Abstract
This cohort study investigates whether MUC16 variation could be a useful biomarker for immune checkpoint inhibitor (ICI) therapy.
Introduction
We and others have demonstrated that patients with cancer with a high tumor variation burden have derived encouraging benefits from immune checkpoint inhibitor (ICI) therapy.1,2 The oncogene MUC16 (OMIM 606154) encodes cancer antigen 125, which has shown robust prognostic ability as well as critical involvement in the regulation of tumor variation burden and immune cellular dysfunction and resistance.3,4 However, there is no current clinical evidence of the association of MUC16 variation with ICI therapy benefit. This comprehensive pancancer cohort study investigates whether MUC16 variation could be a useful biomarker for ICI therapy.
Methods
The study protocol was approved by the ethics committee of the Sun Yat-sen Memorial Hospital of Sun Yat-sen University. The requirement for informed consent of study participants was waived because the human data were obtained from publicly available data sets. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
We collected clinical and MUC16 nonsynonymous variant data of 2129 patients treated with ICI and 10 812 patients treated without ICI from cBioPortal, PubMed, and The Cancer Genome Atlas. The median (interquartile range) follow-up of included patients was 21.0 (10.8-40.0) months. Overall survival (OS) and progression-free survival (PFS) were the primary outcomes, which were computed using the Kaplan Meier method and assessed with the log-rank test. The hazard ratios (HRs) were calculated by the Cox regression model. The tumor variant burden in MUC16 wild-type vs variant tumors were compared with Wilcoxon rank sum tests. All analyses were performed from September 18 to October 1, 2019, using R version 3.4.4 (R Project for Statistical Computing). Statistical significance was set at P < .05, and all tests were 2-tailed.
Results
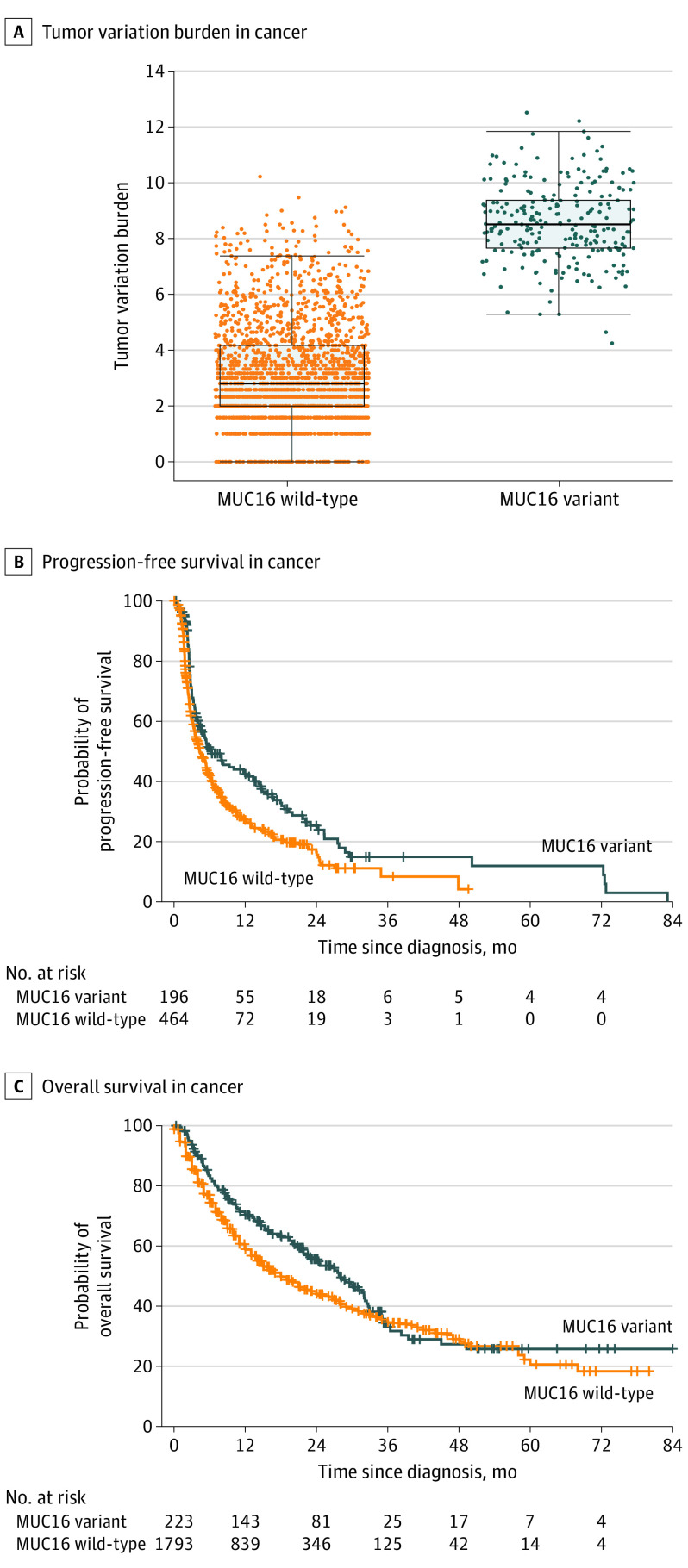
A total of 2129 patients who received ICI therapy were included (252 [11.8%] with MUC16 variant; median [interquartile range] age, 63.0 [54.0-71.0] years; 1255 [58.9%] men), of whom 595 (28.0%) had melanoma, 510 (24.0%) had non–small cell lung cancer (NSCLC), and 1024 (48.1%) had 12 other cancer types. The median (interquartile range) tumor variation burden was significantly higher in the group with MUC16 variant tumors than in the group with MUC16 wild-type tumors (8.5 [7.7-9.4] vs 2.8 [2.0-4.2]; P < .001) (Figure 1A). Compared with patients with MUC16 wild-type tumors, those with MUC16 variant tumors had significantly longer PFS (HR, 0.70; 95% CI, 0.57-0.87; P < .001) (Figure 1B) and OS (HR, 0.79; 95% CI, 0.65-0.96; P = .02) (Figure 1C). However, among 10 812 patients with any cancer (2081 [19.2%] with MUC16 variant) who did not receive ICI therapy, there was no difference in OS between MUC16 variant and wild-type groups (HR, 1.06; 95% CI, 0.97-1.15; P = .19).
Figure 1. Association of MUC16 Variation With Tumor Variant Burden and Survival With Immunotherapy in Pancancer.
A, The dots indicate the level of tumor variant burden for each patient. The box covers the interquartile range, with the upper and lower horizontal lines indicating upper and lower quartiles, respectively. The horizontal line inside the box indicates the median. The ends of the two lines outside the box indicate the highest and lowest data points, and the dots outside of these indicate outliers. B and C, A total of 2129 patients treated with ICI therapy were included in our pancancer analysis, of whom 675 (31.7%) had available data for progression-free survival (B) and 2016 (94.7%) had available data for overall survival (C). The crossing whiskers indicate censoring of data.
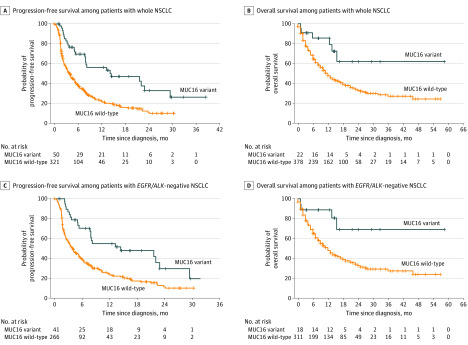
Next, we found the clinical usefulness of MUC16 variant status was most prominent in patients with NSCLC who received ICI therapy. Of 510 patients with NSCLC, 50 (9.8%) had MUC16 variant tumors and, compared with patients with MUC16 wild-type tumors, had higher median (interquartile range) tumor variant burden (8.3 [7.5-9.3] vs 3.2 [2.3-4.4]; P < .001) and longer PFS (HR, 0.42; 95% CI, 0.28-0.64; P < .001) (Figure 2A) and OS (HR, 0.38; 95% CI, 0.17-0.86; P = .02) (Figure 2B). Moreover, among patients with EGFR (OMIM 131550)/ALK (OMIM 105590)–negative NSCLC, MUC16 variation was associated with longer PFS (HR, 0.45; 95% CI, 0.29-0.70; P < .001) (Figure 2C) and OS (HR, 0.30; 95% CI, 0.11-0.80; P = .01) (Figure 2D). We found a significant PFS improvement in MUC16 variant vs wild-type tumors among patients with EGFR or ALK variant NSCLC (HR, 0.32; 95% CI 0.11-0.92; P = .03). However, among patients receiving ICI therapy who had cancers other than NSCLC, we found no significant difference in OS (HR, 0.88; 95% CI, 0.72-1.07; P = .24) or PFS (HR, 1.08; 95% CI, 0.80-1.46; P = .60) between MUC16 variant and wild-type tumors. We also assessed 481 patients with NSCLC (192 [40.0%] with MUC16 variant) who did not receive ICI therapy and found no difference in OS between MUC16 variant and wild-type tumors (HR, 0.94; 95% CI, 0.71-1.25; P = .66).
Figure 2. Association of MUC16 Variant With Survival With Immunotherapy in Non–Small Cell Lung Cancer (NSCLC).
Of 50 patients with NSCLC who were treated with ICI therapy, 22 (44.0%) had available data for overall survival and 50 had available data for progression-free survival. Among 378 patients with NSCLC who had MUC16 wild-type tumors, 321 patients (84.9%) had available data for overall survival and 321 (84.9%) had available data for progression-free survival. The crossing whiskers indicate censoring of data.
Discussion
Using a large data set, we conducted the first study, to our knowledge, to report that MUC16 could be a clinically meaningful biomarker for ICI therapy. The tumor variant burden is an important determinant of tumor antigenicity, and a gene variation is essential if it contributes greatly to affect the whole tumor variation profile.5 We found a significant association of MUC16 variation with elevated tumor variant burden and prolonged PFS and OS during ICI treatment in pancancer and specifically in NSCLC, suggesting that MUC16 might be an important component of the immunogenetic landscape and should be integrated into multiomics for precise selection of patients to receive ICI.
Furthermore, MUC16 could be a potential therapeutic target to enhance ICI treatment outcomes. Adoptive transfer of MUC16-targeted T cells has resulted in powerful antitumor activity in vivo.6 It is conceivable that concurrently targeting MUC16 and ICs might provide synergistic immune response. The study limitations include potential random variability in the context of an exploratory analysis contributed by NSCLC and our inability to clarify the mechanisms underlying the interaction between MUC16 variation and ICI. Future prospective trials with a larger sample size and a longer follow-up period are needed to validate the pancancer applicability of MUC16 variation status and characterize how MUC16 variations interact with the immune system to affect the benefit of ICI therapy.
References
- 1.Yu Y, Zeng D, Ou Q, et al. Association of survival and immune-related biomarkers with immunotherapy in patients with non-small cell lung cancer: a meta-analysis and individual patient-level analysis. JAMA Netw Open. 2019;2(7):e196879. doi: 10.1001/jamanetworkopen.2019.6879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small cell lung cancer. N Engl J Med. 2019;381(21):2020-2031. doi: 10.1056/NEJMoa1910231 [DOI] [PubMed] [Google Scholar]
- 3.Li X, Pasche B, Zhang W, Chen K. Association of MUC16 mutation with tumor mutation load and outcomes in patients with gastric cancer. JAMA Oncol. 2018;4(12):1691-1698. doi: 10.1001/jamaoncol.2018.2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Felder M, Kapur A, Gonzalez-Bosquet J, et al. MUC16 (CA125): tumor biomarker to cancer therapy, a work in progress. Mol Cancer. 2014;13:129. doi: 10.1186/1476-4598-13-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong ZY, Zhong WZ, Zhang XC, et al. Potential predictive value of TP53 and KRAS mutation status for response to PD-1 blockade immunotherapy in lung adenocarcinoma. Clin Cancer Res. 2017;23(12):3012-3024. doi: 10.1158/1078-0432.CCR-16-2554 [DOI] [PubMed] [Google Scholar]
- 6.Chekmasova AA, Rao TD, Nikhamin Y, et al. Successful eradication of established peritoneal ovarian tumors in SCID-Beige mice following adoptive transfer of T cells genetically targeted to the MUC16 antigen. Clin Cancer Res. 2010;16(14):3594-3606. doi: 10.1158/1078-0432.CCR-10-0192 [DOI] [PMC free article] [PubMed] [Google Scholar]