Key Points
Question
Does an 18-month intervention incorporating behavioral counseling, care coordination, and care management reduce cardiovascular risk in adults with serious mental illness, a population at extremely high risk of cardiovascular disease morbidity and mortality?
Findings
In this randomized clinical trial enrolling 269 participants with serious mental illness and at least 1 cardiovascular risk factor, the intervention group participants had a 12.7% relative reduction in the 10-year probability of a cardiovascular event, compared with the control group.
Meaning
These findings support the use of a behavioral counseling, care coordination, and care management intervention to substantially reduce cardiovascular health disparities in this high-risk population.
This randomized clinical trial examines the effectiveness of an 18-month multifaceted intervention incorporating behavioral counseling, care coordination, and care management for overall cardiovascular risk reduction in adults with serious mental illness.
Abstract
Importance
Persons with serious mental illness have a cardiovascular disease mortality rate more than twice that of the overall population. Meaningful cardiovascular risk reduction requires targeted efforts in this population, who often have psychiatric symptoms and cognitive impairment.
Objective
To determine the effectiveness of an 18-month multifaceted intervention incorporating behavioral counseling, care coordination, and care management for overall cardiovascular risk reduction in adults with serious mental illness.
Design, Setting, and Participants
This randomized clinical trial was conducted from December 2013 to November 2018 at 4 community mental health outpatient programs in Maryland. The study recruited adults with at least 1 cardiovascular disease risk factor (hypertension, diabetes, dyslipidemia, current tobacco smoking, and/or overweight or obesity) attending the mental health programs. Of 398 participants screened, 269 were randomized to intervention (132 participants) or control (137 participants). Data collection staff were blinded to group assignment. Data were analyzed on the principle of intention to treat, and data analysis was performed from November 2018 to March 2019.
Interventions
A health coach and nurse provided individually tailored cardiovascular disease risk reduction behavioral counseling, collaborated with physicians to implement appropriate risk factor management, and coordinated with mental health staff to encourage attainment of health goals. Programs offered physical activity classes and received consultation on serving healthier meals; intervention and control participants were exposed to these environmental changes.
Main Outcomes and Measures
The primary outcome was the change in the risk of cardiovascular disease from the global Framingham Risk Score (FRS), which estimates the 10-year probability of a cardiovascular disease event, from baseline to 18 months, expressed as percentage change for intervention compared with control.
Results
Of 269 participants randomized (mean [SD] age, 48.8 [11.9] years; 128 men [47.6%]), 159 (59.1%) had a diagnosis of schizophrenia or schizoaffective disorder, 67 (24.9%) had bipolar disorder, and 38 (14.1%) had major depressive disorder. At 18 months, the primary outcome, FRS, was obtained for 256 participants (95.2%). The mean (SD) baseline FRS was 11.5% (11.5%) (median, 8.6%; interquartile range, 3.9%-16.0%) in the intervention group and 12.7% (12.7%) (median, 9.1%; interquartile range, 4.0%-16.7%) in the control group. At 18 months, the mean (SD) FRS was 9.9% (10.2%) (median, 7.7%; interquartile range, 3.1%-12.0%) in the intervention group and 12.3% (12.0%) (median, 9.7%; interquartile range, 4.0%-15.9%) in the control group. Compared with the control group, the intervention group experienced a 12.7% (95% CI, 2.5%-22.9%; P = .02) relative reduction in FRS at 18 months.
Conclusions and Relevance
An 18-month behavioral counseling, care coordination, and care management intervention statistically significantly reduced overall cardiovascular disease risk in adults with serious mental illness. This intervention provides the means to substantially reduce health disparities in this high-risk population.
Trial Registration
ClinicalTrials.gov Identifier: NCT02127671
Introduction
Persons with serious mental illness, such as schizophrenia, bipolar disorder, and major depressive disorder, encompass a high-risk group, with a cardiovascular mortality rate more than twice that of the overall population and increased prevalence of all modifiable cardiovascular risk factors and behaviors.1,2,3,4,5,6,7,8,9,10 This population urgently needs interventions to address their multiple co-occurring risk factors, including diabetes, hypertension, dyslipidemia, tobacco smoking, and obesity.11 Because persons with serious mental illness often face complex issues, including persistent psychiatric symptoms, executive function impairment, social isolation, poverty, and substance use, tailored health interventions addressing these barriers are needed.12,13,14,15,16
Although behavioral weight loss and tobacco smoking cessation interventions adapted for persons with serious mental illness have shown efficacy in randomized trials,17,18,19,20,21 there is a dearth of evidence on comprehensive programs that target their multiple cardiovascular risk factors. To date, despite increasing interest in integrating physical health care into the mental health settings where many people with serious mental illness receive most of their care,22 studies of interventions in which specialty mental health organizations are responsible for physical health care coordination and management have shown either no or minimal improvements in cardiovascular risk factors.23,24,25,26,27 In contrast, care management and care coordination interventions have shown efficacy in improving cardiovascular risk factors in the overall population.28,29
In 2010, the American Heart Association established strategic goals to decrease cardiovascular disease mortality by 20% and improve overall cardiovascular health to ideal levels by 2020 for all Americans.30 Unless effective interventions are identified and implemented for persons with serious mental illness, it is likely that this population will continue to lag behind the nation in cardiovascular health. The purpose of our trial was to determine the effectiveness of an 18-month comprehensive intervention for overall cardiovascular risk reduction in adults with serious mental illness.
Methods
Study Design
We conducted a randomized clinical trial in 4 outpatient psychiatric rehabilitation programs and affiliated clinics within a large community mental health organization in Maryland. Institutional review boards at Johns Hopkins University and Sheppard Pratt Health System and an independent data safety and monitoring board approved the protocol (Supplement 1). All participants provided written informed consent after receiving a complete description of the study. This trial follows the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Participants
Adults (aged ≥18 years) with hypertension, diabetes, dyslipidemia, current tobacco smoking, and/or overweight or obesity were eligible. Broad eligibility criteria aimed to enroll a population representative of persons with serious mental illness attending community mental health programs. Exclusion criteria were minimal and mainly related to safety (eTable 1 in Supplement 2). Study staff recruited participants through presentations at study sites and referrals from mental health program staff.
Randomization and Masking
Individual participants were randomly assigned to intervention or control groups. The trial statistician computer-generated randomization allocation sequences. Randomization was stratified by study site and sex; assignments were generated with variable block sizes of 4 and 2. The study coordinator received the randomization assignment and communicated the group assignment to each participant. Participants and intervention staff were aware of group assignment; however, data collection staff and investigators were masked until trial end.
Intervention Group
The intervention design drew from behavioral self-management concepts and social cognitive theory.31,32 To promote health behavior change, the intervention team used motivational interviewing and solution-focused therapy.33,34 Sessions were tailored to minimize the impact of memory and executive function deficits by breaking concepts into small components, repeating topics, and modeling and practicing skills.35 The design also incorporated approaches from care coordination and care management.36,37
The multifaceted intervention was delivered by heath coaches and a nurse and consisted of individually tailored cardiovascular risk reduction education and counseling sessions, collaboration with physicians for evidence-based management of cardiovascular health risk, and coordination with mental health staff to advocate for and encourage participants to reach individual cardiovascular health goals (eAppendix in Supplement 2).21,38,39,40,41,42,43,44 Intervention target goals were adapted from the American Heart Association’s Life’s Simple 7.45
Health coaches were based at the community mental health organization where they conducted individual-level coaching sessions. Sessions were 20 to 30 minutes long and were held weekly for the first 6 months and at least every 2 weeks thereafter. For participants with multiple risk factors, the participant and health coach collaboratively identified the primary focus for each session. The health coaches considered the status of each risk factor to guide discussion and used a patient-centered approach to help the participant identify the most impactful behavior change on which to focus between sessions. The health coaches’ location at the community mental health organization provided frequent opportunities for informal contacts, including follow-up with participants on progress toward selected behavioral change targets and communication with mental health program staff to support health goals. The health coaches interacted regularly with the nurse to collaborate on optimal care for each participant’s cardiovascular risk factors.
The nurse met with participants who required cardiovascular risk factor education (eg, diabetes or hypertension) and medication-related counseling. The nurse joined the participant as needed on selected physician visits to advocate for evidence-based treatment and monitoring for diabetes, hypertension, dyslipidemia, and tobacco dependence. The nurse’s interaction with physicians’ and office staff also included sharing information on participant risk factors, facilitating scheduling of and follow-up for appointments and laboratory testing, and communication with other health care practitioners as needed. The health coaches assisted in coordination efforts, including communication with mental health staff and caregivers around participant cardiovascular health.
The intervention supported individual participants’ goals with a point system to reward session attendance and to incentivize recommended behavior change. For example, to encourage smoking cessation, individuals trying to quit received points for reduction in expired carbon monoxide concentration.46 Points were exchanged for small reward items.
Study staff received initial and follow-up training, including regular observation for quality assurance. Implementation of standardized materials and procedures supported intervention fidelity. In addition to the individual-level intervention, we encouraged changes in site environments to support healthy behaviors.
We partnered with the community mental health organization and provided resources and training for them to deliver group physical activity classes for all program attendees, regardless of study enrollment. A study dietician also consulted with program kitchen staff to recommend healthier breakfast and lunch options at sites.
Control Group
As mental health program attendees, control group participants were exposed to the aforementioned environmental changes. They did not receive an individual-level intervention.
Outcomes
The prespecified, primary outcome was the change in the risk of a cardiovascular event from the global Framingham Risk Score47 from baseline to 18 months, expressed as a percentage change for the intervention group compared with the control group. The global Framingham Risk Score estimates the 10-year probability of a cardiovascular disease event (coronary heart disease, cerebrovascular event, peripheral artery disease, or heart failure). The Framingham Risk Score is used in primary care to assess overall cardiovascular risk, providing clinicians with quantitative information to aid in targeted lowering of risk factors.48,49,50 We used relative change in the Framingham Risk Score because it corresponded to the American Heart Association’s goal to reduce cardiovascular mortality by 20%.30 Other study outcomes included changes in the global Framingham Risk Score at 6 months and individual modifiable score components at 6 and 18 months (systolic blood pressure, total cholesterol, high-density lipoprotein, diabetes status, and tobacco smoking status), as well as diastolic blood pressure, fasting blood glucose, glycated hemoglobin A1C, low-density lipoprotein, triglycerides, and body mass index (calculated as weight in kilograms divided by height in meters squared).
Data Collection
Data collection staff performed assessments in person. Blood pressure, body mass index, fasting blood chemical levels, exhaled carbon monoxide, and other study variables including surveillance for safety and adverse events were obtained at baseline and at 6 and 18 months. For tobacco smoking, cessation was determined by self-report and was confirmed by exhaled carbon monoxide level less than 7 ppm.51,52 Sociodemographic characteristics and medication information were obtained from participant self-report and program records, and psychiatric diagnoses were abstracted from program records. Follow-up data collection was completed in November 2018.
Statistical Analysis
Analyses were conducted according to the intention to treat principle. The primary analysis was conducted through a likelihood-based repeated measures mixed-effects regression model with mean of log-transformed global Framingham Risk Score as a function of intervention group assignment, study visit indicators (6 and 18 months, with baseline as reference), and intervention-by-visit interaction terms as the main model predictors, adjusting for sex and study site. A 3-by-3 unstructured variance-covariance matrix was used for the 3 repeated outcome measures per participant. All data were used in the analysis, with any missing data included using the statistical software’s designated missing indicator and treated as missing at random in modeling. The same modeling approach was used to examine the continuous variables for secondary outcomes and to explore the heterogeneity of treatment effects on the primary outcome among prespecified subgroups, including sex, race, cardiovascular risk score at baseline, and psychiatric diagnosis, by incorporating appropriate 2-way and 3-way interaction terms between main model predictors and subgroup indicators. The intervention’s effect on relative change of the American College of Cardiology and American Heart Association Cardiovascular Disease Risk Score53 was also explored using this approach. Generalized estimating equations were used to estimate the population mean treatment effects on binary variables among the risk score components (ie, hypertension medication use, diabetes, and smoking status) over time. Mean models incorporated the same predictors as primary outcome analyses, with statistical inferences based on robust standard errors using the unstructured working correlation. For mixed-effects modeling, t and F tests were used, and Z and χ2 tests were used in generalized estimating equation modeling results. All tests were 2-sided, with P < .05 considered statistically significant. SAS statistical software version 9.4 (SAS Institute) was used for analyses. Data analysis was performed from November 2018 to March 2019. The enrollment target was 250 participants, with assumed 10% loss to follow-up, a baseline global Framingham Risk Score of 11%, and relative risk reduction in the control group of 5%, providing 80% power to detect a 10.7% reduction in the ratio between intervention and control groups of change in relative global Framingham Risk Score at 18 months.
Results
Study Participants
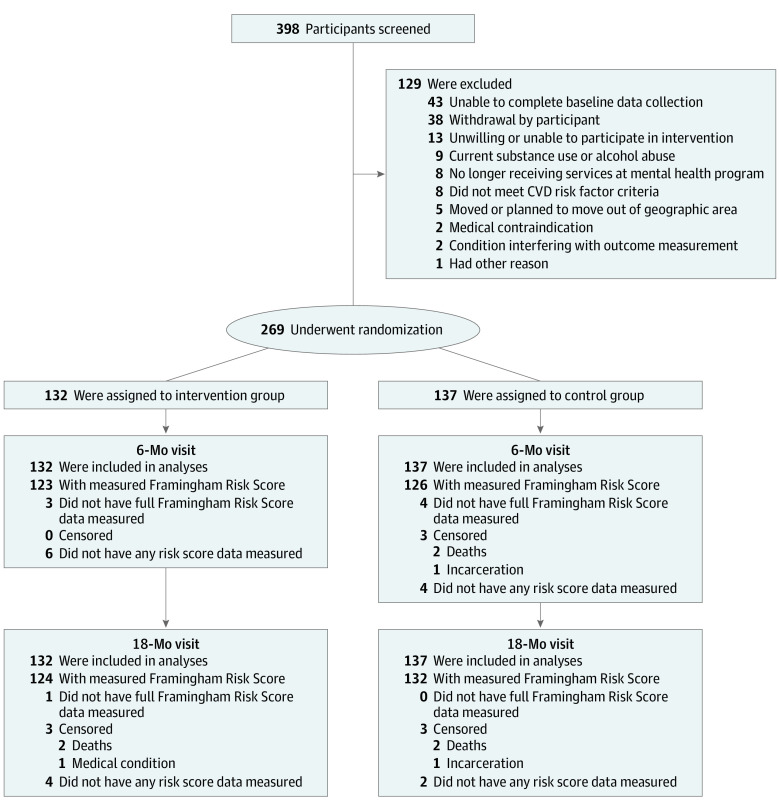
Three hundred ninety-eight participants were screened, and 269 underwent randomization: 132 participants were randomized to the intervention group, and 137 participants were randomized to the control group (Figure 1). The mean (SD) age was 48.8 (11.9) years; 128 participants (47.6%) were men, and 124 participants (46.1%) were black (Table 1). Two hundred thirty-two participants (86.2%) were unable to work or had disability, and 153 (56.9%) lived in supported housing. A total of 159 (59.1%) had schizophrenia or schizoaffective disorder, 67 (24.9%) had bipolar disorder, and 38 (14.1%) had major depressive disorder. The mean (SD) number of psychotropic medications was 3.6 (1.9). Two hundred forty-two participants (90%) had overweight or obesity, 142 (52.8%) had hypertension, 93 (34.6%) had diabetes, 175 (65.1%) had dyslipidemia, and 138 (51.3%) smoked tobacco at baseline, with more than 85% having 2 or more risk factors for cardiovascular disease. Complete measures comprising the global Framingham Risk Score were obtained from 256 participants (95.2%) at 18 months.
Figure 1. Screening, Randomization, and Follow-up of Study Participants.
CVD indicates cardiovascular disease.
Table 1. Baseline Characteristics of the Study Participants.
| Characteristic | Participants, No. (%) | |
|---|---|---|
| Intervention (n = 132) | Control (n = 137) | |
| Age, mean (SD), y | 48.5 (10.8) | 49.1 (12.9) |
| Male | 62 (47.0) | 66 (48.2) |
| Race | ||
| White | 67 (50.8) | 68 (49.6) |
| Black or African American | 61 (46.2) | 63 (46.0) |
| Asian | 1 (0.8) | 4 (2.9) |
| Native Hawaiian or other Pacific Islander | 1 (0.8) | 0 |
| Other race | 2 (1.5) | 2 (1.5) |
| Hispanic or Latino | 2 (1.5) | 4 (2.9) |
| Not high school graduate | 29 (22.0) | 37 (27.0) |
| Never married | 98 (74.2) | 85 (62.0) |
| Lives in residential program or with caregiver | 76 (57.6) | 77 (56.2) |
| Unable to work or receiving disability | 112 (84.9) | 120 (87.6) |
| Health insurance | 130 (98.5) | 135 (98.5) |
| Medicaid | 125 (94.7) | 129 (94.2) |
| Medicare | 68 (51.5) | 67 (48.9) |
| Regular physician | 126 (95.5) | 129 (94.2) |
| Routine physical examination in the past year | 113 (85.6) | 122 (92.4) |
| Psychiatric diagnoses | ||
| Schizophrenia | 40 (30.3) | 41 (29.9) |
| Schizoaffective disorder | 46 (34.9) | 32 (23.4) |
| Bipolar disorder | 25 (18.9) | 42 (30.7) |
| Major depression | 20 (15.2) | 18 (13.1) |
| Other psychotic disorder | 1 (0.8) | 4 (2.9) |
| History of alcohol or other substance use disordera | 69 (52.3) | 69 (50.4) |
| All medications, mean (SD), No. | 9.4 (5.7) | 10.4 (5.4) |
| Psychotropic medications, mean (SD), No. | 3.5 (1.9) | 3.6 (1.8) |
| Antipsychotic | ||
| Any | 106 (80.3) | 114 (83.2) |
| Second generation | 86 (65.2) | 103 (75.2) |
| Clozapine or olanzapine | 29 (22.0) | 34 (24.8) |
| Lithium or mood stabilizer | 68 (51.5) | 82 (59.9) |
| Antidepressant | 86 (65.2) | 88 (64.2) |
| Psychiatric measures, mean (SD), score | ||
| Behavior and Symptom Identification Scale–24b | 1.1 (0.7) | 1.2 (0.7) |
| Center for Epidemiologic Studies Depression Scalec | 20.8 (11.8) | 19.9 (12.0) |
| Cardiovascular risk factors, No.d | ||
| 1 | 14 (10.6) | 20 (14.6) |
| 2 | 37 (28.0) | 23 (16.8) |
| 3 | 39 (29.6) | 47 (34.3) |
| 4 | 33 (25.0) | 34 (24.8) |
| 5 | 9 (6.8) | 13 (9.5) |
Determined according to the Behavior and Symptom Identification Scale–24, the Addiction Severity Index, and diagnoses captured during medical chart abstraction.
Overall summary scores on the Behavior and Symptom Identification Scale–24 range from 0 to 4, with higher scores indicating greater severity of symptoms.
Scores on the Center for Epidemiologic Studies Depression Scale range from 0 to 60, with higher scores indicating more depressive symptoms; a score of 16 points is considered to be a cutoff point for depression.
Includes body mass index (calculated as weight in kilograms divided by height in meters squared) greater than or equal to 25, hypertension, diabetes, dyslipidemia, and tobacco smoking.
Cardiovascular Risk Reduction
The mean (SD) baseline 10-year global Framingham Risk Score (ie, the 10-year probability of a cardiovascular event) was 11.5% (11.5%) (median, 8.6%; interquartile range [IQR], 3.9% to 16.0%) in the intervention group and 12.7% (12.7%) (median, 9.1%; IQR, 4.0% to 16.7%) in the control group (eTable 2 in Supplement 2). At 18 months, cross-sectional estimates of mean (SD) risk scores based on observed data for the intervention and control groups were 9.9% (10.2%) (median, 7.7%; IQR, 3.1% to 12.0%) and 12.3% (12.0%) (median, 9.7%; IQR, 4.0% to 15.9%), respectively. The percentage relative reduction in risk score within the intervention group was 11.2% (95% CI, 3.9% to 18.5%; P = .003) from baseline at 18 months, whereas the percentage relative increase in risk from baseline was 1.4% (95% CI, −8.6% to 5.7%; P = .69) in controls at 18 months, according to mixed-effects analyses.
For the primary study outcome, the net percentage reduction in the 10-year global Framingham Risk Score for the intervention group compared with control at 18 months was 12.7% (95% CI, 2.5%-22.9%; P = .02) (Figure 2; eTable 2 in the Supplement 2), which would correspond to an absolute risk reduction of 1.5% and a number needed to treat of 66 in a population with our 12.1% baseline risk. Analyses with the American College of Cardiology and American Heart Association Risk Score showed similar results with net percentage reduction in 10-year cardiovascular risk of 13.2% (95% CI, 0.5%-25.8%; P = .04) for the intervention compared with control at 18 months (eFigure 1 and eTable 3 in Supplement 2). Results did not differ by subgroups of age, baseline cardiovascular risk, sex, race, and psychiatric diagnosis (all P for interaction >.05; eFigure 2 in Supplement 2).
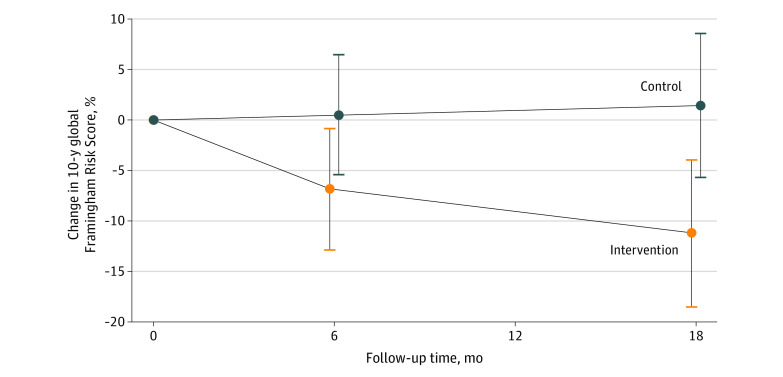
Figure 2. Percentage Change in 10-Year Global Framingham Risk Score Over Time According to Study Group.
The Global Framingham Risk Score reflects the 10-year probability of a cardiovascular event. The numbers of participants in the intervention group were 132 at baseline, 123 at 6 months, and 124 at 18 months. The numbers of participants in the control group were 137 at baseline, 126 at 6 months, and 132 at 18 months. Percentage change estimates (circles) and 95% CIs (error bars) are derived from mixed-effects repeated measures analysis using all available data from all randomized participants. Compared with the control group, the intervention group experienced a mean relative reduction in Framingham Risk Score of 12.7% (95% CI, 2.5%-22.9%; P = .02).
Individual Cardiovascular Risk Factors
Table 2 shows global Framingham Risk Score components and other relevant cardiovascular risk factors. Each risk score component measured in continuous scale (mean [SD] at 18 months in the intervention vs control group: systolic blood pressure, 116.5 [12.9] mm Hg vs 120.2 [16.2] mm Hg; total cholesterol, 171.8 [38.3] mg/dL vs 177.7 [48.7] mg/dL; and high-density lipoprotein levels, 50.7 [14.8] mg/dL vs 51.0 [17.7] mg/dL; to convert cholesterol to millimoles per liter, multiply by 0.0259) and the other continuous risk factor measures had a point estimate favoring the intervention compared with control at 18 months, although between-group differences were not statistically significant. For binary risk score components, absolute changes in prevalence for both diabetes and medication for hypertension also were both nonsignificant for intervention compared with control at 18 months. Tobacco smoking rates declined in the intervention group such that the absolute change in prevalence for those who smoked tobacco at 18 months relative to baseline was −11.81% (95% CI, −18.32% to −5.31%; P = .004) in the intervention group, compared with −1.32% (95% CI, −5.78% to 3.14%; P = .64) in controls, a −10.50% net change in prevalence (95% CI, −18.39% to −2.59%; P = .009) over 18 months. This translates to a 21% relative reduction in smoking prevalence over 18 months (ratio of prevalence ratios [intervention to control] = 0.79; 95% CI, 0.67 to 0.95; P = .01). Exploratory analyses suggest that although smoking cessation was a major contributor to risk reduction at 18 months, changes in blood pressure and lipids also affected overall risk reduction (data not shown).
Table 2. Outcomes for Individual Cardiovascular Risk Factors According to Study Group.
| Outcome | Intervention group | Control group | Between-group difference, mean (95% CI)a | ||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Change, mean (95% CI)a | Mean (SD) | Change, mean (95% CI)a | ||||
| Baseline | 18 mo | Baseline | 18 mo | ||||
| Continuous outcomes | |||||||
| Blood pressure, mm Hg | |||||||
| Systolicb | 118.1 (13.0) | 116.5 (12.9) | −3.9 (−1.5 to 0.8) | 120.1 (14.5) | 120.2 (16.2) | 0.2 (−2.1 to 2.5) | −1.8 (−5.1 to 1.5) |
| Diastolic | 75.3 (8.2) | 73.9 (9.2) | −3.0 (−1.5 to 0.1) | 74.9 (9.3) | 74.7 (11.1) | 0.2 (−1.3 to 1.7) | −1.7 (−3.8 to 0.5) |
| Total cholesterol, mg/dLb | 178.9 (42.1) | 171.8 (38.3) | −7.7 (−14.2 to −1.3) | 181.1 (41.6) | 177.7 (48.7) | −3.4 (−9.7 to 2.9) | −4.3 (−13.3 to 4.7) |
| Low-density lipoprotein, mg/dL | 101.9 (36.8) | 93.7 (33.5) | −8.5 (−13.9 to −3.3) | 101.0 (33.4) | 97.3 (35.9) | −3.2 (−8.4 to 1.9) | −5.4 (−12.7 to 2.0) |
| High-density lipoprotein, mg/dLb | 49.2 (15.3) | 50.7 (14.8) | 1.5 (−0.5 to 3.5) | 50.2 (19.3) | 51.0 (17.7) | 0.5 (−1.4 to 2.4) | 1.0 (−1.8 to 3.8) |
| Trigylcerides, mg/dL | 140.2 (74.3) | 141.0 (76.9) | 0.6 (−14.8 to 16.0) | 156.6 (106.7) | 149.0 (111.1) | −7.5 (−22.5 to 7.6) | 8.1 (−13.4 to 29.6) |
| Fasting glucose, mg/dL | 106.5 (36.7) | 106.7 (45.2) | 0.5 (−6.7 to 7.6) | 110.6 (36.9) | 117.4 (60.0) | 8.1 (1.1 to 15.0) | −7.6 (−17.6 to 2.4) |
| Glycated hemoglobin A1C, % | 6.0 (1.2) | 5.9 (1.3) | −0.1 (−0.3 to 0.1) | 6.3 (1.4) | 6.2 (1.8) | −0.0 (−0.2 to 0.1) | −0.0 (−0.3 to 0.2) |
| Body mass indexc | 34.4 (7.8) | 33.7 (7.7) | −0.8 (−1.3 to −0.2) | 32.9 (6.6) | 32.1 (6.6) | −0.9 (−1.4 to −0.4) | 0.1 (−0.7 to 0.9) |
| Binary outcomes | |||||||
| Medication for hypertension, No. (%)b | 63 (47.7) | 70 (53.0) | 7.99 (1.16 to 14.81)d | 65 (47.5) | 66 (48.2) | −1.87 (−5.04 to 8.80)d | 6.11 (−3.61 to 15.84)e |
| Diabetes, No. (%)b | 42 (31.8) | 46 (34.9) | 4.74 (1.11 to 8.36)d | 51 (37.2) | 60 (43.8) | 8.51 (3.89 to 13.14)d | −3.78 (−9.65 to 2.10)e |
| Tobacco smoking, No. (%)b | 65 (49.2) | 47 (35.6) | −11.81 (−18.32 to −5.31)d | 73 (53.3) | 67 (48.9) | −1.32 (−5.78 to 3.14)d | −10.50 (−18.39 to −2.59)e |
SI conversion factors: to convert cholesterol to millimoles per liter, multiply by 0.0259; glycated hemoglobin A1C to proportion of total hemoglobin, multiply by 0.01; triglycerides to millimoles per liter, multiply by 0.0113; and glucose to millimoles per liter, multiply by 0.0555.
Mixed-effects repeated measures model-based estimates.
Components of the Global Framingham Risk Score.
Body mass index is calculated as weight in kilograms divided by height in meters squared.
Data are absolute change in prevalence, mean (95% CI), calculated with generalized estimating equations–based population-average estimates.
Data are net change in prevalence, mean (95% CI), calculated with generalized estimating equations–based population-average estimates.
Intervention Participation
Table 3 displays the median number of intervention contacts for intervention group participants. Health coach sessions were recommended weekly for the first 6 months and then biweekly for months 7 to 18 (51 sessions); the median number of sessions completed over the 18-month intervention was 38 (IQR, 27-49 sessions). Nurse sessions were conducted as needed with participants who had hypertension, diabetes, dyslipidemia or were interested in pharmacotherapeutic smoking cessation aids with a median of 3.5 sessions (IQR, 2-7 sessions). Among participants for whom the nurse accompanied them to a physician appointment, the median number of appointments was 2 (IQR, 1-3 sessions).
Table 3. Types of Contacts, by Study Period, in the Intervention Group.
| Contact type | Period | ||
|---|---|---|---|
| 1-6 mo | 7-18 mo | 1-18 mo | |
| Health coach behavioral counseling sessionsa | |||
| Participants with contacts, No. | 130 | 123 | 131 |
| Sessions, median (IQR), No. | 20 (14-24) | 20 (10-26) | 38 (27-49) |
| Nurse contactsb | |||
| Nurse-participant sessions | |||
| Participants with contacts, No. | 68 | 50 | 84 |
| Sessions, median (IQR), No. | 3 (2-5.5) | 2 (1-3) | 3.5 (2-7) |
| In-person physician visits with study nurse | |||
| Participants with contacts, No. | 46 | 65 | 79 |
| Visits, median (IQR), No. | 1 (1-2) | 1 (1-3) | 2 (1-3) |
| Study nurse contacts with physician or office staff by telephone, email, or fax | |||
| Participants with contacts, No. | 42 | 95 | 98 |
| Contacts, median (IQR), No. | 1 (1-2) | 2 (1-4) | 3 (1-5) |
Abbreviation: IQR, interquartile range.
Medians and IQRs are based on 132 participants.
Medians and IQRs are based on participants with contacts.
Adverse Events
There were 2 deaths each in the intervention and control groups. No medical events were considered probably or definitely study related. Fifteen percent of intervention participants and 22% of controls reported a medical hospitalization, and 8% of participants in the intervention group and 7% of participants in the control group reported a psychiatric hospitalization (eTable 4 in the Supplement 2).
Discussion
In adults with serious mental illness attending community mental health programs, an 18-month behavioral counseling, care coordination, and care management intervention statistically significantly reduced the estimated 10-year risk of a cardiovascular disease event measured by the global Framingham Risk Score. Rates of tobacco smoking were statistically significantly reduced in the intervention group compared with the control group, and although between-group differences were not statistically significant, the direction of change for blood pressure and lipid risk score components were toward improvement in the intervention group.
The magnitude of estimated overall cardiovascular risk reduction in this trial is substantial and comparable to prior studies in the general population.29,54,55,56,57,58 Our observed relative risk reduction of 12.7% corresponds to an absolute risk reduction of 1.5% in a population with our 12.1% baseline risk. Our study included a broad range of participants with any cardiovascular risk factor, including those with controlled risk factors; in populations with higher baseline risk, we would expect the intervention to produce even greater absolute risk reduction. Still, the level of cardiovascular risk reduction in this trial corresponds to a number needed to treat of 66, which is in range with trials of antihypertensive therapy and primary prevention with statins.59,60,61,62 Exploratory analyses suggest that although smoking cessation was a major contributor to risk reduction at 18 months, changes in blood pressure and lipids also affected overall risk reduction.
In contrast to our findings, care coordination interventions to date have not resulted in cardiovascular risk improvements for persons with serious mental illness, despite a widespread increase in programs incorporating physical health programs into mental health settings.26 In the US, recent studies evaluated behavioral health homes where primary care coordination and management are embedded in specialty mental health settings; of the 2 randomized trials,23,24 1 showed no effects on cardiovascular risk factors, and 1 showed small decreases in risk among those with laboratory data in the intervention group compared with the control group. In Denmark, the CHANGE trial25 tested care coordination plus lifestyle coaching compared with care coordination and control with null results. The low-intensity lifestyle coaching in the studies’ interventions and the Danish health system’s high quality may explain the lack of improvement in cardiovascular risk in previous trials.
Strengths and Limitations
Our trial has several strengths and important features. First, the study enrolled a diverse group of adults with serious mental illness from community-based programs with a range of diagnoses and psychotropic regimens. Despite their young age, participants composed a high-risk group; more than 85% of participants had at least 2 cardiovascular risk factors. Second, we achieved very high follow-up rates for outcome data. Third, the intervention incorporated strategies to address multiple cardiovascular risk factors to facilitate health behavior change. Fourth, having health coaches embedded in the community mental health programs approximated real-world implementation.
Our trial also has limitations. First, the outcome was a risk score rather than actual clinical events. However, risk scores are considered meaningful proxies of clinical outcomes, are feasible in trial settings, and are used to estimate intervention effects in research and clinical practice.29,50,55,56,57,58,63 Second, the Framingham Risk Score has limitations (eg, it was developed in a predominantly white population); however, it was the most widely used score available at trial initiation, and we found similar results using the newer American College of Cardiology and American Heart Association risk score. Third, the trial was designed with power to detect the primary outcome, with only limited power for changes in individual cardiovascular risk factors. Fourth, although the intervention was embedded in a community mental health organization and the intervention team worked closely with mental health staff and practitioners, there was no formal relationship with primary care practitioners, and the intervention team could not prescribe medications. Thus, although the nurse advocated for evidenced-based treatment, it is possible that, in a more formal integrated care structure, the intervention could have resulted in even greater risk reduction.
Conclusions
Results from our trial support the use of a tailored intervention to address cardiovascular risk factors, embedded in routine outpatient specialty mental health care for adults with serious mental illness. In the US, through the Affordable Care Act Medicaid Health Home Waiver, behavioral health homes placing primary care coordination and management in specialty mental health settings have been implemented in 17 states and the District of Columbia.26 These health homes or similar integrated care programs could be a vehicle for implementing the intervention. Organizations may choose to target persons with serious mental illness with higher baseline cardiovascular risk.
These findings show that a behavioral counseling, care coordination, and care management intervention embedded in routine outpatient specialty mental health care can significantly reduce overall cardiovascular risk in adults with serious mental illness. This intervention provides the means to substantially reduce health disparities in this high-risk population.
Trial Protocol
eAppendix. Synopsis of Intervention
eTable 1. Trial Enrollment Criteria
eTable 2. Global Framingham Risk Score by Randomized Group at Each Visit (Cross-Sectional) and Model-Based Percent Longitudinal Change of Global Framingham Risk Score Over Time
eFigure 1. Percent Change in ACC/AHA Risk Score Over Time According to Study Group
eTable 3. ACC/AHA Risk Score by Randomized Group at Each Visit and Model-Based Percent Longitudinal Change of ACC/AHA Risk Score Over Time
eFigure 2. Model-Based Percent Change in Global Framingham Risk Score by Subgroups of Sex, Race, Cardiovascular Risk at Baseline, and Psychiatric Diagnosis
eTable 4. Overnight Hospitalizations, Deaths and Medical Events for Intervention and Control Participants
Data Sharing Statement
References
- 1.Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123-1131. doi: 10.1001/archpsyc.64.10.1123 [DOI] [PubMed] [Google Scholar]
- 2.Goff DC, Sullivan LM, McEvoy JP, et al. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res. 2005;80(1):45-53. doi: 10.1016/j.schres.2005.08.010 [DOI] [PubMed] [Google Scholar]
- 3.Olfson M, Gerhard T, Huang C, Crystal S, Stroup TS. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. 2015;72(12):1172-1181. doi: 10.1001/jamapsychiatry.2015.1737 [DOI] [PubMed] [Google Scholar]
- 4.Correll CU, Druss BG, Lombardo I, et al. Findings of a U.S. national cardiometabolic screening program among 10,084 psychiatric outpatients. Psychiatr Serv. 2010;61(9):892-898. doi: 10.1176/ps.2010.61.9.892 [DOI] [PubMed] [Google Scholar]
- 5.De Hert M, van Winkel R, Van Eyck D, et al. Prevalence of diabetes, metabolic syndrome and metabolic abnormalities in schizophrenia over the course of the illness: a cross-sectional study. Clin Pract Epidemiol Ment Health. 2006;2:14. doi: 10.1186/1745-0179-2-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298(15):1794-1796. doi: 10.1001/jama.298.15.1794 [DOI] [PubMed] [Google Scholar]
- 7.McClave AK, McKnight-Eily LR, Davis SP, Dube SR. Smoking characteristics of adults with selected lifetime mental illnesses: results from the 2007 National Health Interview Survey. Am J Public Health. 2010;100(12):2464-2472. doi: 10.2105/AJPH.2009.188136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lasser KE. Update in smoking and mental illness: a primary care perspective. J Dual Diagn. 2009;5(2):191-196. doi: 10.1080/15504260902869121 [DOI] [Google Scholar]
- 9.Ziedonis D, Hitsman B, Beckham JC, et al. Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report. Nicotine Tob Res. 2008;10(12):1691-1715. doi: 10.1080/14622200802443569 [DOI] [PubMed] [Google Scholar]
- 10.Casagrande SS, Anderson CAM, Dalcin A, et al. Dietary intake of adults with serious mental illness. Psychiatr Rehabil J. 2011;35(2):137-140. doi: 10.2975/35.2.2011.137.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu NH, Daumit GL, Dua T, et al. Excess mortality in persons with severe mental disorders: a multilevel intervention framework and priorities for clinical practice, policy and research agendas. World Psychiatry. 2017;16(1):30-40. doi: 10.1002/wps.20384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mueser KT, McGurk SR. Schizophrenia. Lancet. 2004;363(9426):2063-2072. doi: 10.1016/S0140-6736(04)16458-1 [DOI] [PubMed] [Google Scholar]
- 13.Dickerson FB, Brown CH, Daumit GL, et al. Health status of individuals with serious mental illness. Schizophr Bull. 2006;32(3):584-589. doi: 10.1093/schbul/sbj048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spivak S, Cullen B, Eaton WW, Rodriguez K, Mojtabai R. Financial hardship among individuals with serious mental illness. Psychiatry Res. 2019;282:112632. doi: 10.1016/j.psychres.2019.112632 [DOI] [PubMed] [Google Scholar]
- 15.Olfson M, Mechanic D, Hansell S, Boyer CA, Walkup J. Prediction of homelessness within three months of discharge among inpatients with schizophrenia. Psychiatr Serv. 1999;50(5):667-673. doi: 10.1176/ps.50.5.667 [DOI] [PubMed] [Google Scholar]
- 16.Perkins R, Rinaldi M. Unemployment rates among patients with long-term mental health problems: a decade of rising unemployment. Psychiatr Bull. 2002;26(8):295-298. doi: 10.1192/pb.26.8.295 [DOI] [Google Scholar]
- 17.Daumit GL, Dickerson FB, Wang NY, et al. A behavioral weight-loss intervention in persons with serious mental illness. N Engl J Med. 2013;368(17):1594-1602. doi: 10.1056/NEJMoa1214530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green CA, Yarborough BJ, Leo MC, et al. The STRIDE weight loss and lifestyle intervention for individuals taking antipsychotic medications: a randomized trial. Am J Psychiatry. 2015;172(1):71-81. doi: 10.1176/appi.ajp.2014.14020173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartels SJ, Pratt SI, Aschbrenner KA, et al. Clinically significant improved fitness and weight loss among overweight persons with serious mental illness. Psychiatr Serv. 2013;64(8):729-736. doi: 10.1176/appi.ps.003622012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520. doi: 10.1016/S0140-6736(16)30272-0 [DOI] [PubMed] [Google Scholar]
- 21.Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311(2):145-154. doi: 10.1001/jama.2013.285113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horvitz-Lennon M, Kilbourne AM, Pincus HA. From silos to bridges: meeting the general health care needs of adults with severe mental illnesses. Health Aff (Millwood). 2006;25(3):659-669. doi: 10.1377/hlthaff.25.3.659 [DOI] [PubMed] [Google Scholar]
- 23.Druss BG, von Esenwein SA, Glick GE, et al. Randomized trial of an integrated behavioral health home: the Health Outcomes Management and Evaluation (HOME) study. Am J Psychiatry. 2017;174(3):246-255. doi: 10.1176/appi.ajp.2016.16050507 [DOI] [PubMed] [Google Scholar]
- 24.Druss BG, von Esenwein SA, Compton MT, Rask KJ, Zhao L, Parker RM. A randomized trial of medical care management for community mental health settings: the Primary Care Access, Referral, and Evaluation (PCARE) study. Am J Psychiatry. 2010;167(2):151-159. doi: 10.1176/appi.ajp.2009.09050691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Speyer H, Christian Brix Nørgaard H, Birk M, et al. The CHANGE trial: no superiority of lifestyle coaching plus care coordination plus treatment as usual compared to treatment as usual alone in reducing risk of cardiovascular disease in adults with schizophrenia spectrum disorders and abdominal obesity. World Psychiatry. 2016;15(2):155-165. doi: 10.1002/wps.20318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy KA, Daumit GL, Stone E, McGinty EE. Physical health outcomes and implementation of behavioural health homes: a comprehensive review. Int Rev Psychiatry. 2018;30(6):224-241. doi: 10.1080/09540261.2018.1555153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scharf DM, Schmidt Hackbarth N, Eberhart NK, et al. General medical outcomes from the primary and behavioral health care integration grant program. Psychiatr Serv. 2016;67(11):1226-1232. doi: 10.1176/appi.ps.201500352 [DOI] [PubMed] [Google Scholar]
- 28.Haskell WL, Berra K, Arias E, et al. Multifactor cardiovascular disease risk reduction in medically underserved, high-risk patients. Am J Cardiol. 2006;98(11):1472-1479. doi: 10.1016/j.amjcard.2006.06.049 [DOI] [PubMed] [Google Scholar]
- 29.Ma J, Berra K, Haskell WL, et al. Case management to reduce risk of cardiovascular disease in a county health care system. Arch Intern Med. 2009;169(21):1988-1995. doi: 10.1001/archinternmed.2009.381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lloyd-Jones DM, Hong Y, Labarthe D, et al. ; American Heart Association Strategic Planning Task Force and Statistics Committee . Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586-613. doi: 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 31.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Prentice-Hall; 1986. [Google Scholar]
- 32.Watson DL, Tharp RG. Self-Directed Behavior: Self-Modification for Personal Adjustment. 5th ed Brooks/Cole Publishing Co; 1989. [Google Scholar]
- 33.McCracken S, Corrigan P. Motivational interviewing for medication adherence in individuals with schizophrenia In: Arkowitz H, Westra H, Miller W, Rollnick S, eds. Motivational Interviewing in the Treatment of Psychological Problems. Guilford Press; 2007:249-276. [Google Scholar]
- 34.Gingerich WJ, Eisengart S. Solution-focused brief therapy: a review of the outcome research. Fam Process. 2000;39(4):477-498. doi: 10.1111/j.1545-5300.2000.39408.x [DOI] [PubMed] [Google Scholar]
- 35.Velligan DI, Bow-Thomas CC, Huntzinger C, et al. Randomized controlled trial of the use of compensatory strategies to enhance adaptive functioning in outpatients with schizophrenia. Am J Psychiatry. 2000;157(8):1317-1323. doi: 10.1176/appi.ajp.157.8.1317 [DOI] [PubMed] [Google Scholar]
- 36.Agency for Healthcare Research and Quality Care management: implications for medical practice, health policy, and health services research. Published April 2015. Accessed August 20, 2018. https://www.ahrq.gov/professionals/prevention-chronic-care/improve/coordination/caremanagement/index.html
- 37.Agency for Health Care Research and Quality Care coordination. Published June 2014. Accessed August 20, 2018. https://www.ahrq.gov/ncepcr/care/coordination.html
- 38.Dalcin AT, Jerome GJ, Appel LJ, et al. Need for cardiovascular risk reduction in persons with serious mental illness: design of a comprehensive intervention. Front Psychiatry. 2019;9:786. doi: 10.3389/fpsyt.2018.00786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143-3421. doi: 10.1161/circ.106.25.3143 [DOI] [PubMed] [Google Scholar]
- 40.Stone NJ, Robinson JG, Lichtenstein AH, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25)(suppl 2):S1-S45. doi: 10.1161/01.cir.0000437738.63853.7a [DOI] [PubMed] [Google Scholar]
- 41.Chobanian AV, Bakris GL, Black HR, et al. ; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee . The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2572. doi: 10.1001/jama.289.19.2560 [DOI] [PubMed] [Google Scholar]
- 42.Buchanan RW, Kreyenbuhl J, Kelly DL, et al. ; Schizophrenia Patient Outcomes Research Team (PORT) . The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71-93. doi: 10.1093/schbul/sbp116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.American Diabetes Association Standards of medical care in diabetes: 2013. Diabetes Care. 2013;36(1)(suppl):S11-S66. doi: 10.2337/dc13-S011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313(7):687-694. doi: 10.1001/jama.2015.280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.American Heart Association My life check: life's simple 7. Published 2018. Accessed May 14, 2020. https://www.heart.org/en/healthy-living/healthy-lifestyle/my-life-check--lifes-simple-7
- 46.Heinssen RK. The cognitive exoskeleton: environmental interventions in cognitive rehabilitation In: Corrigan PW, Yudofsky SC, eds. Cognitive Rehabilitation for Neuropsychiatric disorders. American Psychiatric Press; 1996:395-423. [Google Scholar]
- 47.D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743-753. doi: 10.1161/CIRCULATIONAHA.107.699579 [DOI] [PubMed] [Google Scholar]
- 48.Sheridan SL, Viera AJ, Krantz MJ, et al. ; Cardiovascular Health Intervention Research and Translation Network Work Group on Global Coronary Heart Disease Risk . The effect of giving global coronary risk information to adults: a systematic review. Arch Intern Med. 2010;170(3):230-239. doi: 10.1001/archinternmed.2009.516 [DOI] [PubMed] [Google Scholar]
- 49.Shillinglaw B, Viera AJ, Edwards T, Simpson R, Sheridan SL. Use of global coronary heart disease risk assessment in practice: a cross-sectional survey of a sample of U.S. physicians. BMC Health Serv Res. 2012;12:20. doi: 10.1186/1472-6963-12-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kannel WB, D’Agostino RB, Sullivan L, Wilson PW. Concept and usefulness of cardiovascular risk profiles. Am Heart J. 2004;148(1):16-26. doi: 10.1016/j.ahj.2003.10.022 [DOI] [PubMed] [Google Scholar]
- 51.SRNT Subcommittee on Biochemical Verification Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149-159. doi: 10.1080/14622200210123581 [DOI] [PubMed] [Google Scholar]
- 52.Karelitz JL, Michael VC, Perkins KA. Analysis of agreement between expired-air carbon monoxide monitors. J Smok Cessat. 2017;12(2):105-112. doi: 10.1017/jsc.2015.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25)(suppl 2):S49-S73. doi: 10.1161/01.cir.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- 54.Chen ST, Maruthur NM, Appel LJ. The effect of dietary patterns on estimated coronary heart disease risk: results from the Dietary Approaches to Stop Hypertension (DASH) trial. Circ Cardiovasc Qual Outcomes. 2010;3(5):484-489. doi: 10.1161/CIRCOUTCOMES.109.930685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maruthur NM, Wang NY, Appel LJ. Lifestyle interventions reduce coronary heart disease risk: results from the PREMIER Trial. Circulation. 2009;119(15):2026-2031. doi: 10.1161/CIRCULATIONAHA.108.809491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lönnberg L, Ekblom-Bak E, Damberg M. Reduced 10-year risk of developing cardiovascular disease after participating in a lifestyle programme in primary care. Ups J Med Sci. Published online February 20, 2020. doi: 10.1080/03009734.2020.1726533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Egan BM, Li J, Fleming DO, et al. Impact of implementing the 2013 ACC/AHA cholesterol guidelines on vascular events in a statewide community-based practice registry. J Clin Hypertens (Greenwich). 2016;18(7):663-671. doi: 10.1111/jch.12727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwalm JD, McCready T, Lopez-Jaramillo P, et al. A community-based comprehensive intervention to reduce cardiovascular risk in hypertension (HOPE 4): a cluster-randomised controlled trial. Lancet. 2019;394(10205):1231-1242. doi: 10.1016/S0140-6736(19)31949-X [DOI] [PubMed] [Google Scholar]
- 59.Wright JM, Musini VM. First-line drugs for hypertension. Cochrane Database Syst Rev. 2009;(3):CD001841. doi: 10.1002/14651858.CD001841.pub2 [DOI] [PubMed] [Google Scholar]
- 60.Rossignol M, Labrecque M, Cauchon M, Breton MC, Poirier P. Number of patients needed to prescribe statins in primary cardiovascular prevention: mirage and reality. Fam Pract. 2018;35(4):376-382. doi: 10.1093/fampra/cmx124 [DOI] [PubMed] [Google Scholar]
- 61.Mortensen MB, Nordestgaard BG. Statin use in primary prevention of atherosclerotic cardiovascular disease according to 5 major guidelines for sensitivity, specificity, and number needed to treat. JAMA Cardiol. 2019;4(11):1131-1138. doi: 10.1001/jamacardio.2019.3665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pearce KA, Furberg CD, Psaty BM, Kirk J. Cost-minimization and the number needed to treat in uncomplicated hypertension. Am J Hypertens. 1998;11(5):618-629. doi: 10.1016/S0895-7061(97)00488-3 [DOI] [PubMed] [Google Scholar]
- 63.Enserro DM, Vasan RS, Xanthakis V. Twenty-year trends in the American Heart Association cardiovascular health score and impact on subclinical and clinical cardiovascular disease: the Framingham Offspring Study. J Am Heart Assoc. 2018;7(11):e008741. doi: 10.1161/JAHA.118.008741 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix. Synopsis of Intervention
eTable 1. Trial Enrollment Criteria
eTable 2. Global Framingham Risk Score by Randomized Group at Each Visit (Cross-Sectional) and Model-Based Percent Longitudinal Change of Global Framingham Risk Score Over Time
eFigure 1. Percent Change in ACC/AHA Risk Score Over Time According to Study Group
eTable 3. ACC/AHA Risk Score by Randomized Group at Each Visit and Model-Based Percent Longitudinal Change of ACC/AHA Risk Score Over Time
eFigure 2. Model-Based Percent Change in Global Framingham Risk Score by Subgroups of Sex, Race, Cardiovascular Risk at Baseline, and Psychiatric Diagnosis
eTable 4. Overnight Hospitalizations, Deaths and Medical Events for Intervention and Control Participants
Data Sharing Statement