Abstract
The changing profile of lifestyles and their intricate relationships with smoking indicate the importance of accounting for smoking status when assessing cancer preventability. We assessed the association of body mass index, weight change, alcohol intake, and physical activity with risk of total carcinoma among 53,195 smokers and 62,842 nonsmokers in two prospective cohorts. Then, leveraging the national prevalence estimates, we calculated the population attributable risk (PAR) for healthy lifestyle defined as body mass index ≥18.5 and <27.5 kg/m2, mid-life weight change of ≤20 pounds, no or moderate alcohol drinking (≤1 and 2 drinks/day for women and men, respectively), and weekly moderate or vigorous physical activity of at least 150 minutes. The PAR (95% CI) for healthy lifestyle was 18% (14–22%) in nonsmokers and 14% (10–19%) in smokers among women, and 20% (12–27%) in nonsmokers and 11% (5–17%) in smokers among men. While adiposity accounted for a substantially higher proportion of carcinoma cases in nonsmokers than smokers (16% versus 2% in women, 15% versus 2% in men), alcohol contributed more in smokers than nonsmokers (7% versus 3% in women, 8% versus 1% in men). When more strict criteria were used to define healthy lifestyle, the PAR estimates further increased (for women: 37% in smokers and 32% in nonsmokers; for men: 15% and 24%, respectively). In conclusion, lifestyle modification has great potential to reduce cancer risk in both smokers and nonsmokers. Weight control and reducing alcohol consumption should be prioritized for cancer prevention in nonsmokers and smokers, respectively.
Keywords: lifestyle modification, smoking, population attributable risk, primary prevention
INTRODUCTION
Cancer is the second leading cause of death in the United States, with more than 0.6 million cancer deaths estimated to occur in 2019.1 Although cancer mortality has experienced steady decline in the past few decades, the decrease has lagged far behind the decrease in heart disease mortality (68% versus 18%, respectively, from 1969 to 2013).2 As a consequence, cancer is expected to surpass heart disease as the leading cause of death in the US by 2020,3 highlighting the need for a greater emphasis on cancer prevention.4
Lifestyle plays an important role in cancer. Substantial evidence supports that approximately 40–60% of cancer deaths in the US may be prevented by modification of major lifestyle risk factors, including smoking, alcohol use, high body weight, and physical inactivity.5, 6 Diet is also important, although the strongest effect of diet is likely incorporated into its effect on weight control.7 Smoking is the single most important risk factor and may account for over one-quarter of cancer deaths in the US.8, 9 For other lifestyle factors, existing estimates of attributable risk are largely based on the entire population, with smokers and nonsmokers combined.10 Yet, the effect of health behaviors could differ by smoking status.
There are several reasons why it is important to assess the influence of other lifestyle factors according to smoking status. First, intricate relationships exist between smoking and other lifestyle factors. While smokers are less likely to gain weight and develop obesity than nonsmokers, heavy smoking is associated with a clustering of unhealthy behaviors, including poor diet, heavy alcohol drinking, and physical inactivity. As a result, there can be intractable residual confounding by smoking when assessing cancer risk according to these other lifestyle factors, thereby leading to erroneous estimates of attributable risk.11 Second, the metabolic effects of cigarettes promote visceral adiposity and insulin resistance that increase cancer risk.12 Therefore, studies that mixed smokers and nonsmokers may not be able to capture the full spectrum of the adverse effect of adiposity, the second leading cause of cancer death after smoking.6 Third, because smoking causes a selective cluster of cancers, the cancer profile in smokers is different from that in nonsmokers, raising questions about the applicability of a single estimate of preventability for total cancer based on the combination of smokers and nonsmokers. Finally, the risk factor profile has changed dramatically over time. While cigarette smoking in the US has decreased by about 50% between 1964 and 2012,13 the prevalence of obesity has more than doubled during the same time period.14 Taken together, these reasons highlight the importance and necessity of accounting for smoking status when evaluating the influence of other lifestyle factors on cancer risk.
Therefore, we assessed the effect modification by smoking for the association with cancer risk of other major lifestyle factors, including adiposity, weight change, alcohol drinking, and physical activity, in two large US cohort studies. Leveraging these effect estimates, we then calculated the population attributable risk (PAR), among smokers and nonsmokers separately, for each of the factors individually and in combination based on the most recent prevalence data from the National Health and Nutrition Examination Survey (NHANES). The PAR can be interpreted as the proportion of cancer cases that would not occur in the US if all individuals adopted the specified healthy lifestyle.
METHODS AND MATERIALS
Study population
We drew data from the Nurses’ Health Study (NHS), an ongoing cohort that enrolled 121,700 registered female nurses aged 30–55 years in 1976,15 and the Health Professionals Follow-up Study (HPFS), a cohort of 51,529 male health professionals aged 40–75 years enrolled in 1986.16 In both cohorts, participants completed a detailed questionnaire about their medical history and lifestyle at baseline, and every two years thereafter, with over 90% of follow-up. We collected dietary information using validated food frequency questionnaires (FFQs) every four years.
For the current study, we excluded participants who had cancer or missing data on smoking, body weight, physical activity, and alcohol drinking at baseline. Given the unknown relationship of underweight with cancer risk, we also excluded individuals with a BMI below 18.5 kg/m2. Therefore, a total of 74,850 women and 41,187 men were included in the analysis.
The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health, and those of participating registries as required.
Exposure assessment
Detailed description of exposure assessment is provided in the Supplementary Methods. For the NHS and HPFS, we asked participants about their smoking status, height, weight, and leisure-time physical activity through the biennial questionnaires. Because physical activity is a protective factor for cancer, we considered physical inactivity as the exposure in our analysis. We calculated adulthood weight change using the baseline weight and the recalled weight at age 18 years in the NHS and at age 21 years in the HPFS. All exposure assessments have been validated in previous studies in the two cohorts. We derived the national prevalence data of lifestyle factors from the NHANES 2015–2016.17 To facilitate PAR calculation, we restricted the analysis to 4,243 NHANES respondents who were aged at least 35 years old, the minimum age of the NHS and HPFS.
Outcome ascertainment
The primary outcomes of the study were incidence of total carcinomas, which included all cancers other than those in the skin, brain, and lymphatic and haematopoietic tissues. We excluded these cancers because they likely have other strong environmental causes than the ones considered in the current study, such as UV exposure, infections, and irradiation.5 Given the concern about overdiagnosis for indolent prostate cancer by prostate-specific antigen screening,18 we only included fatal prostate cancer in our analysis.
In the NHS and HPFS, participants were asked on biennial questionnaires if they had any diagnosis of cancer in the past two years. For those who reported yes, we asked for permission to acquire their medical records and pathologic reports. Study physicians, blinded to exposure information, reviewed medical records to confirm cancer diagnosis. When medical records were not available, we searched the state cancer registries to confirm diagnosis.
Statistical analysis
Details about statistical analysis are provided in the Supplementary Methods. We first assessed the association of BMI, weight change, alcohol, and physical activity with cancer incidence according to smoking status in the NHS and HPFS using multivariable Cox proportional hazards regression. Nonsmokers included never smokers or past smokers with packyears of <5.5 We calculated the P for interaction for the product term between the lifestyle factors (continuous) and smoking (binary) by Wald test. We then calculated the PAR, among smokers and nonsmokers separately, for a healthy lifestyle, which was defined as BMI ≥18.5 and <27.5 kg/m2, adulthood weight change of no more than 20 pounds (9 kg), weekly moderate or vigorous physical activity of at least 150 minutes that requires the expenditure of at least 3 metabolic equivalents per hour,19 and no or moderate alcohol drinking (≤1 drink/day for women, ≤2 drinks/day for men) as recommended by the 2015–2020 Dietary Guidelines for Americans.20 We calculated the exposure prevalence according to sex and smoking status in the NHANES.21 Using the prevalence (Pi) at the exposure category i and the corresponding HR estimates derived from the NHS and HPFS (HRi), we calculated the PAR for each lifestyle factor using the following approximate formula: .6 We then assessed the joint contribution of multiple lifestyle factors and calculated the 95% CI of the PAR using a simulation method.22
Data availability
NHANES data can be found in: https://www.cdc.gov/nchs/nhanes/index.htm. For the NHS/HPFS cohorts, further information including the procedures to obtain and access data is described at https://www.nurseshealthstudy.org/researchers (nhsaccess@channing.harvard.edu) and https://sites.sph.harvard.edu/hpfs/for-collaborators/.
RESULTS
Our study included 34,204 smokers and 40,646 nonsmokers in women of the NHS, and 18,991 smokers and 22,196 nonsmokers in men of the HPFS. As shown in Table 1, compared to nonsmokers, smokers were more likely to drink alcohol and regularly use aspirin, and were less likely to exercise and take multivitamins. Smokers gained less weight during early adulthood than nonsmokers in women; however, in men smokers gained more weight than nonsmokers, possibly due to the higher proportion of past smokers in men than in women (77% vs. 65%).
Table 1.
Age-standardized participant characteristics according to smoking status in the NHS and HPFS cohorts1
| Women |
Men |
|||
|---|---|---|---|---|
| Smokers (n=34,204) | Nonsmokers (n=40,646) | Smokers (n=18,991) | Nonsmokers (n=22,196) | |
| Age, year | 60.4 (11.4) | 60.5 (11.8) | 65.1 (11.0) | 63.1 (11.5) |
| Family history of cancer, %2 | 54 | 55 | 34 | 35 |
| Pack-years of smoking | 28.7 (19.3) | 0.5 (1.2) | 27.1 (18.0) | 0.4 (1.2) |
| Current smoking status, % | ||||
| Never smokers | 0 | 78 | 0 | 87 |
| Past smokers | ||||
| Quit <5 years ago | 11 | 2 | 16 | 1 |
| Quit 6–9 years ago | 8 | 1 | 10 | 1 |
| Quit ≥10 years ago | 45 | 19 | 51 | 10 |
| Missing quit time | 1 | 0 | 0 | 1 |
| Current smokers | ||||
| 1–14 cigarettes/day | 12 | 0 | 8 | 0 |
| 15–24 cigarettes/day | 14 | 0 | 6 | 0 |
| ≥25 cigarettes/day | 8 | 0 | 5 | 0 |
| Missing cigarettes/day | 1 | 0 | 4 | 0 |
| Body mass index, kg/m2 | 24.7 (4.1) | 25.0 (4.4) | 25.9 (3.3) | 25.5 (3.2) |
| Weight at early adulthood, pounds3 | 129 (20.3) | 125 (18.0) | 162 (23.4) | 161 (22.3) |
| Weight change, pounds4 | 11.3 (20.6) | 16.0 (20.4) | 15.8 (18.9) | 13.2 (17.2) |
| Physical activity, minutes/week | 157 (130) | 160 (130) | 192 (229) | 222 (241) |
| Alcohol intake, g/day | 8.2 (10.9) | 4.4 (7.4) | 14.3 (15.7) | 8.4 (11.3) |
| Multivitamin use, % | 52 | 55 | 42 | 45 |
| Regular use of aspirin, % | 51 | 48 | 56 | 49 |
Abbreviations: HPFS, Health Professionals Follow-up Study; NHS, Nurses’ Health Study.
Updated information throughout follow-up was used to calculate the mean for continuous variables and percentage for categorical variables, unless otherwise specified. All variables are age-standardized except age.
Defined as having a diagnosis of any cancer among parents or siblings.
Early adulthood represents age of 18 years in women and age of 21 years in men.
Weight change was calculated as the change in weight from age 18 to 1976 in women and from age 21 to 1981 in men.
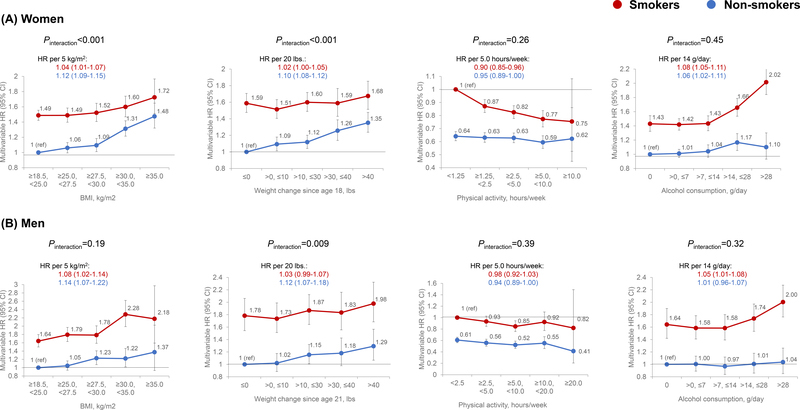
Figure 1 shows the association of lifestyle factors with total carcinoma incidence according to smoking status. We documented 7,889 cases in smokers and 7,206 in nonsmokers in women, and 2,881 and 1,908 in men, respectively. While smokers generally had a higher risk than nonsmokers, the positive associations for BMI and weight change were stronger in nonsmokers than smokers. For example, the multivariable HRs of carcinoma incidence per 5 kg/m2 increment in BMI in women were 1.04 (95% CI, 1.01–1.07) for smokers and 1.12 (95% CI, 1.09–1.15) for nonsmokers (Pinteraction<0.001). No statistically significant interaction with smoking was detected for physical activity or alcohol (Pinteraction>0.05 in both sexes).
Figure 1.
Associations of BMI, weight change, physical activity, and alcohol intake with incidence of total carcinoma according to smoking status in women (A) and men (B). Multivariable Cox proportional hazards regression was used to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs) after adjusting for age, calendar year, family history of cancer, pack-years of smoking, multivitamin use, and regular use of aspirin. In addition, we performed mutual adjustment for BMI, alcohol, and physical activity; and adjusted for body weight at 18 years for women and 21 for men for the analysis on weight change.
Table 2 shows the PAR estimates of total carcinoma incidence, in smokers and nonsmokers separately. The prevalence of ever smoking (including past and current smoking) in the NHANES was 56% in men and 39% in women. The combined PAR was 18% (95% CI, 14–22%) in nonsmoking women and 14% (95% CI, 10–19%) in smoking women, and 20% (95% CI, 12–27%) in nonsmoking men and 11% (95% CI, 5–17%) in smoking men. For individual factors, adiposity as assessed by high BMI and adulthood weight gain accounted for a substantially higher proportion of incident carcinoma cases in nonsmokers (16% in women and 15% in men) than smokers (2%), whereas alcohol contributed more in smokers (10% in women and 8% in men) than nonsmokers (6% in women and 1% in men). For physical activity, a higher PAR was observed in smokers than nonsmokers among women (7% versus 3%); for men while the PAR was higher in nonsmokers than smokers (6% versus 3%), the confidence intervals largely overlapped.
Table 2.
Prevalence of healthy lifestyles in the US and PAR estimates of total carcinoma incidence1
| Definition for healthy lifestyle | Smokers |
Nonsmokers |
|||
|---|---|---|---|---|---|
| Prevalence | PAR (95% CI) | Prevalence | PAR (95% CI) | ||
| Women | |||||
| Adiposity2 | BMI of ≥18.5, <27.5 and weight change≤20 pounds | 28% | 2% (−1 to 6%) | 34% | 16% (12 to 19%) |
| Physical activity3 | ≥150 minutes/week | 30% | 8% (5 to 11%) | 34% | 2% (−1 to 5%) |
| Alcohol intake | ≤1 drink/day | 56% | 7% (5 to 9%) | 77% | 3% (1 to 6%) |
| Total | 3% | 14% (10 to 19%) | 9% | 18% (14 to 22%) | |
| Men | |||||
| Adiposity2 | BMI of ≥18.5, <27.5 and weight change≤20 pounds | 35% | 2% (−3 to 7%) | 35% | 15% (8 to 22%) |
| Physical activity3 | ≥150 minutes/week | 33% | 3% (−2 to 8%) | 44% | 6% (1 to 11%) |
| Alcohol intake | ≤2 drinks/day | 64% | 8% (2 to 13%) | 79% | 1% (−7 to 9%) |
| Total | 7% | 11% (5 to 17%) | 15% | 20% (12 to 27%) | |
Abbreviations: BMI, body mass index; CI, confidence interval; NHANES, National Health and Nutrition Examination Survey; PAR, population attributable risk.
Prevalence was estimated based on the NHANES 2015–2016 data.
Weight change was calculated as the change in body weight since age 25 years.
Physical activity was calculated as the sum of moderate or vigorous activities.
To more closely approach the theoretical maximum of preventability for cancer for these lifestyle factors, we performed a sensitivity analysis based on more strict criteria for healthy lifestyle, defined as BMI ≥18.5 and <25.0 kg/m2, adulthood weight change of no more than 10 pounds, weekly moderate or vigorous physical activity of at least 300 minutes, and no alcohol drinking for women and no more than one drink/day for men (Supplementary Table 2). The PAR further increased in both women (37% in smokers and 32% in nonsmokers) and men (15% in smokers and 24% in nonsmokers).
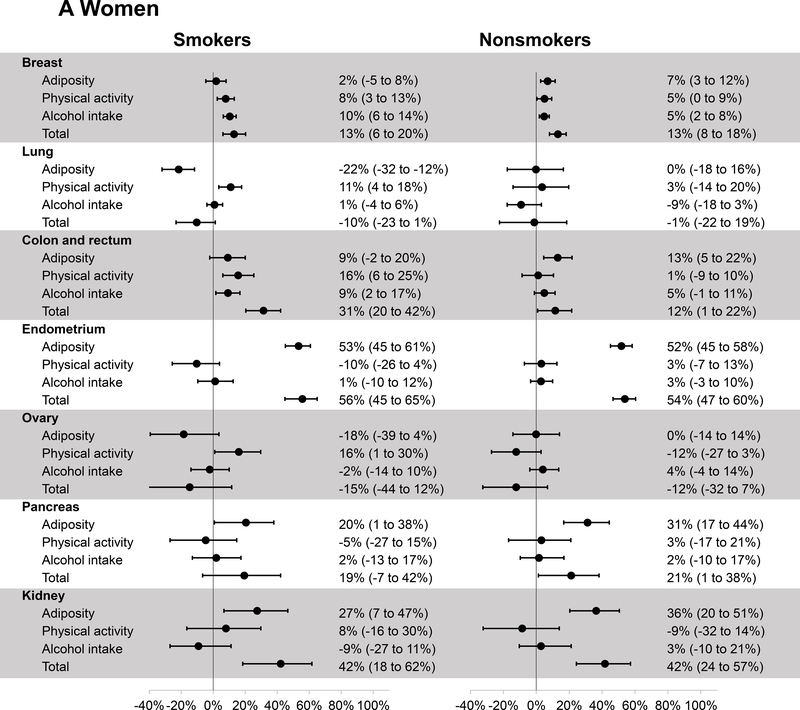
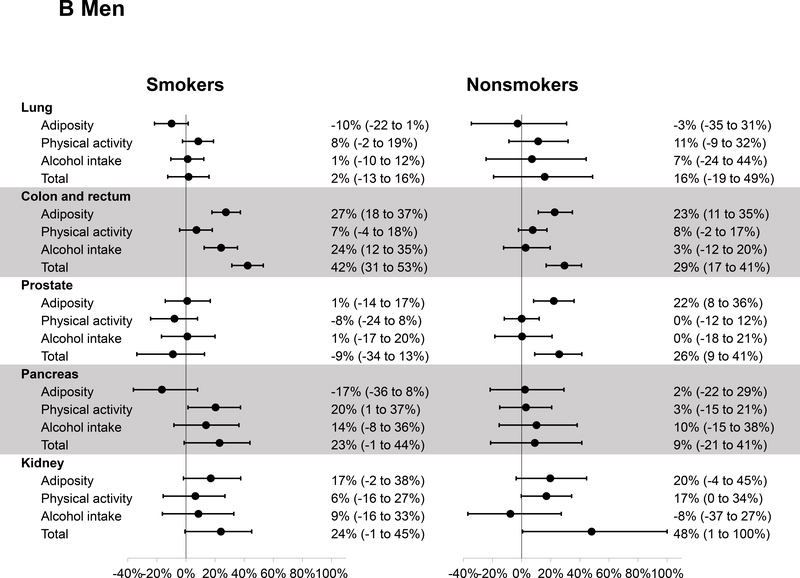
Figure 2 presents the PAR estimates for major individual cancers. The number of cases for each cancer in smokers and nonsmokers is provided in Supplementary Table 1. In women, a positive PAR was observed for all cancers except for lung and ovarian cancers. The estimates of total PAR were similar in smokers and nonsmokers for cancers of the breast (both 13%), endometrium (56% and 54%), pancreas (19% and 21%), and kidney (both 42%). A higher PAR for colorectal cancer was observed in smokers than nonsmokers (31% versus 12%), mainly driven by the PAR associated with physical activity in smokers (16%). In men, the healthy lifestyle was associated with a higher PAR in smokers than nonsmokers for colorectal cancer (42% versus 29%) and pancreatic cancer (23% versus 9%), whereas a higher PAR was observed in nonsmokers than smokers for kidney cancer (48% versus 24%) and a positive PAR was observed in nonsmokers only for prostate cancer (26%).
Figure 2.
Estimates of population attributable risk and 95% confidence interval for the incidence of individual cancers related to a healthy lifestyle according to smoking status in women (A) and men (B). The criteria for healthy lifestyle were as follows: BMI of ≥18.5, <27.5 kg/m2 and weight change of ≤20 pounds (adiposity); physical activity of ≥150 minutes/week; alcohol intake of no more than 1 drink/day in women and 2 drinks/day in men.
DISCUSSION
To the best of our knowledge, this is the first study to comprehensively evaluate the smoking-independent potential of lifestyle modification for cancer prevention in the US. We found that at least 10–15% and 15–20% of carcinoma cases in smokers and nonsmokers, respectively, can be potentially prevented by maintenance of a healthy lifestyle. These estimates further increased, particularly in women (37% in smokers and 32% in nonsmokers), when more strict criteria were used to define healthy lifestyle. Of note, the major contributors of PAR differed between smokers and nonsmokers: while BMI and weight gain were the driving force in nonsmokers, heavy alcohol intake in both sexes and physical inactivity in women contributed to majority of excess carcinoma cases in smokers. These findings support that lifestyle modification, independent of smoking control, has great potential for cancer prevention, and have implications for setting priorities for cancer control initiatives according to smoking, the single most important risk factor for cancer.
Obesity has been implicated as a causal factor for 12 types of cancer.23 A recent study indicated that excess BMI represented the second leading cause of cancer in the US after cigarette smoking and accounted for 5% and 11% of incident cancer cases in women and men, respectively.6 However, these estimates were based on a combination of smokers and nonsmokers. Also, the estimates did not account for the effect of adult weight gain that may better reflect excess body fat than attained BMI, because increases in body weight during adulthood depend on accumulation of fat more than of lean body mass.7, 24 In the current study, we analyzed both BMI and adulthood weight change according to smoking status. Consistent with previous data,25, 26 we found that BMI and weight change were more strongly associated with cancer risk in nonsmokers than smokers, with a much higher PAR estimate for total carcinoma (2% versus 16% in women, and 2% versus 15% in men). These findings may partly reflect the negative confounding by smoking on the BMI-cancer relationship, because smokers tend to have lower BMI and are at higher risk of developing cancer. On the other hand, although keeping BMI relatively low, smoking can in fact promote visceral adiposity and insulin resistance that increase cancer risk.12 Therefore, BMI is a less effective surrogate of metabolic abnormalities among smokers than it is among nonsmokers. In fact, the complex relationships between smoking and body weight, the two major risk factors for cancer with opposing patterns of prevalence, provide strong rationale for assessing their independent contributions to cancer burden.11 Our findings indicate the predominant importance of weight control for cancer prevention in nonsmokers.
Heavy alcohol drinking has been linked to increased risk of several cancer types, including cancers of the breast, oral cavity, pharynx, larynx, liver, esophagus, and colorectum.23 Alcohol intake is estimated to contribute 3–7% of all cancer cases and deaths.6, 27 Because high alcohol drinkers are more likely to smoke, there has been concern that some of the alcohol effect on cancer may be due to residual confounding by smoking. In line with previous studies,28, 29 we found that increasing alcohol intake was more strongly associated with higher cancer risk in smokers than nonsmokers, although the test for multiplicative interaction did not achieve statistical significance. Accordingly, the PAR associated with heavy alcohol drinking appeared to be higher in smokers than nonsmokers (7% versus 3% in women, 8% versus 1% in men). Although residual confounding by smoking cannot be ruled out completely, substantial evidence supports a synergistic effect between alcohol drinking and cigarette smoking in cancers for which both risk factors are causal.30, 31 Such interaction effect is biologically plausible, since alcohol may act as a solvent to promote penetration of carcinogens in cigarette smoke through the mucosa of upper aerodigestive organs.32 Our findings underscore the importance of limiting alcohol intake for cancer prevention, especially in smokers.
Physical activity is known to reduce risk of colon, breast, and endometrial cancers.23 A recent pooled analysis found that physical activity was associated with lower risk of 10 types of cancer independently of BMI.33 For most of the associations, stratified analyses did not reveal any modification by smoking except lung cancer, for which the inverse association was restricted to smokers. Similar heterogeneity for lung cancer has been reported in a meta-analysis.34 Given the known effect of heavy smoking on respiratory function and physical capability, the intensity of smoking is likely to differ between frequent exercisers and individuals who do not exercise. Therefore, residual confounding by smoking is likely to contribute, at least partly, to the inverse association between physical activity and lung cancer in smokers. On the other hand, given the benefit of exercise on physical health and tumor-intrinsic factors,35 it is possible that high physical activity may be particularly beneficial among smokers by reducing the exposure to carcinogenic agents in tobaccos, improving the capacity of resistance mechanisms, and mitigating the adverse metabolic consequences of smoking. In the current study, we found that physical activity was more strongly associated with lower cancer risk among smokers than nonsmokers in women, with a PAR estimate of 8% and 2%, respectively, whereas an opposite pattern was found in men.
Our study has several strengths. Leveraging the rich data of the two large cohorts with repeated lifestyle assessments and long-term follow-up, we systematically assessed the influence of major lifestyle factors on cancer risk according to smoking. By further integrating with the NHANES data, we provided rigorous evaluation for the potential impact of lifestyle modification on cancer prevention at the national scale. In addition, unlike prior PAR studies that have only used BMI as the indicator for adiposity, we also examined adulthood weight change, which has been associated with higher cancer risk independent of baseline body weight.26, 36–39
Some limitations of the study are noteworthy. First, all the NHS and HPFS participants were health professionals, which limited the generalizability of our findings. However, the consistency of our relative risk estimates with prior meta-studies or pooled analyses from different populations supports the reproducibility of our findings. Also, the homogeneity of the study population helps minimize any residual confounding. In addition, our analysis is based on a low-risk group achieving certain goals (low adiposity, low alcohol, and adequate physical activity); although proportionally there may be more health professionals who achieve these goals, it is likely that non-health professionals achieving the same goals would experience similar benefit in cancer risk. Second, we selected the four most prevalent risk factors with suggestive effect modification by smoking but did not include other risk factors, such as diet. However, the predominant effects of diet on cancer are believed to be mediated through affecting body weight that would have already been captured by our PAR estimates.7, 40 Indeed, we found in the NHANES that individuals who met the criteria for healthy lifestyle in Table 2 had a better diet and were generally much more likely to achieve the goal for healthy eating based on the recommendations by the World Cancer Research Fund and American Institute for Cancer Research (Supplementary Table 3). Third, for analysis of individual cancer types, we focused on major cancers and were unable to examine other less common cancers due to the limited number of cases. Finally, because not all covariate data are available in the NHANES, we were unable to use the multivariable HRs for calculation of the partial PARs.41 Instead, we applied the multivariable HRs to the crude PAR formula, as done in other studies.6, 42 Although this approach is considered biased, recent methodological evidence has shown that the bias is limited in settings typically encountered in epidemiology.43
In conclusion, we found that approximately 10–15% and 15–20% of cancer cases in smokers and nonsmokers, respectively, might be prevented by maintaining a BMI between 18.5 and 27.5, avoiding adulthood weight gain of no more than 20 pounds, avoiding heavy alcohol consumption, and exercising at a moderate or vigorous intensity for at least 150 minutes every week. While weight control should be prioritized for cancer prevention in nonsmokers, reducing alcohol consumption may have a great potential to reduce cancer risk in smokers.
Supplementary Material
Novelty and Impact.
We performed the first comprehensive assessment of cancer preventability by lifestyle modification according to smoking status. Our findings indicate that the major contributors of population attributable risk differed between smokers and nonsmokers: while adiposity was the driving force in nonsmokers, heavy alcohol intake and physical inactivity contributed to majority of excess carcinoma cases in smokers. These results have implications for setting priorities for cancer control initiatives according to smoking.
Acknowledgement
We would like to thank the participants and staff of the Nurses’ Health Study and the Health Professionals Follow-up Study for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Funding
This work was supported by the World Cancer Research Fund International [Commissioned research project 2018 to E.L. Giovannucci], the American Cancer Society [Grant number MRSG-17-220-01 – NEC to M. Song], and the U.S. National Institutes of Health (NIH) grants [K99 CA215314 and R00 CA215314 to M. Song; P01 CA87969 to M.J. Stampfer and E.Giovannucci; UM1 CA186107 to M.J. Stampfer; P01 CA55075 to W.C. Willett and E.Giovannucci; UM1 CA167552 to W.C. Willett and L.A. Mucci].
Role of the sponsor
The funders had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- FFQ
food frequency questionnaire
- HPFS
Health Professionals Follow-up Study
- NHANES
National Health and Nutrition Examination Survey
- NHS
Nurses’ Health Study
- PAR
population attributable risk
Footnotes
Conflict of interest: None.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019. [DOI] [PubMed] [Google Scholar]
- 2.Ma J, Ward EM, Siegel RL, Jemal A. Temporal Trends in Mortality in the United States, 1969–2013. JAMA 2015;314: 1731–9. [DOI] [PubMed] [Google Scholar]
- 3.Weir HK, Anderson RN, Coleman King SM, Soman A, Thompson TD, Hong Y, Moller B, Leadbetter S. Heart Disease and Cancer Deaths - Trends and Projections in the United States, 1969–2020. Prev Chronic Dis 2016;13: E157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song M, Vogelstein B, Giovannucci EL, Willett WC, Tomasetti C. Cancer prevention: Molecular and epidemiologic consensus. Science 2018;361: 1317–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song M, Giovannucci E. Preventable Incidence and Mortality of Carcinoma Associated With Lifestyle Factors Among White Adults in the United States. JAMA Oncol 2016;2: 1154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Islami F, Goding Sauer A, Miller KD, Siegel RL, Fedewa SA, Jacobs EJ, McCullough ML, Patel AV, Ma J, Soerjomataram I, Flanders WD, Brawley OW, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin 2017. [DOI] [PubMed] [Google Scholar]
- 7.World Cancer Research Fund / American Institute for Cancer Research, Diet, nutrition, physical activity: Energy balance and body fatness. Continuous Update Project Expert Report 2018., 2018.
- 8.Jacobs EJ, Newton CC, Carter BD, Feskanich D, Freedman ND, Prentice RL, Flanders WD. What proportion of cancer deaths in the contemporary United States is attributable to cigarette smoking? Ann Epidemiol 2015;25: 179–82 e1. [DOI] [PubMed] [Google Scholar]
- 9.Lortet-Tieulent J, Goding Sauer A, Siegel RL, Miller KD, Islami F, Fedewa SA, Jacobs EJ, Jemal A. State-Level Cancer Mortality Attributable to Cigarette Smoking in the United States. JAMA Intern Med 2016;176: 1792–8. [DOI] [PubMed] [Google Scholar]
- 10.Schottenfeld D, Beebe-Dimmer JL, Buffler PA, Omenn GS. Current perspective on the global and United States cancer burden attributable to lifestyle and environmental risk factors. Annu Rev Public Health 2013;34: 97–117. [DOI] [PubMed] [Google Scholar]
- 11.Song M, Giovannucci E. Estimating the Influence of Obesity on Cancer Risk: Stratification by Smoking Is Critical. J Clin Oncol 2016;34: 3237–9. [DOI] [PubMed] [Google Scholar]
- 12.Cena H, Fonte ML, Turconi G. Relationship between smoking and metabolic syndrome. Nutr Rev 2011;69: 745–53. [DOI] [PubMed] [Google Scholar]
- 13.National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon Generaled. Atlanta (GA): Centers for Disease Control and Prevention (US), 2014. [PubMed] [Google Scholar]
- 14.Fryar CD, Carroll MD, Ogden CL. Prevalence of Overweight, Obesity, and Severe Obesity Among Adults Aged 20 and Over: United States, 1960–1962 Through 2015–2016: National Center for Health Statistics, 2018.
- 15.Rimm EB, Giovannucci EL, Willett WC, Colditz GA, Ascherio A, Rosner B, Stampfer MJ. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet 1991;338: 464–8. [DOI] [PubMed] [Google Scholar]
- 16.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health 1997;6: 49–62. [DOI] [PubMed] [Google Scholar]
- 17.CDC National Center for Health Statistics. National Health and Nutrition Examination Survey, 2009–2010. http://wwwn.cdc.gov/Nchs/Nhanes/Search/nhanes09_10.aspx. Accessed November 3, 2015.
- 18.Etzioni R, Penson DF, Legler JM, di Tommaso D, Boer R, Gann PH, Feuer EJ. Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst 2002;94: 981–90. [DOI] [PubMed] [Google Scholar]
- 19.Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The Physical Activity Guidelines for Americans. JAMA 2018;320: 2020–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U.S. Department of Health and Human Services and U.S. Department of Agriculture, 2015–2020 Dietary Guidelines for Americans. 8th Edition. Available at http://health.gov/dietaryguidelines/2015/guidelines/, 2015.
- 21.Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, Curtin LR. National health and nutrition examination survey: analytic guidelines, 1999–2010. Vital and health statistics Series 2, Data evaluation and methods research 2013: 1–24. [PubMed] [Google Scholar]
- 22.Greenland S Interval estimation by simulation as an alternative to and extension of confidence intervals. Int J Epidemiol 2004;33: 1389–97. [DOI] [PubMed] [Google Scholar]
- 23.World Cancer Research Fund / American Institute for Cancer Research, Food, Nutrition, Physical Activity, and Cancer: a Global Perspective. Continuous Update Project Expert Report 2018., 2018.
- 24.Iyengar NM, Arthur R, Manson JE, Chlebowski RT, Kroenke CH, Peterson L, Cheng TD, Feliciano EC, Lane D, Luo J, Nassir R, Pan K, et al. Association of Body Fat and Risk of Breast Cancer in Postmenopausal Women With Normal Body Mass Index: A Secondary Analysis of a Randomized Clinical Trial and Observational Study. JAMA Oncol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348: 1625–38. [DOI] [PubMed] [Google Scholar]
- 26.Song M, Hu FB, Spiegelman D, Chan AT, Wu K, Ogino S, Fuchs CS, Willett WC, Giovannucci EL. Adulthood Weight Change and Risk of Colorectal Cancer in the Nurses’ Health Study and Health Professionals Follow-up Study. Cancer Prev Res (Phila) 2015;8: 620–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Praud D, Rota M, Rehm J, Shield K, Zatonski W, Hashibe M, La Vecchia C, Boffetta P. Cancer incidence and mortality attributable to alcohol consumption. Int J Cancer 2016;138: 1380–7. [DOI] [PubMed] [Google Scholar]
- 28.Cao Y, Willett WC, Rimm EB, Stampfer MJ, Giovannucci EL. Light to moderate intake of alcohol, drinking patterns, and risk of cancer: results from two prospective US cohort studies. BMJ 2015;351: h4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allen NE, Beral V, Casabonne D, Kan SW, Reeves GK, Brown A, Green J, Million Women Study C. Moderate alcohol intake and cancer incidence in women. J Natl Cancer Inst 2009;101: 296–305. [DOI] [PubMed] [Google Scholar]
- 30.Pelucchi C, Gallus S, Garavello W, Bosetti C, La Vecchia C. Alcohol and tobacco use, and cancer risk for upper aerodigestive tract and liver. Eur J Cancer Prev 2008;17: 340–4. [DOI] [PubMed] [Google Scholar]
- 31.Hashibe M, Brennan P, Chuang SC, Boccia S, Castellsague X, Chen C, Curado MP, Dal Maso L, Daudt AW, Fabianova E, Fernandez L, Wunsch-Filho V, et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev 2009;18: 541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boffetta P, Hashibe M. Alcohol and cancer. The Lancet Oncology 2006;7: 149–56. [DOI] [PubMed] [Google Scholar]
- 33.Moore SC, Lee IM, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, Keadle SK, Arem H, Berrington de Gonzalez A, Hartge P, Adami HO, Blair CK, et al. Association of Leisure-Time Physical Activity With Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA Intern Med 2016;176: 816–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmid D, Ricci C, Behrens G, Leitzmann MF. Does smoking influence the physical activity and lung cancer relation? A systematic review and meta-analysis. Eur J Epidemiol 2016;31: 1173–90. [DOI] [PubMed] [Google Scholar]
- 35.Hojman P, Gehl J, Christensen JF, Pedersen BK. Molecular Mechanisms Linking Exercise to Cancer Prevention and Treatment. Cell Metab 2018;27: 10–21. [DOI] [PubMed] [Google Scholar]
- 36.Rosner B, Eliassen AH, Toriola AT, Chen WY, Hankinson SE, Willett WC, Berkey CS, Colditz GA. Weight and weight changes in early adulthood and later breast cancer risk. Int J Cancer 2017;140: 2003–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dickerman BA, Ahearn TU, Giovannucci E, Stampfer MJ, Nguyen PL, Mucci LA, Wilson KM. Weight change, obesity and risk of prostate cancer progression among men with clinically localized prostate cancer. Int J Cancer 2017;141: 933–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Warren Andersen S, Wen W, Gao YT, Lan Q, Rothman N, Ji BT, Yang G, Xiang YB, Shu XO, Zheng W. Prospective cohort study of general and central obesity, weight change trajectory and risk of major cancers among Chinese women. Int J Cancer 2016;139: 1461–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keum N, Greenwood DC, Lee DH, Kim R, Aune D, Ju W, Hu FB, Giovannucci EL. Adult weight gain and adiposity-related cancers: a dose-response meta-analysis of prospective observational studies. J Natl Cancer Inst 2015;107. [DOI] [PubMed] [Google Scholar]
- 40.Giovannucci E A framework to understand diet, physical activity, body weight, and cancer risk. Cancer causes & control : CCC 2018;29: 1–6. [DOI] [PubMed] [Google Scholar]
- 41.Spiegelman D, Hertzmark E, Wand HC. Point and interval estimates of partial population attributable risks in cohort studies: examples and software. Cancer causes & control : CCC 2007;18: 571–9. [DOI] [PubMed] [Google Scholar]
- 42.Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT, Lancet Physical Activity Series Working G. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 2012;380: 219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong BHW, Peskoe SB, Spiegelman D. The effect of risk factor misclassification on the partial population attributable risk. Stat Med 2018;37: 1259–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
NHANES data can be found in: https://www.cdc.gov/nchs/nhanes/index.htm. For the NHS/HPFS cohorts, further information including the procedures to obtain and access data is described at https://www.nurseshealthstudy.org/researchers (nhsaccess@channing.harvard.edu) and https://sites.sph.harvard.edu/hpfs/for-collaborators/.