Abstract
Background
To explore the association between the ratio of tricuspid annular plane systolic excursion (TAPSE) and pulmonary arterial systolic pressure (PASP), and long- and short-term outcomes in mechanically ventilated septic shock patients.
Methods
Septic shock patients admitted to the intensive care unit (ICU) were screened for enrollment. Echocardiographic parameters including TAPSE and tricuspid regurgitation velocity, haemodynamic and respiratory parameters, and prognostic data were obtained.
Results
One hundred eighteen subjects were enrolled in this study, among whom 75 survived and 43 died at the one-year follow-up. ROC curve analysis revealed that the TAPSE/PASP ratio was able to assess one-year all-cause mortality with an area under the curve of 0.817 (95% CI: 0.739–0.896, p < 0.001) and the optimal cutoff value was 0.50 mm/mmHg. Kaplan-Meier survival analysis showed that one-year all-cause mortality was significantly higher in patients with TAPSE/PASP ≤0.5 mm/mmHg than in patients with TAPSE/PASP > 0.5 mm/mmHg (log-rank 32.934, p < 0.001). According to the Cox regression survival analyses, the TAPSE/PASP ratio was independently associated with one-year all-cause mortality (HR 0.007, 95% CI:0.000–0.162, p = 0.002) and ICU mortality (HR 0.027, 95% CI:0.001–0.530, p = 0.017). According to the multivariable analysis, the TAPSE/PASP ratio was an independent variable associated with mechanical ventilation (MV) duration (standard coefficient − 0.240, p = 0.010).
Conclusion
The TAPSE/PASP ratio demonstrated prognostic value for one-year all-cause mortality, ICU mortality and MV duration in mechanically ventilated septic shock patients.
Keywords: Echocardiography, Tricuspid annular plane systolic excursion, Pulmonary arterial pressure, Septic shock, Prognosis
Introduction
Septic shock is a major health problem, affecting millions of people around the world each year and having high morbidity and mortality [1]. Septic cardiomyopathy, which is usually diagnosed via echocardiography, has a high incidence in septic shock patients. Although the definition of septic cardiomyopathy is based on the left ventricular ejection fraction (LVEF), both ventricles can be affected [2, 3]. Furthermore, it has been increasingly recognized that right ventricular (RV) systolic dysfunction is associated with long-term prognosis in septic patients [4, 5].
The right ventricle (RV) is anatomically and functionally different from the left ventricle and is more prone to be affected by the alterations in afterload [6]. In addition to the decrease in intrinsic RV contractile function in septic shock, an increase in RV afterload is also common, resulting from complications including acute respiratory distress syndrome (ARDS), concomitant LV dysfunction, or positive pressure ventilation [7–9]. Winkelhorst and his colleagues, in a recent study, indicated that RVEF was associated with one-year mortality in septic patients. Their data also showed that the pulmonary arterial pressure was significantly higher in patients with low RVEF than in patients with high RVEF [5]. Thus, a combined assessment of RV systolic function and its afterload by right ventricular-pulmonary arterial coupling would potentially provide additional physiological information in septic patients.
The ratio of tricuspid annular plane systolic excursion (TAPSE) and pulmonary arterial systolic pressure (PASP) is deemed as an indicator of right ventricular-pulmonary arterial coupling [10]. TAPSE is a simple and reproducible parameter of RV systolic function with low interobserver variability, even in patients with raised right-sided pressures [11, 12]. PASP can be reliably determined from the peak tricuspid regurgitation velocity in the majority of patients [13]. The TAPSE/PASP ratio was found to be associated with mortality in patients with pulmonary arterial hypertension or heart failure [10, 14–16]. However, whether TAPSE/PASP ratio is related to the prognosis of septic shock patients has not been investigated. We hypothesize that right ventricular-pulmonary arterial coupling can also be compromised in septic shock and that the TAPSE/PASP ratio is of prognostic value among these patients. Accordingly, the aim of the present study was to explore the association between the TAPSE/PASP ratio and the long- and short-term prognoses of mechanically ventilated septic shock patients.
Patients and methods
Study population
This prospective observational study was conducted at a tertiary hospital intensive care unit (ICU). Patients admitted from 1 May 2017 to 1 October 2018 were screened for enrolment within the first 24 h after admission.
We enrolled septic shock patients who were on mechanical ventilation. Septic shock was defined as sepsis with persisting hypotension requiring vasopressors to maintain a mean arterial pressure equal to or above 65 mmHg and having a serum lactate level above 2 mmol/L despite adequate volume resuscitation [17].
Patients were excluded if they had any of the following criteria: age less than 18 years; acute coronary syndrome within 1 week; rhythm characteristics of atrial fibrillation; prosthetic valves or valvular diseases such as severe mitral, aortic or tricuspid stenosis or regurgitation; moderate to severe chronic pulmonary hypertension; an inadequate echocardiographic images for measurement; no monitoring of central venous pressure (CVP); without informed consent; withholding of life support; and loss of follow up.
The study was conducted in compliance with the Declaration of Helsinki and was approved by the ethics committee of our institution (Approval No. ZS-1422). Informed consent was obtained from the next of kin.
Echocardiography
Echocardiograms were recorded within the first 24 h of ICU admission using an echocardiograph (X-Porte, SonoSite, USA) with a 2.5-MHz phased-array probe. Images were saved for offline analysis. Two physicians who were experienced in echocardiography performed the echo examination. Electrocardiograms were recorded continuously during the examination. Three cardiac cycles were analysed and averaged. M-mode and Doppler echocardiographic measurements were taken according to standard protocols.
TAPSE was obtained in the apical 4-chamber view by positioning the M-mode cursor along the lateral part of the tricuspid valve ring and measuring the difference between the highest and lowest points of the M-mode sinusoid wave [18]. The right ventricular outflow tract (RVOT) dimensions were obtained from the parasternal short-axis view at the level of the aortic root. The velocity of tricuspid regurgitation (TR) was measured in the RV inflow view, apical 4-chamber view and aortic short-axis view via continuous wave Doppler, and the highest value was chosen. The pulmonary arterial systolic pressure (PASP) was calculated by the following equation: PASP = 4 × (TR velocity)2 + CVP [13]. RVOT fractional shortening (RVOT-FS) was calculated as (dimension at end-diastole – end-systole)/end-diastole [19]. The left ventricular ejection fraction (LVEF) was obtained using a modified biplane Simpson’s method from the apical two- and four-chamber views. The mitral annular plane systolic excursion (MAPSE) was obtained from the apical 4-chamber view by positioning the cursor along the lateral mitral ring. The mitral e’ velocity was measured with tissue Doppler imaging by placing the sample volume on the lateral and medial mitral annulus, and the averaged value from both annuli was chosen.
Other parameters collected
Demographic information and the diagnosis, Acute Physiology and Chronic Health Evaluation (APACHE) II score, Sequential Organ Failure Assessment (SOFA) score, comorbidities, maximum norepinephrine (NE) dose, arterial blood lactate level at ICU admission and the timing of the echo examination were collected for all patients. Each patient’s heart rate (HR), mean arterial pressure (MAP), CVP, total fluid infusion, positive end-expiratory pressure (PEEP), and plateau pressure (Pplat) were also collected at the time of the echo examination.
Endpoints
The primary outcome was one-year all-cause mortality. The secondary outcomes included ICU mortality, ICU length of stay, and mechanical ventilation (MV) duration. One-year mortality was obtained via a telephone survey of the relatives. The other data were obtained from the medical records.
Statistical analyses
The statistical analysis was performed using the statistical software package SPSS 13.0 (SPSS, Inc., Chicago, Illinois, USA). Continuous variables are expressed as the mean ± SD or as the median and the interquartile range. Categorical variables are presented as frequencies and percentages. The distributions of the continuous values were assessed for normality by the Kolmogorov-Smirnov test. Differences among groups were assessed by Student’s unpaired t test, the Mann-Whitney U test, the Kruskal-Wallis test, the chi-squared test, or Fisher’s exact test, as appropriate. Spearman’s correlation coefficients and their corresponding p values were calculated to assess the variable relationships. Receiver operating characteristic (ROC) curve analysis was used to identify the optimal cutoff value of the TAPSE/PASP ratio in the assessment of one-year all-cause mortality. Cumulative survival curves of the one-year follow-up were estimated with the Kaplan-Meier method, and the effect of the TAPSE/PASP ratio on the survival probability was compared between groups using a log-rank test. Prognostic factors for one-year all-cause mortality and ICU mortality were determined using the Cox regression model. The following variables were considered for the survival analysis: age, APACHEII, SOFA, NE dose, Pplat, TAPSE, PASP, TAPSE/ PASP ratio, RVOT-FS, LVEF, MAPSE, and e’. The variables that had p < 0.25 in the univariable model were included in the multivariable model and the hazard ratio was calculated, together with its 95% confidence intervals. Given the collinearity between TAPSE, PASP and the TAPSE/PASP ratio, separate Cox regression models were performed. Multivariable linear regression analysis was performed to assess the independent associations of the general characteristics and the echocardiographic parameters with MV duration and ICU length of stay. As MV duration and ICU length of stay did not fit a Gaussian distribution, a logarithm was taken. Variables were assessed for collinearity prior to inclusion in the model. Intra-observer and interobserver variabilities in TAPSE, RVOT-FS, LVEF, MAPSE, and e’ were assessed in 20 randomly selected patients and were tested using both paired t tests and intraclass correlation coefficients (ICCs). An ICC > 0.8 was considered excellent agreement. Two-tailed p < 0.05 was considered significant.
Results
Measurement variability
The intra-observer variabilities in TAPSE, RVOT-FS, LVEF, MAPSE and mitral e’ velocity were minimal. The interobserver variability analysis revealed that the ICCs for TAPSE, TR velocity, RVOT-FS, LVEF, MAPSE, and e’ were 0.966 (95% CI: 0.915–0.987),0.926 (95% CI: 0.743–0.974) 0.910 (95% CI: 0.744–0.970), 0.900 (95% CI: 0.759–0.960), 0.959 (95% CI: 0.891–0.985), and 0.924 (95% CI: 0.863–0.979), respectively.
General characteristics of all patients
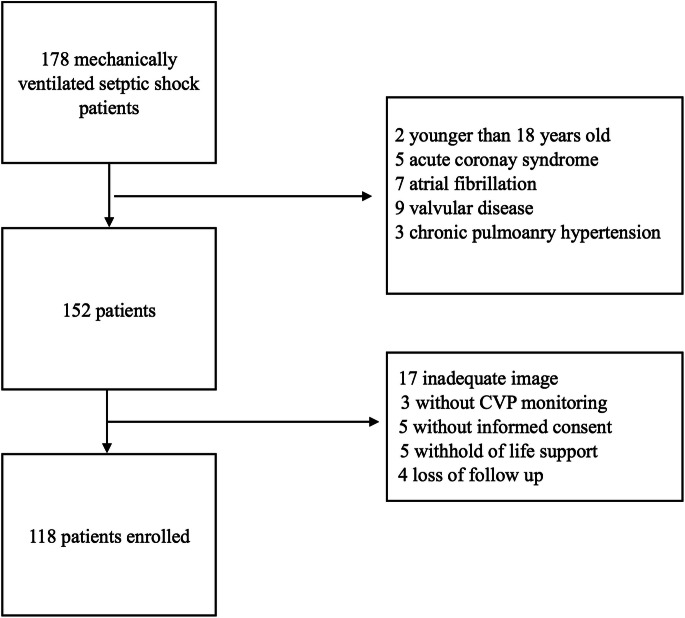
A total of 178 patients were screened for enrolment. Sixty patients were excluded because of diagnoses that could be confounding factors, inadequate images, a lack of informed consent, or withholding of life support or because they were lost to follow-up (Fig. 1). One hundred eighteen patients were enrolled in this study, among whom 75 survived and 43 died at the one-year follow-up. The general characteristics are listed in Table 1. The survivors and non-survivors had similar ages, sex proportions, diagnoses and comorbidities. Compared to survivors, non-survivors had higher APACHE II scores (p < 0.001) and SOFA scores (p < 0.001) as well as higher maximum NE doses (p = 0.001).
Fig. 1.
Flow chart of the study
Table 1.
General characteristics
| Categories | Survivors (n = 75) | Non-survivors (n = 43) | p value |
|---|---|---|---|
| Age (yr) | 59.3 ± 15.3 | 67.6 ± 14.1 | 0.004 |
| Sex (male, %) | 47 (62.6%) | 29 (67.4%) | 0.590 |
| APACHE II | 18 (14, 22) | 26 (19, 30) | < 0.001 |
| SOFA | 12 (10, 13) | 14 (12, 16) | < 0.001 |
| Diagnosis (n, %) | |||
| Pneumonia | 15 (20.0%) | 13 (30.2%) | 0.208 |
| Abdominal infection | 38 (50.7%) | 17 (39.5%) | 0.250 |
| Biliary tract infection | 4 (5.3%) | 2 (4.7%) | 0.863 |
| CRBSI | 4 (5.3%) | 2 (4.7%) | 0.863 |
| Cellulitis | 10 (13.3%) | 5 (11.6%) | 0.802 |
| Others* | 4 (5.3%) | 4 (9.3%) | 0.405 |
| Comorbidities | |||
| HTN | 36 (48.0%) | 25 (58.1%) | 0.284 |
| DM | 28 (37.3%) | 22 (51.2%) | 0.143 |
| CAD | 14 (18.7%) | 13 (30.2%) | 0.147 |
| CKD | 5 (6.7%) | 6 (14.0%) | 0.194 |
| COPD | 7 (9.3%) | 8 (18.6%) | 0.151 |
| Timing of echo (hr from admission) | 12 (8,18) | 13 (9, 16) | 0.905 |
| Maximum NE dose (μg/kg/min) | 0.36 (0.18, 0.75) | 0.81 (0.40, 1.61) | 0.001 |
| Fluid administered (ml) | 2560 (1366, 3140) | 2880 (1629, 3530) | 0.766 |
| Lactate (mmol/L) | 3.5 (2.7, 4.2) | 4.0 (3.3, 4.9) | 0.185 |
| MV duration (hr) | 108 (67, 240) | 203 (105, 340) | 0.051 |
| ICU length of stay (day) | 7 (4, 12) | 10 (6, 15) | 0.233 |
TAPSE tricuspid annular plane systolic excursion, APACHE acute physiology and chronic health evaluation, SOFA sequential organ failure assessment, UTI urinary tract infection, CRBSI catheter related bloodstream infection, HTN hypertension, DM diabetes mellitus, CAD coronary arterial disease, CKD chronic kidney dysfunction, COPD chronic obstructive pulmonary disease, NE norepinephrine, ICU intensive care unit
*Others including urinary tract infection, intracranial, mediastinum infections
Comparison of haemodynamic, respiratory and echocardiographic parameters between the survivors and non-survivors
The survivors and non-survivors had similar HR, MAP and PEEP levels. The Pplat level was higher in non-survivors than in survivors (p = 0.031). The CVP level was higher in non-survivors than in survivors, but the difference was not statistically significant (p = 0.074). Compared to survivors, non-survivors had lower TAPSE (p < 0.001), higher PASP (p = 0.003) and a lower TAPSE/PASP ratio (p < 0.001); non-survivors also had lower RVOT-FS (p = 0.042), MAPSE (p = 0.012) and mitral e’ velocity values (p = 0.043) (Table 2).
Table 2.
Haemodynamic, respiratory and echocardiographic parameters of the two groups
| Survivors (n = 75) | Non-survivors (n = 43) | p Value | |
|---|---|---|---|
| HR (bpm) | 89 (80, 101) | 107 (78, 116) | 0.174 |
| MAP (mmHg) | 82 ± 4 | 80 ± 9 | 0.407 |
| CVP (mmHg) | 9 (8, 12) | 10 (9, 13) | 0.074 |
| PEEP (cmH2O) | 5 (5, 8) | 6 (5, 8) | 0.440 |
| Pplat (cmH2O) | 18 (16, 20) | 19 (17, 22) | 0.031 |
| TAPSE (mm) | 19.5 ± 5.1 | 15.1 ± 4.8 | < 0.001 |
| PASP (mmHg) | 32.0 ± 9.6 | 37.9 ± 11.0 | 0.003 |
| TAPSE/PASP (mm/mmHg) | 0.61 (0.50, 0.81) | 0.39 (0.31, 0.53) | < 0.001 |
| RVOT-FS (%) | 43 (34, 55) | 38 (30, 48) | 0.042 |
| LVEF (%) | 58 ± 13 | 57 ± 15 | 0.244 |
| MAPSE (mm) | 14.6 ± 4.6 | 9.9 ± 0.8 | 0.012 |
| e’ (cm/s) | 8.3 (6.9–10.6) | 7.5 (4.6, 9.1) | 0.043 |
HR heart rate, MAP mean arterial pressure, CVP central venous pressure, PEEP positive end-expiratory pressure, Pplat plateau pressure, TAPSE tricuspid annular plane systolic excursion, PASP pulmonary arterial systolic pressure, RVOT-FS right ventricular outflow tract fractional shortening, LVEF left ventricular ejection fraction, MAPSE mitral annular plane systolic excursion; e’: mitral e’ velocity
Correlation between TAPSE and PASP and the relationship between the TAPSE/PASP ratio and TAPSE, RVOT-FS, LVEF, and MAPSE
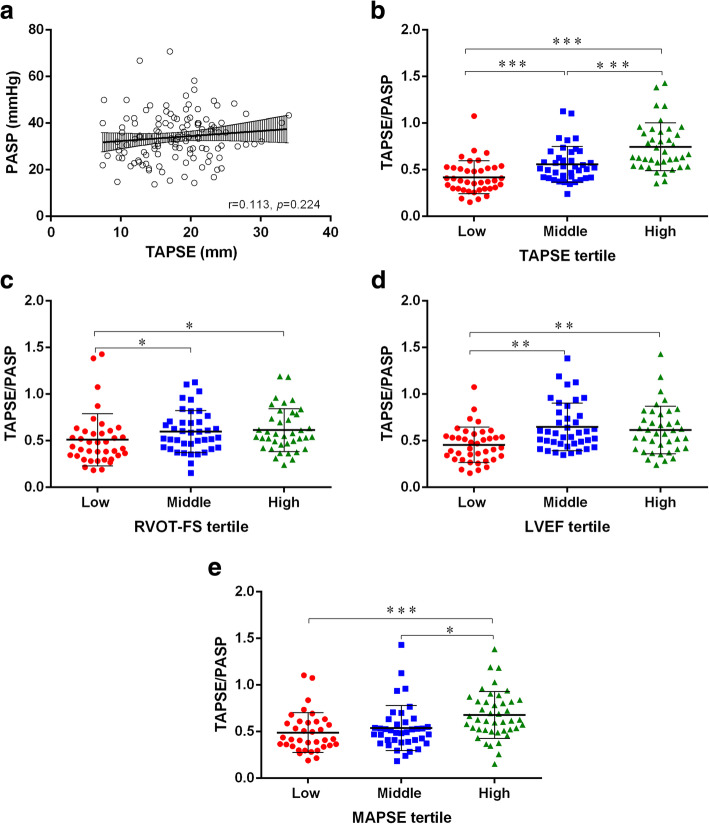
TAPSE was not associated with PASP (r = 0.113, p = 0.224) (Fig. 2a). We divided patients into three tertiles according to TAPSE, RVOT-FS, LVEF and MAPSE. The TAPSE/PASP ratio was able to differentiate between tertiles of TAPSE (Fig. 2b). Patients with low RVOT-FS had a lower TAPSE/PASP ratio than those with middle and high RVOT-FS (Fig. 2c). Patients with low LVEF had a lower TAPSE/PASP ratio than those with middle and high LVEF (Fig. 2d). Compared with the high tertile, the low MAPSE tertile demonstrated a significantly lower TAPSE/PASP ratio (Fig. 2e).
Fig. 2.
Correlation between TAPSE and PASP, relationships of TAPSE/PASP ratio with TAPSE, RVOT-FS, LVEF, and MAPSE. a. Correlation between the TAPSE and PASP. TAPSE was not associated with PASP, r = 0.113, p = 0.224. b. Relationship of TAPSE/PASP ratio with TAPSE tertiles (low: TAPSE ≤15.0 mm; middle: TAPSE 15.1 mm–19.9 mm; high: TAPSE ≥20.0 mm). c. Relationship of TAPSE/PASP ratio with RVOT-FS tertiles (low: RVOT-FS ≤ 34%; middle: RVOT-FS 35–47%; high: RVOT-FS ≥ 48%). d. Relationship of TAPSE/PASP ratio with LVEF tertiles (low: LVEF ≤50%; middle: LVEF 51–62%; high: LVEF ≥63%). e. Relationship of TAPSE/PASP ratio with MAPSE tertiles (low: MAPSE ≤11.0 mm; middle: MAPSE 11.1 mm–14.0 mm; high: MAPSE ≥14.1 mm). Lines in b-e indicate median and interquartile range, * p < 0.05, ** p < 0.01, *** p < 0.001(Kruskal-Wallis test). TAPSE: tricuspid annular plane systolic excursion; PASP: pulmonary arterial systolic pressure; RVOT-FS: right ventricular outflow tract fractional shortening; LVEF: left ventricular ejection fraction; MAPSE: mitral annular plane systolic excursion
Primary outcome
To determine the cutoff value of the TAPSE/PASP ratio in the assessment of one-year all-cause mortality, ROC curves were generated (Fig. 3). The area under the curve (AUC) for the TAPSE/PASP ratio in order to assess one-year all-cause mortality was 0.817 (95% CI: 0.739–0.896, p < 0.001) and the optimal cutoff value was 0.50 mm/mmHg (Table 3).
Fig. 3.
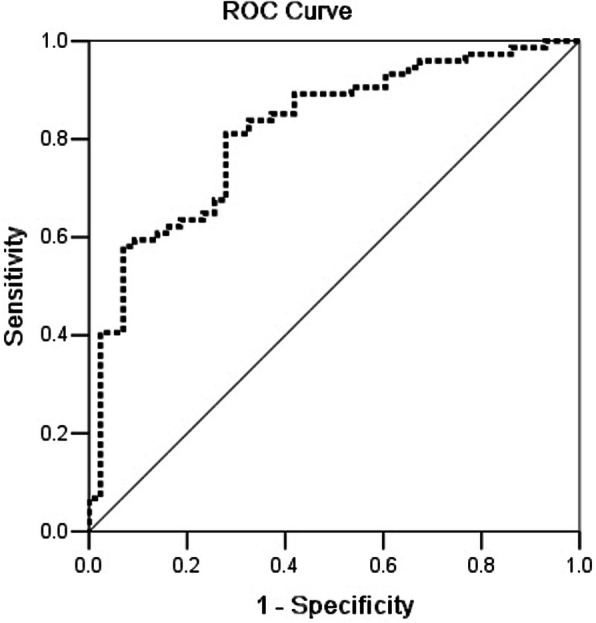
ROC curve analysis of TAPSE/PASP ratio for one-year all-cause mortality. The area under the curve was 0.817 (95% CI: 0.739–0.896, p < 0.001). TAPSE: tricuspid annular plane systolic excursion; PASP: pulmonary arterial systolic pressure
Table 3.
ROC analysis of TAPSE/PASP ratio in the prediction of one-year all-cause mortality
| AUC | 95%CI | p | Optimal cutoff | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|---|---|
| TAPSE/PASP (mm/mmHg) | 0.817 | 0.739–0.896 | < 0.001 | 0.50 | 74.3% | 72.1% | 60.4% | 83.1% |
TAPSE tricuspid annular plane systolic excursion, PASP pulmonary arterial systolic pressure, AUC area under curve, PPV positive predictive value, NPV negative predictive value
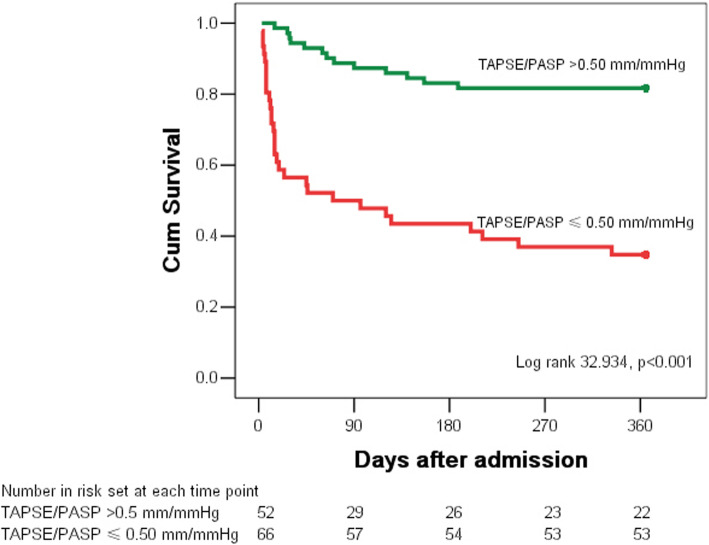
At the one-year follow-up, 19.7% (13/66) of patients with TAPSE/PASP > 0.50 mm/mmHg died; whereas 57.7% (30/52) of patients with TAPSE/PASP ≤0.50 mm/mmHg died. The Kaplan-Meier curves for estimated survival showed that one-year all-cause mortality was significantly higher in patients with TAPSE/PASP ≤0.50 mm/mmHg than in patients with TAPSE/PASP > 0.50 mm/mmHg (log-rank: 32.934, p < 0.001) (Fig. 4).
Fig. 4.
The Kaplan-Meier curves for estimated survival showed that one-year all-cause mortality was significantly higher in patients with TAPSE/PASP ≤0.50 mm/mmHg than in patients with TAPSE/PASP > 0.50 mm/mmHg (log-rank: 32.934, p < 0.001). TAPSE: tricuspid annular plane systolic excursion; PASP: pulmonary arterial systolic pressure
According to the Cox regression survival analysis, after adjusting for age, APACHE II, SOFA, NE dose, Pplat, RVOT-FS, MAPSE, LVEF and e’, the TAPSE/PASP ratio was independently associated with one-year all-cause mortality (HR 0.007, 95% CI:0.000–0.162, p = 0.002) (Table 4).
Table 4.
Factors associated with one-year all-cause mortality
| Hazard Ratio | 95%CI | p Value | |
|---|---|---|---|
| Univariable analysis | |||
| Age | 1.030 | 1.008–1.053 | 0.009 |
| APACHEII | 1.087 | 1.050–1.125 | < 0.001 |
| SOFA | 1.202 | 1.089–1.327 | < 0.001 |
| NE dose | 1.897 | 1.348–2.671 | 0.001 |
| Pplat | 1.101 | 1.018–1.192 | 0.017 |
| CVP | 1.083 | 0.992–1.182 | 0.074 |
| TAPSE | 0.269 | 0.144–0.502 | < 0.001 |
| PASP | 1.047 | 1.018–1.077 | 0.001 |
| TAPSE/PASP | 0.006 | 0.001–0.044 | < 0.001 |
| RVOT-FS | 0.980 | 0.956–1.004 | 0.096 |
| MAPSE | 0.350 | 0.141–0.870 | 0.024 |
| LVEF | 0.987 | 0.965–1.009 | 0.239 |
| e’ | 0.882 | 0.792–0.982 | 0.022 |
| Multivariable analysis 1 | |||
| LVEF | 1.019 | 0.985–1.055 | 0.272 |
| APACHEII | 1.064 | 1.008–1.123 | 0.025 |
| PASP | 1.046 | 1.012–1.081 | 0.008 |
| TAPSE | 0.248 | 0.083–0.741 | 0.013 |
| Multivariable analysis 2 | |||
| LVEF | 1.018 | 0.986–1.051 | 0.271 |
| APACHEII | 1.058 | 1.006–1.113 | 0.029 |
| SOFA | 1.168 | 1.027–1.327 | 0.018 |
| TAPSE/PASP | 0.007 | 0.000–0.162 | 0.002 |
APACHE acute physiology and chronic health evaluation, SOFA sequential organ failure assessment, NE norepinephrine, Pplat plateau pressure, CVP central venous pressure, TAPSE tricuspid annular plane systolic excursion, PASP pulmonary arterial systolic pressure, RVOT-FS right ventricular outflow tract fractional shortening, LVEF left ventricular ejection fraction, MAPSE mitral annular plane systolic excursion; e’: mitral e’ velocity
Secondary outcomes
The ICU mortality rates in patients with TAPSE/PASP > 0.50 mm/mmHg and patients with TAPSE/PASP ≤0.50 mm/mmHg were 6.1% (4/66) and 40.4% (21/52), respectively. The Cox regression analysis showed that the TAPSE/PASP ratio was independently associated with ICU mortality (HR 0.027, 95% CI:0.001–0.530, p = 0.017) (Supplemental Table 1). According to the multivariable analysis, the TAPSE/PASP ratio was not an independent variable associated with ICU length of stay but was independently associated with MV duration (standard coefficient − 0.240, p = 0.010) (Supplemental Tables 2, 3).
Discussion
In this study, we assessed the TAPSE/PASP ratio in mechanically ventilated septic shock patients and investigated its association with the prognosis of these patients. We observed that the TAPSE/PASP ratio was an independent predictor of one-year all-cause mortality. We also noticed that the TAPSE/PASP ratio was associated with ICU mortality and MV duration in this cohort of patients.
Our study exclusively enrolled mechanically ventilated septic shock patients. This is different from prior studies, which also incorporated patients who were not in a shock state and those who were not on mechanical ventilation [4, 5, 20–22]. Although the ICU houses fewer than 10% of total hospital beds, ICU care accounts for one-third of total health care costs [23]. Furthermore, ICU survivors, particularly those with longer MV durations and ICU stays, often suffer from ICU-acquired weakness and physical dysfunction [24, 25]. Thus, studies regarding the MV duration, ICU length of stay and long-term survival of mechanically ventilated septic shock patients are necessary to improve the short-term and long-term prognoses and reduce the costs of these patients. General assessment scores of illness severity, such as SOFA and APACHE scores, may not be sensitive enough to reflect the prognosis of patients with different heart functions [4, 26]. Our results showed that, if APACHE II and SOFA scores as well as other echocardiographic variables were included in the same model, the TAPSE/PASP ratio was still of prognostic value in these patients.
The TAPSE/PASP ratio reflects the interaction between RV systolic function and its afterload. As a feasible and reproducible RV function parameter, TAPSE correlated well with RV ejection fraction [11]. TAPSE appears to be reproducible and has been proven to be a strong predictor of prognosis in heart failure and critically ill patients [26–28]. Interestingly, PASP was also found to be related to the prognosis of heart failure patients [9, 29]. In comparison with LV, RV was more sensitive to the afterload alteration [30]. Several factors might have contributed to the increase in PASP in septic shock patients. Apparently, positive pressure ventilation increases pulmonary vascular resistance [8]. Furthermore, ARDS is a common complication of severe sepsis and septic shock [7]. Even with lung protection ventilation, ARDS can challenge the RV with an incidence in acute cor pulmonale (ACP) as high as 25% [31, 32]. The frequency of LV dysfunction in septic patients can reach 40%, which might result in the increase in PASP via elevated left atrial pressure [2, 3].
Several prior studies reported the association of RV function and the prognosis of ICU patients [4, 5, 26, 33]. However, the prognostic value of the TAPSE/PASP ratio for sepsis and septic shock patients has not been reported. The present study showed that the correlation between TAPSE and PASP was rather low in septic shock patients. This is consistent with a prior study that found that TAPSE and maximal tricuspid regurgitation pressure gradient were not related in a group of heart failure patients [34]. Since both TAPSE and PASP can be affected in septic patients, the TAPSE/PASP ratio has the potential to result in a cumulative risk prediction. Therefore, the TAPSE/PASP ratio deserves more attention in the management of septic shock patients.
We found that the TAPSE/PASP ratio was lower in patients with low LVEF or MAPSE than in patients with high LVEF or MAPSE. Nevertheless, the TAPSE/PASP ratio did not discriminate the three LVEF and MAPSE tertiles as well as it did with the TAPSE tertile. This study also demonstrated that LV systolic function was not associated with prognosis among these patients, which is in line with previous studies [35, 36]. Given that septic cardiomyopathy was diagnosed by LV systolic function, this study indicates that RV function should be taken into consideration in the diagnosis and management of septic cardiomyopathy. Some researchers have reported that left ventricular-arterial uncoupling is common in septic shock patients and has been deemed as a parameter of left ventricular performance [37, 38]. Although few studies have been performed on the association of left ventricular-arterial coupling and the long-term prognosis of septic patients, we speculate that right ventricular-pulmonary arterial coupling may be more clinically relevant in these patients. Future studies are warranted to elucidate this speculation.
Limitations
This study has several limitations. First, this study was conducted at a single centre, and the sample size was limited. Second, although we incorporated mitral e’ velocity, the LV diastolic function was not fully evaluated. Third, given the nature of this one-time echocardiographic examination, we cannot rule out pre-existing RV dysfunction that could have affected the prognosis of these septic patients. Fourth, the exclusion of patients without TR measurements would cause selection bias. However, the PASP measurement from TR velocity was feasible in most cases. Prior studies have also reported the high obtainment rate of TR measurements [14, 29]. In addition, instead of estimating the CVP from the inferior vena cava diameter, we were able to measure the CVP from a central venous catheter, which would increase the accuracy of PASP estimation.
Conclusion
The TAPSE/PASP ratio demonstrated prognostic value for one-year all-cause mortality, ICU mortality and MV duration in mechanically ventilated septic shock patients.
Supplementary information
Additional file 1: Supplemental Table 1. Factors associated with ICU mortality. Supplemental Table 2. Significant independent relation of MV duration with other variables. Supplemental Table 3. Significant independent relation of ICU length of stay with other variables.
Acknowledgements
This study was performed at Critical Care Department of Peking Union Medical College Hospital. We would like to thank Prof. Chengli Shen, from Division of Surgical Oncology, the Ohio State University Wexner Medical Centre for his kind suggestions on the statistical issue.
Abbreviations
- ICU
intensive care unit
- MV
mechanical ventilation
- TAPSE
tricuspid annular plane systolic excursion
- TR
tricuspid regurgitation
- PASP
pulmonary arterial systolic pressure
- RV
right ventricle
- RVOT-FS
right ventricular outflow tract fractional shortening
- LVEF
left ventricular ejection fraction
- MAPSE
mitral annular plane systolic excursion
- APACHE
acute physiology and chronic health evaluation
- SOFA
sequential organ failure assessment
- HR
heart rate
- MAP
mean arterial pressure
- CVP
central venous pressure
- ICC
intraclass correlation coefficient
- PEEP
positive end-expiratory pressure
- Pplat
plateau pressure
- NE
norepinephrine
Authors’ contributions
H Zhang conceived and designed the study, obtained and interpreted data, performed the statistical analysis, and drafted the manuscript. H Lian analyzed data, performed the statistical analysis and revised the manuscript. Q Zhang obtained data and revised manuscript. X Chen revised the manuscript; X Wang revised the manuscript. D Liu designed the study and revised the manuscript. All authors read and approved the final manuscript.
Authors’ information
None.
Funding
Nil
Availability of data and materials
All datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was approved by the ethics committee of Peking Union Medical College Hospital, Beijing, China (Approval No. ZS-1422). Written informed consent was obtained from the next of kin of each patient.
Consent for publication
Informed consent was obtained from the next of kin.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hongmin Zhang, Email: ozohom@163.com.
Hui Lian, Email: lianhui@pumch.cn.
Qing Zhang, Email: zhqmt@163.com.
Xiukai Chen, Email: xic91@pitt.edu.
Xiaoting Wang, Email: syzzyx2015@sina.com.
Dawei Liu, Email: dwliu2015@sina.com.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12947-020-00198-y.
References
- 1.Liu V, Escobar GJ, Greene JD, Soule J, Whippy A, Angus DC, et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA. 2014;312:90–92. doi: 10.1001/jama.2014.5804. [DOI] [PubMed] [Google Scholar]
- 2.Pulido JN, Afessa B, Masaki M, Yuasa T, Gillespie S, Herasevich V, et al. Clinical spectrum, frequency, and significance of myocardial dysfunction in severe sepsis and septic shock. Mayo Clin Proc. 2012;87:620–628. doi: 10.1016/j.mayocp.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vieillard-Baron A. Septic cardiomyopathy. Ann Intensive Care. 2011;1:6. doi: 10.1186/2110-5820-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vallabhajosyula S, Kumar M, Pandompatam G, Sakhuja A, Kashyap R, Kashani K, et al. Prognostic impact of isolated right ventricular dysfunction in sepsis and septic shock: an 8-year historical cohort study. Ann Intensive Care. 2017;7:94. doi: 10.1186/s13613-017-0319-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winkelhorst JC, Bootsma IT, Koetsier PM, de Lange F, Boerma EC. Right ventricular function and long-term outcome in sepsis: a retrospective cohort study. Shock. 2019. [DOI] [PubMed]
- 6.Sanz J, Sánchez-Quintana D, Bossone E, Bogaard HJ, Naeije R. Anatomy, function, and dysfunction of the right ventricle: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:1463–1482. doi: 10.1016/j.jacc.2018.12.076. [DOI] [PubMed] [Google Scholar]
- 7.Mikkelsen ME, Shah CV, Meyer NJ, Gaieski DF, Lyon S, Miltiades AN, et al. The epidemiology of acute respiratory distress syndrome in patients presenting to the emergency department with severe sepsis. Shock. 2013;40:375–381. doi: 10.1097/SHK.0b013e3182a64682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bronicki RA, Anas NG. Cardiopulmonary interaction. Pediatr Crit Care Med. 2009;10:313–322. doi: 10.1097/PCC.0b013e31819887f0. [DOI] [PubMed] [Google Scholar]
- 9.Guazzi M, Labate V. Pulmonary hypertension in heart failure patients: pathophysiology and prognostic implications. Curr Heart Fail Rep. 2016;13:281–294. doi: 10.1007/s11897-016-0306-8. [DOI] [PubMed] [Google Scholar]
- 10.Guazzi M, Dixon D, Labate V, Beussink-Nelson L, Bandera F, Cuttica MJ, et al. RV contractile function and its coupling to pulmonary circulation in heart failure with preserved ejection fraction: stratification of clinical phenotypes and outcomes. JACC Cardiovasc Imaging. 2017;10:1211–1221. doi: 10.1016/j.jcmg.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 11.Kaul S, Tei C, Hopkins JM, Shah PM. Assessment of right ventricular function using two-dimensional echocardiography. Am Heart J. 1984;107:526–531. doi: 10.1016/0002-8703(84)90095-4. [DOI] [PubMed] [Google Scholar]
- 12.Hammarström E, Wranne B, Pinto FJ, Puryear J, Popp RL. Tricuspid annular motion. J Am Soc Echocardiogr. 1991;4:131–139. doi: 10.1016/S0894-7317(14)80524-5. [DOI] [PubMed] [Google Scholar]
- 13.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Tello K, Axmann J, Ghofrani HA, Naeije R, Narcin N, Rieth A, et al. Relevance of the TAPSE/PASP ratio in pulmonary arterial hypertension. Int J Cardiol. 2018;266:229–235. doi: 10.1016/j.ijcard.2018.01.053. [DOI] [PubMed] [Google Scholar]
- 15.Guo X, Lai J, Wang H, Tian Z, Wang Q, Zhao J, et al. Predictive value of non-invasive right ventricle to pulmonary circulation coupling in systemic lupus erythematosus patients with pulmonary arterial hypertension. Eur Heart J Cardiovasc Imaging. 2019. [DOI] [PubMed]
- 16.Guazzi M, Bandera F, Pelissero G, Castelvecchio S, Menicanti L, Ghio S, et al. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis. Am J Physiol Heart Circ Physiol. 2013;305:H1373–H1381. doi: 10.1152/ajpheart.00157.2013. [DOI] [PubMed] [Google Scholar]
- 17.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for Sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forfia PR, Fisher MR, Mathai SC, Housten-Harris T, Hemnes AR, Borlaug BA, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med. 2006;174:1034–1041. doi: 10.1164/rccm.200604-547OC. [DOI] [PubMed] [Google Scholar]
- 19.Lindqvist P, Henein M, Kazzam E. Right ventricular outflow-tract fractional shortening: an applicable measure of right ventricular systolic function. Eur J Echocardiogr. 2003;4:29–35. doi: 10.1053/euje.4.1.29. [DOI] [PubMed] [Google Scholar]
- 20.Rolando G, Espinoza ED, Avid E, Welsh S, Pozo JD, Vazquez AR, et al. Prognostic value of ventricular diastolic dysfunction in patients with severe sepsis and septic shock. Rev Bras Ter Intensiva. 2015;27:333–339. doi: 10.5935/0103-507X.20150057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orde SR, Pulido JN, Masaki M, Gillespie S, Spoon JN, Kane GC, et al. Outcome prediction in sepsis: speckle tracking echocardiography based assessment of myocardial function. Crit Care. 2014;18:R149. doi: 10.1186/cc13987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harmankaya A, Akilli H, Gul M, Akilli NB, Ergin M, Aribas A, et al. Assessment of right ventricular functions in patients with sepsis, severe sepsis and septic shock and its prognostic importance: a tissue Doppler study. J Crit Care. 2013;28:1111.e7–1111.e11. doi: 10.1016/j.jcrc.2013.07.059. [DOI] [PubMed] [Google Scholar]
- 23.Shorr AF. An update on cost-effectiveness analysis in critical care. Curr Opin Crit Care. 2002;8:337–343. doi: 10.1097/00075198-200208000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Desai SV, Law TJ, Needham DM. Long-term complications of critical care. Crit Care Med. 2011;39:371–379. doi: 10.1097/CCM.0b013e3181fd66e5. [DOI] [PubMed] [Google Scholar]
- 25.de Jonghe B, Lacherade JC, Sharshar T, Outin H. Intensive care unit-acquired weakness: risk factors and prevention. Crit Care Med. 2009;37:S309–S315. doi: 10.1097/CCM.0b013e3181b6e64c. [DOI] [PubMed] [Google Scholar]
- 26.Gajanana D, Seetha Rammohan H, Alli O, Romero-Corral A, Purushottam B, Ponamgi S, et al. Tricuspid annular plane systolic excursion and its association with mortality in critically ill patients. Echocardiography. 2015;32:1222–1227. doi: 10.1111/echo.12926. [DOI] [PubMed] [Google Scholar]
- 27.Smith JL, Bolson EL, Wong SP, Hubka M, Sheehan FH. Three-dimensional assessment of two-dimensional technique for evaluation of right ventricular function by tricuspid annulus motion. Int J Cardiovasc Imaging. 2003;19:189–197. doi: 10.1023/A:1023655705807. [DOI] [PubMed] [Google Scholar]
- 28.Ghio S, Recusani F, Klersy C, Sebastiani R, Laudisa ML, Campana C, et al. Prognostic usefulness of the tricuspid annular plane systolic excursion in patients with congestive heart failure secondary to idiopathic or ischemic dilated cardiomyopathy. Am J Cardiol. 2000;85:837–842. doi: 10.1016/S0002-9149(99)00877-2. [DOI] [PubMed] [Google Scholar]
- 29.Shalaby A, Voigt A, El-Saed A, Saba S. Usefulness of pulmonary artery pressure by echocardiography to predict outcome in patients receiving cardiac resynchronization therapy heart failure. Am J Cardiol. 2008;101:238–241. doi: 10.1016/j.amjcard.2007.07.064. [DOI] [PubMed] [Google Scholar]
- 30.Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right ventricular function in cardiovascular disease, part I: anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation. 2008;117:1436–1448. doi: 10.1161/CIRCULATIONAHA.107.653576. [DOI] [PubMed] [Google Scholar]
- 31.Repessé X, Charron C, Vieillard-Baron A. Right ventricular failure in acute lung injury and acute respiratory distress syndrome. Minerva Anestesiol. 2012;78:941–948. [PubMed] [Google Scholar]
- 32.Repessé X, Charron C, Vieillard-Baron A. Acute cor pulmonale in ARDS: rationale for protecting the right ventricle. Chest. 2015;147:259–265. doi: 10.1378/chest.14-0877. [DOI] [PubMed] [Google Scholar]
- 33.Wiersema R, Koeze J, Hiemstra B, Pettilä V, Perner A, Keus F, et al. Associations between tricuspid annular plane systolic excursion to reflect right ventricular function and acute kidney injury in critically ill patients: a SICS-I sub-study. Ann Intensive Care. 2019;9:38. doi: 10.1186/s13613-019-0513-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kjaergaard J, Akkan D, Iversen KK, Køber L, Torp-Pedersen C, Hassager C. Right ventricular dysfunction as an independent predictor of short- and long-term mortality in patients with heart failure. Eur J Heart Fail. 2007;9:610–616. doi: 10.1016/j.ejheart.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Li J, Ning B, Wang Y, Li B, Qian J, Ren H, et al. The prognostic value of left ventricular systolic function and cardiac biomarkers in pediatric severe sepsis. Medicine (Baltimore) 2019;98:e15070. doi: 10.1097/MD.0000000000015070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang SJ, Nalos M, McLean AS. Is early ventricular dysfunction or dilatation associated with lower mortality rate in adult severe sepsis and septic shock? A meta-analysis. Crit Care. 2013;17:R96. doi: 10.1186/cc12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guarracino F, Ferro B, Morelli A, Bertini P, Baldassarri R, Pinsky MR. Ventriculoarterial decoupling in human septic shock. Crit Care. 2014;18:R80. doi: 10.1186/cc13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guarracino F, Bertini P, Pinsky MR. Cardiovascular determinants of resuscitation from sepsis and septic shock. Crit Care. 2019;23:118. doi: 10.1186/s13054-019-2414-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplemental Table 1. Factors associated with ICU mortality. Supplemental Table 2. Significant independent relation of MV duration with other variables. Supplemental Table 3. Significant independent relation of ICU length of stay with other variables.
Data Availability Statement
All datasets used and analyzed during the current study are available from the corresponding author on reasonable request.