Abstract
Thymic carcinoma is a rare malignant disease with no standard systemic chemotherapy. The purpose of the present study was to investigate tumor‐infiltrating immune cells (TIIC) in the tumor microenvironment (TME), focusing on the impact of TIIC and program death‐ligand 1 (PD‐L1) expression on clinical outcomes in thymic cancer. Patients with thymic carcinoma resected between 1973 and 2017 were investigated. The tissue specimens were analyzed through immunohistochemical staining to elucidate the prognostic effects of TIIC, their ratios and PD‐L1 in a preliminary cohort (n = 10). The density of TIIC as well as PD‐L1 expression was evaluated in intraepithelial and tumor‐stromal areas on the representative whole section of tumors. The immune factors showing significant association with disease‐free survival (DFS) were evaluated in the total cohort (n = 42). TIIC in the preliminary population showed no significant difference between the two groups. However, CD8, CD20, CD204, FOXP3 and CD20/CD204 ratio demonstrated a tendency to act as predictive markers for recurrence. In the total cohort, significant differences were observed for CD8+, CD20+ and CD204+ cells in tumor islets, and for CD8+, CD20+ and FOXP3+ cells as well as the CD8/CD204 and CD20/CD204 ratios in the stroma, indicating their prognostic effect. The prognostic effect of the PD‐L1 expression in tumor cells could not be established, possibly because of intratumoral heterogeneity. CD8, CD20 and CD204 positive TIIC in stroma were identified as possible better prognostic biomarkers, considering the heterogeneity of other biomarkers. The present study paves the way for exploring strategies of combination immunotherapy targeting B cell immunity in thymic carcinoma.
Keywords: CD20, CD204, M2 macrophages, thymic cancer, tumor‐infiltrating immune cells
The present study revealed that CD8+, CD20+ and CD204+ tumor‐infiltrating immune cells in cancer stroma might be prognostic biomarkers, considering the heterogeneity of other biomarkers, including PD‐L1 expression on tumor cells in thymic carcinoma.
1. INTRODUCTION
Thymic cancer is a rare malignant disease, occurring in approximately 0.02 of 100 000 person‐years. 1 , 2 The disease was advanced in approximately 30% of patients at the time of diagnosis, although resection surgery or chemoradiation therapy is definitive treatment for localized thymic carcinoma. 3 Advanced and recurrent thymic carcinoma have a poor prognosis, and chemotherapy has been used to achieve prolonged disease control in such cases. 4 Several retrospective studies and phase 2 clinical trials have been conducted, intending to demonstrate the efficacy of cytotoxic agents and targeted drugs. 5 , 6 , 7 However, standard chemotherapy regimens have not been established. The recent development of immune‐checkpoint inhibitors (ICI), to block program death‐1 (PD‐1) or program death‐ligand 1 (PD‐L1), has succeeded in many types of solid tumors. 8 , 9 Among thymic carcinoma patients, conflicting results of the efficacy of anti–PD‐1 antibody, pembrolizumab and nivolumab have been reported in phase 2 clinical trials. 10 , 11
The attempts to correlate PD‐L1 expression on tumor cells and the efficacy of ICI have shown conflicting results in several cancer types. In non–small cell lung cancer (NSCLC) patients, PD‐L1 expression on tumor cells was regarded as the predictive marker of anti–PD‐1 antibodies, nivolumab and pembrolizumab. However, this was not the case in all the other cancer types and the other ICI, validating the threshold in many settings. 12 , 13 Although previous studies report PD‐L1 expression in thymic epithelial tumors, the significance of PD‐L1 expression on tumor cells as a predictive biomarker for ICI has not yet been evaluated. 14 , 15 , 16
The tumor microenvironment (TME), a complex immune network, consisting of tumor‐infiltrating immune cells (TIIC), tumor cells and stroma cells, contributes to tumor biology and therapeutic response to ICI. Previous reports have shown the positive prognostic effects of TIIC, including CD8+ cells, FOXP3+ cells and CD20+ cells in several cancer types. 17 , 18 , 19 , 20 , 21 , 22 , 23 The complex mechanisms of immune systems remain to be completely elucidated. However, it has been indicated that effector cells that attack the tumor cells, including CD8+ cells, are a predictive marker for ICI. 24 The suppressor cells inhibiting antitumor activity affect tumor progression and drug efficacy, and FOXP3+ T cells and CD204+ M2 macrophages are representative suppressor cells that are reported as negative prognostic factors. 25 , 26 Considering the function of the cells and factors that comprise the TME, it has been indicated that the balance of the effector cells and suppressor cells might play an important role in the TME and could be both prognostic and predictive biomarkers for ICI. 19 In addition, the significance of TIIC in patient prognosis varies in different cancer types. 27 , 28 , 29 , 30 In thymic cancer patients, previous studies have reported that the effector cells, including CD8+ cells and CD4+ cells, are positive prognostic factors; however, the results are conflicting, and few findings of suppressor cells have been revealed. 31 , 32
In several solid tumors, the distribution site of TIIC has an influence on the prognostic and predictive power. In breast cancer patients, the ratio of TIIC in stroma lesions was correlated to patient prognosis. 21 Among head and neck tumors, esophageal cancer and squamous cell lung cancer, the ratios of TIIC, CD3+, CD4+, CD8+, CD25+ and FOXP3+ cells are reported as positive prognosis factors. 33 , 34 , 35 A few studies report a correlation between the distribution site of TIIC, tumor cells or stroma and the efficacy of ICI.
This is the first comprehensive report to investigate the effects of TIIC including CD4+, CD8+, CD20+, CD56+, CD66b+, CD204+ and FOXP3+ cells, PD‐L1 expression, and the balance of effector and suppressor immune cells in tumor islets or stroma among resectable thymic carcinoma. Considering the heterogeneity of tumor characteristics, six to eight sites from each specimen were selected, followed by counting the number of immune cells (maximum, mean and median) to find the most consistent method for evaluating immune factors.
2. METHODS
2.1. Patients and specimens
The patients with thymic carcinoma who underwent surgical resection between 1973 and 2017 at the National Cancer Center Hospital, Tokyo, Japan satisfying the following criteria were evaluated in the present study: (a) those with pathologically diagnosed thymic cancer; (b) those for whom medical records of treatment outcome were available; and (c) those for whom formalin‐fixed paraffin‐embedded tumor tissue samples were available. The patients who had double primary cancer underwent biopsy surgery only, and those with insufficient specimens were excluded from the study. The study was conducted following the epidemiological study guidelines of the Ministry of Health Labor and Welfare of Japan and approved by the institutional review board of the National Cancer Center. Individual consent was obtained from patients; however, for the deceased patients and their relatives, the study design was disclosed on the website of the National Cancer Center.
The study population was divided into two cohorts: the preliminary cohort (n = 10) and the total cohort (n = 42). The preliminary cohort consisted of the patients who underwent resection between July 2012 and January 2015. The cohort included two groups: the recurrent group (n = 5) and the non–recurrent group (n = 5). The total cohort, including the cases in the preliminary cohort, consisted of 42 patients, with 26 recurrent and 16 non–recurrent patients. Based on the results in the preliminary cohort, candidate factors for prognostic markers were selected and further investigation was conducted in the total cohort.
We reviewed H&E slides of the archived primary tumors and selected a representative block in each case. Considering the heterogeneity of immune factors, we selected 6‐8 areas (1.511 mm2) from whole section slides in each case. Each area was selected according to the location (central or peripheral), the frequency of TIIC and the presence of necrosis. To overcome the heterogeneity of immune cells, we count not only the total numbers but also those according to the locations (i.e., tumor islet or stroma), and calculate the mean, median and maximum numbers in each case (Figure 1). Subsequently, we calculated the ratio of each factor and investigated their influence on disease‐free survival (DFS). Representative blocks, slides and areas were selected and analyzed by independent medical doctors, including pathologists.
FIGURE 1.
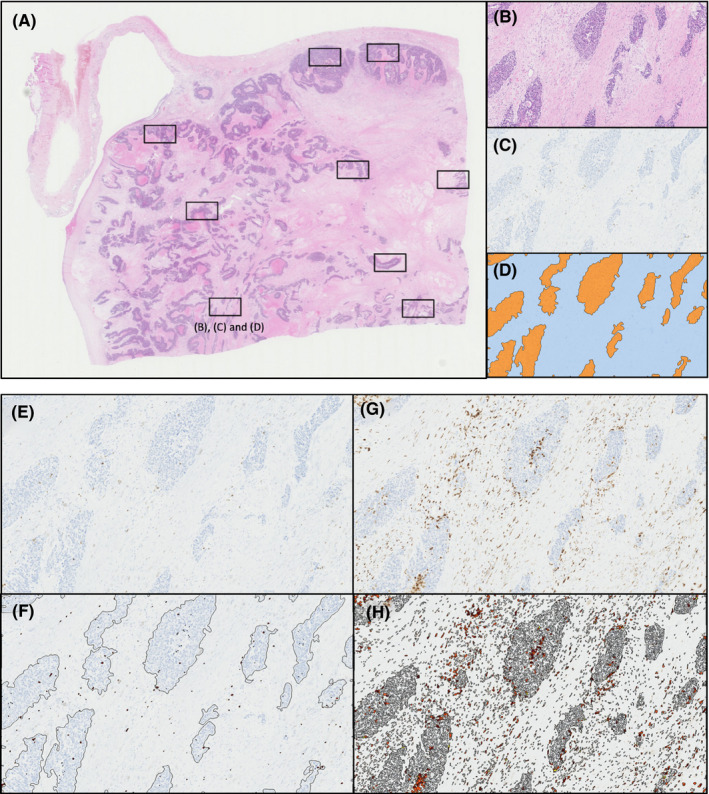
Representative images of selected areas from each specimen. A, Area detection image of H&E. B, Representative image of tumor islet and stroma of H&E. C‐D, CD8+ cell density. D, Orange‐colored area represents tumor islet and blue‐colored area represents stroma. E‐H, Positive immune cells of CD8+ as representative images of immune factors: relatively low frequency in (E) and (F); high frequency in (G) and (H)
2.2. Immunohistochemistry
Immunohistochemical staining was performed on the representative whole sections of formalin‐fixed, paraffin‐embedded tissue following the previous protocols of Oguro et al 36 with the use of autostainers (Ventana BenchMark ULTRA platform [Ventana] and Autostainer Link 48 [Dako]). The primary antibodies used in this study are listed in Table S1. We assessed the TIIC, including CD4, CD8, CD20, CD56, CD66b, CD204 and FOXP3, and the expression of PD‐L1 expression in tumor cells.
2.3. Evaluation of Immunohistochemistry
The microscopic images were scanned and digitized using a NanoZoomer Digital Pathology system (NDP; Hamamatsu Photonics). The density of the immune‐labeled cells was analyzed using Tissue Studio (Definiens). The H&E stained slides were viewed at a magnification of 40× to count the number of TIIC. For the purpose of evaluation of intratumoral heterogeneity, six to eight independent areas of the same size (1.512 mm2) were randomly selected from different locations, including central and peripheral sites of tumors, hot and cold spots of TIIC, and locations with and without necrosis. Subsequently, the numbers of positive cells per mm2 in clusters of tumor cells (tumor islets) and the stroma between tumor islets (stroma) were counted using the Definiens Tissue Studio v4.0 (Definiens).
The expression of PD‐L1 in tumor cells as a percentage of total tumor cells showing PD‐L1 membrane staining (TC value) and the percentage of TIIC (IC value) was scored as described previously using SP142 clone assay (Ventana Medical Systems). 12 The scoring was as follows: TC3 ≥ 50%, TC2 ≥ 5% and < 50%, TC1 ≥ 1% and < 5%, and TC0 < 1%; IC3 ≥ 10%, IC2 ≥ 5% and < 10%, IC1 ≥ 1% and <5%, and IC0 < 1%. To assess the heterogeneity of PD‐L1 expression, we measured the maximum, mean and minimum scores in each specimen. All immunohistochemical (IHC) parameters were analyzed and cross‐verified by independent doctors, including pathologists.
2.4. Statistical analysis
The mean, median and SD values were calculated for each group and analyzed with the χ2‐test or Fisher’s exact test. A P‐value of <0.05 was considered significant. Histograms were used to represent the count of TIIC (maximum, median and mean) in tumor islets and stroma, and were evaluated according to the TC and IC scores in PD‐L1 expression analysis. Univariate analysis (Pearson’s product‐moment correlation coefficient) was conducted among each factor and ratio. Survival analyses were conducted using the Kaplan‐Meier method, and statistically significant differences between survival curves were assessed by the log‐rank test. All statistical analyses were performed using SPSS version 19 (SPSS).
3. RESULTS
3.1. Patient characteristics
A total of 51 patients underwent resection surgery for thymic carcinoma during the study period, of which 42 were eligible. Among these, 26 and 16 patients had recurrence and non–recurrence of the thymic carcinoma, respectively. The demographic and clinical characteristics of the patients are summarized in Table 1. Of all the factors, Masaoka’s staging status was significantly higher in the relapsed cohort (P = 0.006).
TABLE 1.
Demographic and clinical characteristics of the patients
| Characteristics | Patients, n (%) | P‐value | |
|---|---|---|---|
| Non–recurrence (n = 16) | Recurrence (n = 26) | ||
| Age (mean, 95% confidence interval) | 56.3 (50.9‐61.8) | 57.2 (51.4‐63.0) | 0.833 |
| Gender | |||
| Male | 8 (50%) | 12 | 0.814 |
| Female | 8 (50%) | 14 | |
| Histological type | |||
| Squamous | 14 | 23 | 0.928 |
| Other types | 2 | 3 | |
| Masaoka‐Koga surgical Stage | |||
| I | 1 | 0 | 0.006 |
| II | 7 | 2 | |
| III | 7 | 22 | |
| IV | 1 | 2 | |
| Preoperative therapy | |||
| None | 15 | 23 | 0.474 |
| Chemotherapy | 1 | 2 | |
| Radiation | 0 | 1 | |
| Treatment after operation | |||
| None | 6 | 19 | 0.093 |
| Chemotherapy | 0 | 0 | |
| Radiation | 9 | 6 | |
| Chemoradiation | 0 | 1 | |
| Treatment after recurrence | |||
| None | NA | 7 | |
| Surgery | NA | 2 | |
| Chemotherapy | NA | 6 | |
| Radiation | NA | 9 | |
| Chemoradiation | NA | 2 | |
Abbreviation: NA, not applicable.
3.2. Analysis of the preliminary cohort
The immune factors evaluated in the preliminary cohort showed no significant difference between the two groups (Table S2). However, CD8, CD20, CD204 and FOXP3 positive cells demonstrated a tendency to act as predictive markers for recurrence (P‐value ranged from 0.182 to 0.577). Furthermore, the CD20 to CD204 ratio (mean numbers in stroma region) was found to be significantly higher in the non–recurrent group (P = 0.029). Based on these results, the immune factors CD8+, CD20+, CD204+ and FOXP3+ were evaluated in the total cohort.
3.3. Analysis of total cohort
The data on DFS of each subset in the total cohort are presented in Table 2. The log‐rank test revealed significant differences between the groups for all the factors, at least for one parameter. The ratios between the selected factors also showed significant differences, as identified through the log‐rank test. These factors and ratios indicated that high CD8+ and CD20+ in tumor islets as well as stroma, low CD204+ in tumor islets, high FOXP3+, high CD8+/CD204+ and high CD20+/CD204+ in stroma could be prognostic factors for tumor recurrence. It was observed that the immune factors in stroma and their mean number were most significantly associated with the clinical prognosis.
TABLE 2.
Disease‐free survival of intraepithelial and stromal lymphocyte subsets and their ratios in the total cohort
| High | Low | Log‐rank | |||
|---|---|---|---|---|---|
| Median (d) | (95% CI) | Median (d) | (95% CI) | P‐value | |
| CD8 | |||||
| Tumor islets | |||||
| Max | 58.88 | 0.00‐210.6 | 21.60 | 10.93‐32.27 | 0.217 |
| Med | 145.20 | 20.75‐269.65 | 16.50 | 6.16‐26.84 | 0.037 |
| Mean | 39.85 | 17.10‐173.30 | 5.28 | 6.16‐26.84 | 0.074 |
| Stromal | |||||
| Max | 95.50 | 0.00‐195.40 | 14.60 | 6.79‐22.41 | 0.034 |
| Med | 145.20 | 72.17‐218.23 | 14.60 | 8.34‐20.86 | 0.004 |
| Mean | 145.20 | 16.13‐274.27 | 14.60 | 8.34‐20.86 | 0.004 |
| CD20 | |||||
| Tumor islets | |||||
| Max | 23.40 | 0.00‐144.32 | 27.80 | 3.89‐51.72 | 0.565 |
| Med | 109.70 | 70.09‐149.31 | 20.70 | 7.24‐34.16 | 0.027 |
| Mean | 23.40 | 0.00‐144.32 | 27.80 | 3.89‐51.72 | 0.565 |
| Stromal | |||||
| Max | 145.20 | 0.00‐352.56 | 19.30 | 6.08‐32.52 | 0.002 |
| Med | 109.70 | 0.00‐231.80 | 21.60 | 5.31‐37.89 | 0.096 |
| Mean | 145.20 | 0.00‐352.56 | 19.30 | 6.08‐32.52 | 0.002 |
| CD204 | |||||
| Tumor islets | |||||
| Max | 45.70 | 0.00‐137.72 | 23.40 | 14.12‐32.68 | 0.617 |
| Med | 95.20 | 19.78‐170.62 | 21.60 | 10.56‐32.64 | 0.046 |
| Mean | 95.20 | 6.77‐183.62 | 23.40 | 14.38‐32.42 | 0.122 |
| Stromal | |||||
| Max | 23.90 | 15.10‐32.70 | 38.80 | 0.00‐79.08 | 0.957 |
| Med | 20.70 | 5.22‐36.18 | 95.20 | 2.09‐188.31 | 0.275 |
| Mean | 23.90 | 15.82‐31.98 | 49.40 | 0.00‐104.63 | 0.632 |
| FOXP3 | |||||
| Tumor islets | |||||
| Max | 109.70 | 15.34‐204.07 | 23.40 | 18.36‐28.44 | 0.108 |
| Med | 109.70 | 0.00‐296.41 | 23.90 | 14.26‐33.54 | 0.480 |
| Mean | 109.70 | 15.34‐204.07 | 23.40 | 18.36‐28.44 | 0.108 |
| Stromal | |||||
| Max | 145.20 | 1.67‐288.73 | 19.30 | 6.05‐32.56 | 0.030 |
| Med | 109.70 | 29.78‐189.63 | 19.30 | 9.35‐29.25 | 0.060 |
| Mean | 145.20 | 1.58‐288.82 | 19.30 | 9.35‐29.25 | 0.022 |
| CD8/204 | |||||
| Tumor islets | |||||
| Max | 38.80 | 0.00‐110.79 | 19.30 | 6.32‐32.29 | 0.341 |
| Med | 95.20 | 4.78‐185.62 | 19.30 | 5.843‐32.757 | 0.066 |
| Mean | 95.20 | 0.00‐203.97 | 19.30 | 5.84‐32.76 | 0.162 |
| Stromal | |||||
| Max | 95.20 | 0.00‐197.577 | 21.60 | 10.63‐32.57 | 0.406 |
| Med | 145.20 | 15.94‐274.46 | 16.50 | 6.04‐26.96 | 0.016 |
| Mean | 95.20 | 1.60‐188.80 | 12.60 | 4.56‐20.64 | 0.029 |
| CD20/204 | |||||
| Tumor islets | |||||
| Max | 45.70 | 0.00‐162.09 | 21.60 | 11.52‐31.68 | 0.277 |
| Med | 95.20 | 0.00‐194.952 | 20.70 | 7.24‐34.16 | 0.029 |
| Mean | 45.70 | 0.00‐162.09 | 21.60 | 11.52‐31.68 | 0.283 |
| Stromal | |||||
| Max | 145.20 | 39.73‐250.67 | 12.60 | 7.56‐17.64 | 0.02 |
| Med | 109.70 | 31.88‐187.52 | 14.60 | 4.13‐25.01 | 0.03 |
| Mean | 145.20 | 80.82‐209.58 | 12.60 | 7.560‐17.640 | <0.01 |
Abbreviation: CI, confidence interval.
Furthermore, the results for the mean number were consistent with those of the maximum number. Compared to the results of the single immune factor, their ratios were found to be stable, with good reproducibility among regions (i.e. tumor islets and stroma), and values (maximum, mean and median). Kaplan‐Meier curves of these factors and ratios in stroma (mean) are described in Figure 2. The results demonstrated that the high mean numbers of stromal CD8+ (P = 0.04), CD20+ (P = 0.002) and FOXP3+ (P = 0.022) cells were significantly associated with favorable prognosis and high CD204+ cell density tended to be correlated with poor prognosis (P = 0.632). In addition, a strong association between high levels of the ratios of CD8+/CD204+ (P = 0.029) and CD20+/CD204+ (P < 0.001) in stroma and favorable prognosis was observed. Existence of a positive correlation was observed between the median number of stromal CD8+ and CD20+, CD8+ and FOXP3+, and CD20+ and FOXP3+ (Figure 3). Considering the influence of the treatment strategy, the patients who relapsed after surgery were divided into two groups: those who received surgery before 2000 and those who received surgery after 2000. The Kaplan‐Meier analysis revealed no significant difference between the groups (Figure S1). The histograms of the immune factors in the total cohort are presented in Figure S2.
FIGURE 2.
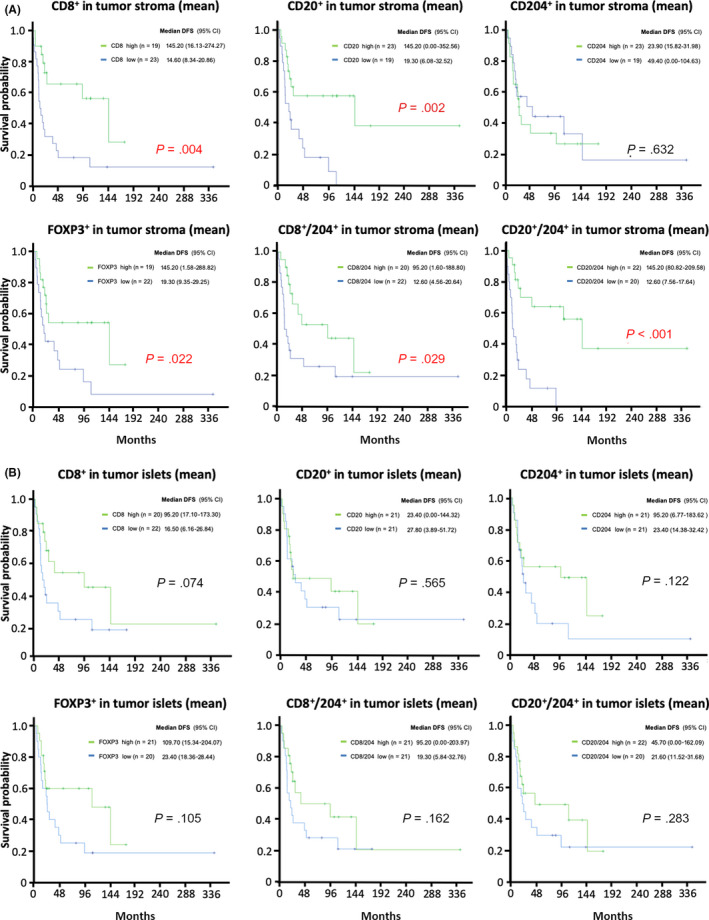
Kaplan‐Meier curves of tumor‐infiltrating immune cells. Kaplan‐Meier curves of CD8+, CD20+, CD204+, FOXP3+, and the ratio of CD8+ to CD204+, and CD20+ to CD204+ in (A) stroma (mean) and (B) islets (mean) are shown. CI, confidence interval; DFS, disease‐free survival
FIGURE 3.
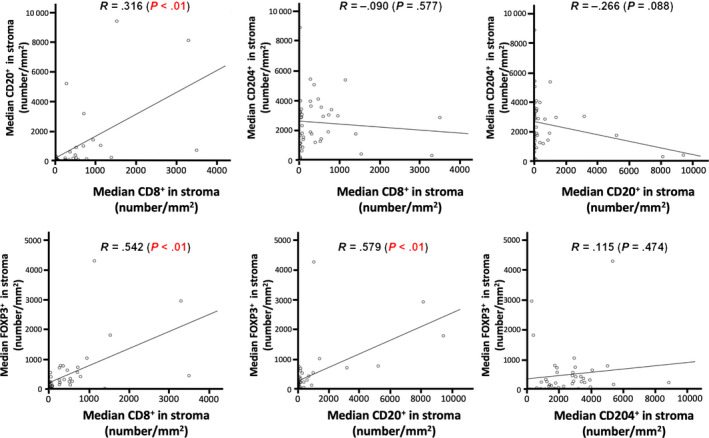
Correlation between tumor‐infiltrating immune cells. Correlation charts with regression lines among CD8+, CD20+, CD204+ and FOXP3+ are shown
3.4. Analysis of program death‐ligand 1 expression in the total cohort
The DFS of patients according to the expression levels of the PD‐L1 are summarized in Table S3. It was observed that the mean TC showed a significant relationship with DFS. However, no significant difference among the other parameters was observed. In terms of the number and frequency, this demonstrated low coincidence among maximum, minimum and mean scores. The combined scores of TC and IC values also did not reveal any significant prognostic effect (see Table 3). The correlation of immune factors CD8+ and CD20+ with PD‐L1 expression is shown in Figure S3. The results indicated that the density of CD8+ cells had a strong relationship with PD‐L1 expression. Kaplan‐Meier curves of PD‐L1 expression and CD8+ cell density, and PD‐L1 expression and CD20+ cell density revealed no significant relationship between these markers (Figure S4).
TABLE 3.
Relationship between disease‐free survival and PD‐L1 expression
| n | Median (mo) | CI (95%) | P‐value | |
|---|---|---|---|---|
| Max | ||||
| TC 0 and IC 0 | 2 | 11.60 | NA | 0.860 |
| TC 1/2/3 or IC 1/2/3 | 6 | 12.50 | 0.00‐54.15 | |
| TC 2/3 or IC 2/3 | 10 | 38.80 | 0.00‐77.97 | |
| TC 3 or IC 3 | 24 | 23.90 | 13.87‐33.93 | |
| Mean | ||||
| TC 0 and IC 0 | 5 | 12.60 | 10.45‐14.75 | 0.109 |
| TC 1/2/3 or IC 1/2/3 | 6 | 12.50 | 8.18‐16.82 | |
| TC 2/3 or IC 2/3 | 19 | 109.70 | 11.96‐207.44 | |
| TC 3 or IC 3 | 12 | 17.80 | 13.05‐22.55 | |
| Min | ||||
| TC 0 and IC 0 | 11 | 12.50 | 10.77‐14.23 | 0.404 |
| TC 1/2/3 or IC 1/2/3 | 6 | 23.60 | 0.00‐92.84 | |
| TC 2/3 or IC 2/3 | 17 | 109.70 | 0.00‐223.68 | |
| TC 3 or IC 3 | 8 | 12.30 | 0.00‐32.67 |
Abbreviation: CI, confidence interval.
4. DISCUSSION
Tumor‐infiltrating immune cells are an indication of the host immune reaction to tumor antigens. Here, we evaluated the association between the amount of intraepithelial and stromal TIIC, their ratios and PD‐L1 expression level with thymic carcinomas through IHC, and identified B cells (CD20+) and M2 macrophage (CD204+) as the prognostic factors. In addition, the ratios of CD8+ to CD204+ and CD20+ to CD204+ in stroma lesions were found to have significant prognostic effects. The results strongly suggested that the balance of effector cells and suppressor cells might play an important role in tumor immunity in thymic cancer.
Several studies show the importance of effector immune cells in the TME. Among effector cells, the presence of CD8+ tumor‐infiltrating cells was found to be significantly associated with prognosis in many cancer types. 37 Higher infiltration of CD8+ cells was related to favorable prognosis in NSCLC colorectal cancer and thymic carcinoma. 22 , 23 , 31 The present study also reported the importance of CD8+ cells, especially in stroma, in thymic carcinoma patients. In addition to the prognostic role, the CD8+ cells in local tumor sites have been reported to be associated with tumor mutation burden and treatment outcome of immune‐checkpoint inhibitors. 17 Considering the conflicting efficacy of PD‐1 blockade in thymic carcinoma, pembrolizumab (overall response rate 22.5%) and nivolumab (overall response rate 0.0%), further evaluation of CD8+ cell density as a prognostic or predictive biomarker for ICI is warranted, particularly in pre–treatment and post–treatment analysis. 10 , 11
The low density of CD20+ cells has been shown to be a favorable prognostic marker in colorectal and lung cancer. 37 , 38 However, in the present study, the higher density of CD20+ cells was found to be significantly associated with favorable prognosis in thymic carcinoma. The results are supported by Shim et al 31 , who report a significant correlation between the density of stromal CD20+ cells and favorable prognosis in thymic carcinoma. These results indicate that the discrepancy of the impact of CD20+ cells in thymic malignancy is different compared to other cancer types and the density of CD20+ TIIC should be investigated in stroma lesions. The observed discrepancy could be related to the function of thymus as a primary lymphoid organ of the immune system, which may affect antitumor immune responses in thymic carcinoma patients. Previous studies reported that tumor‐infiltrating CD20+ B cells activated tumor‐infiltrating CD8+ T cells and have a role of antigen presentation. 39 , 40 These results indicate the functional role of CD20+ cells in the TME, and the positive correlation with CD8+ cells and CD20+ cells in stroma has been associated with patient survival in other cancer types.
The M2 macrophage has angiogenic and immunosuppressive molecules affecting tumor immune systems. 41 Furthermore, tumor‐infiltrating M2 macrophages were associated with poor treatment outcomes in renal cell carcinoma, melanoma, leiomyosarcoma and follicular lymphoma. 42 , 43 Among several markers for macrophages, CD204‐expressing M2 macrophages were prognostic factors in urothelial cell carcinoma of the bladder, esophageal squamous cell carcinoma, pancreatic cancer and NSCLC. 44 , 45 , 46 , 47 We observed a similar tendency of prognosis, indicating the importance of CD204+ M2 macrophages in the TME.
The data from the present study predicted the tendency of FOXP3+ cells as predictive markers for the recurrence of thymic carcinoma. Earlier reports have shown the importance of immune suppressor cells as prognostic factors and predictive markers for cancer therapies in many cancer types. FOXP3+ cells recognized as regulatory T cells (Treg) have an important function, especially in the TME. Higher infiltration of FOXP3+ cells in intra–tumor or peri–tumor sites has been reported as both poor and favorable prognostic markers in several cancer types. 27 , 28 , 29 In our study, positive FOXP3+ cells was correlated with better prognosis, which appeared to be contradictory. However, as shown in Figure 3, there were also linear increases between CD8+ cells and FOXP3+ cells, and between CD20+ cells and FOXP3+ cells. These results indicated that the increase of FOXP3+ cells was accompanied with the increase of effector cells, and was a consequence of response to inflammatory antitumor response by effector cells. Our understanding is that regulatory T cells have been increasing as immunosuppressive responses following antitumor immune responses such as cytotoxic CD8+ T cell infiltration and B cell infiltration. Further analysis is warranted. However, our study might provide new insight into the function of tumor‐infiltrating FOXP3+ cells, and further analysis is warranted.
In the TME, the association of the balance of effector cells and immune‐suppressive cells with prognosis has been reported. In thymic carcinoma, the ratio of CD4+/CD8+ tumor‐infiltrating cells has been shown to have prognosis effects. 32 To our knowledge, this study is the first report demonstrating the prognostic effect of the ratios of CD8+/CD204+ and CD20+/CD204+ tumor‐infiltrating cells and their significant association with the overall DFS. The significance of the ratio of CD8+/CD204+ TIIC has also been reported in esophageal carcinoma and uterine cervical adenocarcinoma. 48 , 49 Compared to the single immune cells, their ratios were found to be consistent among regions (tumor islets and stroma) and values (maximum, mean and median) in this study.
An appropriate evaluation method for the distribution and heterogeneity of TIIC, both effector cells and suppressor cells, has not been established. It has been reported that the lesions (tumor islets, stroma or both) related to prognosis are different depending on the cancer type (e.g., tumor islets for head and neck cancer and stroma for breast cancer) and the optimal lesions for reliable assessment have not been established yet in many cancer types. 21 , 50 However, Shim et al 31 report the relationship between effector immune cells (CD4, CD8 and CD20 positive cells) in stroma and their favorable prognosis. To overcome the problem of heterogeneity in analysis, the importance of the lesions for evaluation and the balance between effector and suppressor cells should be validated in further studies including other cancer types.
Several studies report on the frequency and the association of PD‐L1 expression with clinical information in thymic carcinoma. 14 , 16 However, its prognostic effect for the recurrence of thymic carcinoma is not conclusive. Here, we evaluated the data of PD‐L1 expression from whole section slides to overcome heterogeneity, and observed discrepancy among the two cohorts, representing non–reproducibility of expression levels of PD‐L1 as the biomarker in thymic carcinoma, and, thus, could not establish the obvious clinical significance of PD‐L1 expression as a prognostic factor for thymic carcinoma. In most of the previous reports, histological characteristics of immune factors, including PD‐L1 expression and TIIC, were assessed either through tissue microarray or selection of representative fields with highly integrated immune cells from whole section slides, leading to selection biases.
Furthermore, it was reported that the influence of different characters of anti–PD‐L1 antibody clones (DAKO 28‐8, DAKO 22C3, Ventana SP263 and Ventana SP142) in NSCLC was relatively small but are still not a consequence. 51 Conversely, TIIC in stroma, especially the ratios of CD8+/CD204+ cells and CD20+/CD204+ cells, demonstrated high reproducibility in this study. In addition, we observed that the mean and maximum data had relatively high concordance rates in each group, suggesting that the heterogeneity of these immune factors of TIIC was relatively lower than that of the expression levels of PD‐L1 on tumor cells (Table 2).
This study has some limitations, as it was retrospectively performed and had a relatively small sample size. Due to the development of chemotherapy regimens in recurrent thymic carcinoma, survival time after recurrence might have improved. We did not observe any significant difference between the groups receiving the treatment before and after 2000 in the total cohort (Figure S1). However, considering the rarity of the disease, this report is one of the most extensive retrospective analyses, with comprehensive immunological analysis by IHC staining using the patient data covering 40 years at the National Cancer Center Hospital in Japan. Further prospective analysis to investigate the significance of TIIC (CD8, CD20 and CD204 positive cells) as predictive markers for immune‐checkpoint inhibitors is warranted, and the results of this study may lead to the development of a new strategy of combination therapy targeting B cell immunity.
In conclusion, the present study revealed that CD8+, CD20+ and CD204+ TIIC in cancer stroma might be prognostic biomarkers, considering the heterogeneity of other biomarkers, including PD‐L1 expression on tumor cells in thymic carcinoma.
DISCLOSURE
The authors declare no potential conflicts of interest for this study. This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Supporting information
Figure S1
Figure S2
Figure S3
Figure S4
Table S1
Table S2
Table S3
ACKNOWLEDGEMENT
The authors thank Aya Kuchiba for advising on the statistical analysis.
Sato J, Kitano S, Motoi N, et al. CD20+ tumor‐infiltrating immune cells and CD204+ M2 macrophages are associated with prognosis in thymic carcinoma. Cancer Sci. 2020;111:1921–1932. 10.1111/cas.14409
REFERENCES
- 1. Gatta G, van der Zwan JM, Casali PG et al Rare cancers are not so rare: the rare cancer burden in Europe. Eur J Cancer. 2011;47:2493‐2511. [DOI] [PubMed] [Google Scholar]
- 2. Hishida T, Nomura S, Yano M et al Long‐term outcome and prognostic factors of surgically treated thymic carcinoma: results of 306 cases from a Japanese nationwide database study. Eur J Cardio‐Thoracic Surg. 2016;49:835‐841. [DOI] [PubMed] [Google Scholar]
- 3. Ruffini E, Detterbeck F, van Raemdonck D et al Thymic carcinoma: A cohort study of patients from the European society of thoracic surgeons database. J Thorac Oncol. 2014;9:541‐548. [DOI] [PubMed] [Google Scholar]
- 4. Girard N, Lal R, Wakelee H, Riely GJLP. Chemotherapy definitions and policies for thymic malignancies. J Thorac Oncol. 2011;6:S1749‐S1755. [DOI] [PubMed] [Google Scholar]
- 5. Lemma GL, Lee J‐W, Aisner SC et al Phase II study of carboplatin and paclitaxel in advanced thymoma and thymic carcinoma. J Clin Oncol. 2011;29:2060‐2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hirai F, Yamanaka T, Taguchi K et al A multicenter phase II study of carboplatin and paclitaxel for advanced thymic carcinoma: WJOG4207L. Ann Oncol. 2015;26:363‐368. [DOI] [PubMed] [Google Scholar]
- 7. Thomas A, Rajan A, Berman A et al Sunitinib in patients with chemotherapy‐refractory thymoma and thymic carcinoma: an open‐label phase 2 trial. Lancet Oncol. 2015;16:177‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Borghaei H, Paz‐Ares L, Horn L et al Nivolumab versus docetaxel in advanced nonsquamous non–small‐cell lung cancer. N Engl J Med. 2015;373:1627‐1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reck M, Rodríguez‐Abreu D, Robinson AG et al Pembrolizumab versus chemotherapy for PD‐L1‐positive non–small‐cell lung cancer. N Engl J Med. 2016;375:1823‐1833. [DOI] [PubMed] [Google Scholar]
- 10. Giaccone G, Kim C, Thompson J et al Pembrolizumab in patients with thymic carcinoma: a single‐arm, single‐centre, phase 2 study. Lancet Oncol. 2018;19:347‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Katsuya Y, Horinouchi H, Seto T et al Single‐arm, multicentre, phase II trial of nivolumab for unresectable or recurrent thymic carcinoma: PRIMER study. Eur J Cancer. 2019;113:78‐86. [DOI] [PubMed] [Google Scholar]
- 12. Rittmeyer A, Barlesi F, Waterkamp D et al Atezolizumab versus docetaxel in patients with previously treated non–small‐cell lung cancer (OAK): a phase 3, open‐label, multicentre randomised controlled trial. Lancet. 2017;389:255‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bellmunt J, de Wit R, Vaughn DJ et al Pembrolizumab as second‐line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376:1015‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brown JA, Dorfman DM, Ma F‐R et al Blockade of programmed death‐1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2014;170:1257‐1266. [DOI] [PubMed] [Google Scholar]
- 15. Padda SK, Riess JW, Schwartz EJ et al Diffuse high intensity PD‐L1 staining in thymic epithelial tumors. J Thorac Oncol. 2015;10:500‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Katsuya Y, Fujita Y, Horinouchi H, Ohe Y, Watanabe S‐I, Tsuta K. Immunohistochemical status of PD‐L1 in thymoma and thymic carcinoma. Lung Cancer. 2015;88:154‐159. [DOI] [PubMed] [Google Scholar]
- 17. Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Article molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160:48‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang L, Conejo‐Garcia JR, Katsaros D et al Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203‐213. [DOI] [PubMed] [Google Scholar]
- 19. Mazzaschi G, Madeddu D, Falco A et al Low PD‐1 expression in cytotoxic CD8 � tumor‐in fi ltrating lymphocytes confers an immune‐privileged tissue microenvironment in NSCLC with a prognostic and predictive value. Clin Cancer Res. 2018;24(2):407‐420. [DOI] [PubMed] [Google Scholar]
- 20. Sjöberg E, Frödin M, Lövrot J et al A minority‐group of renal cell cancer patients with high infiltration of CD20+B‐cells is associated with poor prognosis. Br J Cancer. 2018;119:840‐846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Salgado R, Denkert C, Demaria S et al Thompson EA10, Symmans WF11, Richardson AL12, Brock J13, Criscitiello C14, Bailey H15, Ignatiadis M16, Floris G17, Sp LS. The evaluation of tumor‐infiltrating lymphocytes (TILs) in breast cancer The evaluation of tumor‐infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Galon J, Costes A, Sanchez‐Cabo F et al Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(September):1960‐1964. [DOI] [PubMed] [Google Scholar]
- 23. Koh J, Go H, Keam B et al Clinicopathologic analysis of programmed cell death‐1 and programmed cell death‐ligand 1 and 2 expressions in pulmonary adenocarcinoma: comparison with histology and driver oncogenic alteration status. Mod Pathol. 2015;28:1154‐1166. [DOI] [PubMed] [Google Scholar]
- 24. Herbst RS, Soria J‐C, Kowanetz M et al Predictive correlates of response to the anti–PD‐L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563‐567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bates GJ, Fox SB, Han C et al Quantification of regulatory T cells enables the identification of high‐risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24:5373‐5380. [DOI] [PubMed] [Google Scholar]
- 26. Chen W, Konkel JE. Development of thymic Foxp3 + regulatory T cells : TGF‐ β matters. Eur J Immunol. 2015;45:958‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gu‐Trantien C, Loi S, Garaud S et al CD4+ follicular helper T cell infiltration predicts breast cancer survival Find the latest version : CD4+ follicular helper T cell infiltration predicts breast cancer survival. J Clin invest. 2013;123:2873‐2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Teschendorff AE, Gomez S, Arenas A et al Improved prognostic classification of breast cancer defined by antagonistic activation patterns of immune response pathway modules. BMC Cancer. 2010;10:604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Curiel TJ, Coukos G, Zou L et al Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942‐949. [DOI] [PubMed] [Google Scholar]
- 30. Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889‐896. [DOI] [PubMed] [Google Scholar]
- 31. Shim HS, Byun CS, Bae MK et al Prognostic effect of stromal lymphocyte infiltration in thymic carcinoma. Lung Cancer. 2011;74:338‐343. [DOI] [PubMed] [Google Scholar]
- 32. Yokoyama S, Miyoshi H, Nakashima K et al Prognostic value of programmed death ligand 1 and programmed death 1 expression in thymic carcinoma. Clin Cancer Res. 2016;22:4727‐4734. [DOI] [PubMed] [Google Scholar]
- 33. Nguyen N, Bellile E, Thomas D et al Tumor infiltrating lymphocytes and survival in patients with head and neck squamous cell carcinoma. Head Neck. 2016;38:1074‐1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suzuki K, Kadota K, Sima CS et al Clinical impact of immune microenvironment in stage I lung adenocarcinoma: tumor interleukin‐12 receptor β2 ( IL‐12R β2), IL‐7R, and stromal FoxP3/CD3 ratio are independent predictors of recurrence. J Clin Oncol. 2013;31:490‐498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kayser G, Schulte‐Uentrop L, Sienel W et al Stromal CD4/CD25 positive T‐cells are a strong and independent prognostic factor in non–small cell lung cancer patients, especially with adenocarcinomas. Lung Cancer. 2012;76:445‐451. [DOI] [PubMed] [Google Scholar]
- 36. Oguro S, Ino Y, Shimada K et al Clinical significance of tumor‐infiltrating immune cells focusing on BTLA and Cbl‐b in patients with gallbladder cancer. Cancer Sci. 2015;106:1750‐1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fridman WH, Pagès F, Sautès‐Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298‐306. [DOI] [PubMed] [Google Scholar]
- 38. Gentles AJ, Newman AM, Liu CL et al The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21:938‐945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nielsen JS, Sahota RA, Milne K et al CD20+ tumor‐infiltrating lymphocytes have an atypical CD27 ‐ memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin Cancer Res. 2012;18:3281‐3292. [DOI] [PubMed] [Google Scholar]
- 40. DiLillo DJ, Yanaba K, Tedder TF. B cells are required for optimal CD4+ and CD8+ T cell tumor immunity: therapeutic B cell depletion enhances B16 melanoma growth in mice. J Immunol. 2010;184:4006‐4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Qian B, Pollard JW. Review macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Komohara Y, Hasita H, Ohnishi K et al Macrophage infiltration and its prognostic relevance in clear cell renal cell carcinoma. Cancer Sci. 2011;102:1424‐1431. [DOI] [PubMed] [Google Scholar]
- 43. Jensen TO, Schmidt H, Møller HJ et al Macrophage markers in serum and tumor have prognostic impact in American joint committee on cancer stage I/II melanoma. J Clin Oncol. 2009;27:3330‐3337. [DOI] [PubMed] [Google Scholar]
- 44. Wang B, Liu H, Dong X et al High CD204+ tumor‐infiltrating macrophage density predicts a poor prognosis in patients with urothelial cell carcinoma of the bladder. Oncotarget. 2015;6:20204‐20214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shigeoka M, Urakawa N, Nakamura T et al Tumor associated macrophage expressing CD204 is associated with tumor aggressiveness of esophageal squamous cell carcinoma. Cancer Sci. 2013;104:1112‐1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sugimoto M, Mitsunaga S, Yoshikawa K et al Prognostic impact of M2 macrophages at neural invasion in patients with invasive ductal carcinoma of the pancreas. Eur J Cancer. 2014;50:1900‐1908. [DOI] [PubMed] [Google Scholar]
- 47. Ohtaki Y, Ishii G, Nagai K et al Stromal macrophage expressing CD204 is associated with tumor aggressiveness in lung adenocarcinoma. J Thorac Oncol. 2010;5:1507‐1515. [DOI] [PubMed] [Google Scholar]
- 48. Hatogai K, Kitano S, Fujii S et al Comprehensive immunohistochemical analysis of tumor microenvironment immune status in esophageal squamous cell carcinoma. Oncotarget. 2016;7:47252‐47264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kawachi A, Yoshida H, Kitano S, Ino Y, Kato T, Hiraoka N. Tumor‐associated CD204 + M2 macrophages are unfavorable prognostic indicators in uterine cervical adenocarcinoma. Cancer Sci. 2018;109:863‐870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Balermpas P, Michel Y, Wagenblast J et al Tumour‐infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. Br J Cancer. 2014;110:501‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Büttner R, Gosney JR, Skov BG et al Programmed death‐ligand 1 immunohistochemistry testing: a review of analytical assays and clinical implementation in non–small‐cell lung cancer. J Clin Oncol. 2017;35:3867‐3876. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Figure S3
Figure S4
Table S1
Table S2
Table S3


