Abstract
Tumor‐infiltrating immune cells play a crucial role in tumor progression and response to treatment. However, the limited studies on infiltrating immune cells have shown inconsistent and even controversial results for osteosarcoma (OS). In addition, the dynamic changes of infiltrating immune cells after neoadjuvant chemotherapy are largely unknown. We downloaded the RNA expression matrix and clinical information of 80 OS patients from the TARGET database. CIBERSORT was used to evaluate the proportion of 22 immune cell types in patients based on gene expression data. M2 macrophages were found to be the most abundant immune cell type and were associated with improved survival in OS. Another cohort of pretreated OS samples was evaluated by immunohistochemistry to validate the results from CIBERSORT analysis. Matched biopsy and surgical samples from 27 patients were collected to investigate the dynamic change of immune cells and factors before and after neoadjuvant chemotherapy. Neoadjuvant chemotherapy was associated with increased densities of CD3+ T cells, CD8+ T cells, Ki67 + CD8+ T cells and PD‐L1+ immune cells. Moreover, HLA‐DR‐CD33+ myeloid‐derived suppressive cells (MDSC) were decreased after treatment. We determined that the application of chemotherapy may activate the local immune status and convert OS into an immune “hot” tumor. These findings provide rationale for investigating the schedule of immunotherapy treatment in OS patients in future clinical trials.
Keywords: CIBERSORT, neoadjuvant chemotherapy, osteosarcoma, tumor‐infiltrating immune cells, tumor‐infiltrating lymphocytes
Host anti–tumor immune response boosted by neoadjuvant chemotherapy. Following neoadjuvant chemotherapy, CD3+ T cells increased significantly and there was a trend of increased cytotoxic T cells. CD8+ T cells in both tumor center and stroma also increased remarkably. Importantly, activated CD8+ T cells, defined as Ki67 + CD8+ T cells, were more abundant in post–chemotherapy samples, and were negatively correlated with the proliferation ability of tumor cells.

Abbreviations
- ADM
Adriamycin
- DDP
cisplatin
- FFPE
formalin‐fixed paraffin‐embedded
- ICI
immune checkpoint inhibitor
- IFO
ifosfamide
- IHC
immunohistochemistry
- MTX
methotrexate
- OS
osteosarcoma
- TIL
tumor‐infiltrating lymphocyte
- TIME
tumor immunologic microenvironment
1. INTRODUCTION
Osteosarcoma (OS) is the most common malignancy of bone, which primarily affects children and adolescents. Neoadjuvant chemotherapy combined with surgery and adjuvant chemotherapy has been the standard treatment strategy for patients with locally high‐grade resectable OS. 1 , 2 Advancement of diagnostic modalities and aggressive chemotherapy, most significantly doxorubicin, cisplatin, ifosfamide (IFO) and high dose methotrexate‐based chemotherapy, has improved the survival rate dramatically. The disease‐free survival rates of patients with localized OS at present reaches nearly 70% at specialized sarcoma centers. 3 , 4 Despite the great success of this multimodal therapy, patients with relapsed or metastatic disease still have a dismal survival rate, of 30% or less. 5 Hence, identifying new therapeutic targets to improve outcomes for these patients is of great urgency.
Immunotherapy, especially use of immune checkpoint inhibitors (ICI), has profoundly changed the landscape of treatment in oncology. 6 Unfortunately, many patients and tumor types still do not respond to ICI. The success of immunotherapy depends on the active interaction between tumor cells and the host immune system. Chen and Mellman 7 first described the tumor‐host interaction as the cancer immunity cycle. In general, ICIs are hypothesized to be more effective in tumors that have T‐cell responses, including cells with CD8+, programmed cell death 1 (PD‐1)/programmed cell death ligand 1 (PD‐L1)+ and Granzyme B+ (GZMB+) expression, as well as other markers of active immune response. 8 However, infiltration of suppressive components, such as regulatory T cells (Tregs) and myeloid‐derived suppressive cells (MDSC), can compromise the host immune response, thus limiting the benefit of immunotherapies. 9 , 10 Therefore, researchers are focusing on identifying methods to transform the immune‐suppressive tumor immunologic microenvironment (TIME) to sensitize tumors to current forms of immunotherapy. 11 To achieve this end, it is critical to have exact and thorough knowledge of the TIME, including the heterogeneity of the composition and distribution. 8 , 12 , 13
Osteosarcoma is not considered a “hot tumor.” 14 However, evaluation of tumor‐infiltrating lymphocytes (TIL) in OS s varies in different sarcoma centers. Palmerini and colleagues 15 reported that most cases presented with TIL with CD3+ (90%), CD8+ (86%) and Tia‐1+ (73%) in 129 localized OS patients, and the authors also reported that PD‐L1 expression was barely expressed in tumor cells but that 22% of TIL showed PD‐1 expression. Another study reported OS with low TIL, including failure of effective antitumor response (absence of GZMB+ cells), and few cells exhibiting immunotherapeutic targets, such as CTLA‐4 and PD‐1. 16 In contrast, Shen and colleagues 17 reported that PD‐L1 is highly expressed in a subset of OS patients, and positively correlated with the density of TIL. Despite the above heterogeneous results, a recent clinical trial (SARC 028 study) showed only a limited response rate (5%) of pembrolizumab (anti–PD‐1 antibody) in advanced OS. 18 Thus, combinatory or induction treatment is required to enhance the pre–existing immune response to sensitize OS to immunotherapy.
Although historically regarded as immunosuppressive, compelling preclinical and clinical evidence has shown that chemotherapy may stimulate an anticancer immune response under certain situations. 19 It has been reported that agents, such as anthracyclines, cyclophosphamide and cisplatin, boost host immune response through multiple mechanisms. 20 Cisplatin induces major histocompatibility complex (MHC) class I expression in tumor cells, which is associated with presentation of tumor antigens and killing by cytotoxic T cells. 21 In addition, several studies have illustrated that low dose cyclophosphamide depletes Tregs, one of the major immunosuppressive cells in TIME, thereby restoring the function of T cells. 22 , 23 Anthracyclines, such as doxorubicin, have been demonstrated to eliminate MDSC 24 and directly cause immunogenic cell death. 25 A previous study in ovarian cancer showed that platinum‐based and taxane‐based neoadjuvant chemotherapy increases infiltration of CD3+, CD8+, CD8 + TIA‐1+, PD‐1+ and CD20+ TIL, thus augmenting pre–existing immune responses. 26 More recently, the TONIC trial, which was primarily designed to explore the impact of induction chemotherapy or irradiation on the TIME of triple‐negative breast cancer, showed that short‐term doxorubicin and cisplatin may induce a more favorable TIME and increase sensitivity to PD‐1 blockade. 27 Therefore, the immunomodulatory effect of chemotherapy highlights the potential benefits of combining traditional chemotherapy with immunotherapy. Although there are increasing reports demonstrating the TIME of OS, none of these studies have indicated whether the effect of chemotherapy is positive or negative.
In this study, we first evaluated the composition of TIME in OS using RNA‐sequencing data from a public database. To address the immune effect of neoadjuvant chemotherapy in OS patients, we compared the change in immune infiltrates before and after neoadjuvant treatment using matched biopsy and surgical specimens. We also investigated the correlation between immune infiltrations and the response to neoadjuvant chemotherapy.
2. MATERIALS AND METHODS
2.1. Gene expression and clinical dataset
The mRNA expression profiles (transcripts per kilobase million [TPM] values), based on RNA sequencing (RNA‐seq) data of patients with OS (n = 80), were obtained from the Therapeutically Applicable Research to Generate Effective Treatments (TARGET, https://ocg.cancer.gov/programs/target) database. Corresponding clinical characteristics, including gender, tumor region and distant metastasis, were collected. The overall survival of patients was calculated.
2.2. Assessment of immune infiltration
The CIBERSORT analytical tool (https://cibersort.stanford.edu/), which was developed by Bindea et al, was used to infer the relative proportions of 22 types of infiltrating immune cells in complex tissues based on normalized gene expression data. 28 We used CIBERSORT to analyze the immune landscape of the OS microenvironment based on the TARGET RNA‐seq data. The analysis was performed using the LM22 signature matrix at 100 permutations.
2.3. Patients and samples
A total of 27 OS patients treated at Sun Yat‐sen University Cancer Center between 2018 and 2019 were enrolled in this study. Patients who had been treated previously, including with surgery, chemotherapy or radiotherapy, were excluded. All patients received biopsies and proceeded with neoadjuvant MAPI (methotrexate [MTX] 12 g/m2, ADM 40 mg/m2 × 2 days + DDP 100 mg/m2 and IFO 2.5 g/m2 × 4 days) chemotherapy, and then underwent surgical resection of primary tumors. RECIST1.1 criteria was used to evaluate the response of neoadjuvant chemotherapy based on the change in tumor volume according to MRI, as previously reported. 29 , 30 Formalin‐fixed paraffin‐embedded (FFPE) tissues of matched pre–neoadjuvant and post–neoadjuvant chemotherapy tumors were retrieved from the Department of Pathology. Only tissues with sufficient tumor and stromal components were selected for further study. The present study was approved by the Institutional Ethical Board of the Sun Yat‐sen University Cancer Center. Written informed consent for use of tumor specimens and clinical information was obtained from all patients.
2.4. Immunohistochemistry and immunofluorescence
Immunohistochemistry (IHC) was performed on 4‐μm sections from FFPE tissues using a Ventana Discovery XT automated system (Ventana Medical Systems) as previously reported. 31 Slides were dewaxed using the automated system and then subjected to heat‐induced antigen retrieval. After nonspecific blocking (goat serum, ZSGB‐BIO), slides were incubated with specific antibody overnight at 4°C. Detailed information regarding the antibodies used in this study is provided in Table S1. After incubation with secondary antibodies (ZSGB‐BIO), slides were detected with DAB staining, and counterstained with hematoxylin. For double‐color IHC, primary antibodies were co–incubated, and then processed with a polymer dual‐stain kit (Mo/HRP + Rb/AP, DS‐0001; ZSGB‐BIO), according to the manufacturer’s instructions. Immunofluorescence (IF) was performed on FFPE as previously described. 32 After incubation with primary rabbit anti–human HLA‐DR and mouse anti–human CD33, secondary fluorescent antibodies (Abcam) were used. Then sections were mounted with ProLong Diamond Antifade Mountant with DAPI (Invitrogen). A paraffin‐embedded human placenta section was used as a positive control for PD‐L1 and CD33 staining, human NK/T‐cell lymphoma for Granzyme B, and normal human tonsil for the other markers. The scoring methods of IHC and immunofluorescence are provided in Appendix S1.
2.5. Statistical analysis
Only cases with CIBERSORT P < 0.05 were selected for subsequent analysis. Differential fractions of the 22 immune cell types were evaluated by Wilcoxon test. Kaplan‐Meier survival curves with log‐rank test were used to estimate the correlation between immune cell types and OS. The cutoff value (the most significant split based on the log‐rank test) was evaluated with the use of maxstat (Maximally Selected Rank Statistics) R package. Change in infiltrating immune cell densities between pre–neoadjuvant and post–neoadjuvant chemotherapy tumor samples was compared using the Wilcoxon matched‐pairs test with Pratt’s method. Differences in MHC I, PD‐L1+ and PD‐1 expression were analyzed using Fisher’s exact test. Pearson correlation analysis was used to evaluate the correlations between Ki67 + CD8+ T cells and Ki67+ tumor cells. Statistical analyses were performed using R version 3.6.0. and GraphPad Prism 7 (GraphPad). P‐values (two‐sided) <0.05 were considered significant.
3. RESULTS
3.1. Profiles of immune cell infiltration in osteosarcoma
We first investigated the infiltration landscape of immune cells in OS in the TARGET cohort using the CIBERSORT algorithm. The results obtained from 80 OS tissues are summarized in Figure 1A,B, and the detailed values for distributions are provided in Table 1. The profile of infiltrated immune cells varied among patients. M2 macrophages were the most abundant immune cell type (24 ± 13%) in the OS microenvironment. M2 macrophages, M0 macrophages, resting CD4+ T cells and naïve B cells were the top four highest infiltrating fractions in OS tissues. Moreover, memory B cells, monocytes, activated dendritic cells, activated mast cells, eosinophils and neutrophils were scarcely infiltrated. In addition, there were no significant differences regarding the gender or the primary tumor site of patients (Figure 1B).
FIGURE 1.
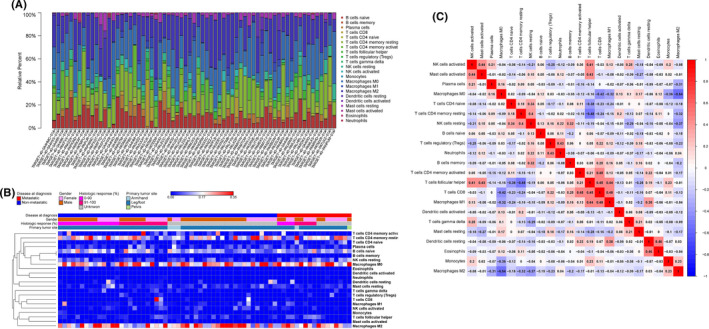
The landscape of infiltrating immune cells in osteosarcoma (OS). A, Bar plot of the fractions of 22 immune cell types in OS from TARGET database, with CIBERSORT P < 0.05, n = 80. B, Heat map of infiltrating immune cells from TARGET database, on a blue–red scale indicated in the color key. C, Correlation matrix of the densities of 22 immune cells in the TARGET cohort
TABLE 1.
Fraction of 22 infiltrating immune cells in osteosarcoma from TARGET database analyzed by CIBERSORT
| Immune cell type | Fraction (%, mean ± SD) |
|---|---|
| B cells naïve | 0.11 ± 0.06 |
| B cells memory | 0 |
| Plasma cells | 0.03 ± 0.04 |
| T cells CD8 | 0.01 ± 0.06 |
| T cells CD4 naïve | 0.01 ± 0.03 |
| T cells CD4 memory resting | 0.17 ± 0.1 |
| T cells CD4 memory activated | 0.03 ± 0.07 |
| T cells follicular helper | 0.02 ± 0.03 |
| T cells regulatory (Tregs) | 0.01 ± 0.02 |
| T cells gamma delta | 0.01 ± 0.02 |
| Natural killer cells resting | 0.04 ± 0.04 |
| Natural killer cells activated | 0.01 ± 0.02 |
| Monocytes | 0 |
| Macrophages M0 | 0.23 ± 0.1 |
| Macrophages M1 | 0.02 ± 0.04 |
| Macrophages M2 | 0.24 ± 0.13 |
| Dendritic cells resting | 0.01 ± 0.04 |
| Dendritic cells activated | 0 |
| Mast cells resting | 0.03 ± 0.03 |
| Mast cells activated | 0 |
| Eosinophils | 0 |
| Neutrophils | 0 |
To further explore the underlying relationships among different immune cells in OS tissue, we evaluated the correlation between every two types of immune cells. As shown in Figure 1C, the two most relevant immune cells were M0 macrophages and M2 macrophages, with an R value of −0.54. Of note, M0 macrophages were also negatively associated with CD8+ T cells (R = −0.42). The most positively correlated cells with CD8+ T cells were M1 macrophages with an R‐value of 0.48. CD8+ T cells were also positively associated with both activated memory CD4+ T cells and follicular helper T cells (R = 0.44).
3.2. Clinical significance of infiltrating immune cells
We next investigated the correlation of the fractions of immune cells with clinical information extracted from the TARGET database. The histological response to neoadjuvant chemotherapy, as defined by tumor necrosis, is an important prognostic factor in OS patients. 33 We observed that a higher proportion of regulatory T cells (Tregs) indicated good histological response (P = 0.005). Of note, patients with a good response tended to be infiltrated with less M2 macrophages, although not statistically significantly (P = 0.081, Figure 2A). Patients with metastatic disease were infiltrated with higher density of naïve CD4+ T cells (P = 0.032) and resting NK cells (P = 0.037), while no significant difference was found within other immune cell types (Figure 2B). As shown in Figure 2C, a higher fraction of M1 macrophages (P = 0.03), M2 macrophages (P = 0.03) and follicular helper T cells (P = 0.02) indicated a favorable prognosis. In contrast, a higher fraction of resting NK cells (P = 0.003), plasma cells (P = 0.04) and naïve CD4 T cells (P = 0.01) was associated with poorer survival.
FIGURE 2.
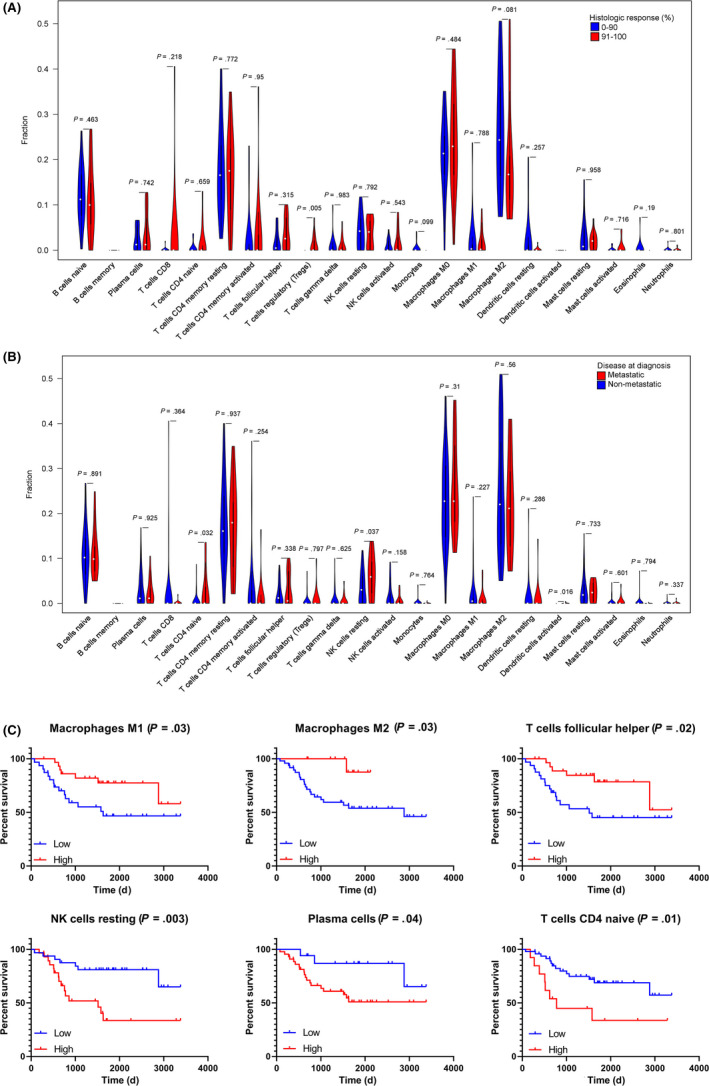
Clinical correlation of infiltrating immune cells in TARGET cohort. A, The quantified contrast of the proportion of immune cells between patients with lung metastatic and non‐metastatic disease. B, The quantified contrast of the proportion of immune cells between patients with good (91%‐100% tumor necrosis rate) and poor (0%‐90%) histologic response. C, Kaplan‐Meier survival curves with log‐rank test show the overall survival in the high‐density and low‐density immune cells. The figure shows the six immune cell types associated with overall survival (P < 0.05)
3.3. Patient characteristics
A cohort of patients with matched pre–neoadjuvant and post–neoadjuvant chemotherapy tumor tissues was included for analysis. The clinical characteristics are summarized in Table 2. Most of the patients were classified as Enneking stage IIB (22, 81.5%). All patients received at least three cycles of neoadjuvant chemotherapy. Among these patients, 8 (29.7%) experienced an objective response (partial response, PR), 9 (33.35) had stable disease (SD), while 5 (18.5%) patients had progressive disease (PD).
TABLE 2.
Characteristics of 27 OS patients with matched pre–neoadjuvant and post–neoadjuvant chemotherapy samples
| Variables | N (%) |
|---|---|
| Age at diagnosis, y | |
| <14 | 13 (48.1) |
| ≥14 | 14 (51.9) |
| Gender | |
| Male | 18 (66.7) |
| Female | 9 (33.3) |
| Enneking stage | |
| IIA | 1 (3.7) |
| IIB | 22 (81.5) |
| III | 4 (14.8) |
| Cycles of neoadjuvant chemotherapy | |
| 3 | 2 (7.4) |
| 4 | 16 (59.3) |
| 5 | 1 (3.7) |
| 6 | 8 (29.6) |
| Treatment response | |
| PR | 8 (29.7) |
| SD | 9 (33.3) |
| PD | 5 (18.5) |
| NA | 5 (18.5) |
Abbreviations: NA, not available; OS, osteosarcoma; PD, progressive disease; PR, partial response; SD, stable disease.
3.4. Tumor‐infiltrating T cells increase following neoadjuvant chemotherapy
In the pre–neoadjuvant chemotherapy samples, CD68+ macrophages were identified to be the most abundant immune cell type, with a median density of 15.8 and 23 cells/HPF in tumor center and stroma, respectively. CD3+ T cells were found in almost all cases (26/27). The density of CD3+ T cells varied widely among patients, with a median density of 5 cells/HPF (0‐42 cells/HPF). CD8+ T cells were more prevalent in stroma (4 cells/HPF) than tumor center (1.8 cells/HPF). Detailed statistics of infiltrating immune cells are presented in Table S2.
Following neoadjuvant chemotherapy, the density of CD8 + T cells increased remarkably, both in tumor center and stroma (Figure 3A). Meanwhile, the amount of CD68+ macrophages did not change significantly either in tumor center or stroma. Infiltrated CD3+ T cells increased from a median density of 5 to 17.2 cells/HPF (P < 0.001, Figure 3B). No significant change in density was observed in CD4+ and CD20+ cells (Figure S1A,B). To assess the change in cytotoxic T cells, we analyzed the expression of granzyme B. Although not significant, there was a trend of increase in GZMB+ cells (from a median of 1.4 to 3 cells/HPF, P = 0.088, Figure S1C). To evaluate the activity of CD8+ T cells recruited into tumors, we performed double‐color IHC using antibody to Ki67 and CD8. The density of Ki67 + CD8+ cells increased significantly in post–chemotherapy samples (P = 0.008, Figure 3C). Moreover, the percentage of Ki67 + tumor cells was negatively correlated with Ki67 + CD8+ T cells (r = −0.58, P = 0.002, Figure 3D). In addition, we evaluated the expression of MHC I by tumor cells. MHC I was positive in 51.9% and 40.7% of pre–neoadjuvant and post–neoadjuvant chemotherapy tissues, respectively (P = 0.59, Table S3).
FIGURE 3.
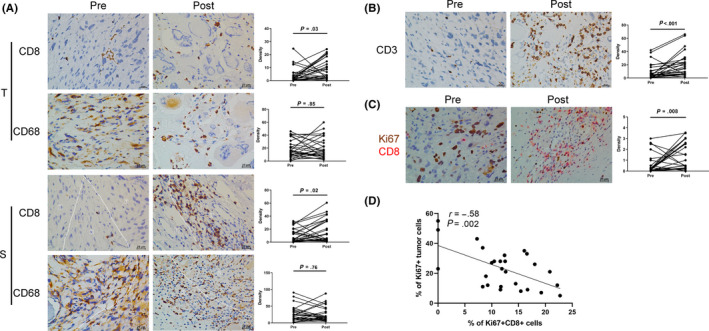
Comparison of infiltrating lymphocytes densities before and after neoadjuvant chemotherapy. A, Representative immunohistochemistry (IHC) images and statistical analysis of CD8 and CD68 in tumor center (T) and stroma (S). B, Representative IHC images and statistical analysis of CD3. C, Representative double‐color IHC images and quantification of Ki67 + CD8+ before and after neoadjuvant chemotherapy in OS tissues. Densities are presented as the average number of cells in five random HPF. P‐values were calculated using the Wilcoxon matched‐pairs test with Pratt’s method, n = 27. D, Correlation between percentage of Ki67 + CD8+ cells in CD8+ T cells and Ki67+ tumor cells
3.5. Neoadjuvant chemotherapy alters the immunosuppressive components of TIME
Chemotherapy has been shown to change the immunosuppressive status of the tumor environment. Thus, we next investigated the change in immunosuppressive cells and factors induced by neoadjuvant chemotherapy. The density of MDSC (HLA‐DR‐CD33+) cells decreased from a median of 4.2 to 3.4 cells/HPF (P = 0.01, Figure 4A). Treg cells (FOXP3 + CD4+) showed no significant change after neoadjuvant chemotherapy (Figure 4B). Consistent Smeland et al (2019), 34 we found that PD‐L1 expression was relatively low both in tumor cells (18.5%) and infiltrating immune cells (IC, 7.4%) before neoadjuvant chemotherapy. A similar result was observed for the expression of PD‐1 in TIL (7.4%). The positivity of PD‐L1 in immune cells increased after chemotherapy, from 7.4% to 33.3% (Figure 4C,D, P = 0.039). However, the expression pattern of PD‐L1 in tumor cells or PD‐1 in TIL remained unchanged (Table S3).
FIGURE 4.
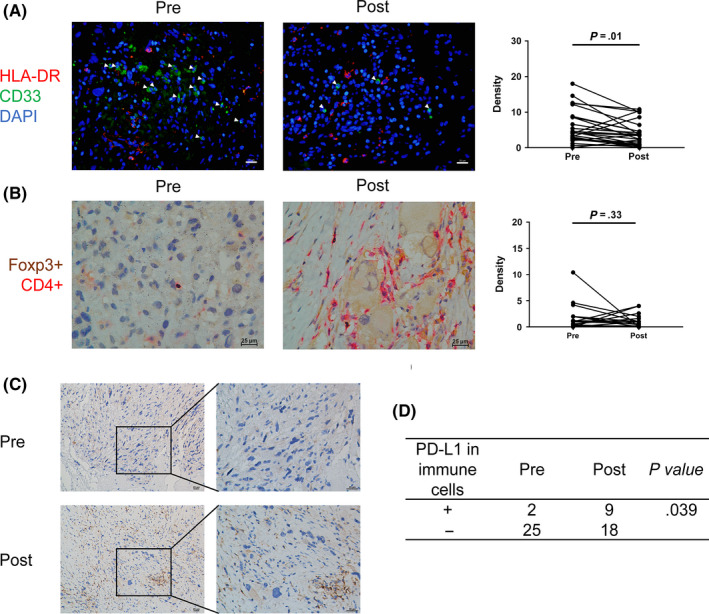
Immunosuppressive components before and after neoadjuvant chemotherapy. A, Representative IF images and quantification of HLA‐DR‐CD33+ cells before and after neoadjuvant chemotherapy. White triangle indicates HLA‐DR‐CD33+ cells. B, Representative double‐color immunohistochemistry (IHC) images and quantification of Foxp3 + CD4+ before and after neoadjuvant chemotherapy. C, Representative IHC images of programmed cell death ligand 1 (PD‐L1) expression in immune cells before and after neoadjuvant chemotherapy. D, Statistical analysis of the positivity of PD‐L1+ immune cells using Fisher’s exact test
To investigate the influence of the intensity of chemotherapy, we analyzed the correlation between cycles of chemotherapy and immunogenic change. Increases of CD3+ cells and reductions of HLA‐DR‐CD33+ cells were seen in both groups of patients with different cycles of chemotherapy. However, significant increases in CD8 + cells were observed only in patients receiving 5‐6 cycles of chemotherapy (P = 0.005, Figure S2). No significant change of other immune cells was found in either group of patients (data not shown).
3.6. Correlation between infiltrating immune cells and response to neoadjuvant treatment
In the pre–neoadjuvant biopsy samples, a low density of CD3+ T cells (P = 0.04) or MDSC (P = 0.004) was associated with a good pathological response (Figure 5A,B). No significant correlation was seen in pre–neoadjuvant CD8+ T cells (Figure 5C). In the post–neoadjuvant surgical samples, more infiltrating CD4+ T cells indicates a good pathological response (P = 0.02, Figure 5D). In contrast, more GZMB+ cells or MDSC is correlated with a poor response (Figure 5E,F). No significant relationship was observed for other immune cells either in pre–neoadjuvant or post–neoadjuvant treatment samples.
FIGURE 5.
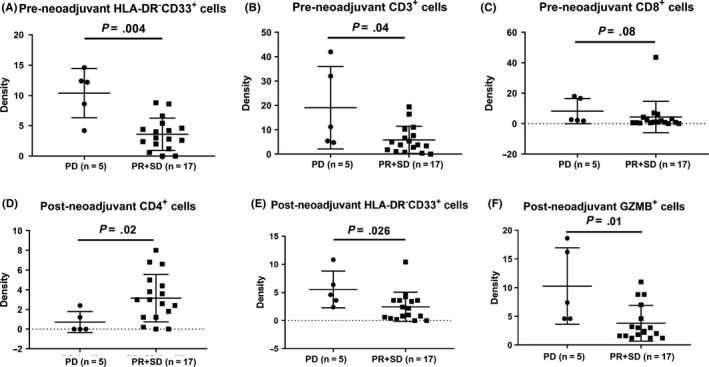
Comparison of the densities of tumor‐infiltrating immune cells in responders (partial response [PR] and stable disease [SD]) and non‐responders (progressive disease [PDf]). A‐C, Analysis in pre–neoadjuvant specimens, including myeloid‐derived suppressive cells (MDSC; HLA‐DR‐CD33+), CD3+ and CD8+ cells. D‐F, Results in post–neoadjuvant specimens, including CD4+, MDSC and GZMB+ cells. P‐values are calculated using the Wilcoxon test
4. DISCUSSION
Infiltrated immune cells in the tumor microenvironment play a vital role in tumorigenesis and tumor progression. 8 , 35 Therefore, understanding the immune microenvironmental status and the fractions of infiltrated immune cells may help optimize treatment strategies. Published results of the immune microenvironment of OS vary across different studies. 15 , 17 , 34 , 36 The limits of immunohistochemistry processing and analyzing methods may account for such discrepancies. In the present study, for the first time, we described a comprehensive atlas of infiltrated immune cell types in OS tissues based on public RNA expression data from the TARGET database, using the CIBERSORT algorithm. In accordance with previous reports, 37 macrophages were the predominant infiltrated immune cells in OS tissue, while CD8+ T cells were relatively infrequent. These results implied that OS is an immune “cold” tumor, which may partly explain the low response rate (5%) of pembrolizumab monotherapy in advanced OS in a recent clinical trial. 18 Because research has indicated that immune checkpoint inhibitors tend to be less efficient in immunologically “cold” tumors, 38 it is important to explore combination strategies that can convert OS into an immunologically “hot” tumor.
Infiltrated immune cells, as markers of the host immune response, are strong prognostic indicators of response to therapy and overall survival. 39 , 40 We found that infiltrated macrophages, both M1 and M2 subtypes, were associated with improved survival in OS, which was consistent with other studies. 41 , 42 Intriguingly, patients with good histologic response to neoadjuvant chemotherapy tended to have less M2 macrophages, although not statistically significantly (P = 0.081). Apart from histological response, tumor location, patient gender and metastatic status at diagnosis are identified as predictors of survival in OS patients. 33 , 43 Because various stages of patients from the TARGET database were involved in the analysis, such factors might contribute to the discrepancy between histologic response and survival prediction of M2 macrophages. In addition, another study has shown that infiltration of macrophages is associated with poorer 5‐year event‐free survival. 44 Owing to the heterogeneity of tumor‐associated macrophages, the mechanism underlying this correlation and the therapeutic significance of macrophages in OS warrant further study. A higher amount of naïve CD4+ T cells and resting NK cells was correlated with lung metastasis and poor survival. Resting NK cells are generally less lytic against target cells, which indicates week anti–tumor innate immunity. 45 Recent research showed that tumor‐infiltrated naïve CD4+ T could develop into Tregs, and, thus, contribute to immunosuppression in breast cancer. 46 Further research is needed to demonstrate the mechanisms of recruitment and function of this immune cell type in OS. Follicular helper CD4+ T cells, a subset of CD4+ T cells, have been reported to play a protective role in breast cancer and colorectal cancer, 47 , 48 which is consistent with the present study. In contrast, plasma cells are related to poor prognosis. Tumor‐educated plasma cells have been revealed to promote tumor progression through pathological IgG. 49 Although correlation analysis showed that a higher proportion of regulatory T cells indicated good histological response, and activated dendritic cells were associated with metastatic disease, the fractions and positive rates of both types of immune cells were too small to reflect the actual correlation. Therefore, these results should be interpreted with caution. In our present cohort, the density of MDSC in either pre–neoadjuvant or post–neoadjuvant chemotherapy was negatively correlated with treatment response. MDSC have emerged as a key contributor to tumor angiogenesis, drug resistance and promotion of tumor metastases. 50 Therefore, MDSC might be a promising immunotherapeutic target in OS. While pre–neoadjuvant CD3+ and post–neoadjuvant GZMB+ cells were more abundant in patients with PD, the deviation in the PD group (n = 5) was obviously too large. Therefore, a larger cohort is needed to better illustrate their clinical relevance.
Despite the great success of immune checkpoint blockades since 2014, most patients still do not respond to this type of immunotherapy. Possible mechanisms of resistance include defective tumor cell antigenicity owing to loss of MHC expression, lack of infiltrating lymphocytes, suppressive immune cells (e.g. Tregs and MDSC) and upregulation of other checkpoint molecules. 38 Numerous efforts are underway to identify potential treatment strategies with synergetic effects to “prime” patients for better immunological response. Among these strategies, chemotherapeutics are gaining increasing interest not only because they are fundamental treatment approaches for most tumors but also because of their complex influence on the TIME. Chemotherapeutic agents may activate host immune response by inducing tumor cell death and releasing tumor‐associated antigens. 19 , 20 In addition, chemotherapy can directly reprogram the suppressive immune microenvironment by depleting Tregs or MDSC. 21 , 24
To clarify the influence of chemotherapy on the immune microenvironment of OS, we analyzed the matched pre–neoadjuvant and post–neoadjuvant chemotherapy tumor tissues using IHC for a broad panel of immune cells. We also investigated the expression patterns of PD‐1 and PD‐L1. To our knowledge, this is the first study analyzing the transformation of immunological status after neoadjuvant chemotherapy in OS. We found that following neoadjuvant chemotherapy, CD3+ T cells increased significantly and there was a trend of increased cytotoxic T cells. CD8+ T cells in both tumor center and stroma also increased remarkably. Importantly, activated CD8+ T cells, defined as Ki67 + CD8+ T cells, were more abundant in post–chemotherapy samples, and were negatively correlated with the proliferation ability of tumor cells. Such results indicated that host anti–tumor immune response was boosted, and functional anti–tumor CD8+ T cells were recruited into the tumor microenvironment following neoadjuvant chemotherapy. In addition, we found that cycles of chemotherapy did not influence the immunogenic effects on CD3+ and HLA‐DR‐CD33+ cells. It is established that cytotoxic drugs target all replicating cells, thus having a negative effect on hematopoietic cells in the bone marrow. One possible explanation is that granulocyte colony stimulating factor (G‐CSF) is mandatory following IFO/cisplatin/doxorubicin to help white blood cells recover. In addition, Das et al 51 investigated the change in T‐cell phenotypes during chemotherapy in children with solid tumors and lymphomas. Interestingly, they found that the potential of T cells to respond to ex vivo stimulation remained steady over time in OS. These factors may partially explain why immune cells would be recruited into the tumor microenvironment after intense chemotherapy in OS.
The limited efficacy of pembrolizumab monotherapy in OS is primarily due to its low tumor mutation burden and immunogenic potential. 18 , 52 Our results, however, indicate that neoadjuvant chemotherapy can notably prime anti–tumor T‐cell responses and also alleviate the immunosuppressive condition by depleting immunosuppressive MDSC. Noteworthily, increased positivity of PD‐L1 in immune cells was observed. Previous research has shown that higher levels of PD‐L1 in immune cells is significantly associated with the likelihood of response to anti–PD‐L1 antibody. 53 Immunotherapies such as checkpoint inhibitors are believed to augment and prolong the immune responses activated by the immunogenic chemotherapy. However, the schedule of immunotherapy and chemotherapy would profoundly influence the outcomes of such combined treatment. 54 In the TONIC trial, anti–PD‐1 therapy was given following the induction treatment to achieve a synergic effect. 27 Preclinical and clinical studies have shown improved benefit of neoadjuvant immunotherapy treatment in cancer. 55 , 56 , 57 Thus, we suppose that immunotherapy, such as use of ICI, may be more effective in neoadjuvant settings in OS patients, and should be used concomitant with or following neoadjuvant chemotherapy before surgery resection. It is noteworthy that such immune‐modulatory effects are complicated. For example, Taxol has been reported to improve efficacy of ICI by activating M1 macrophages. However, prolonged exposure to taxol induces M2 macrophage polarization and restrains therapeutic response. 58 A phase II trial has assessed the scheduling of ipilimumab in combination with paclitaxel and carboplatin for patients with stage IV NSCLC. In this trial, ipilimumab was given either concurrently or sequentially following chemotherapy. Interestingly, an improvement in progression‐free survival was observed in the phased but not the concurrent arm comparing to chemotherapy alone. 59 Owing to such complicated effects of chemotherapy, it is necessary to optimize the timing of immunotherapy treatment in future clinical trials.
Several limitations of this study should be mentioned when interpreting these results. First, this was a retrospective study with inevitable selection bias. Second, the heterogeneous distribution of immune cells in tumor tissue remains unresolved, although we calculated the average number from five different fields. Third, due to the rareness of OS, the cohort was relatively small, which restrained the power of statistical analysis. Finally, owing to the short follow up in this cohort, we did not evaluate the association between the dynamic change in immune cells post–neoadjuvant chemotherapy and overall survival.
In summary, we described the distribution atlas of 22 immune cell types in OS primary tissues based on RNA expression data from the TARGET database, using the CIBERSORT algorithm. M2 macrophages were found to be the most abundant immune cell type and were associated with improved survival in OS. Application of neoadjuvant chemotherapy converted OS into an immunological “hot” tumor. The clinical impact of this transformation needs to be verified in future clinical trials of immunotherapy in OS.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Supporting information
Fig S1
Fig S2
Table S1
Table S2
Table S3
Appendix S1
ACKNOWLEDGMENTS
We thank Dr Xinke Zhang and Dr Linzhi Kong (Department of Pathology, SYSUCC) for IHC scoring. The raw data of this study were uploaded into the Research Data Deposit public platform (www.ressearchdata.org.cn). The RDD number for this dataset is RDDB2019000732. This study was supported by the National Natural Science Foundation of China (81872268, 81702665 and 81872183) and the Fundamental Research Funds for the Central Universities (19ykpy189).
Deng C, Xu Y, Fu J, et al. Reprograming the tumor immunologic microenvironment using neoadjuvant chemotherapy in osteosarcoma. Cancer Sci. 2020;111:1899–1909. 10.1111/cas.14398
Chuangzhong Deng, Yanyang Xu, Jianchang Fu and Xiaojun Zhu contributed equally to this work.
Contributor Information
Qinglian Tang, Email: tangql@sysucc.org.cn.
Wenlin Huang, Email: huangwl@sysucc.org.cn.
Jin Wang, Email: wangjinbs@sysucc.org.cn.
REFERENCES
- 1. Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: current treatment and a collaborative pathway to success. J Clin Oncol. 2015;33:3029‐3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115:1531‐1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bacci G, Mercuri M, Longhi A, et al. Grade of chemotherapy‐induced necrosis as a predictor of local and systemic control in 881 patients with non‐metastatic osteosarcoma of the extremities treated with neoadjuvant chemotherapy in a single institution. Eur J Cancer. 2005;41:2079‐2085. [DOI] [PubMed] [Google Scholar]
- 4. Meyers PA, Schwartz CL, Krailo MD, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival–a report from the Children's Oncology Group. J Clin Oncol. 2008;26:633‐638. [DOI] [PubMed] [Google Scholar]
- 5. Bielack SS, Kempf‐Bielack B, Branscheid D, et al. Second and subsequent recurrences of osteosarcoma: presentation, treatment, and outcomes of 249 consecutive cooperative osteosarcoma study group patients. J Clin Oncol. 2009;27:557‐565. [DOI] [PubMed] [Google Scholar]
- 6. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen DS, Mellman I. Oncology meets immunology: the cancer‐immunity cycle. Immunity. 2013;39:1‐10. [DOI] [PubMed] [Google Scholar]
- 8. Binnewies M, Roberts EW, Kersten K, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Draghiciu O, Lubbers J, Nijman HW, Daemen T. Myeloid derived suppressor cells—an overview of combat strategies to increase immunotherapy efficacy. Oncoimmunology. 2015;4:e954829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression – implications for anticancer therapy. Nature reviews Clinical oncology. 2019;16:356‐371. [DOI] [PubMed] [Google Scholar]
- 11. Jain A, Zhang Q, Toh HC. Awakening immunity against cancer: a 2017 primer for clinicians. Chin J Cancer. 2017;36:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137‐148. [DOI] [PubMed] [Google Scholar]
- 13. Riaz N, Havel JJ, Makarov V, et al. Tumor and microenvironment evolution during Immunotherapy with Nivolumab. Cell. 2017;171:e916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18:197‐218. [DOI] [PubMed] [Google Scholar]
- 15. Palmerini E, Agostinelli C, Picci P, et al. Tumoral immune‐infiltrate (IF), PD‐L1 expression and role of CD8/TIA‐1 lymphocytes in localized osteosarcoma patients treated within protocol ISG‐OS1. Oncotarget. 2017;8:111836‐111846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alves PM, de Arruda JAA, Arantes DAC, et al. Evaluation of tumor‐infiltrating lymphocytes in osteosarcomas of the jaws: a multicenter study. Virchows Arch. 2019;474:201‐207. [DOI] [PubMed] [Google Scholar]
- 17. Shen JK, Cote GM, Choy E, et al. Programmed cell death ligand 1 expression in osteosarcoma. Cancer Immunol Res. 2014;2:690‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tawbi HA, Burgess M, Bolejack V, et al. Pembrolizumab in advanced soft‐tissue sarcoma and bone sarcoma (SARC028): a multicentre, two‐cohort, single‐arm, open‐label, phase 2 trial. Lancet Oncol. 2017;18:1493‐1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen G, Emens LA. Chemoimmunotherapy: reengineering tumor immunity. Cancer Immunol Immunother. 2013;62:203‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39:74‐88. [DOI] [PubMed] [Google Scholar]
- 21. de Biasi AR, Villena‐Vargas J, Adusumilli PS. Cisplatin‐induced antitumor immunomodulation: a review of preclinical and clinical evidence. Clin Cancer Res. 2014;20:5384‐5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scurr M, Pembroke T, Bloom A, et al. Low‐dose cyclophosphamide induces antitumor T‐cell responses, which associate with survival in metastatic colorectal cancer. Clin Cancer Res. 2017;23:6771‐6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao J, Cao Y, Lei Z, Yang Z, Zhang B, Huang B. Selective depletion of CD4+CD25+Foxp3+ regulatory T cells by low‐dose cyclophosphamide is explained by reduced intracellular ATP levels. Can Res. 2010;70:4850‐4858. [DOI] [PubMed] [Google Scholar]
- 24. Alizadeh D, Trad M, Hanke NT, et al. Doxorubicin eliminates myeloid‐derived suppressor cells and enhances the efficacy of adoptive T‐cell transfer in breast cancer. Can Res. 2014;74:104‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Casares N, Pequignot MO, Tesniere A, et al. Caspase‐dependent immunogenicity of doxorubicin‐induced tumor cell death. J Exp Med. 2005;202:1691‐1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lo CS, Sanii S, Kroeger DR, et al. Neoadjuvant chemotherapy of ovarian cancer results in three patterns of tumor‐infiltrating lymphocyte response with distinct implications for immunotherapy. Clin Cancer Res. 2017;23:925‐934. [DOI] [PubMed] [Google Scholar]
- 27. Voorwerk L, Slagter M, Horlings HM, et al. Immune induction strategies in metastatic triple‐negative breast cancer to enhance the sensitivity to PD‐1 blockade: the TONIC trial. Nat Med. 2019;25:920‐928. [DOI] [PubMed] [Google Scholar]
- 28. Newman AM, Liu CL, Green MR, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12:453‐457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228‐247. [DOI] [PubMed] [Google Scholar]
- 30. Bajpai J, Gamnagatti S, Kumar R, et al. Role of MRI in osteosarcoma for evaluation and prediction of chemotherapy response: correlation with histological necrosis. Pediatr Radiol. 2011;41:441‐450. [DOI] [PubMed] [Google Scholar]
- 31. Deng C, Li Z, Guo S, et al. Tumor PD‐L1 expression is correlated with increased TILs and poor prognosis in penile squamous cell carcinoma. Oncoimmunology. 2016;6:e1269047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gide TN, Silva IP, Quek C, et al. Close proximity of immune and tumor cells underlies response to anti–PD‐1 based therapies in metastatic melanoma patients. Oncoimmunology. 2020;9:1659093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smeland S, Bielack SS, Whelan J, et al. Survival and prognosis with osteosarcoma: outcomes in more than 2000 patients in the EURAMOS‐1 (European and American Osteosarcoma Study) cohort. Eur J Cancer. 1990;2019:36‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sundara YT, Kostine M, Cleven AH, Bovee JV, Schilham MW, Cleton‐Jansen AM. Increased PD‐L1 and T‐cell infiltration in the presence of HLA class I expression in metastatic high‐grade osteosarcoma: a rationale for T‐cell‐based immunotherapy. Cancer Immunol Immunother. 2017;66:119‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dai J, Lu YI, Roca H, et al. Immune mediators in the tumor microenvironment of prostate cancer. Chin J Cancer. 2017;36:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fritzsching B, Fellenberg J, Moskovszky L, et al. CD8(+)/FOXP3(+)‐ratio in osteosarcoma microenvironment separates survivors from non‐survivors: a multicenter validated retrospective study. OncoImmunology. 2015;4:e990800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Inagaki Y, Hookway E, Williams KA, et al. Dendritic and mast cell involvement in the inflammatory response to primary malignant bone tumours. Clinical Sarcoma Res. 2016;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kon E, Benhar I. Immune checkpoint inhibitor combinations: current efforts and important aspects for success. Drug Resist Updat. 2019;45:13‐29. [DOI] [PubMed] [Google Scholar]
- 39. Denkert C, von Minckwitz G, Darb‐Esfahani S, et al. Tumour‐infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19:40‐50. [DOI] [PubMed] [Google Scholar]
- 40. Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. 2019;19:133‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dumars C, Ngyuen J‐M, Gaultier A, et al. Dysregulation of macrophage polarization is associated with the metastatic process in osteosarcoma. Oncotarget. 2016;7:78343‐78354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Buddingh EP, Kuijjer ML, Duim RAJ, et al. Tumor‐infiltrating macrophages are associated with metastasis suppression in high‐grade osteosarcoma: a rationale for treatment with macrophage activating agents. Clin Cancer Res. 2011;17:2110‐2119. [DOI] [PubMed] [Google Scholar]
- 43. Whelan JS, Jinks RC, McTiernan A, et al. Survival from high‐grade localised extremity osteosarcoma: combined results and prognostic factors from three European Osteosarcoma Intergroup randomised controlled trials. Ann Oncol. 2012;23:1607‐1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Koirala P, Roth ME, Gill J, et al. Immune infiltration and PD‐L1 expression in the tumor microenvironment are prognostic in osteosarcoma. Sci Rep. 2016;6:30093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006;107:159‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Su S, Liao J, Liu J, et al. Blocking the recruitment of naive CD4(+) T cells reverses immunosuppression in breast cancer. Cell Res. 2017;27:461‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bindea G, Mlecnik B, Tosolini M, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782‐795. [DOI] [PubMed] [Google Scholar]
- 48. Gu‐Trantien C, Loi S, Garaud S, et al. CD4(+) follicular helper T cell infiltration predicts breast cancer survival. J Clin Investig. 2013;123:2873‐2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gu Y, Liu Y, Fu LI, et al. Tumor‐educated B cells selectively promote breast cancer lymph node metastasis by HSPA4‐targeting IgG. Nat Med. 2019;25:312‐322. [DOI] [PubMed] [Google Scholar]
- 50. Gabrilovich DI. Myeloid‐derived suppressor cells. Cancer Immunol Res. 2017;5:3‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Das RK, Vernau L, Grupp SA, Barrett DM. Naive T‐cell deficits at diagnosis and after chemotherapy impair cell therapy potential in pediatric cancers. Cancer Discov. 2019;9:492‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gröbner SN, Worst BC, Weischenfeldt J, et al. The landscape of genomic alterations across childhood cancers. Nature. 2018;555:321‐327. [DOI] [PubMed] [Google Scholar]
- 53. Herbst RS, Soria J‐C, Kowanetz M, et al. Predictive correlates of response to the anti–PD‐L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563‐567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rothschilds AM, Wittrup KD. What, why, where, and when: bringing timing to immuno‐oncology. Trends Immunol. 2019;40:12‐21. [DOI] [PubMed] [Google Scholar]
- 55. Amaria RN, Reddy SM, Tawbi HA, et al. Neoadjuvant immune checkpoint blockade in high‐risk resectable melanoma. Nat Med. 2018;24:1649‐1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cloughesy TF, Mochizuki AY, Orpilla JR, et al. Neoadjuvant anti–PD‐1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25:477‐486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liu J, Blake SJ, Yong MCR, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov. 2016;6:1382‐1399. [DOI] [PubMed] [Google Scholar]
- 58. Garassino MC, Torri V, Colombo MP, Sica A. Choosing the best chemotherapy agent to boost immune checkpoint inhibition activity. Can Res. 2018;78:5729‐5730. [DOI] [PubMed] [Google Scholar]
- 59. Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first‐line treatment in stage IIIB/IV non‐small‐cell lung cancer: results from a randomized, double‐blind, multicenter phase II study. J Clin Oncol. 2012;30:2046‐2054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Table S1
Table S2
Table S3
Appendix S1


