Abstract
Introduction:
Camptocormia is defined as an involuntary, marked flexion of the thoracolumbar spine appearing during standing or walking and resolving in the supine position or when leaning against a wall. However, there is no established agreement on the minimum degree of forward flexion needed to diagnose camptocormia. Likewise, the current definition does not categorize camptocormia on the basis of the bending fulcrum.
Methods:
We performed a survey among movement disorders experts to identify camptocormia using images of patients with variable degrees and types of forward trunk flexion by fulcrum (upper and lower fulcra). We tested the subsequently generated diagnostic criteria in a sample of 131 consecutive patients referred for evaluation of postural abnormalities.
Results:
Experts reached full consensus on lower camptocormia (L1-Sacrum, hip flexion) with a bending angle ≥30° and upper camptocormia (C7 to T12-L1) with a bending angle ≥45°. This definition detected camptocormia in 9/131 consecutive PD patients (2 upper/7 lower) but excluded camptocormia in 71 patients considered to have camptocormia by the referring neurologist.
Conclusions:
Camptocormia can be defined as “an involuntary flexion of the spine appearing during standing or walking and resolving in the supine position of at least 30° at the lumbar fulcrum (LI-Sacrum, hip flexion, i.e. lower camptocormia) and/or at least 45° at the thoracic fulcrum (C7 to T12-L1, i.e. upper camptocormia)”. Strict criteria for camptocormia are met by 7% of patients with abnormal posture. The ascertainment of upper and lower camptocormia subtypes could improve the validity of epidemiological studies and assist future therapeutic trials.
Keywords: Camptocormia, Bent spine syndrome, Stooped posture, Postural abnormalities, Back pain
1. Introduction
Camptocormia is one of the most known and disabling postural abnormalities in patients with Parkinson’s disease (PD) or atypical parkinsonism [1]. Camptocormia is defined as an involuntary, marked flexion of the thoracolumbar spine appearing during standing or walking and resolving in the supine position or when leaning against a wall [1,2]. There is no established agreement on the minimum degree of forward flexion needed to diagnose camptocormia. The lack of diagnostic criteria for camptocormia may explain the high variability in prevalence figures reported in the literature, ranging from 3 to 17.6% in largely unselected cohorts [3–9]. Most studies have based the diagnosis of camptocormia on the angle of forward bending, with cut-off values ranging between 15 and 45° [3–7,10–16]. In addition, the term camptocormia is often incorrectly used in clinical settings to denote a simple stooped posture in PD [17].
An additional issue is the fulcrum of trunk flexion as the term “thoracolumbar” does not provide a useful anchor to define camptocormia. Some authors have sub-classified camptocormia into upper and lower types depending on the fulcrum of the bending angle: at a point between the lower thoracic and upper lumbar spine (upper fulcrum), or at the hip joint (lower fulcrum) [18–20]. Although this classification can be useful for the understanding of the pathophysiology and management of camptocormia (e.g., to help determine the most overactive muscles), the differences – if any – between upper and lower types remain unclear [2].
Future epidemiological and intervention studies would benefit greatly from having clear criteria for the presence or absence of camptocormia. Therefore, we performed a two-phase study, first establishing a consensus-based definition of camptocormia, and then testing this in a large sample of consecutive PD patients.
2. Methods
First, we performed a survey among eight movement disorders experts by showing the lateral view of 11 PD patients with a variable combination of trunk flexion (either upper or lower fulcrum, and with variable bending degrees for each). All patients presented a flexion of the thoracolumbar spine appearing during standing or walking and resolving in the supine position, no patient had other causes for postural abnormalities, e.g. musculoskeletal disorders. Each expert – blind to others’ diagnoses – was invited to make a diagnosis (yes/no) of camptocormia. Diagnoses with complete agreement among experts were taken into account to draft a definition of camptocormia, which was later shared and discussed by email with experts and remaining authors till a definition satisfactory to all was achieved.
Second, we tested the generated proposed criteria in a cohort of 131 PD patients enrolled consecutively (73 men, 58 women, mean age: 71.0 ± 8.2 years; mean disease duration: 9.4 ± 5.2 years) who were referred to our attention for the evaluation of postural abnormalities.
PD was diagnosed according with the United Kingdom Parkinson’s Disease Society Brain Bank criteria [21]. Exclusion criteria were as follows: 1) concomitant neurologic disease known to affect posture; 2) a history of major spinal surgery or muscle and/or skeletal diseases; 3) treatment with drugs with potential capacity to induce postural deformities (neuroleptics other than clozapine or quetiapine and antiemetics with the exception of domperidone) in the 6 months before enrolment; and 4) clinical characteristics consistent with a diagnosis of atypical parkinsonism [22]. The study was approved by the local ethical committee (Verona, Italy), and all participants signed an informed consent form before participation.
All patients underwent a systematic evaluation in a single session and were evaluated on their usual drug treatment, during the on-medication phase (Table 1). Trunk deviation was measured by means of a wall goniometer and expressed in degrees in keeping with methodology previously published [23]. Upper and lower camptocormia was measured according to the clinical method used by Furusawa et al. using C7, fulcrum of trunk deviation and the vertical line for the upper camptocormia and C7, sacrum and the vertical line for lower camptocormia (Fig. 1A–B) [19].
Table 1.
Comparison of the demographic and clinical features of 131 PD patients with camptocormia, FTB and NP (significant differences are bold-typed).
| Camptocormia (N = 9) |
FTB (N = 71) |
NP (N = 51) |
Camptocormia vs. FTB (P Value) |
Camptocormia vs. NP (P Value) |
FTB vs. NP (P Value) |
|
|---|---|---|---|---|---|---|
| Male/Female | 5 (55.6)/4 (44.4) | 39 (54.9)/32 (45.1) | 29 (56.9)/22 (43.1) | 1.000 | 1.000 | .832 |
| Age | 74.7 ± 2.9 | 71.8 ± 7.1 | 69.3 ± 9.9 | .034 | .050 | .155 |
| Body Mass Index | 24.6 ± 2.8 | 25.5 ± 4.0 | 25.8 ± 3.9 | .802 | .404 | .271 |
| Age at PD onset | 66.7 ± 4.5 | 61.7 ± 9.1 | 60.8 ± 11.3 | .013 | .011 | .645 |
| Disease Duration | 8 ± 4.6 | 10.2 ± 5.5 | 8.4 ± 4.8 | .258 | .884 | .066 |
| H&Y stage | 2.9 ± 0.7 | 2.7 ± 0.8 | 2.3 ± 0.7 | .326 | .013 | .002 |
| Right-side onset | 3 (33.3) | 25 (35.2) | 27 (52.9) | 1.000 | .472 | .051 |
| Left-side onset | 2 (22.2) | 32 (45.1) | 17 (33.4) | .288 | .705 | .192 |
| Bilateral onset | 4 (44.5) | 14 (19.7) | 7 (13.7) | .109 | .050 | .387 |
| Tremor typea | 2 (22.2) | 23 (32.4) | 26 (51) | .712 | .155 | .039 |
| Rigid-akinetic typea | 7 (77.8) | 29 (40.8) | 20 (39.2) | .071 | .065 | .856 |
| Mixed typea | 0 | 19 (26.8) | 5 (9.8) | .106 | 1.000 | .020 |
| Clinical asymmetryb | 7 (77.8) | 32 (45.1) | 30 (58.8) | .084 | .460 | .134 |
| UPDRS Ic | 2.9 ± 1.0 | 3.3 ± 2.3 | 2.8 ± 2.8 | .824 | .456 | .293 |
| UPDRS IIc | 19.7 ± 5.0 | 14.0 ± 8.0 | 10.5 ± 7.7 | .041 | .001 | .008 |
| UPDRS IIIc | 34.1 ± 8.4 | 28.9 ± 9.9 | 24.7 ± 10.7 | .135 | .006 | .010 |
| UPDRS IVc | 1.5 ± 1.9 | 3.6 ± 3.7 | 3.5 ± 3.8 | .103 | .164 | .727 |
| UPDRS Totalc | 58.2 ± 14.0 | 49.9 ± 18.0 | 41.4 ± 20.5 | .184 | .006 | .004 |
| PDQ8 | 11.3 ± 4.5 | 10.8 ± 6.0 | 8.3 ± 5.1 | .604 | .083 | .027 |
| Years between PD onset and drugs introduction | 1.7 ± 1.3 | 1.7 ± 3.8 | 1.1 ± 1.0 | .430 | .163 | .396 |
| LD monotherapy (onset) | 3 (33.3) | 29 (40.8) | 21 (41.2) | .734 | .729 | .971 |
| DAs monotherapy (onset) | 3 (33.3) | 23 (32.4) | 22 (43.1) | 1.000 | .772 | .225 |
| LD + DAs (onset) | 2 (22.2) | 10 (14.1) | 0 | .617 | .020 | .005 |
| Other anti-PD drugsd (onset) | 1 (11.2) | 9 (12.7) | 8 (15.7) | 1.000 | 1.000 | .636 |
| LD monotherapy (ongoing) | 1 (11.1) | 12 (16.9) | 10 (19.6) | 1.000 | 1.000 | .701 |
| DAs monotherapy (ongoing) | 1 (11.1) | 1 (1.4) | 1 (2) | .214 | .280 | 1.000 |
| LD + DAs (ongoing) | 6 (66.7) | 31 (43.7) | 15 (29.4) | .290 | .054 | .109 |
| Other anti-PD drugsd (ongoing) | 1 (11.1) | 27 (38) | 25 (49) | .150 | .064 | .226 |
| LEDDe | 528.1 ± 341.6 | 625.5 ± 253.5 | 483.9 ± 279.4 | .229 | .604 | .001 |
| Comorbiditiesf | 7 (77.8) | 52 (73.2) | 29 (56.9) | 1.000 | .293 | .059 |
| Associated Medical Conditionsg | 6 (66.7) | 40 (56.3) | 24 (47.1) | .726 | .472 | .311 |
| Back pain (yes) | 6 (66.7) | 50 (70.4) | 35 (68.6) | 1.000 | 1.000 | .832 |
| VAS Back painh | 6.0 ± 3.0 | 5.9 ± 2.2 | 5.9 ± 2.3 | 1.000 | .894 | .917 |
| History of falls | 3 (33.3) | 15 (21.1) | 7 (13.7) | .413 | .163 | .294 |
| Forward falls | 2 | 10 | 3 | .617 | .158 | .148 |
| Backward falls | 1 | 3 | 2 | .386 | .391 | 1.000 |
| Sidewise falls | 0 | 2 | 2 | 1.000 | 1.000 | 1.000 |
All values are mean ± standard deviation or N (%).
Abbreviations:
defined according to: [23];
clinical asymmetry was calculated as the differences between the lateralized scores of UPDRS-III items 20–26 of the UPDRS (a difference of ≥4 points was considered indicative of motor asymmetry) [27];
on medication;
anticholinergics, MAO-B inhibitors, amantadine or tolcapone;
defined according to [24];
heart diseases, malignancies, diabetes, hypertension, mental disorders, obesity, metabolic disorders, cerebrovascular diseases, physical trauma;
osteoporosis, arthrosis, rheumatic diseases, otovestibular disorders;
Back pain intensity was determined and graded on a visual analog scale graded from 0 (no pain at all) to 10 (excruciating pain); DAs: Dopamine Agonists; FTB: forward trunk bending; H&Y: Hoehn and Yahr; LD: L-Dopa; LEDD: L-dopa equivalent daily dose; NP: normal posture; PD: Parkinson’s disease; UPDRS: Unified Parkinson’s Disease Rating Scale.
Fig. 1.
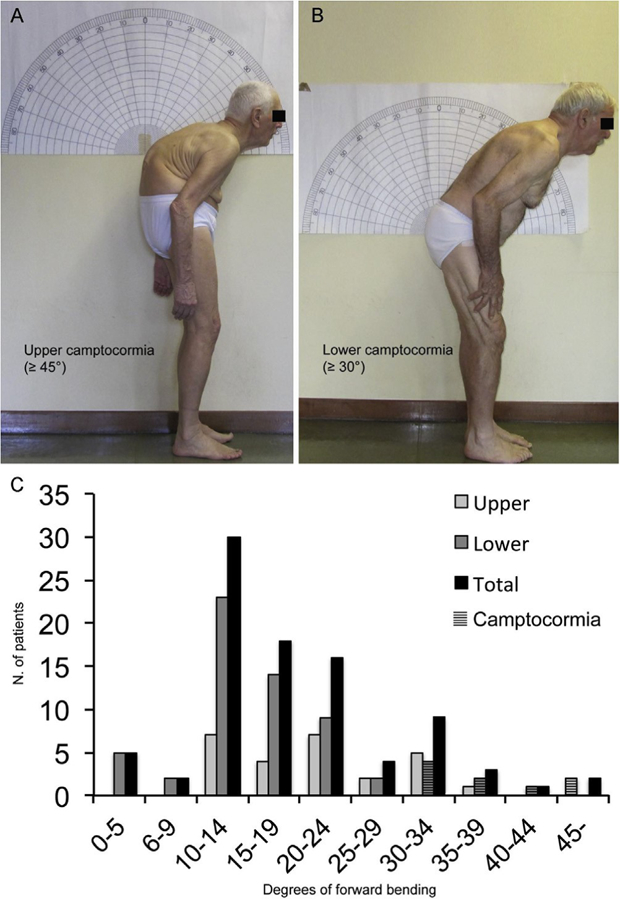
Two example of upper (A) and lower camptocormia (B) according to the definitions reached by full consensus by the panel of experts. Upper and lower camptocormia was measured according to the clinical method used by Furusawa et al. using C7, fulcrum of trunk deviation and the vertical line for the upper camptocormia and C7, sacrum and the vertical line for lower camptocormia [19]. This goniometric modality to quantify postural deformities was chosen due to the relatively easy way to quantify trunk angles during a routine visit and its ability to distinguish two spinal regions (thoracic and lumbar/sacral) which contribute differently to spinal motion [24]. The lumbar/sacral region was considered as a single functional unit because a strict relationship between the pelvic tilt and lumbar lordosis exists during standing posture. Indeed, increasing of the degrees of anterior pelvic tilt increases the angle of lumbar lordosis, and vice versa [25]. Panel C shows the angle distribution of patients with FTB and camptocormia.
Patients were divided into three groups: 1) “camptocormia” when fulfilling the diagnostic criteria generated by the consensus panel; 2) “forward trunk bending” (FTB) when patients did not fulfill the diagnostic criteria but were diagnosed with camptocormia by the referring physician; and 3) patients with “normal posture” (NP).
3. Data analysis
Absolute and relative frequencies were calculated for categorical data and tested by χ2 tests after checking the minimum acceptable number of expected frequencies (> 5) otherwise the Fisher’s Exact test was used. Non-normality of continuous variables was assessed by visual inspection of distribution and confirmed by Shapiro-Wilk test. When several continuous variables were normally distributed, the comparisons across groups (camptocormia vs. FTB vs. NP) were performed with the t-test for independent samples. The homogeneity of variances was tested using the Levene’s test for equality of variances. When continuous variables were not normally distributed, the comparisons across groups were performed using nonparametric Mann-Whitney U tests. All tests were bilateral at p < 0.05. Statistical analysis was carried out using the SPSS for Mac statistical package, version 20.0.
4. Results
A complete (100%) agreement was reached between the experts for the following definition of camptocormia: “an involuntary flexion of the spine appearing during standing or walking and resolving in the supine position of at least 30° at the lumbar fulcrum (L1-Sacrum, hip flexion, ie. lower camptocormia) and/or at least 45° at the thoracic fulcrum (C7 to T12-L1, ie. upper camptocormia)” (Fig. 1A–B).
When testing this new tentative definition in a sample of 131 PD patients, nine patients were considered to be affected by camptocormia (two upper and seven lower), yielding a prevalence of 6.9%. Seventy-one patients were identified as FTB (mean bending angle of 16 ± 7.1° and fulcra ranging from T4 to L5-S1) and 51 as NP. Fig. 1C depicts the angle distribution of patients with FTB and camptocormia: a bimodal distribution is present, particularly for the lower fulcrum group.
Patients with camptocormia were older than the FTB group (p = 0.034), had a later age at PD onset than those with FTB (p = 0.013) or NP (p = 0.011), and a higher score on the UPDRS II than the FTB or NP groups (p = 0.041 and p = 0.001). Compared to NP, patients with camptocormia they also had a higher score in the UPDRS III (p = 0.006), total UPDRS scores I-IV (p = 0.006), H&Y stage (p = 0.013), and were more likely to be treated with a combination of L-dopa and dopamine agonists (p = 0.02). Finally, compared to NP, FTB patients had higher scores in terms of H&Y (p = 0.02), UPDRS-II (p = 0.008), UPDRS-III (p = 0.01), UPDRS-Total (p = 0.004), PDQ8 (p = 0.027) and phenotypically were more often of the mixed type (p = 0.02) and less often of the tremor type (p = 0.039). FTB were more commonly treated with a combination of L-dopa and dopamine agonists at onset and had higher LEDD than NP (p = 0.005 and p = 0.001, respectively; Table 1).
5. Discussion
Our consensus-based approach defined lower camptocormia as an involuntary flexion of the spine during standing or walking of at least 30° at the lumbar fulcrum (L1-Sacrum, hip flexion) and upper camptocormia of at least 45° at the thoracic fulcrum (C7 to T12-L1), in both cases resolving in the supine position. Noteworthy, camptocormia cannot be diagnosed on the basis of the bending angle alone but this study explored the issue of bending degrees and fulcra as previous definitions did not take these important features into account.
Using these definitions, we found a combined prevalence of camptocormia within the lower range of previously reported figures in a cohort of patients specifically evaluated for postural impairments [3–9]. In addition, we found that the term ‘camptocormia’ is often applied to genetically indicate a stooped posture, designated as FTB in this study. Furthermore, we compared the clinical and demographic features of PD patients with camptocormia, FTB and NP and found that both patients with camptocormia and those with FTB had greater disability, poorer performance of activities of daily living (ADLs) and a lower quality of life than PD patients with NP. Therefore, although the bimodal distribution of bending angles in our sample might suggest that camptocormia patients are distinct from those with FTB, the apparent similarities between these two groups support a more conservative view of a continuum between FTB and camptocormia. Whether FTB patients represent ‘sub-threshold camptocormia’ or will develop camptocormia with disease progression is still an open question given the lack of longitudinal follow-up. Intriguingly, FTB and camptocormia were more commonly treated at onset with a combination of L-dopa and dopamine agonists compared with NP, as previously reported in PD patients with Pisa syndrome [23].
Our results are in line with the findings reported by Margraf and collaborators [26]. In fact, 90% of their camptocormia patients needed walking aids and 93% reported specific disabilities attributed to camptocormia [26]. Since patients with bending angles greater than 30° were more severely affected in ADLs, the authors identified this angle as their diagnostic cut-off [26]. However, the angle of forward bending alone is not sufficient to define camptocormia [2]. The identification of two spine levels to measure camptocormia was proposed by Furusawa et al. [18], but cut-off angles remained undefined. Our survey enabled us to identify two different angles for the two levels of camptocormia with important implications for diagnostic and therapeutic purposes. Criteria for upper and lower camptocormia are associated with different muscles involved during electromyographic investigations: the upper subtype is associated with bilateral overactivity of abdominal external and internal oblique as well as rectus abdominis muscles [2,18–20] while the lower subtype is associated with combined activation of rectus abdominis and iliopsoas muscle [2].
A possible limitation of our approach is that we did not take into consideration the subjective complaint of involuntary bending, which was considered a key criterion for camptocormia in a previous study [26]. Nevertheless, patients may be unaware of their posture or might not complain about their abnormal posture until it interferes with their mobility or vision, especially if the onset of the deformity is gradual [1]. Awareness may be greater in the rare patients with sub-acute onset, developing significant flexion over days to months. The delay in patients’ awareness might correspondingly delay the deployment of therapeutic interventions, which have been summarized elsewhere [1]. Likewise, we did not consider the presence of back pain or L-dopa responsiveness in the definition of camptocormia since these features are inconsistently seen in PD patients with disorders of posture [1].
Another limitation of our approach is the lack of a radiological measurement of spine angles, as proposed by others [27,28]. However, camptocormia should be evaluated not only in a static condition, but also dynamically. In fact, evaluating the sagittal trunk incline during walking is more accurate than radiological measures in showing the detrimental effects of camptocormia on ambulation and quality of life [29]. Previous epidemiological studies used a procedure similar to the one used in our sample although they did not differentiate between upper and lower camptocormia [5,6,9]. In addition, a wall goniometer was used to assess the degrees of trunk lateral bending in other studies on Pisa syndrome [23]. Although practical and suitable for screening large samples, we realize that using a wall goniometer in a clinical setting might underestimate bending angles depending on the extent of compensatory posturing of the knees and ankles. For this reason, future studies should investigate which angles better describe trunk position in space and also compare reliability and accuracy of different methods to measure them (e.g. clinical vs. software-based). Finally, the proposed definition carries all the weaknesses of expert-derived observations, particularly the subjective and arbitrary nature of diagnosis our sample patients with camptocormia.
In conclusion, our proposed diagnostic criteria for camptocormia may assist clinicians in properly identifying camptocormia from other postural problems and tailoring an individualized therapeutic strategy [30]. This definition of camptocormia would be expected to improve the accuracy of epidemiological studies and inclusion criteria for future interventional trials. Nevertheless, less severe degrees of forward trunk bending – even thought not classified as camptocormia – must be monitored and promptly treated to prevent the progression, worsening and to avoid that the deformity becomes irreversible.
Acknowledgments
Authors are grateful to Profs Guenther Deuschl, Nir Giladi and Jens Volkmann for their contribution to the initial expert-based definition of camptocormia.
References
- [1].Doherty KM, van de Warrenburg BP, Peralta MC, Silveira-Moriyama L, Azulay JP, Gershanik OS, Bloem BR, Postural deformities in Parkinson’s disease, Lancet Neurol 10 (2011) 538–549. [DOI] [PubMed] [Google Scholar]
- [2].Srivanitchapoom P, Hallett M, Camptocormia in Parkinson’s disease: definition, epidemiology, pathogenesis and treatment modalities, J. Neurol. Neurosurg. Psychiatry 87 (2016) 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lepoutre AC, Devos D, Blanchard-Dauphin A, Pardessus V, Maurage CA, Ferriby D, Hurtevent JF, Cotten A, Destée A, Defebvre L, A specific clinical pattern of camptocormia in Parkinson’s disease, J. Neurol. Neurosurg. Psychiatry 77 (2006) 1229–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ashour R, Jankovic J, Joint and skeletal deformities in Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy, Mov. Disord 21 (2006) 1856–1863. [DOI] [PubMed] [Google Scholar]
- [5].Tiple D, Fabbrini G, Colosimo C, Ottaviani D, Camerota F, Defazio G, Berardelli A, Camptocormia in Parkinson disease: an epidemiological and clinical study, J. Neurol. Neurosurg. Psychiatry 80 (2009) 145–148. [DOI] [PubMed] [Google Scholar]
- [6].Abe K, Uchida Y, Notani M, Camptocormia in Parkinson’s disease, Parkinsons Dis 2010 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Seki M, Takahashi K, Koto A, Mihara B, Morita Y, Isozumi K, Ohta K, Muramatsu K, Gotoh J, Yamaguchi K, Tomita Y, Sato H, Nihei Y, Iwasawa S, Suzuki N, Keio Parkinson’s Disease Database, Camptocormia in Japanese patients with Parkinson’s disease: a multicenter study, Mov. Disord 26 (2011) 2567–2571. [DOI] [PubMed] [Google Scholar]
- [8].Yoritaka A, Shimo Y, Takanashi M, Fukae J, Hatano T, Nakahara T,Miyamato N, Urabe T, Mori H, Hattori N, Motor and non-motor symptoms of 1453 patients with Parkinson’s disease: prevalence and risks, Park. Relat Disord 19 (2013) 725–731. [DOI] [PubMed] [Google Scholar]
- [9].Song W, Guo X, Chen K, Huang R, Zhao B, Cao B, Chen Y, Shang HF, Camptocormia in Chinese patients with Parkinson’s disease, J. Neurol. Sci 337 (2014) 173–175. [DOI] [PubMed] [Google Scholar]
- [10].Djaldetti R, Mosberg-Galili R, Sroka H, Merims D, Melamed E, Camptocormia (bent spine) in patients with Parkinson’s disease-characterization and possible pathogenesis of an unusual phenomenon, Mov. Disord 14 (1999) 443–447. [DOI] [PubMed] [Google Scholar]
- [11].Azher SN, Jankovic J, Camptocormia: pathogenesis, classification, and response to therapy, Neurology 65 (2005) 355–359. [DOI] [PubMed] [Google Scholar]
- [12].Bloch F, Houeto JL, Tezenas du Montcel S, Bonneville F, Etchepare F, Welter ML, Rivaud-Pechoux S, Hahn-Barma V, Maisonobe T, Behar C, Lazennec JY, Kurys E, Amulf I, Bonnet AM, Agid Y, Parkinson’s disease with camptocormia, J. Neurol. Neurosurg. Psychiatry 77 (2006) 1223–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bonneville F, Bloch F, Kurys E, du Montcel ST, Welter ML, Bonnet AM, Agid Y, Dormont D, Houeto JL, Camptocormia and Parkinson’s disease: MR imaging, Eur. Radiol 18 (2008) 1710–1719. [DOI] [PubMed] [Google Scholar]
- [14].Sako W, Nishio M, Maruo T, Shimazu H, Matsuzaki K, Tamura T, Mure H, Ushio Y, Nagahiro S, Kaji R, Goto S, Subthalamic nucleus deep brain stimulation for camptocormia associated with Parkinson’s disease, Mov. Disord 24 (2009) 1076–1079. [DOI] [PubMed] [Google Scholar]
- [15].Margraf NG, Wrede A, Rohr A, Schulz-Schaeffer WJ, Raethjen J, Eymess A, Volkmann J, Mehdom MH, Jansen O, Deuschl G, Camptocormia in idiopathic Parkinson’s disease: a focal myopathy of the paravertebral muscles, Mov. Disord 25 (2010) 542–551. [DOI] [PubMed] [Google Scholar]
- [16].Spuler S, Krug H, Klein C, Medialdea IC, Jakob W, Ebersbach G, Gruber D, Hoffmann KT, Trottenberg T, Kupsch A, Myopathy causing camptocormia in idiopathic Parkinson’s disease: a multidisciplinary approach, Mov. Disord 25 (2010) 552–559. [DOI] [PubMed] [Google Scholar]
- [17].Richer P, Meige H, Etude morphologique sur la Maladie de Parkinson, Nouvelle Iconographie de la Salpetriére 8 (1895) 361–371. [Google Scholar]
- [18].Furusawa Y, Mukai Y, Kobayashi Y, Sakamoto T, Murata M, Role of the external oblique muscle in upper camptocormia for patients with Parkinson’s disease, Mov. Disord 27 (2012) 802–803. [DOI] [PubMed] [Google Scholar]
- [19].Furusawa Y, Mukai Y, Kawazoe T, Sano T, Nakamura H, Sakamoto C, Iwata Y, Wakita M, Nakata Y, Kamiya K, Kobayashi Y, Sakamoto T, Takiyama Y, Murata M, Long-term effect of repeated lidocaine injections into the external oblique for upper camptocormia in Parkinson’s disease, Park. Relat. Disord 19 (2013) 350–354. [DOI] [PubMed] [Google Scholar]
- [20].Furusawa Y, Hanakawa T, Mukai Y, Aihara Y, Taminato T, Iawata Y, Takei T, Sakamoto T, Murata M, Mechanism of camptocormia in Parkinson’s disease analyzed by tilt table-EMG recording, Park. Relat Disord 21 (2015) 765–770. [DOI] [PubMed] [Google Scholar]
- [21].Gelb DJ, Oliver E, Gilman S, Diagnostic criteria for Parkinson disease, Arch. Neurol 56 (1999) 33–39. [DOI] [PubMed] [Google Scholar]
- [22].Wenning GK, Krismer F, Poewe W, New insights into atypical parkinsonism, Curr. Opin. Neurol 24 (2011) 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tinazzi M, Fasano A, Geroin C, Morgante F, Ceravolo R, Rossi S, Thomas A, Fabbrini G, Bentivoglio A, Tamma F, Cossu G, Modugno N, Zappia M, Volontfc MA, Dallocchio C, Abbruzzese G, Pacchetti C, Marconi R, Defazio G, Canesi M, Cannas A, Pisani A, Mirandola R, Barone P, Vitale C, Italian Pisa Syndrome Study Group, Pisa syndrome in Parkinson disease: an observational multicenter Italian study, Neurology 85 (2015) 1769–1779. [DOI] [PubMed] [Google Scholar]
- [24].Hsu CJ, Chang YW, Chou WY, Chiou CP, Chang WN, Wong CY, Measurement of spinal range of motion in healthy individuals using an electromagnetic tracking device, J. Neurosurg. Spine 8 (2008) 135–142. [DOI] [PubMed] [Google Scholar]
- [25].Levine D, Whittle MW, The effects of pelvic movement on lumbar lordosis in the standing position, J. Orthop. Sports Phys. Ther 24 (1996) 130–135. [DOI] [PubMed] [Google Scholar]
- [26].Margraf NG, Granert O, Hampel J, Wrede A, Schulz-Schaeffer WJ, Deuschl G, Clinical definition of camptocormia in Parkinson’s disease, Mov Disord Clin Pract 4 (2017) 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Margraf NG, Rohr A, Granert O, Hampel J, Drews A, Deuschl G, MRI of lumbar trunk muscles in patients with Parkinson’s disease and camptocormia, J. Neurol 262 (2015) 1655–1664. [DOI] [PubMed] [Google Scholar]
- [28].Margraf NG, Wrede A, Deuschl G, Schulz-Schaeffer WJ, Pathophysiological concepts and treatment of camptocormia, J. Parkinson’s Dis 6 (2016) 485–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].de Sèze MP, Guillaud E, Slugacz L, Cazalets JR, An examination of camptocormia assessment by dynamic quantification of sagittal posture, J. Rehabil. Med 47 (2015) 72–79. [DOI] [PubMed] [Google Scholar]
- [30].Wijemanne S, Jimenez-Shahed J, Improvement in dystonic camptocormia following botulinum toxin injection to the external oblique muscle, Park. Relat. Disord 20 (2014) 1106–1107. [DOI] [PubMed] [Google Scholar]


