Abstract
Purpose of Review
There is increasing evidence indicating an association between several risk factors and worse prognosis in patients with coronavirus disease 2019 (COVID-19), including older age, hypertension, heart failure, diabetes, and pulmonary disease. Hypertension is of particular interest because it is common in adults and there are concerns related to the use of renin-angiotensin system (RAS) inhibitors in patients with hypertension infected with COVID-19. Levels of angiotensin-converting enzyme 2 (ACE2), a protein that facilitates entry of coronavirus into cells, may increase in patients using RAS inhibitors. Thus, chronic use of RAS inhibition could potentially lead to a more severe and fatal form of COVID-19. In this review, we provide a critical review to the following questions: (1) Does hypertension influence immunity or ACE2 expression favoring viral infections? (2) Are the risks of complications in hypertension mediated by its treatment? (3) Is aging a major factor associated with worse prognosis in patients with COVID-19 and hypertension?
Recent Findings
Despite the potential involvement of immune responses in the pathogenesis of hypertension, there is no evidence supporting that hypothesis that hypertension or RAS inhibitors contributes to unfavorable outcomes in viral infections. Future investigations adopting a strict protocol for confirming hypertension status as well as assessing associated comorbidities that may influence outcomes are necessary. From the therapeutic perspective, recombinant ACE2 may serve as a potential therapy, but relevant studies in humans are lacking. Definitive evidence regarding the use of RAS inhibitors in patients with COVID-19 is needed; 5 randomized trials examining this issue are currently underway.
Summary
There is no current scientific support for claiming that hypertension or its treatment with RAS inhibitors contribute to unfavorable outcomes in COVID-19.
Keywords: Hypertension, COVID-19, Prognosis, Treatment, Outcomes, RAS inhibition
Introduction
Coronavirus disease 2019 (COVID-19), a systemic disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2), is quickly becoming one of the biggest challenges the global population has faced since World War II [1]. As of May 12, 2020, 4,215,514 cases have been reported with 287,158 confirmed deaths, which represents an overall mortality rate of 6.81% [2••]. It is well known that these numbers are not precise due to the low availability of diagnostic tests needed to assess the true numbers infected [3–5]. In addition, many patients have minor symptoms and may not seek testing [3–4].
Recent reports [6–7] describe several risk factors associated with major complications in COVID-19 such as severe acute respiratory syndrome, mechanical ventilation, and death. Among these risk factors (including older age and several comorbidities), the presence of hypertension has been consistently reported as a marker of worse prognosis in patients with COVID-19 [6,7]. This is a very alarming situation, considering that up to 46% of the adult population is classified as having hypertension [8]. Hypertension was common in patients infected in previous coronavirus and influenza outbreaks [9], but the potential prognostic implications of having hypertension have never been highlighted as they are in the current coronavirus outbreak. However, most of this concern is not related to hypertension per se, but the pathophysiology and mechanism of infection of coronavirus in the cells.
In this brief review, we will discuss three major topics highlighting the current knowledge and uncertainties around the potential role of hypertension in the COVID-19 pandemic.
Does Hypertension Influence Immunity or Angiotensin-Converting Enzyme 2 (ACE2) Expression Favoring Viral Infections?
Growing evidence suggests the potential involvement of innate (natural killer cells and γ/δ T cells) and adaptive (B and T lymphocytes, as well as dendritic and monocyte/macrophages) immune responses in the pathogenesis of hypertension. Vascular inflammation is the most common pathway, favoring arterial remodeling and increased blood pressure [10, 11]. Once activated, the immune system seems to play a significant role in the end-organ damage associated with hypertension [12,13]. Overall, the contribution of the immune system, particularly T-lymphocytes, to hypertension is widely accepted, but the mechanistic processes preceding activation of immune cells are poorly understood [14]. One of the possible mechanisms is related to the increase of sympathetic activation in a complex scenario because sympathoexcitation may induce both suppression or excitatory effects of the T-lymphocytes [14]. Specifically, evidence suggests that increased sympathetic outflow may directly attenuate the ability of naive T-lymphocytes to become fully activated while also exacerbating the inflammatory effects of activated T-lymphocytes [14]. However, the clinical relevance of these findings is still unclear. Despite the use of lymphocyte-targeted immunosuppressant drugs shown to attenuate both experimental [15] and human hypertension [16], the lack of robust and consistent data prevents any current indications for treating hypertension with these therapies [8].
Does the immunology of hypertension fuel a predisposition to infections? To the best of our knowledge, no large prospective cohorts have shown that patients with hypertension are more susceptible to infection diseases or unfavorable outcomes than observed in normotensive patients.
Another critical concern is related to ACE2, a protein that facilitates the entrance of SARS-COV-2 into cells [17,18]. Theoretically, increased ACE2 expression may be potentially harmful for COVID-19 infection. To date, there is no definitive evidence that hypertension is associated with increased ACE2 expression and whether this expression may contribute to poor outcomes in COVID-19 [19]. A recent publication found that ACE2 expression was elevated in the lungs of patients with COVID-19 that presented with comorbidities, such as hypertension, when compared with control participants [20]. However, due to the cross-sectional design, no cause-effect can be determined. While we acknowledge the need for additional research in this area, there is no evidence that hypertension per se predisposes patients with COVID-19 to a poor prognosis.
Are the Risks of Complications in Hypertension Mediated by Its Treatment?
This question has gained a lot of attention in recent weeks because of the potential impact on the management of hypertension. Recent publications reviewed the role of renin-angiotensin system (RAS) inhibitors in COVID-19 infection [.21•, 22•,23] ACE2, an enzyme with a physiological role in RAS activity, plays a crucial role in COVID-19 infection. A recent report postulated that the use of RAS inhibitors, one of the most prescribed classes in hypertension, may alter ACE2 expression and influence the virulence of COVID-19 infection in China [24]. However, there is no definitive evidence indicating harmful effects of RAS inhibitors. Despite RAS inhibitors being recommended as one of the first-line hypertension treatments [8], the use of these drugs in China is quite low, and it was estimated that RAS inhibitors were used in 25–30% of treated patients [25]. This flat rate may have implications for the study power of previous reports. Animal and pre-clinical studies evaluating ACE2 levels of expression in different tissues with the use of RAS inhibitors have demonstrated conflicting data [ 21•, 22•,23]. Angiotensin-converting enzyme inhibitors (ACEi) do not inhibit ACE2 (ACE and ACE2 are different enzymes), making the harmful effect of this class unlikely [22•]. Angiotensin II type 1 receptor blockers (ARB) have been shown to upregulate ACE2 in experimental animals [26,27], but these findings do not seem to translate into clinical observations, including in the setting of COVID-19. One of the first reports in humans evaluated 112 patients with COVID-19 and previous cardiovascular diseases [28]. In this retrospective study from China, the use of RAS inhibitors was not associated with higher morbidity and mortality [28].
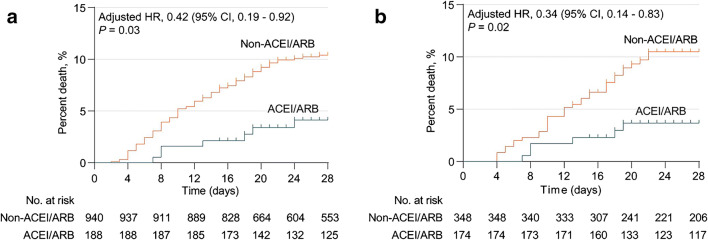
More recently, 6 observational studies shed some light on this issue and called for more definitive research in this area [29•, 30, 31, 32••, 33••, 34••]. A retrospective multicenter analysis of 1128 patients with hypertension diagnosed with COVID-19 compared patients taking an ACEi/ARB (n = 188) with those not taking these drugs (n = 940) [29•]. In the mixed-effect Cox model, treating site as a random effect and after adjusting for age, sex, comorbidities, and in-hospital medications, the detected risk for all-cause mortality was lower in those taking an ACEi/ARB group versus those not taking an ACEi/ARB (Fig. 1a). The results of a propensity score matched analysis performed in a subgroup of patients indicated that use of ACEi/ARBs, compared with other antihypertensive drugs, was also associated with decreased mortality (Fig. 1b). Of note, the percentage of diuretics and beta-blockers used was higher in patients taking ACEi/ARB, which may represent potential residual factors [29•].
Fig. 1.
Kaplan-Meier curves for the cumulative probability of COVID-19 mortality during 28-day follow-up in ACEI/ARB or non-ACEI/ARB cohort among patients with hypertension in a unmatched model and b propensity score matched model. Reproduced with permission from Zhang et al. Circ Res Apr 17:2020 [29•]. doi/10.1161/CIRCRESAHA.120.317134
A retrospective single-center study evaluated 126 patients with COVID-19 patients and preexisting hypertension at Hubei Provincial Hospital of Traditional Chinese Medicine [30]. The patients were allocated to either the ACEi/ARBs group (n = 43) or the non-ACEi/ARBs group (n = 83) according to their antihypertensive medication. A total of 125 age- and sex-matched COVID-19 patients without hypertension were randomly selected as non-hypertension controls. Among patients with COVID-19 and hypertension, those who received either ACEi/ARBs or non-ACEi/ARBs had comparable blood pressure. However, the ACEi/ARB group had significantly lower concentrations of C-reactive protein and procalcitonin compared with the non-ACEi/ARB group, but there were no significant differences in the proportion of critical patients (9.3% vs. 22.9%; p = 0.061) or rates of death (4.7% vs. 13.3%; p = 0.216) [30].
A prospective single center study including 5700 hospitalized patients with COVID-19 in the New York City area showed that the mortality rates for patients with hypertension not taking ACEIs or ARBs, taking ACEIs, and taking ARBs were similar: 26.7%, 32.7%, and 30.6%, respectively [30]. However, as noted by the authors, the results are unadjusted for known confounders, including age, sex, race, ethnicity, socioeconomic status indicators, and comorbidities such as diabetes, chronic kidney disease, and heart failure [31].
Using an observational database from 8910 patients admitted in 169 hospitals in Asia, Europe, and North America, Mehran and colleagues [32••] confirmed previous observations suggesting that underlying cardiovascular disease (but not hypertension) was associated with an increased risk of in-hospital death in patients hospitalized with COVID-19. No increased risk of in-hospital death was found to be associated with the use of ACE inhibitors (2.1% vs. 6.1%; odds ratio, 0.33; 95% CI, 0.20 to 0.54) or the use of ARBs (6.8% vs. 5.7%; odds ratio, 1.23; 95% CI, 0.87 to 1.74).
In a population-based case-control study in the Lombardy region of Italy including 6272 case patients and 30,759 matched beneficiaries of the Regional Health Service (controls) according to sex, age, and municipality of residence, the use of ACE inhibitors and ARBs was more frequent in patients with COVID-19 than in controls because of their higher prevalence of cardiovascular disease. However, there was no evidence that ACE inhibitors or ARBs was associated with the increased risk of COVID-19 [33••].
Finally, Reynolds and colleagues [34••] studied the relation between previous treatment with ACEi, ARBs, beta-blockers, calcium-channel blockers, or thiazide diuretics and the likelihood of a COVID-19 positive test result as well as the infection severity (defined as intensive care, mechanical ventilation, or death) in these patients. Among 12,594 patients who were tested for COVID-19, they found no significant association between five common classes of antihypertensive medications and a positive test for COVID-19 or greater infection severity.
Collectively, the available clinical evidence points to a neutral or even beneficial effect on outcomes in patients with COVID-19 receiving ACEi/ARB, but the observational nature of these investigations prevents any definitive conclusion.
Is Aging a Major Factor Associated with Worse Prognosis in Patients with COVID-19 and Hypertension?
As previously noted [6,7], elderly patients are at high risk for complications related to COVID-19 and are a group for which social distancing has been identified as a top priority. Aging is also a well-established risk factor for developing hypertension [8]. Indeed, there is a progressive increase in the prevalence of hypertension in parallel to age stratification [35]. According to population statistics, the prevalence of hypertension may reach > 60% in the elderly [35]. In this scenario, it is reasonable to expect that hypertension will be a frequent “red flag warning” in the statistics about complications and deaths from COVID-19 [7].
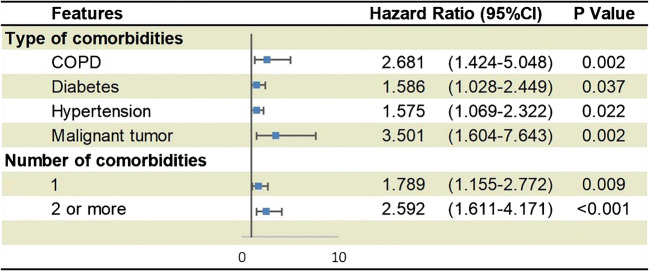
A recent national analysis from China that included 1590 hospitalized patients from 575 hospitals suggested a potential role of hypertension as an important comorbidity in COVID-19 [36]. The authors evaluated the risk of serious adverse outcomes (the composite endpoint consisted of admission to intensive care unit, or invasive ventilation, or death) stratified by comorbidity status. After adjusting for age and smoking status, chronic obstructive pulmonary disease, diabetes, malignancy, and hypertension were risk factors for the composite endpoint. Moreover, the number of comorbidities also predicted outcomes (Fig. 2). However, because comorbidities were self-reported by patients on admission [36], these results should be interpreted with caution. It is conceivable that several patients are not aware of their hypertension status. Supporting this concept, 269 patients reported a medical history of hypertension in this investigation [37], which represents 16.9%, which is lower than indicated by the adult population in China [25]. Indeed, for the mean age of patients in the study (48 years), the prevalence of hypertension in China is 29.6% [25]. Therefore, reports to date have not rigorously accounted for age or other key factors that contribute to health as potential confounders in risk prediction [21•].
Fig. 2.
Predictors of composite endpoints in hospitalized patients with COVID-19 in 575 hospitals in China. Comorbidities were determined based on the patient’s self-reported on admission. Reproduced with permission from Guan et al. Eur Resp J 2020 DOI: 10.1183/13993003.00547-2020. [36] COPD: Chronic obstructive pulmonary disease
Perspectives
The relevance of discussing and investigating whether hypertension is or is not a prognostic marker in the COVID-19 pandemic is clear. From the epidemiologic/economic perspective, it is crucial to stablish the real risk factors for priorities from social distancing regardless of their age even in several strategic areas including healthcare professionals. In this scenario, future investigations addressing the impact of hypertension on prognosis may have significant value when adopting a strict protocol to confirm the hypertension status, medications under chronic use, and associated comorbidities including overweight/obesity (reported by some investigations as a predictor of poor outcomes in COVID-19) [28,37] and chronic kidney and pulmonary disease. We share the opinion that not only age but also other relevant variables should be adjusted for to clarify the potential role of hypertension as an independent risk factor in the COVID-19.
From the treatment perspective, it is important to determine the role of hypertension treatments such as ACEi/ARBs on the susceptibility to viral infections. Particular attention should be devoted to the role of ACE2 overexpression in these patients. In this sense, recombinant ACE2, gene-delivery of ACE2, angiotensin 1–7 analogs, and G-coupled protein receptor of angiotensin- [1–7] (Mas receptor) agonists enhance ACE2 action and may serve as potential target therapies, but relevant studies in humans are lacking [23]. We also need definitive evidence regarding the use of RAS inhibitors during COVID-19 infection. Currently, there are 5 ongoing randomized trials registered with ClinicalTrials.gov aiming to test the impact of ACEi and/or ARBs in patients with COVID-19 (Table 1). Four of them are focusing on the safety issues of these classes in the acute phase (3 studies) and in the chronic scenario (1 study). Interestingly, the PRAETORIAN-COVID trial is testing whether an ARB (valsartan) may be protective in patients with severe COVID-19 without hypertension. The rationale for this study is based on the assumption that during acute lung injury, the alveolar ACE2 appears to be downregulated [38]. ARBs would truly upregulate membrane-bound ACE2, thereby supporting a protective rather than deleterious effect in COVID-19.
Table 1.
Summary of the trials designed to evaluate the impact of ACEi and/or ARB in patients with COVID-19
| ClinicalTrials.gov registration/trial name | Country | Sample size/follow-up | Comparator | Primary endpoint |
|---|---|---|---|---|
| NCT04330300 / CORONACION trial | Ireland | 2414/12 months | Switch BP medications (at physician discretion—usually diuretics/calcium channel blockers) vs. continue ACEi or ARB | Number of COVID-19 positive participants who die, require intubation in ICU, or require hospitalization for non-invasive ventilation |
| NCT04338009 / REPLACECOVID trial | USA | 152/28 days | Discontinuation vs. continuation ACEi/ARBs | Global rank score that ranks: (1) time to death, (2) number of days under mechanical ventilation or ECMO, (3) number of days under renal replacement therapy or pressor/inotropic therapy, and (4) a modified SOFA score |
| NCT04329195 ACORES-2 trial | France | 554/28 days | Discontinuation vs. continuation ACEi/ARBs | Time to clinical improvement (improvement of 2 points on a 7-category ordinal scale or live discharge from the hospital, whichever comes first) |
| NCT04335786 PRAETORIAN-COVID trial | Netherlands | 651/14 days | Valsartan (as a preventive strategy) vs. placebo | First occurrence of intensive care unit admission, mechanical ventilation, or death. |
| NCT04364893 BRACE-CORONA trial | Brazil | 500/30 days | Discontinuation vs. continuation ACEi/ARBs | Days alive and outside the hospital; calculated for each included patient; calculation will be from date of randomization to 30 days post-randomization |
ACEi angiotensin-converting enzyme inhibitors, ARB angiotensin receptor blocker, BP blood pressure, COVID-19 coronavirus disease 2019, ECMO extracorporeal membrane oxygenation, ICU intensive care unit, SOFA Sequential Organ Failure Assessment, USA United States of America
Taken together, the evidence so far is unclear about whether hypertension or its treatment is a real risk factor in COVID-19 infection. Future investigations are necessary to clarify this complex and multifaceted puzzle.
Author Contributions
Study concept and design: Dr. Drager.
Literature review, analysis, and interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: All authors.
Compliance with Ethical Standards
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Resistant Hypertension
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Chakraborty I, Maity P. COVID-19 outbreak: migration, effects on society, global environment and prevention. Sci Total Environ. 2020;728:138882. doi: 10.1016/j.scitotenv.2020.138882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.•• Johns Hopkins Coronavirus Center. https://coronavirus.jhu.edu/data. Very useful tool for checking the world statistics about COVID-19.
- 3.Niehus R, De Salazar PM, Taylor AR, Lipsitch M. Using observational data to quantify bias of traveller-derived COVID-19 prevalence estimates in Wuhan, China. Lancet Infect Dis. 2020. 10.1016/S1473-3099(20)30229-2. [DOI] [PMC free article] [PubMed]
- 4.De Salazar PM, Niehus R, Taylor A, Buckee CO, Lipsitch M. Identifying locations with possible undetected imported severe acute respiratory syndrome coronavirus 2 cases by using importation predictions. Emerg Infect Dis. 2020; Mar 26(7). 10.3201/eid2607.200250 [DOI] [PMC free article] [PubMed]
- 5.Salathé M, Althaus CL, Neher R, Stringhini S, Hodcroft E, Fellay J, Zwahlen M, Senti G, Battegay M, Wilder-Smith A, Eckerle I, Egger M, Low N. COVID-19 epidemic in Switzerland: on the importance of testing, contact tracing and isolation. Swiss Med Wkly. 2020;150:w20225. doi: 10.4414/smw.2020.20225. [DOI] [PubMed] [Google Scholar]
- 6.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, Ji R, Wang H, Wang Y, Zhou Y. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC, Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Sr, Williamson JD, Wright JT., Jr 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. 2018;71(6):e13–e115. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 9.Al-Baadani AM, Elzein FE, Alhemyadi SA, Khan OA, Albenmousa AH, Idrees MM. Characteristics and outcome of viral pneumonia caused by influenza and Middle East respiratory syndrome-coronavirus infections: a 4-year experience from a tertiary care center. Ann Thorac Med. 2019;14:179–185. doi: 10.4103/atm.ATM_179_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norlander AE, Madhur MS, Harrison DG. The immunology of hypertension. J Exp Med. 2018;215(1):21–33. doi: 10.1084/jem.20171773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caillon A, Schiffrin EL. Role of inflammation and immunity in hypertension: recent epidemiological, laboratory, and clinical evidence. Curr Hypertens Rep. 2016;18:21. doi: 10.1007/s11906-016-0628-7. [DOI] [PubMed] [Google Scholar]
- 12.Singh MV, Chapleau MW, Harwani SC, Abboud FM. The immune system and hypertension. Immunol Res. 2014;59(1–3):243–253. doi: 10.1007/s12026-014-8548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schiffrin EL. The immune system: role in hypertension. Can J Cardiol. 2013;29:543–548. doi: 10.1016/j.cjca.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Case AJ, Zimmerman MC. Sympathetic-mediated activation versus suppression of the immune system: consequences for hypertension. J Physiol. 2016;594:527–536. doi: 10.1113/JP271516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodríguez-Iturbe B, Pons H, Quiroz Y, Gordon K, Rincón J, Chávez M, Parra G, Herrera-Acosta J, Gómez-Garre D, Largo R, Egido J, Johnson RJ. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from angiotensin II exposure. Kidney Int. 2001;59(6):2222–2232. doi: 10.1046/j.1523-1755.2001.00737.x. [DOI] [PubMed] [Google Scholar]
- 16.Herrera J, Ferrebuz A, MacGregor EG, Rodriguez-Iturbe B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol. 2006;17(12 Suppl 3):S218–S225. doi: 10.1681/ASN.2006080918. [DOI] [PubMed] [Google Scholar]
- 17.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li W, Zhang C, Sui J, Kuhn JH, Moore MJ, Luo S, Wong SK, Huang IC, Xu K, Vasilieva N, Murakami A, He Y, Marasco WA, Guan Y, Choe H, Farzan M. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J. 2005;24:1634–1643. doi: 10.1038/sj.emboj.7600640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel SK, Velkoska E, Freeman M, Wai B, Lancefield TF, Burrell LM. From gene to protein-experimental and clinical studies of ACE2 in blood pressure control and arterial hypertension. Front Physiol. 2014;5:227. doi: 10.3389/fphys.2014.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinto BGG, Oliveira AER, Singh Y, Jimenez L, Goncalves ANA, Ogava RLT, Creighton R, Peron JPS, Nakaya HI. ACE2 expression is increased in the lungs of patients with comorbidities associated with severe COVID-19. MedRxiv. 2020;03(21):20040261. doi: 10.1093/infdis/jiaa332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.• Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020 Apr 23;382(17):1653–1659. NEJMsr2005760 [published online ahead of print, 2020 Mar 30]. Interesting review discussing the current evidence of renin-angiotensin-aldosterone system inhibitors in patients with COVID-19. [DOI] [PMC free article] [PubMed]
- 22.• Danser AHJ, Epstein M, Batlle D. Renin-angiotensin system blockers and the COVID-19 pandemic: at present there is no evidence to abandon renin-angiotensin system blockers. Hypertension. 2020; Jun;75(6):1382–1385. HYPERTENSIONAHA12015082 [published online ahead of print, 2020 Mar 25]. Interesting review detailing the importance of angiotensin-converting enzyme 2 (ACE2), a protein that facilitates entry of coronavirus and the impact of renin-angiotensin-aldosterone system inhibitors. [DOI] [PMC free article] [PubMed]
- 23.Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, Raizada MK, Grant MB, Oudit GY. Angiotensin converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system. Circ Res. 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4):e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z, Chen Z, Zhang L, Wang X, Hao G, Zhang Z, Shao L, Tian Y, Dong Y, Zheng C, Wang J, Zhu M, Weintraub WS, Gao R. China Hypertension Survey Investigators. Status of hypertension in China: results from the China Hypertension Survey, 2012-2015. Circulation. 2018;137:2344–2356. doi: 10.1161/CIRCULATIONAHA.117.032380. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Ye Y, Gong H, Wu J, Yuan J, Wang S, Yin P, Ding Z, Kang L, Jiang Q, Zhang W, Li Y, Ge J, Zou Y. The effects of different angiotensin II type 1 receptor blockers on the regulation of the ACE-AngII-AT1 and ACE2-Ang(1-7)-Mas axes in pressure overload-induced cardiac remodeling in male mice. J Mol Cell Cardiol. 2016;97:180–190. doi: 10.1016/j.yjmcc.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 27.Soler MJ, Ye M, Wysocki J, William J, Lloveras J, Batlle D. Localization of ACE2 in the renal vasculature: amplification by angiotensin II type 1 receptor blockade using telmisartan. Am J Physiol Renal Physiol. 2009;296:F398–F405. doi: 10.1152/ajprenal.90488.2008. [DOI] [PubMed] [Google Scholar]
- 28.Peng YD, Meng K, Guan HQ, et al. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48(0):E004. [DOI] [PubMed]
- 29.• Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J, et al. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020. 10.1161/CIRCRESAHA.120.317134Provocative retrospective data showing that all-cause mortality was lower in those taking an ACEi/ARB group versus those not taking an ACEi/ARB in patients with COVID-19.
- 30.Yang G, Tan Z, Zhou L, Yang M, Peng L, Liu J, et al. Effects of ARBs and ACEIs on virus infection, inflammatory status and clinical outcomes in COVID-19 patients with hypertension: a single center retrospective study. Hypertension. 2020. 10.1161/HYPERTENSIONAHA.120.15143. [DOI] [PubMed]
- 31.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020. 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed]
- 32.•• Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med. 2020. 10.1056/NEJMoa2007621This observational study from 169 hospitals in Asia, Europe, and North America showed that underlying cardiovascular disease (but not hypertension) was associated with an increased risk of in-hospital death in patients hospitalized with COVID-19.
- 33.•• Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020. 10.1056/NEJMoa2006923The use of ACE inhibitors and ARBs was more frequent in patients with COVID-19 than in controls because of their higher prevalence of cardiovascular disease but no association with increased risk for poor outcome in a population-based case-control study in the Lombardy region. [DOI] [PMC free article] [PubMed]
- 34.•• Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, et al. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N Engl J Med. 2020. 10.1056/NEJMoa2008975Interesting observational data showing that the likelihood of a COVID-19 positive test result as well as the infection severity (defined as intensive care, mechanical ventilation, or death) was not related to any major classes of anti-hypertensive treatment. [DOI] [PMC free article] [PubMed]
- 35.Zhang Y, Moran AE. Trends in the prevalence, awareness, treatment, and control of hypertension among young adults in the United States, 1999 to 2014. Hypertension. 2017;70(4):736–742. doi: 10.1161/HYPERTENSIONAHA.117.09801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, Liu XQ, Chen RC, Tang CL, Wang T, Ou CQ, Li L, Chen PY, Sang L, Wang W, Li JF, Li CC, Ou LM, Cheng B, Xiong S, Ni ZY, Xiang J, Hu Y, Liu L, Shan H, Lei CL, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Cheng LL, Ye F, Li SY, Zheng JP, Zhang NF, Zhong NS, He JX, China Medical Treatment Expert Group for Covid-19 Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring). 2020. 10.1002/oby.22831. [DOI] [PMC free article] [PubMed]
- 38.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, Fukamizu A, Hui CC, Hein L, Uhlig S, Slutsky AS, Jiang C, Penninger JM. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]