Abstract
Both ceritinib (CER) and programmed cell death (PD)‐1/PD ligand‐1 (PD‐L1) have brought significant breakthroughs for anaplastic lymphoma kinase (ALK)‐rearranged non‐small‐cell lung cancer (NSCLC). However, the overall clinical efficacy of either CER or PD‐1/PD‐L1 inhibitor monotherapy has been limited to a large extent. In addition, the antitumor effect of combined CER and PD‐L1 inhibitor in ALK‐rearranged NSCLC is not fully understood. In H2228 cells, we examined the tumor killing effect of CER plus PD‐L1 inhibitor in vitro by quantitative RT‐PCR, flow cytometry, ELISA, western blot analysis, PBMC coculture system, and plasmid and transfection experiments. A Ba/F3 (EML4‐ALK‐WT) xenograft mouse model was also utilized to further evaluate the synergistic anticancer effects of CER and PD‐L1 inhibitor in vivo. The coculture system of PBMCs with H2228 cells promotes the expression of PD‐L1 and phospho‐ERK, and combined treatments facilitate lymphocyte proliferation and activation, inhibit PD‐L1 expression, and enhance lymphocyte cytotoxicity and cell death. In the in vivo NSCLC xenograft model, the volumes of tumors treated with CER and PD‐L1 inhibitor in combination were significantly smaller than those treated with CER or PD‐L1 alone. The relative tumor growth inhibitions were 84.9%, 20.0%, and 91.9% for CER, PD‐L1 inhibitor, and CER plus PD‐L1 groups, respectively. Ceritinib could synergize with PD‐1/PD‐L1 blockade to yield enhanced antitumor responses along with favorable tolerability of adverse effects. Ceritinib and PD‐L1 inhibitor combined produced a synergistic antineoplastic efficacy in vitro and in vivo, which provides a key insight and proof of principle for evaluating CER plus PD‐L1 blockade as combination therapy in clinical therapeutic practice.
Keywords: ceritinib, combination, NSCLC, PD‐L1 inhibitor, synergistic efficacy
Co‐culture system of PBMC with H2228 cell promotes the expression of PD‐L1 and phospho‐ERK, and combination treatments facilitate lymphocyte proliferation and activation, inhibit PD‐L1 expression, and enhance lymphocyte cytotoxicity and cell death. Ceritinib can synergize with PD‐1/PD‐L1 blockade to yield enhanced antitumor responses along with favorable tolerability of adverse effects in the in vivo NSCLC xenograft model.

Abbreviations
- ALK
anaplastic lymphoma kinase
- CER
ceritinib
- EML4
echinoderm microtubule‐associated protein like‐4
- ICI
immune checkpoint inhibitor
- irAE
immune‐related adverse event
- LDH
lactate dehydrogenase
- NKG2D
natural‐killer group 2, member D
- NSCLC
non‐small‐cell lung cancer
- ORR
overall response rate
- p‐
phosphorylated
- PD‐1
programmed cell death‐1
- PD‐L1
programmed cell death ligand‐1
- PE
phycoerythrin
- TEAE
treatment‐emerging adverse event
- TKI
tyrosine kinase inhibitor
1. INTRODUCTION
Non‐small‐cell lung cancer, which accounts for approximately 85% of all lung cancer cases, is a devastating disease worldwide. Despite the development of innovative treatments and remarkable recent advances in therapeutic options, the 5‐year survival rate of lung cancer is still estimated to be only 20%. 1 , 2 The WHO evaluated 2 093 876 (11.6%) new cases in 2018, with 1 761 007 deaths (18.4%), and a 5‐year survival rate (17.8%) much lower than other leading cancers (eg, colon and breast) (http://gco.iarc.fr) 3
It is well known that oncogenic drivers play critical roles both in the process of tumorigenesis and tumor cell survival and proliferation. The EML4 and ALK fused oncogene accounts for 3%‐7% of NSCLC patients. The breakthrough and clinical application of EML4‐ALK molecular targeted inhibitors have launched a new era for lung cancer research and personalized treatment, which significantly improves outcome and survival of advanced cancer patients. 4 , 5 , 6 Ceritinib is a second‐generation small molecule TKI of ALK and shows high activity and durable advance events in patients with advanced, ALK‐rearranged NSCLC. 7 Regrettably, in spite of the excellent disease control in the initial stage of therapy, CER fails to prolong the overall survival of these patients, and eventually most patients relapse. Additionally, overall clinical efficacy is substantially limited due to increasing primary or secondary resistance and serious toxicity, which remarkably reduces the benefit and risk ratios for patients with advanced cancer. 8 , 9 , 10 Therefore, from the therapeutic standpoint, it is necessary and pivotal to find surrogate therapeutic strategies to overcome the acquired resistance.
Recently, ICIs, especially PD‐1 and PD‐L1, have transformed therapeutic strategies for NSCLC and significantly improved survival outcomes of advanced cancer patients. 11 Programmed cell death ligand‐1, an immune checkpoint protein expressed on tumor cells and tumor‐infiltrating immune cells, binds to its receptor PD‐1, which mediates anticancer immunosuppression and further ameliorates survival outcomes of advanced cancer patients. 12 , 13 , 14 Anti‐PD‐1/PD‐L1 Abs, for example nivolumab and atezolizumab, block PD‐1/PD‐L1 interactions and enable T cell activation as well as immune system recognition. However, with the increasing use of PD‐1/PD‐L1 inhibitors in clinical practice, several shortcomings have been revealed, and treatment loses effectiveness in many cancer patients due to the PD‐1/PD‐L1 checkpoint blockades. As reported previously, the clinical ORRs to single therapy with PD‐1/PD‐L1 blockade agents are roughly 20%‐30% in patients with solid cancer, 15 , 16 which indicates that further efficacy improvement is required. In addition, although PD‐1/PD‐L1 inhibitors have a certain therapeutic effect on patients with NSCLC, the TEAEs are inevitable and severe. The irAEs due to enhanced T cell reactivity and activation of self‐reactive T cells, such as common side‐effects (eg, fatigue, pruritus, and nausea) and life‐threatening pneumonitis, account for appropriate 14% in grade ≥3 level with broad organ system spectrum. 17 , 18 , 19 Moreover, another aspect to be considered is that innate and acquired resistance, which prevent most cancer patients from reacting to PD‐1/PD‐L1 blockade, are major barriers to therapeutic application, and a large proportion of patients still face disease progression. 19 , 20 , 21 Collectively, monotherapy using PD‐1/PD‐L1 blockade in a small proportion of patients with NSCLC shows limited outcomes, and it is indispensable to explore highly effective therapeutic approaches to overcome the weaknesses discussed above and maximize patients’ clinical benefit‐risk ratios.
A number of phase I trials evaluating this novel therapy combination in patients with advanced NSCLC are currently underway. 22 The combination of TKIs with PD‐1/PD‐L1 blockade could be a favorable alternative solution in clinical treatment practice aimed at controlling possible combined adverse events and ultimately improving the benefit to cancer patients.
To the best of our knowledge, few reports on the synergistic effects or mechanisms of CER combined with PD‐1/PD‐L1 inhibitor, either in vitro or in vivo, have been published because of different pharmacological antitumor mechanisms and unique pharmacodynamic profiles. The objective of the present study is to investigate the combined antitumor effect of CER and PD‐L1 inhibitor in vitro by using flow cytometry, real‐time PCR, and western blot analysis. Furthermore, an EML4‐ALK‐positive NSCLC xenograft model is applied for the purpose of evaluating whether PD‐L1 inhibitor synergistically potentiates the antitumor effects in vivo.
2. MATERIALS AND METHODS
2.1. Chemicals and reagents
Ceritinib (purity 98% or higher, away from light) was provided by Beijing Shikang Synthetic Medicine Technology and serially diluted with 5% methylcellulose. In vivo mAb of anti‐mouse PD‐L1 was obtained from BioXcell and diluted by PBS. RPMI‐1640 medium, DMEM, FBS, 0.25% trypsin‐EDTA, and penicillin‐streptomycin were provided by Thermo Fisher Scientific. Phenylmethanesulfonyl fluoride (100 mM), RIPA lysis buffer, and the enhanced BCA protein assay kit were obtained from Beyotime Biotechnology.
SuperSignal molecular weight protein ladder, West Pico kit, and TRIzol reagent (Invitrogen) were provided by Thermo Fisher Scientific. Phosphorylated ERK1/2 (Thr202/Tyr204) Ab and ERK1/2 ss β‐actin Abs were purchased from Affinity Biosciences. Free HRP secondary Ab was provided by Boster Biological Technology. The FITC anti‐human CD3, FITC‐CD4, PE‐CD8a, PE‐NKG2D, and APC‐anti‐human CD274 (PD‐L1) were all from BioLegend. Glyceraldehyde‐3‐phosphate dehydrogenase was obtained from Hangzhou Xianzhi Biology (lot AB‐P‐R001), and MEK inhibitor (U0126) was purchased from Target Molecule. Ceritinib was diluted to the desired concentrations in RPMI‐1640 medium for in vitro study. Heparinized whole blood was acquired from healthy volunteers and transplant recipients under the guidance of the Beijing Chao‐Yang Hospital Institutional Review Board. Peripheral blood mononuclear cells were obtained by Ficoll‐Hypaque (Amersham Pharmacia Biotech) using the standard density gradient centrifugation method. 23
2.2. Animals
Male C3H Mus musculus (5‐6 weeks old, 24‐28 g) mice were obtained from Beijing Vital River Laboratory Animal Technology. All animals were maintained under specific pathogen‐free conditions at Beijing Chao‐Yang Hospital. All protocols were carried out in accordance with guidelines issued by the Guide for the Care and Use of Laboratory Animals.
2.3. Cell culture
The NSCLC human cell line H2228 (Shanghai Zhong Qiao Xin Zhou Biotechnology) was cultured in RPMI‐1640 medium supplemented with 10% FBS, 100 units/mL penicillin, 100 μg/mL streptomycin, and 2 µg/mL blasticidine (Thermo Fisher Scientific) at 37°C with 5% CO2 and appropriate humidity.
The Ba/F3 immortalized murine bone marrow‐derived pro‐B cell line, which stably expresses the exogenous EML4‐ALK fusion gene, was provided by Beijing Yicon Medical Science and Technology. Ba/F3 (EML4‐ALK‐WT) cells were routinely verified using polyphasic (genotypic and phenotypic) testing, and kept in RPMI‐1640 medium supplemented with 10% FBS, 2 mmol/L L‐glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 20 mmol/L HEPES, and incubated in a humidified atmosphere at 37°C with 5% CO2. Ba/F3 cell medium was complemented with CHO cells’ supernatant (1:2000). All the experiments were undertaken in the exponential phase of the cells.
2.4. Cell colony formation assay
H2228 cells were cocultured with activated PBMCs. Subsequently, an additional 50 µL/well of the growth medium containing different treatments, including anti‐PD‐L1 combined with CER, or either agent alone, or vehicle, was added to the different wells in the H2228 cell coculture system. For the colony formation assay, H2228 cells were plated into a 6‐well plates at a density of 5 × 103 cells/well and incubated with CER at 37°C for 14 days. After removing the supernatant, cells in each well were washed twice with PBS, and the cells attached to the bottom of the plate were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet solution at room temperature for 10 minutes. After the microplate was washed with PBS and dried, the absorbance of each well was determined and quantified spectrophotometrically at 570 nm using a microplate reader. Stained cells were photographed using an Olympus microscope (Olympus).
2.5. Isolation and activation of PBMCs and coculture with H2228 cells
H2228 cells were seeded into 24‐well plates at a density of 3 × 104 cells/well overnight. Human PBMCs were isolated by Ficoll Paque density centrifugation from 50 mL heparinized peripheral blood. For isolation and phenotypic analysis, a standard operation protocol provided by a commercial kit (Tian Jin Hao Yang Biological Manufacture Co.) was executed. Briefly, whole blood was mixed with separation solution with a 1:1 ratio followed by step by step operations. For human PBMC activation, human anti‐CD3 mAb (10 μg/mL, 200 μL/well) and human anti‐CD28 mAb (10 μg/mL, 200 μL/well) were coated on 24‐well cell plates overnight. Thereafter, the lymphocytes were transferred and cultured in RPMI‐1640 medium for an additional 6 days. Next, we collected activated lymphocytes and used them in experiments. 24 Activated PBMCs were coincubated with H2228 cells for 48 hours at the effector : target ratio of 5:1. The PBMC/H2228 cocultured cells were treated with anti‐PD‐L1 Ab (10 μg/mL), CER (1 μmol/L), or vehicle.
2.6. Flow cytometric analysis
For expression of PD‐L1 in H2228 cells, both supernatant and suspension of PBMCs were discarded. After washing with PBS 3 times, the cells were collected by 0.25% trypsin and collected into a flow tube. The cells were washed once with 2 mL PBS and centrifuged at 500 g for 5 minutes, and the supernatant was discarded. The APC‐anti‐human CD274 (2 μL PD‐L1; BioLegend) Ab was added to each tube after vortexing and incubated at room temperature for 20 minutes in the dark. After another centrifugation at 500 g for 5 minutes, the supernatant was discarded. Then 2 mL PBS was added to each tube, vortexed and mixed, centrifuged at 500 g for 5 minutes, then the supernatant was discarded. The cells were resuspended in 0.5 mL PBS and detected by flow cytometry (FACSCanto II; BD Biosciences).
2.7. Fluorescence activated cell sorting
To investigate the expression of NKG2D, CD3, CD4, and CD8 on activated lymphocytes and PD‐L1 on H2228 cells, the cells were incubated for 20 minutes with FITC‐conjugated anti‐CD3 and CD4 mAbs or PE‐conjugated anti‐NKG2D, CD8, and PD‐L1 Ab. Mouse IgG was utilized for isotype control. Thereafter, cells were rinsed twice with PBS and incubated in PBS containing 5% BSA and 0.1% NaN3 and the appropriate concentration of labeled mAb for 1 hour at 4°C. After washing with FACS, the fluorescence intensity or percentage of positive cells was measured using a FACSCalibur flow cytometer (FACSCanto II; BD Biosciences) and analyzed with CellQuest software (BD Biosciences). 24
2.8. Western blot analysis
H2228 cells were seeded into 6‐well plates and incubated with DMSO control and further treated by coculture with PBMCs, U0126, PD‐L1 Ab, or CER for 24 hours. Cells were collected and lysed with RIPA lysis buffer comprising a cocktail of proteinase and phosphatase inhibitors. After centrifugation at 15 000 g for 15 minutes, the protein concentration of the supernatant was measured using BCA protein assay reagent. After incubation with primary/secondary Abs and sufficient washing, the bands were visualized and detected utilizing an ECL agent. The densitometry analysis was carried out using ImageJ2 software. 25
2.9. Quantitative real‐time PCR
Quantitative real‐time PCR experiments were undertaken according to the method described by Tain et al. 26 H2228 cells were washed 3 times using PBS, and RNA was extracted utilizing TRIzol reagent (Invitrogen). RNA was eluted with 100 µL elution buffer and then kept at −80°C until use. The total RNA was isolated and cDNA was synthesized by the GoScript reverse transcription system (Promega). The quantitative RT‐PCR analysis was carried out using an Applied Biosystems 7500 real‐time PCR system to quantify PD‐L1 mRNA expression. Primers for the present study were: PD‐L1 forward, 5′‐TTGCTGAACGCCCCATACAA‐3′ and reverse, 5′‐TCCAGATGACTTCGGCCTTG‐3′; and GAPDH forward, 5′‐TCATTGACCTCAACTACATGG‐3′ and reverse, 5′‐ TCGCTCCTGGAAGATGGTG‐3′. The ratio of target gene expression to GAPDH (internal control) was calculated and the relative expression levels of each gene are shown. Each treatment group was compared with the control group to show the relative mRNA level. The total semiquantitative PCR product was then run on a 2% agarose gel. 27
2.10. Enzyme‐linked immunosorbent assay
The cytoplasm of living cells contains LDH, and when cells are killed by natural killer cells, LDH is released outside the cell. A human lactate dehydrogenase D ELISA detection kit (Wuhan Genemi Biotechnology) was utilized to evaluate the lymphocyte cytotoxicity when PBMCs are cocultured with H2228 cells. Data were analyzed and recorded at 450 nm.
2.11. Plasmid construction, transfection, and stable clone selection
An overexpression vector for ERK was established to evaluate the function of the MAPK signal mechanism pathway. The overexpression vector system was provided by GenePharma. Three treatment groups were created for transfection: H2228 cells plus DMSO, H2228 cells plus CER and vector, H2228 cells plus CER and ERK overexpression. After incubation for 48 hours, CER (1 µM) was added to treatment groups, and cells further incubated for another 24 hours.
2.12. Lymphocyte proliferation analysis
H2228 cells treated with CER or PD‐L1 inhibitor were seeded into 12‐well plates and incubated for 24 hours to allow adherence. Effector cells (activated lymphocytes) at an effector : target ratio of 5:1 were added to the culture and incubated under normoxic conditions or hypoxic conditions for 48 hours. The number of lymphocytes was counted under a light microscope. 24
2.13. Non‐small‐cell lung cancer xenograft model
Ba/F3 (EML4‐ALK‐WT) cells in logarithmic phase were utilized in this study. Adherent cells were digested, harvested, counted, and resuspended in PBS. The density of cells was adjusted to 5.0 × 107 cells/mL. Tumor cell suspension (100 μL/mouse) was implanted carefully s.c. into the right flank of the back of C3H mice. When the tumors reached 100 mm3 approximately 10 days after inoculation, mice were randomized into 4 groups (8 mice/group) and give an i.p. injection or gavage (p.o.) of vehicle (5% methylcellulose), CER (25 mg/kg, p.o., qd × 5), PD‐L1 inhibitor (PDL1, 10 mg/kg, i.p., q2w × 3), and combination (CER, 25 mg/kg, p.o., qd × 5; PDL1, 10 mg/kg, i.p., q2w × 3). The doses were determined on the basis of previous studies. 28 Tumor volume was measured and determined every 2 days by electronic caliper measurements. The calculation method was according to the formula (length × width2)/2, where length represents the larger tumor diameter and width represents the smaller tumor diameter. Mice were monitored twice weekly for body weight and daily for general condition. The percentage of ∆T/C (% of control for ∆growth) was calculated using the following formula: (∆T/∆C) ×100%, where ∆T and ∆C are the changes in tumor volume (∆growth) for the treated and control groups, respectively. 29 The experiment was terminated when the size of tumors in either the treated or control group reached 2000 mm3. After approximately 20 days, mice were killed under deep anesthesia and tumors were immediately harvested and weighed. All animal experiments were approved by the Institutional Committee for Animal Care and Use, Capital Medical University, and were undertaken in good accordance to the institutional guidelines.
2.14. Data and statistical analysis
Data are presented as the mean ± SEM of results from at least 3 independent experiments. Quantitative data were analyzed using the statistics software SPSS 22.0 (IBM). Two‐way ANOVA was used to compare tumor growth inhibition and tumor tissue weight between groups. A P value less than .05 was considered statistically significant.
3. RESULTS
3.1. Ceritinib reduces PD‐L1 expression through the ERK signaling pathway
To investigate the signaling pathway involved in PD‐L1 mediated by CER, the signal inhibitor was used. H2228 cells were serum‐starved for 2 hours and then treated with MEK kinase inhibitor (U0126, 20 µM) for 24 hours. As shown in Figure 1A, the phosphorylation level of ERK1/2, rather than total ERK1/2 protein, decreased after treatment by U0126, which was consistent with the results of PD‐L1 mRNA levels (Figure 1B). Furthermore, the phosphorylation of ERK1/2 was significantly downregulated after treatment by CER, and transfection of ERK overexpression plasmid could restore the downregulation of ERK protein phosphorylation to some extent (Figure 1C). The induction of PD‐L1 by overexpression of ERK was further verified by real‐time PCR (Figure 1D). Taken together, these results were consistent with previous reports, 30 which indicated that inhibiting EML4‐ALK by CER could lower PD‐L1 expression by the ERK signaling pathway.
FIGURE 1.

Ceritinib reduces programmed cell death ligand‐1 (PD‐L1) expression through the ERK signaling pathway in H2228 cells. A, Phosphorylation level of ERK1/2 protein after treatment using ERK inhibitor U0126. GAPDH was utilized to verify equal loading. B, Relative expression level of PD‐L1 mRNA after treatment using ERK inhibitor U0126. C, Phosphorylation level of ERK1/2 protein after transfection of ERK overexpression plasmid (OE). GAPDH was utilized to verify equal loading. D, Protein expression level of PD‐L1, which was stably transfected with control vector plasmid and ERK overexpression plasmid for 24 h. *P < .05, **P < .01. p‐, phosphorylated
3.2. Coculture of PBMCs with H2228 cells promotes expression of PD‐L1 and p‐ERK
It is well known that the PD‐1/PD‐L1 signaling axis plays crucial roles in oncoimmunology. To investigate whether PD‐L1 inhibitor combined with CER could enhance activated PBMC function and PD‐L1 expression, we cocultured H2228 cells with PBMCs. As Figure 2A,B shows, compared with H2228 cells alone, the number of PD‐L1‐positive cells was significantly upregulated after coculturing of PBMCs and H2228 cells for 48 hours. The percentage of PD‐L1 cells of the cocultured group was higher than of the PBMC alone group (P < .01). Next, we evaluated the protein levels of PD‐L1, total ERK1/2, and p‐ERK1/2. We found that the coculture system could promote both the PD‐L1 and p‐ERK1/2 expression levels; however, no significant effects were observed with the total ERK1/2 protein level (Figure 2C). Our results showed that coculture system of PBMCs with H2228 cells can promote the expression of PD‐L1 through p‐ERK1/2 rather than total ERK1/2.
FIGURE 2.
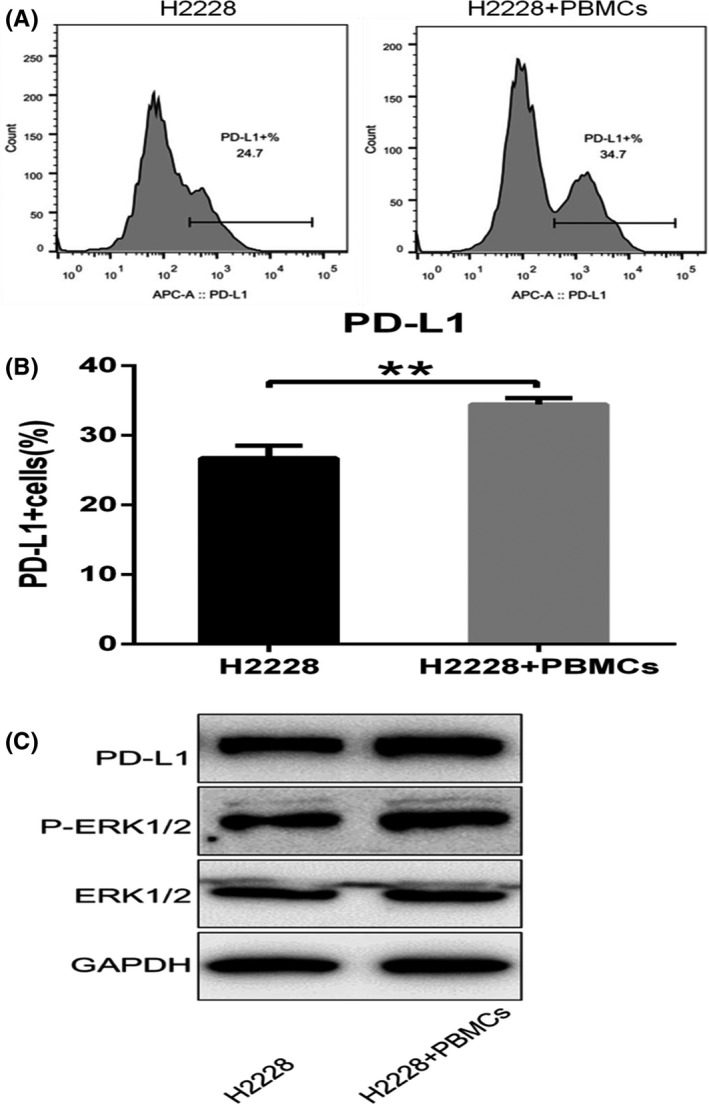
Coculture system of PBMCs with H2228 cells promotes the expression of programmed cell death ligand‐1 (PD‐L1). A, B, Percentage of PD‐L1 cells after coculture of PBMCs with H2228 cells. C, Protein levels of PD‐L1, ERK1/2, and phosphorylated (p‐)ERK1/2 after coculture. **P < .01
3.3. Combined treatment facilitates lymphocyte proliferation and activation
Based on previous results, we further examined the effect of combined CER and PD‐L1 inhibitor on lymphocytes. After coculture with activated PBMCs and H2228 cells, the number of lymphocytes was statistically reduced (P < .01), which indicated the expression of PD‐L1 in H2228 cells affected the proliferation of lymphocytes in activated PBMCs.
We next explored the effect of different treatments on lymphocyte proliferation. As can be seen in Figure 3, the numbers in the PBMC plus H2228 group were reduced significantly. One reason for this phenomenon is that PD‐L1 overexpression of tumor cells could bind to PD‐1 of PBMCs after coculture with H2228 cells, which might contribute to reduce their proliferation ability as well as the functions of lymphocytes. Afterwards, the coculture system was treated with PD‐L1 inhibitor, which inhibits the effect of PD‐L1, and the number of lymphocytes increased. This inhibitory effect was further enhanced by adding CER, which lowered the expression of PD‐L1. Overall, the effects of combined treatment with CER and PD‐L1 inhibitor on lymphocyte proliferation were better than any single investigated group (P < .01).
FIGURE 3.
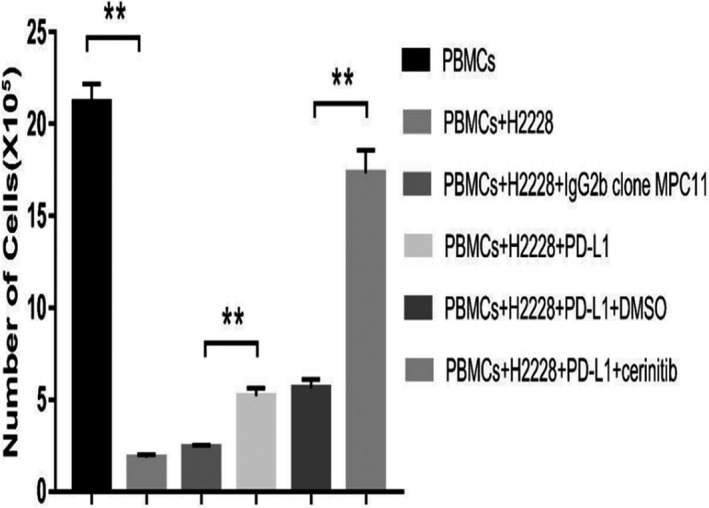
Effect of different treatments on lymphocyte proliferation assay. **P < .01. PD‐L1, programmed cell death ligand‐1
To understand how combined treatment affects lymphocyte function, we tested the lymphocyte cell number after different treatments. As shown in Figure 4, the PD‐L1 mAb not only increased the numbers of CD4‐ and NKG2D‐positive cells but also decreased the number of CD8a cells. However, there was no significant effect on CD3 cells. Furthermore, the combined treatment increased the number of CD3‐, CD4‐, and NKG2D‐positive cells, but reduced the number of CD8a cells. Additionally, the degree of reduction was significantly greater than that of the PD‐L1 inhibitor alone group. Results of lymphocyte activation suggested that combination therapy with EML4‐ALK inhibitor and immunoncology agents could have potential synergistic enhancement by promoting lymphocyte functions.
FIGURE 4.
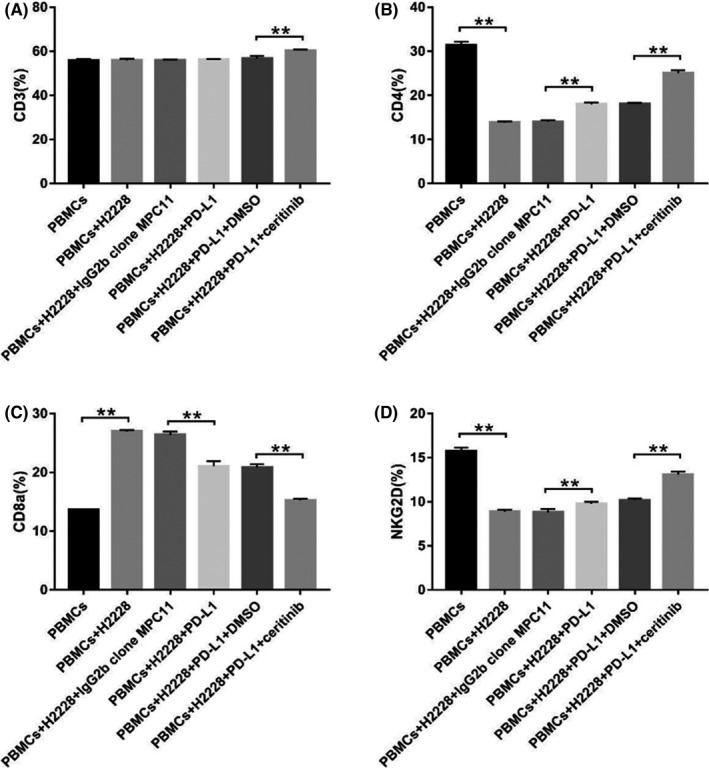
Expression of lymphocytes in H2228 cells after different treatments. A, CD3. B, CD4. C, CD8a. D, natural‐killer group 2, member D (NKG2D). **P < .01
3.4. Combined treatment blocks binding of PD‐1 and PD‐L1, and enhances lymphocyte cytotoxicity
In order to analyze whether combined treatment exerts potential synergic effects, we further investigated the expression of PD‐L1 by flow cytometry. Treatment with PD‐L1 mAb or combined therapy blocked the binding of PD‐1 and PD‐L1 (Figure 5A). The extent of inhibition for the combination group was stronger than that of the single treatment group (Figure 5B).
FIGURE 5.
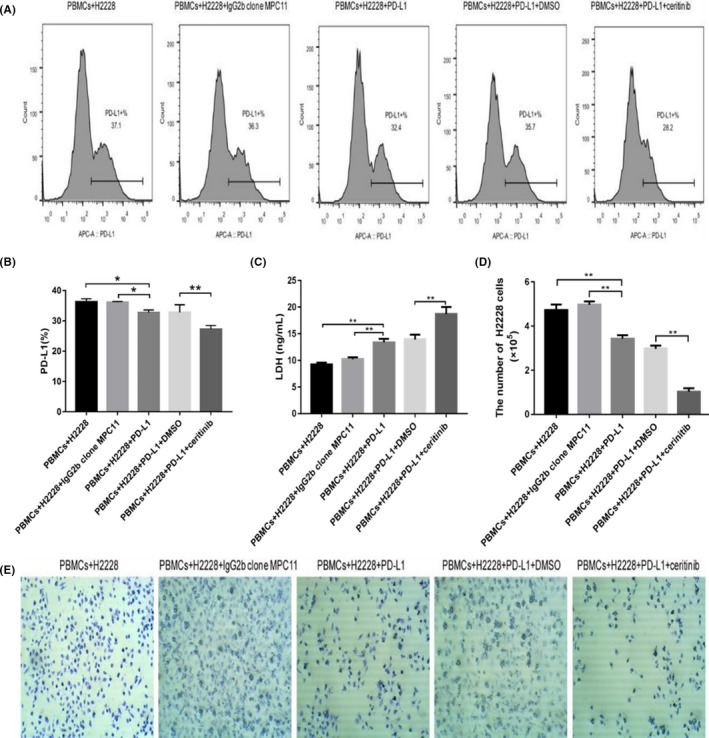
Expression of programmed cell death ligand‐1 (PD‐L1) in H2228 cells, lymphocyte cytotoxicity and cell viability after different treatments. A, Representative flow cytometry results. B, Percentage of PD‐L1 expression. C, Cellular lactate dehydrogenase (LDH) values. D, Quantification of H2228 cells after different treatments by crystal violet staining. E, Photographs of crystal violet staining after different treatments. *P < .05, **P < .01
Results of ELISA showed that both PD‐L1 blockade and combined treatment could increase the LDH level in cell culture supernatant compared with the cocultured control group (Figure 5C). Furthermore, both PD‐L1 mAb and CER can enhance the killing effect of lymphocytes, and the effect of combined therapy is more significant than that of the single treatment group (P < .01), which is consistent with results of cell viability determined by crystal violet staining (Figure 5D,E).
3.5. Ceritinib can synergize with PD‐1/PD‐L1 blockade to trigger an enhanced antitumor response in NSCLC xenograft model
Lower single drug clinical efficacy and acquired drug resistance are 2 major barriers for effective therapy for EML4‐ALK NSCLC patients. As we have observed encouraging antitumor activity indicating that CER combined with PD‐L1 inhibitor could potentiate synergistic antitumor effects in vitro, we hypothesized that CER plus PD‐L1 inhibitor combined treatment could induce enhanced antitumor effects in vivo. Prior to the initiation of animal experiments, we constructed the murine pro‐B cell line Ba/F3, which stably expresses the exogenous EML4‐ALK fusion gene. As expected, in the NSCLC xenograft model, we observed that CER plus PD‐L1 inhibitor treatment and the CER group both significantly attenuated tumor growth compared with the control (P < .001; Figure 6A). In addition, the tumor volumes of any single treatment group were displayed in order to obtain intuitive observations (Figure 6B). No statistically significant differences in body weight were found among any group (Figure 7A,B), suggesting no overt toxicity was observed. For the purpose of further verifying our hypothesis that CER plus PD‐L1 inhibitor could induce enhanced antitumor effects, the mice were killed and tumor tissues were harvested and weighed. As described in Figure 7C,D, combined or single CER treatment could enhance the synergistic antitumor effect compared with the control group, which was consistent with the results of tumor weights. In agreement with previous observations obtained in vitro, synergistic enhancement of CER plus PD‐L1 inhibitor was confirmed, based on the animal experiments.
FIGURE 6.
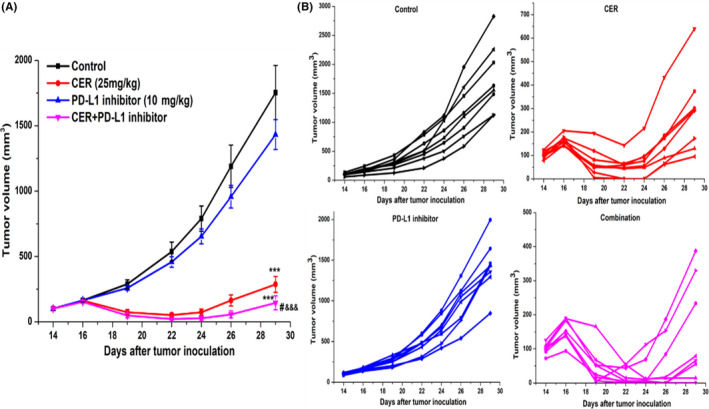
Antitumor activities of combined ceritinib (CER) with programmed cell death ligand‐1 (PD‐L1) inhibitor in Ba/F3 (EML4‐ALK‐WT) tumor xenograft model. A, Relative tumor volume at indicated time points (n = 8). B, Spider plots of each group. Data are shown as mean ± SEM. ***P < .001 vs nontreatment control; #P < .05 vs ceritinib; &&&P < .001 vs PD‐L1 inhibitor
FIGURE 7.
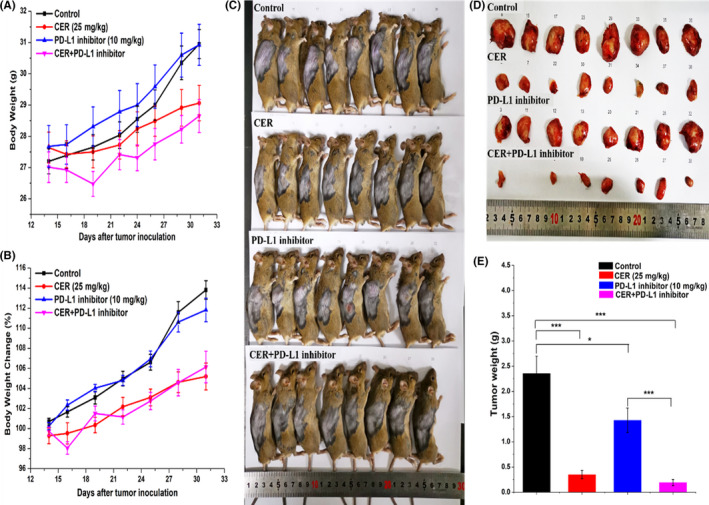
In vivo antitumor efficacy of ceritinib (CER) in combination with programmed cell death ligand‐1 (PD‐L1) inhibitor in Ba/F3 (EML4‐ALK‐WT) tumor xenografts in mice (n = 8). A, Body weight. B, Body weight changes. C, D, Representative tumors harvested from mice killed on the final day of the experiment. E, Tumor weights of different groups. ***P < .001
For efficacy studies in the experimental xenograft model after 12 days, the relative tumor growth inhibitions were 84.9%, 20.0%, and 91.9% for CER, PD‐L1, and CER plus PD‐L1 groups compared with controls, respectively. Both CER and CER plus PD‐L1 treatments could significantly inhibit tumor growth (P < .001, Table 1). In addition, the tumor volumes of the CER plus PD‐L1 inhibitor group were significant smaller than either CER or PD‐L1 treatment (P < .05 and P < .001, respectively, Figure 6A).
TABLE 1.
In vivo tumor growth inhibition profile in Ba/F3 tumor xenografts in mice
| Group | Animals (N) | Tumor weight (g) | Relative tumor proliferation rate (T/C, %) | Tumor cell growth inhibition rate (%) | P a value | P b value |
|---|---|---|---|---|---|---|
| Control | 8 | 2.357 ± 0.340 | – | – | – | – |
| CER | 8 | 0.347 ± 0.083 | 15.1 | 84.9 | .000 | .104 |
| PD‐L1 | 8 | 1.426 ± 0.243 | 80.0 | 20.0 | .140 | .000 |
| CER plus PD‐L1 | 8 | 0.192 ± 0.059 | 8.2 | 91.9 | .000 | – |
Tumor cell growth inhibition rate: 1 − T/C.
–, not applicable; T/C, treatment/control.
Compared with control group.
Compared with ceritinib (CER) plus programmed cell death ligand‐1 (PD‐L1) group.
4. DISCUSSION
Although the therapeutic landscape for advanced NSCLC has changed through the development of increasingly effective and selective ALK inhibitors and ICIs in recent years, NSCLC is still a fatal disease with increasing incidence worldwide. 9 In the last 2 decades, monotherapy with either CER or PD‐1/PD‐L1 inhibitor has produced limited clinical efficacy owing to low selectivity, secondary drug resistance, unexpected adverse effects, or irAEs. Immune checkpoint inhibitors blocking the PD‐1/PD‐L1 pathway provide a standard therapy option for patients with NSCLC and have rapidly been adopted into clinical practice. 31 , 32 However, the response rate of ICIs in unselected populations is 14%‐23%, whereas the response rate in patients with PD‐L1 expression is 16%‐48%. 32 Currently, overcoming acquired drug resistance caused by ALK TKIs and relieving TEAEs due to enhanced immunology is a hot research topic in NSCLC patients. 8 , 9 , 17 , 18 , 19 In some cases, although this efficacy is enhanced, the level of toxicity is still unacceptable, which highlights the need to consider adjusting the dosage, regimen, or combination type to achieve the best treatment. Therefore, combined anticancer therapies have been essential to achieve complete remission and cures for patients with NSCLC. The goal of combination therapy is to take advantage of the complementary action and reduced toxicity.
Recent breakthroughs have led to the development of cancer immunotherapy, which has dramatically changed the treatment algorithm of patients with NSCLC, significantly ameliorating their prognosis. Reportedly, PD‐L1 was expressed in 19.6%‐65.3% of NSCLC cases. 33 A previous study reported that PD‐L1 expression was related with ALK fusion genes in NSCLC cell lines, and overexpression of ALK fusion protein could increase PD‐L1 expression. 34 However, how the PD‐L1 expression was affected by CER in ALK‐positive NSCLC was largely unknown. In the present study, we showed that CER can reduce PD‐L1 expression through the ERK signaling pathway in H2228 cells (Figure 1). Moreover, a coculture system of PBMCs with H2228 cells could promote the expression of PD‐L1 through p‐ERK1/2 rather than total ERK1/2 (Figure 2), which is in line with results reported by Ota et al. 30 Based on the above results, CER can downregulate PD‐L1, thereby enhancing antigenicity and supporting the efficacy of immune‐/target‐therapy combination.
It was recently reported that lymphocytes play a key role in the therapeutic process of NSCLC. The current study further indicated that proliferation and activation of lymphocytes ameliorate the tumor microenvironment as well as the efficiency of immunotherapy (Figures 3 and 4), and the number of lymphocytes significantly strengthens under different processes. Furthermore, both PD‐L1 mAb and CER can enhance the killing effect of lymphocytes, and combined treatment exerts more significant effects than single treatment groups, which was also confirmed by the results of cell viability assays (Figure 5D,E).
One of the key strengths of this study is that we elucidated the synergistic effect of CER and PD‐L1 mAb in an in vitro coculture system. The coculture model is one of the most useful and widely used approaches to investigate immunomodulatory effects, 35 , 36 immunological interactions, 37 drug discovery, 38 and efficacy/toxicity. 39 Adopting a coculture system in in vitro experiments not only evaluates direct interaction between H2228 cells and activated T lymphocytes in PBMCs, which consist of monocytes, macrophages, dendritic cells, and lymphocytes, but also investigates the cross‐talk effect of expressing immune checkpoint proteins and various cytokines. However, considering the cellular or tumor microenvironments, the coculture system cannot completely simulate the complex environment in the body.
Despite several strengths of the present study, a few caveats deserve mention. There is still potential to improve with respect to multiple cell line cross‐validation (eg, same ALK‐positive H3122 cells), although the present study could completely support these in vitro and in vivo robust results. For the purpose of the synergistic mechanism of combined treatment, apoptosis (eg, PI3K/AKT/mTOR and STAT3), western blot analysis (eg, protein lysates from harvested tumors) or PD‐L1 expression in vivo experiments regarding signal pathways could be beneficial to further elucidate the possible mechanisms of synergic effects.
With regard to the NSCLC xenograft model, this is the first study to indicate the in vivo efficacy of CER plus PD‐L1 inhibitor. Although CER has been the first‐line therapy for ALK‐rearrangement‐positive NSCLC, issued by the NCCN Guidelines version 2019, 31 the overall clinical efficacy remains limited due to acquired resistance and severe toxicity. Also, the mean ORR of currently available ICIs is just approximately 30%. 40 As reported by Tang et al in 2018, a total of 2250 active clinical trials are running for ICIs, and 1716 trials are designed to test combining anti‐PD1/PDL1 agents with other cancer therapies. 41 , 42 Undoubtedly, combined therapy could provide alternative solutions to overcome current challenges existing in the procedure of ALK TKIs anti‐PD‐1/PD‐L1 treatment. Figure 6 has indicated that CER plus PD‐L1 inhibitor could not only significantly reduce tumor size, but also exert tolerable toxicity in a xenograft model. These results potentiate strong evidence for clinical therapeutic practice in ALK‐rearranged NSCLC.
In spite of the accumulation of preclinical and clinical data, there are still some problems, such as the optimal dosage and timing of various drugs, the toxicity of combined strategies, and the mechanism of immune resistance to blockade of single‐agent checkpoints. Our preclinical studies showed that when CER is used in combination with anti‐PD‐L1 therapy, tumor cells have a synergistic killing effect, which highlights that reactivation of ERK signals is a hallmark feature of combination therapy. More importantly, a recent open‐label, phase IB, multicenter, dose‐escalation and expansion study provides proof‐of‐concept that both ALKi‐naïve and ALKi‐pretreated patients benefit from the combined strategy of CER plus nivolumab with an ORR range of 25%‐80%, 43 which further confirmed our present in vitro and in vivo results.
Seeking more effective and less toxic treatments for NSCLC patients is an urgent task, not only for preclinical researchers but also for oncology clinicians. Collectively, our study revealed the combined antitumor effect of CER and PC‐L1 inhibitor in vitro and in vivo for the first time, providing insight for new therapeutic options. The combination of CER and PD‐L1 inhibitor has durable effects, along with favorable tolerability, and is applicable to a broad spectrum of cancer types, especially in solid tumors.
In conclusion, this study revealed that combined CER and PD‐1/PD‐L1 inhibitor can exert a synergistic antitumor effect both in vitro and in vivo. The promising results are expected to ameliorate the treatment paradigm. Our results provide a scientific rationale and proof of principle for assessing CER plus PD‐L1 blockade as combined treatment in clinical practice, eventually improving outcomes for ALK‐rearranged NSCLC patients.
CONFLICT OF INTEREST
The authors have no conflict of interest.
ACKNOWLEDGMENTS
This research was supported by the National Natural Science Foundation of China (Grant No. 81703611) and Beijing Hospitals Authority Youth Program (Grant No. QML20180305).
Du P, Hu T, An Z, Li P, Liu L. In vitro and in vivo synergistic efficacy of ceritinib combined with programmed cell death ligand‐1 inhibitor in anaplastic lymphoma kinase‐rearranged non‐small‐cell lung cancer. Cancer Sci. 2020;111:1887–1898. 10.1111/cas.14397
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7‐30. [DOI] [PubMed] [Google Scholar]
- 2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115‐132. [DOI] [PubMed] [Google Scholar]
- 3. Terlizzi M, Colarusso C, Pinto A, Sorrentino R. Drug resistance in non‐small cell lung Cancer (NSCLC): impact of genetic and non‐genetic alterations on therapeutic regimen and responsiveness. Pharmacol Ther. 2019;202:140‐148. [DOI] [PubMed] [Google Scholar]
- 4. Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4‐ALK fusion gene in non‐small‐cell lung cancer. Nature. 2007;448(7153):561‐566. [DOI] [PubMed] [Google Scholar]
- 5. Koivunen JP, Mermel C, Zejnullahu K, et al. EML4‐ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res. 2008;14(13):4275‐4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kwon JH, Kim KJ, Sung JH, et al. Afatinib overcomes pemetrexed‐acquired resistance in non‐small cell lung cancer cells harboring an EML4‐ALK rearrangement. Cells‐Basel. 2019;8(12):1693‐1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nishio M, Felip E, Orlov S, et al. Final overall survival, other efficacy and safety results from ASCEND‐3: phase II study of ceritinib in ALKi‐naive patients with ALK‐rearranged non‐small‐cell lung cancer. J. Thorac Oncol. 2019;370(13):1189‐1197. [DOI] [PubMed] [Google Scholar]
- 8. Du P, Guan Y, An Z, Li P, Liu L. A selective and robust UPLC‐MS/MS method for the simultaneous quantitative determination of anlotinib, ceritinib and ibrutinib in rat plasma and its application to a pharmacokinetic study. Analyst. 2019;144(18):5462‐5471. [DOI] [PubMed] [Google Scholar]
- 9. Gainor JF, Dardaei L, Yoda S, et al. Molecular mechanisms of resistance to first‐ and second‐generation ALK inhibitors in ALK‐rearranged lung cancer. Cancer Discov. 2016;6(10):1118‐1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vasan N, Baselga J, Hyman DM. A view on drug resistance in cancer. Nature. 2019;575(7782):299‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu B, Song Y, Liu D. Recent development in clinical applications of PD‐1 and PD‐L1 antibodies for cancer immunotherapy. J Hematol Oncol. 2017;10(1):174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen L, Han X. Anti‐PD‐1/PD‐L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125(9):3384‐3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dong H, Zhu G, Tamada K, Chen L. B7–H1, a third member of the B7 family, co‐stimulates T‐cell proliferation and interleukin‐10 secretion. Nat Med. 1999;5(12):1365‐1369. [DOI] [PubMed] [Google Scholar]
- 14. Dong H, Strome SE, Salomao DR, et al. Tumor‐associated B7–H1 promotes T‐cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793‐800. [DOI] [PubMed] [Google Scholar]
- 15. Borghaei H, Paz‐Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med. 2015;373(17):1627‐1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med. 2015;373(2):123‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune‐checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16(9):563‐580. [DOI] [PubMed] [Google Scholar]
- 18. Arnaud‐Coffin P, Maillet D, Gan HK, et al. A systematic review of adverse events in randomized trials assessing immune checkpoint inhibitors. Int J Cancer. 2019;145(3):639‐648. [DOI] [PubMed] [Google Scholar]
- 19. Shergold AL, Millar R, Nibbs R. Understanding and overcoming the resistance of cancer to PD‐1/PD‐L1 blockade. Pharmacol Res. 2019;145:104258. [DOI] [PubMed] [Google Scholar]
- 20. Zhao X, Subramanian S. Intrinsic resistance of solid tumors to immune checkpoint blockade therapy. Cancer Res. 2017;77(4):817‐822. [DOI] [PubMed] [Google Scholar]
- 21. Sharma P, Hu‐Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707‐723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Manegold C, Dingemans AC, Gray JE, et al. The potential of combined immunotherapy and antiangiogenesis for the synergistic treatment of advanced NSCLC. J Thorac Oncol. 2017;12(2):194‐207. [DOI] [PubMed] [Google Scholar]
- 23. Boyum A. Separation of leukocytes from blood and BM. Introduction. Scand J Clin Lab Invest Suppl. 1968;97:31‐50. [PubMed] [Google Scholar]
- 24. Onishi H, Fujimura A, Oyama Y, et al. Hedgehog signaling regulates PDL‐1 expression in cancer cells to induce anti‐tumor activity by activated lymphocytes. Cell Immunol. 2016;310:199‐204. [DOI] [PubMed] [Google Scholar]
- 25. Shen J, Wang J, Du J, et al. A novel ALK inhibitor ZYY inhibits Karpas299 cell growth in vitro and in a nude mouse xenograft model and induces protective autophagy. Toxicol Appl Pharm. 2019;383:114781. [DOI] [PubMed] [Google Scholar]
- 26. Tain YL, Lee WC, Wu K, Leu S, Chan J. Targeting arachidonic acid pathway to prevent programmed hypertension in maternal fructose‐fed male adult rat offspring. J Nutr Biochem. 2016;38:86‐92. [DOI] [PubMed] [Google Scholar]
- 27. Breyer J, Wirtz RM, Erben P, et al. FOXM 1 overexpression is associated with adverse outcome and predicts response to intravesical instillation therapy in stage pT 1 non‐muscle‐invasive bladder cancer. BJU Int. 2019;123(1):187‐196. [DOI] [PubMed] [Google Scholar]
- 28. Dhillon S, Clark M. Ceritinib: first global approval. Drugs. 2014;74(11):1285‐1291. [DOI] [PubMed] [Google Scholar]
- 29. Kato Y, Tabata K, Kimura T, et al. Lenvatinib plus anti‐PD‐1 antibody combination treatment activates CD8+ T cells through reduction of tumor‐associated macrophage and activation of the interferon pathway. PLoS ONE. 2019;14(2):e212513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ota K, Azuma K, Kawahara A, et al. Induction of PD‐L1 expression by the EML4‐ALK oncoprotein and downstream signaling pathways in non‐small cell lung cancer. Clin Cancer Res. 2015;21(17):4014‐4021. [DOI] [PubMed] [Google Scholar]
- 31. NCCN Clinical Practice Guidelines in Oncology (NCCN Guideline) . Non‐small cell lung cancer. Verision6. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed 12 August 2019.
- 32. Shukuya T, Carbone DP. Predictive markers for the efficacy of anti‐PD‐1/PD‐L1 antibodies in lung cancer. J Thorac Oncol. 2016;11(7):976‐988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen N, Fang W, Zhan J, et al. Upregulation of PD‐L1 by EGFR activation mediates the immune Escape in EGFR‐driven NSCLC: implication for optional immune targeted therapy for NSCLC patients with EGFR mutation. J Thorac Oncol. 2015;10(6):910‐923. [DOI] [PubMed] [Google Scholar]
- 34. Hong S, Chen N, Fang W, et al. Upregulation of PD‐L1 by EML4‐ALK fusion protein mediates the immune escape in ALK positive NSCLC: Implication for optional anti‐PD‐1/PD‐L1 immune therapy for ALK‐TKIs sensitive and resistant NSCLC patients. Oncoimmunology. 2016;5(3):e1094598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kwon MS, Noh MY, Oh KW, et al. The immunomodulatory effects of human mesenchymal stem cells on peripheral blood mononuclear cells in ALS patients. J Neurochem. 2014;131(2):206‐218. [DOI] [PubMed] [Google Scholar]
- 36. Luukkainen A, Puan KJ, Yusof N, et al. A co‐culture model of PBMC and stem cell derived human nasal epithelium reveals rapid activation of NK and innate T cells upon Influenza A virus infection of the nasal epithelium. Front Immunol. 2018;9:92514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Doumba PP, Nikolopoulou M, Gomatos IP, Konstadoulakis MM, Koskinas J. Co‐culture of primary human tumor hepatocytes from patients with hepatocellular carcinoma with autologous peripheral blood mononuclear cells: study of their in vitro immunological interactions. BMC Gastroenterol. 2013;13(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Berg EL, Hsu Y, Lee JA. Consideration of the cellular microenvironment: physiologically relevant co‐culture systems in drug discovery. Adv Drug Deliver Rev. 2014;69‐70:190‐204. [DOI] [PubMed] [Google Scholar]
- 39. Rose KA, Holman NS, Green AM, Andersen ME, LeCluyse EL. Co‐culture of hepatocytes and Kupffer cells as an in vitro model of inflammation and drug‐induced hepatotoxicity. J Pharm Sci. 2016;105(2):950‐964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. 2019;2(5):e192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tang J, Yu JX, Hubbard‐Lucey VM, et al. Trial watch: the clinical trial landscape for PD1/PDL1 immune checkpoint inhibitors. Nat Rev Drug Discov. 2018;17(12):854‐855. [DOI] [PubMed] [Google Scholar]
- 42. Xin YJ, Hubbard‐Lucey VM, Tang J. Immuno‐oncology drug development goes global. Nat Rev Drug Discov. 2019;18(12):899‐900. [DOI] [PubMed] [Google Scholar]
- 43. Felip E, de Braud FG, Maur M, et al. Ceritinib plus nivolumab in patients with advanced ALK‐rearranged non‐small‐cell lung cancer: results of an open‐label, multicenter, phase 1B study: ceritinib plus nivolumab in ALK‐rearranged NSCLC. J Thorac Oncol. 2019;15:392‐403. [DOI] [PubMed] [Google Scholar]


