Summary
In response to coronavirus disease 2019 (COVID-19), a rapid-cycle in-situ simulation (ISS) programme was developed to facilitate identification and resolution of systems-based latent safety threats. The simulation involved a possible COVID-19 case in respiratory failure, using a mannequin modified to aerosolize phosphorescent secretions. Thirty-six individuals participated in five ISS sessions over 6 weeks, and a further 20 individuals observed these sessions. Debriefing identified latent safety threats from four domains: personnel, personal protective equipment, supply/environment and communication. These threats were addressed and resolved in later iterations. Ninety-four percent of participants felt more prepared to care for a potential case of COVID-19 after the ISS.
Keywords: Simulation, Respiratory infections, COVID-19
Introduction
In early 2020, the World Health Organization declared a public health emergency of international concern in regard to an emerging novel respiratory pathogen, known as severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the cause of coronavirus disease 2019 (COVID-19). SARS-CoV-2 is among the family of zoonotic coronaviruses that includes severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV). While much remains unclear regarding the transmission dynamics of SARS-CoV-2, it does appear that it may spread via aerosolization during procedures [1]. As with previous novel respiratory pathogens, such as SARS-CoV and MERS-CoV, the spread of SARS-CoV-2 is anxiety provoking and tests the agility of healthcare systems to protect both patients and healthcare workers. The first documented case of COVID-19 in Canada created a sense of urgency locally to ensure preparedness for more potential cases [2].
In-situ simulation (ISS) is a process in which simulation exercises are conducted in the clinical workspace by care providers who are on clinical duty using equipment and resources in the workspace [3,4]. Such exercises can aid clinical units in improving team functioning, identifying latent safety threats (LSTs), informing processes, and guiding clinical protocols and care [4,5]. Rapid-cycle simulation has been described previously in medical education as a way to provide real-time feedback and opportunities for learners to practice [6], and there are examples of ISS being used in an iterative fashion to find solutions to LSTs over months to years [7,8]. This article presents a hybrid of these two methods – a rapid-cycle ISS programme – developed for emergency departments (EDs) in the wake of an emerging respiratory pathogen.
The ability of the rapid-cycle ISS programme to uncover and address departmental, organizational and system gaps that present LSTs to both patients and providers in an urgent and time-sensitive fashion was evaluated. Leaders of the physician, nursing and respiratory therapist staff were familiar with the ISS programme at the study institution, and highly supportive of the use of this novel ISS programme to test ED preparedness, particularly as Toronto has historically been a potential area of spread of pathogens from China, as evidenced by the 2003 SARS epidemic [9,10].
Methods
Setting
This study was conducted at an academic, tertiary care academic centre in Toronto, Canada which includes two EDs, collectively seeing over 126,000 patients per year. Simulations were conducted in the ED negative pressure rooms.
Intervention
The ISS team included three physician simulationists, a simulation education specialist, and nurse educators and nurse managers from each site. The case was created using aggregate data from previous febrile respiratory illness outbreaks, namely local data captured from the 2003 SARS outbreak [9].
Given the hospital's SARS experience, the ISS team understood that the highest risk of this emerging respiratory pathogen would be in the case of a critically ill patient presenting to triage and ultimately requiring aerosol-generating procedures in the ED [9,10]. The rapid-cycle ISS programme was created to identify LSTs to staff, and mitigate these with innovative solutions that could subsequently be tested in the next simulation. Although there is no established frequency of simulations to be considered a ‘rapid cycle’, previous iterative ISS studies have been completed on much longer timelines (months to years) [7,8]. This study planned to facilitate one session per week, with time between sessions dedicated to solution generation and implementation.
The case was a febrile, haemodynamically unstable patient in severe respiratory distress, who screened positive at triage for potential COVID-19 exposure based on travel history. A mannequin was modified to aerosolize phosphorescent droplet secretions. At the end of the case, these secretions were visualized on providers using black light. Full details of the case, including the phosphorescent moulage, are published on EMSimCases [11].
Simulation sessions were conducted in standard negative pressure rooms, congruent with institutionally established full personal protective equipment (PPE) (eye protection, N95 mask, gown and gloves) and isolation protocols (airborne, droplet and contact) for a critically ill patient with COVID-19. The simulations were conducted in real-time with clinical staff on duty that day. In accordance with infection control guidelines to minimize team size in potential cases of COVID-19, one physician, one registered nurse and one respiratory therapist participated. Key stakeholders contributed to subsequent formalization and improvement of existing ED processes, including infection prevention and control, infectious diseases, anaesthesia and the ISS team.
Evaluation
Following each session, an ISS physician team member facilitated a 15-min debriefing session. A second ISS team member took notes during the debriefing session, which were utilized to drive solutions between sessions. Each participant completed a brief mixed-methods survey via a Google form. Participants were asked to identify key safety threats, major learning points and outstanding questions via open-ended short answer questions. The survey also included Likert-type questions about perceived preparedness and attitudes towards the ISS programme. Two investigators (EF, KH) independently coded and thematically analysed open-ended survey responses and facilitator debriefing notes to identify emerging themes. These themes were organized by simulation session date to identify persistence and/or resolution of safety threats. The aim was to continue the ISS sessions until no new major modifiable safety threats were identified, and thematic saturation was reached. This quality improvement initiative received an exemption from the institutional research ethics board.
Results
Between 25th January and 5th March 2020, five COVID-19 ISS sessions were completed in a rapid-cycle format as the epidemic was emerging. Thirty-six people participated in the simulation and evaluation, representing a broad team of hospital-wide stakeholders informing guidelines and addressing LSTs that were identified after each simulation. An additional 20 individuals observed the session but did not fully participate in the debrief or complete the survey. Infographics, e-mail notifications, video recording of the simulation and invitations to participate as observers helped broaden the reach of these simulations. Results were also presented at two monthly ED team meetings, as well as at morning safety huddles. After the session on 5th March 2020, investigators reached a consensus that thematic saturation had been achieved, with no new safety threats identified. This coincided with a marked increase in patients in the department requiring isolation precautions, given concerns about imminent community circulation of COVID-19 in Toronto. The first critically ill suspected case of COVID-19 arrived at the study institution less than 1 week after the most recent ISS. The team believed that ISS had prepared them adequately to care for the patient without breaching PPE.
Participants rated the ISS process very positively. Ninety-seven percent of participants agreed that the simulation was relevant to their practice, and 94% felt more prepared to care for a potential case of COVID-19. Only 11% of participants believed that the simulation distracted from patient care.
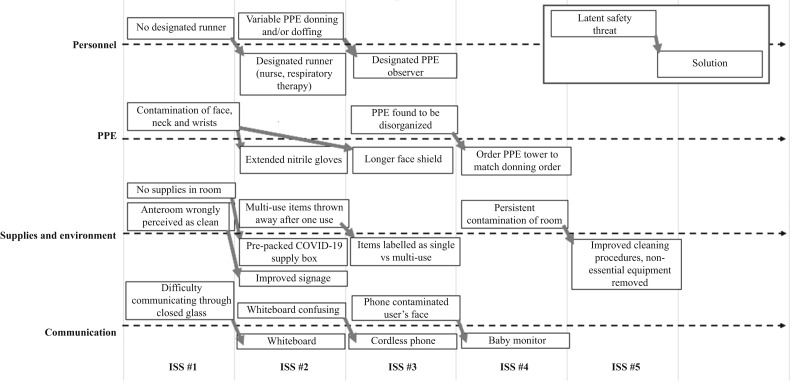
Debriefing identified LSTs from four domains: PPE, personnel, supply/environment and communication. The identified safety threats and iterative solutions are detailed in Figure 1 . When asked separately, the responses to the three open-ended survey questions were highly similar; as such, they were thematically analysed together. The majority of survey results reflected common themes identified in the debriefing sessions. The survey demonstrated that most safety threats were addressed adequately by later iterations, as early issues did not re-emerge in subsequent surveys.
Figure 1.
Iterations of solutions addressing latent safety threats following simulation exercises. PPE, personal protective equipment; COVID-19, coronavirus disease 2019; ISS, in-situ simulation.
PPE
PPE remained a concern for participants across sessions, but evolved from general (e.g. order of donning/doffing) to specific (e.g. wearing shoe covers) over time. Only one respondent remarked explicitly on the emotional response to a novel risk such as COVID-19, yet provider concern about the specifics of personal protection was a dominant theme in debriefing and the survey. After identification, safety threats in the PPE domain were generally resolved with a single session.
Personnel
Survey responses highlighted the specific value of practising in interprofessional teams; for example, by identifying different standard operating procedures between the respiratory therapy group and the nursing staff for the same task. Team-based strategies to address threats were identified in both the debriefings and the survey; for example, pre-selection of a specific staff member to gather any additional supplies for the team inside the isolation room.
Supply/environment
LSTs in this domain were largely resolved with improved signage and preparation of materials [see Appendix 1 (online supplementary material) for materials signage]. The stakeholder analysis for the simulation was an iterative process, and some team members (e.g. housekeeping staff) were invited to participate later in the process, which led to identification of, and solutions for, new safety threats after several sessions had occurred.
Communication
Safety threats in this domain required multiple iterations to address. Several of the solutions trialled posed large new issues; for example, contamination of the user with a portable phone. The team members found that a baby monitor (hands-free communication system) provided sufficient clarity of communication with minimal risk of contamination.
Discussion
ISS allows users to design context- and location-specific scenarios that address specific behaviours (i.e. teamwork, communication), identify system vulnerabilities and implementation challenges, and shape processes of care [3]. This model of rapid-cycle ISS was particularly helpful as it allowed iterative modification of the simulation based on feedback and evolving guidelines, which allowed for incremental improvements over a very short period of time. As new solutions were implemented, the authors were able to quickly disseminate updates to the group via infographics, e-mails, morning departmental safety huddles, and team meetings. As these solutions were trialled in clinical care, it was possible to ascertain feedback from the group to inform further iterative changes in subsequent simulations. The systemization of these changes provides a structure for other sites to navigate changes to their departmental infrastructure and practices. This structure can aid in implementing changes and rationalizing the specific solutions to the care team. Demonstrating how and why these changes have been implemented has aided in the uptake and acceptance of these innovations into clinical practice.
Successes
Personal safety, maintained primarily through proper PPE, was a major theme of this case. The phosphorescent aerosol moulage changed providers' perception of their risk during the resuscitative scenario, and led to high commitment to maintaining infection prevention precautions in the simulated exercise. This high commitment likely uncovered LSTs more quickly than a lower commitment from stakeholders, and very different results may have been obtained without this critical component of the simulation. ISS in a team environment, rather than performing preparedness drills, led to key improvements, particularly in the communication domain. This finding emphasizes the strength and agility of an interprofessional training environment.
Limitations
While the sample size was relatively small, the endpoint of thematic saturation/no major new safety threats identified in the final ISS session was met. Knowledge translation in ISS can be challenging due to the low ratio of participants to overall care providers in the group; however, it was possible to disseminate findings rapidly to stakeholders. Continued sessions may yield new or different results; this was balanced with the relative urgency to share the findings in an expedited fashion. The timing of this ISS was expedited while allowing for procurement of supplies, and within the limitations of scheduling the various facilitators and sessions; however, preparedness for COVID-19 could have been further accelerated and condensed over a shorter time period.
In conclusion, this rapid-cycle ISS programme provides an opportunity to identify and iteratively address LSTs in caring for patients with possible COVID-19 in a time-sensitive fashion. Rapid-cycle ISS is a valuable model to augment departmental preparedness in the wake of emerging epidemics.
Acknowledgements
The authors wish to acknowledge the efforts of Dr Peng Yinhua, who alerted the world to the potential dangers of COVID-19, and ultimately succumbed to the disease. The authors would also like to thank Kathy Bates, Deborah Davies, Elayna Fremes and Camilla Parpia for their assistance in propelling this project forward.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhin.2020.06.020.
Conflict of interest statement
None declared.
Funding sources
None.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Wax R.S., Christian M.D. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anesth Can Anesth. 2020 doi: 10.1007/s12630-020-01591-x. http://link.springer.com/10.1007/s12630-020-01591-x Available at: [last accessed February 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silverstein W.K., Stroud L., Cleghorn G.E., Leis J.A. First imported case of 2019 novel coronavirus in Canada, presenting as mild pneumonia. Lancet. 2020;395:734. doi: 10.1016/S0140-6736(20)30370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrosoniak A., Auerbach M., Wong A.H., Hicks C.M. In situ simulation in emergency medicine: moving beyond the simulation lab: in situ simulation in emergency medicine. Emerg Med Australas. 2017;29:83–88. doi: 10.1111/1742-6723.12705. [DOI] [PubMed] [Google Scholar]
- 4.Cheng A., Grant V., Auerbach M. Using simulation to improve patient safety: dawn of a new era. JAMA Pediatr. 2015;169:419. doi: 10.1001/jamapediatrics.2014.3817. [DOI] [PubMed] [Google Scholar]
- 5.Couto T.B., Barreto J.K.S., Marcon F.C., Mafra A.C.C.N., Accorsi T.A.D. Detecting latent safety threats in an interprofessional training that combines in situ simulation with task training in an emergency department. Adv Simul. 2018;3:23. doi: 10.1186/s41077-018-0083-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taras J., Everett T. Rapid cycle deliberate practice in medical education – a systematic review. Cureus. 2017 Apr 19;9(4):e1180. doi: 10.7759/cureus.1180. http://www.cureus.com/articles/6528-rapid-cycle-deliberate-practice-in-medical-education---a-systematic-review Available at: [last accessed March 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yager P., Collins C., Blais C., O’Connor K., Donovan P., Martinez M. Quality improvement utilizing in-situ simulation for a dual-hospital pediatric code response team. Int J Pediatr Otorhinolaryngol. 2016;88:42–46. doi: 10.1016/j.ijporl.2016.06.026. [DOI] [PubMed] [Google Scholar]
- 8.Barbeito A., Bonifacio A., Holtschneider M., Segall N., Schroeder R., Mark J. In situ simulated cardiac arrest exercises to detect system vulnerabilities. Simul Healthc J Soc Simul Healthc. 2015;10:154–162. doi: 10.1097/SIH.0000000000000087. [DOI] [PubMed] [Google Scholar]
- 9.Basrur S.V., Yaffe B., Henry B. SARS: a local public health perspective. Can J Public Health. 2004;95:22–24. doi: 10.1007/BF03403628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scales D.C., Green K., Chan A.K., Poutanen S.M., Foster D., Nowak K. Illness in intensive care staff after brief exposure to severe acute respiratory syndrome. Emerg Infect Dis. 2003;9:1205–1210. doi: 10.3201/eid0910.030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dharamsi A., Yi S., Hayman K. 2020. Suspected COVID-19.https://emsimcases.com/2020/02/18/suspected-covid-19/ Available at: [last accessed February 2020] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.