of chondrogenesis. Development (Cambridge, England) 2018;145(14) doi: 10.1242/dev.164459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W., Feng Y., Aimaiti Y., Jin X., Mao X., Li D. TGFbeta signaling controls intrahepatic bile duct development may through regulating the Jagged1-Notch-Sox9 signaling axis. J. Cell. Physiol. 2018;233(8):5780–5791. doi: 10.1002/jcp.26304. [DOI] [PubMed] [Google Scholar]
- 17.Hong Y., Chen W., Du X., Ning H., Chen H., Shi R., Lin S., Xu R., Zhu J., Wu S., Zhou H. Upregulation of sex-determining region Y-box 9 (SOX9) promotes cell proliferation and tumorigenicity in esophageal squamous cell carcinoma. Oncotarget. 2015;6(31):31241–31254. doi: 10.18632/oncotarget.5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xi M., Wan S., Hua W., Zhou Y., Jiang W., Hu J. Correlation of SOX9 and NM23 genes with the incidence and prognosis of prostate cancer. Oncol. Lett. 2019;17(2):2296–2302. doi: 10.3892/ol.2018.9828. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Cha B.H., Kim J.H., Kang S.W., Do H.J., Jang J.W., Choi Y.R., Park H., Kim B.S., Lee S.H. Cartilage tissue formation from dedifferentiated chondrocytes by codelivery of BMP-2 and SOX-9 genes encoding bicistronic vector. Cell Transplant. 2013;22(9):1519–1528. doi: 10.3727/096368912X647261. [DOI] [PubMed] [Google Scholar]
- 20.Maneix L., Servent A., Poree B., Ollitrault D., Branly T., Bigot N., Boujrad N., Flouriot G., Demoor M., Boumediene K., Moslemi S., Galera P. Up-regulation of type II collagen gene by 17beta-estradiol in articular chondrocytes involves Sp1/3, Sox-9, and estrogen receptor alpha. J. Mol. Med. (Berlin, Germany) 2014;92(11):1179–1200. doi: 10.1007/s00109-014-1195-5. [DOI] [PubMed] [Google Scholar]
- 21.Lynn F.C., Smith S.B., Wilson M.E., Yang K.Y., Nekrep N., German M.S. Sox9 coordinates a transcriptional network in pancreatic progenitor cells. PNAS. 2007;104(25):10500–10505. doi: 10.1073/pnas.0704054104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lefebvre V., Huang W., Harley V.R., Goodfellow P.N., de Crombrugghe B. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Mol. Cell. Biol. 1997;17(4):2336–2346. doi: 10.1128/mcb.17.4.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosen G., Caparros B., Huvos A.G., Kosloff C., Nirenberg A., Cacavio A., Marcove R.C., Lane J.M., Mehta B., Urban C. Preoperative chemotherapy for osteogenic sarcoma: selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer. 1982;49(6):1221–1230. doi: 10.1002/1097-0142(19820315)49:6<1221::aid-cncr2820490625>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 24.S.M. Hartig, Basic image analysis and manipulation in ImageJ, Current protocols in molecular biology Chapter 14 (2013) Unit14.15. [DOI] [PubMed]
- 25.Ferguson J.L., Turner S.P. Bone cancer: diagnosis and treatment principles. Am. Fam. Physician. 2018;98(4):205–213. [PubMed] [Google Scholar]
- 26.Zhu H., Tang J., Tang M., Cai H. Upregulation of SOX9 in osteosarcoma and its association with tumor progression and patients' prognosis. Diagn. Pathol. 2013;8:183. doi: 10.1186/1746-1596-8-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wehrli B.M., Huang W., De Crombrugghe B., Ayala A.G., Czerniak B. Sox9, a master regulator of chondrogenesis, distinguishes mesenchymal chondrosarcoma from other small blue round cell tumors. Hum. Pathol. 2003;34(3):263–269. doi: 10.1053/hupa.2003.41. [DOI] [PubMed] [Google Scholar]
- 28.Xu L., Jin J., Hu A., Xiong J., Wang D., Sun Q., Wang S. Soft tissue recurrence of giant cell tumor of the bone: prevalence and radiographic features. J. Bone Oncol. 2017;9:10–14. doi: 10.1016/j.jbo.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scherer F. Capturing tumor heterogeneity and clonal evolution by circulating tumor DNA profiling, recent results in cancer research. Fortschritte der Krebsforschung. Progres dans les recherches sur le Cancer. 2020;215:213–230. doi: 10.1007/978-3-030-26439-0_11. [DOI] [PubMed] [Google Scholar]
- 30.Mirzaei A., Tavoosidana G., Rad A.A., Rezaei F., Tavakoli-Yaraki M., Kadijani A.A., Khalili E., Madjd Z. A new insight into cancer stem cell markers: could local and circulating cancer stem cell markers correlate in colorectal cancer? Tumour Biol. 2016;37(2):2405–2414. doi: 10.1007/s13277-015-3989-7. [DOI] [PubMed] [Google Scholar]
- 31.Perdigones N., Murtaza M. Capturing tumor heterogeneity and clonal evolution in solid cancers using circulating tumor DNA analysis. Pharmacol. Ther. 2017;174:22–26. doi: 10.1016/j.pharmthera.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Dylla S.J., Beviglia L., Park I.K., Chartier C., Raval J., Ngan L., Pickell K., Aguilar J., Lazetic S., Smith-Berdan S., Clarke M.F., Hoey T., Lewicki J., Gurney A.L. Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PLoS ONE. 2008;3(6) doi: 10.1371/journal.pone.0002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mirzaei A., Madjd Z., Kadijani A.A., Tavakoli-Yaraki M., Modarresi M.H., Verdi J., Akbari A., Tavoosidana G. Evaluation of circulating cellular DCLK1 protein, as the most promising colorectal cancer stem cell marker, using immunoassay based methods. Cancer Biomarkers. 2016;17(3):301–311. doi: 10.3233/CBM-160642. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki E., Sugimoto M., Kawaguchi K., Pu F., Uozumi R., Yamaguchi A., Nishie M., Tsuda M., Kotake T., Morita S., Toi M. Gene expression profile of peripheral blood mononuclear cells may contribute to the identification and immunological classification of breast cancer patients. Breast Cancer (Tokyo, Japan) 2019;26(3):282–289. doi: 10.1007/s12282-018-0920-2. [DOI] [PubMed] [Google Scholar]
- 35.Tayoun T., Faugeroux V., Oulhen M., Aberlenc A., Pawlikowska P., Farace F. CTC-derived models: a window into the seeding capacity of circulating tumor cells (CTCs) Cells. 2019;8(10) doi: 10.3390/cells8101145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fabbri M., Calin G.A. Epigenetics and miRNAs in human cancer. Adv. Genet. 2010;70:87–99. doi: 10.1016/B978-0-12-380866-0.60004-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.